ABSTRACT
Central corneal thickness (CCT) can be a promising source of glaucoma monitoring and diagnosis. This study evaluated changes in CCT according to experimental adjustment of intraocular pressure (IOP) in canine eyes. To adjust and measure IOP, each eye was cannulated with two 26-gauge needles under inhalant anesthesia. One needle was connected to a pressure transducer, and the other was connected to an adjustable bag of physiologic saline. IOP was stepwise increased from 10 mmHg to 70 mmHg in 10 mmHg increments (Group T). IOP was maintained at 15 mmHg (Group C15), 30 mmHg (Group C30), 45 mmHg (Group C45), 60 mmHg (Group C60) and 75 mmHg (Group C75) during the experiment. CCT was measured with an ultrasonic pachymeter every 10 min after cannulation. There was a significant difference in the effect of time on CCT (P<0.001) and difference in CCT (dCCT; P<0.001) between groups. The CCT of group C15 remained constant during the experiment. However, group T showed an initial decrease and then an increase after passing the lowest point. Group C30 showed decreasing values for 30 min, after which the values remained constant. The values in Group C45 showed no changes for 40 min and then increased. The values in group C60 showed no change for 20 min and then increased. Group C75 showed a steady increase. In conclusion, the CCT showed two core changes according to increased IOP. This study provides essential basic data to enable further investigation into the association of IOP and CCT in dogs.
Keywords: canine, central corneal thickness, glaucoma, intraocular pressure, pachymeter
The canine cornea consists of 5 layers: The epithelium, basement membrane, stroma, Descemet’s membrane and endothelium [21]. The normal thickness of the canine cornea is approximately 550 microns in the central area, but the peripheral cornea is slightly thicker [7]. Both the epithelium and endothelium control corneal hydration, but the endothelium plays a more important role in this task [20]. The pump-leak hypothesis is considered the basic model of corneal hydration control. The cornea consists of two sets of receptors. One senses hydration, and the other modulates the activity of the endothelial pump. In addition, a linking system is needed to connect these two receptors [5].
Because the corneal endothelium is in direct contact with the aqueous humor, corneal thickness can be altered by conditions affecting the aqueous humor. Inflammation of the aqueous humor inhibits the function of the endothelial pump, leading to corneal edema [14]. Intraocular pressure (IOP) is formed by the production and drainage of aqueous humor, and changes in IOP can also affect corneal thickness [24, 25]. Two possible mechanisms of change in central corneal thickness (CCT) according to IOP can be considered. One is associated with the pump function of the corneal endothelium, which can be impaired when the IOP reaches the critical pressure. The other mechanism is the direct affect of elevated IOP on the mechanical properties of the cornea [24].
The ultrasonic pachymeter has disadvantages, such as false results due to indentation during measurement, the risk of infection by direct contact and inaccuracy due to the perpendicularity of the probe placement [11, 22]. However, the ultrasonic pachymeter is the gold standard for CCT measurement, because it has a noninvasive, simple method and high degree of interobserver reproducibility in humans [17]. Since the introduction of the ultrasonic pachymeter, CCT has been investigated in human glaucoma patients. Although this issue remains controversial, patients with a thin CCT have shown a high risk for glaucoma [8].
The current study was designed to investigate CCT acute response to the experimental adjustment of IOP in dogs. In addition, the possibility of CCT as a factor for diagnosis and monitoring of canine glaucoma was investigated.
MATERIALS AND METHODS
Animals studies and preparations: All animal procedures were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of Seoul National University (SNU-111115-4). Both eyes of 25 clinically normal laboratory beagle dogs were used in this study. Complete ophthalmic examinations were performed before the experiment using a rebound tonometer (TonoVet, Tiolat, Helsinki, Finland), Schirmer Tear Test (Schirmer Tear Test, Schering-Plough Animal Health, Kenilworth, NJ, U.S.A.), slit-lamp biomicroscope (Topcon SL-D7, Topcon Corp., Tokyo, Japan) and indirect ophthalmoscope (Vantage, Keeler Instruments Inc., Broomall, PA, U.S.A.) with a 30-diopter indirect lens (Classic BIO Lens, Volk Optical Inc., Mentor, OH, U.S.A.). To investigate changes in CCT according to stepwise increases in IOP compared with a fixed normal IOP, 30 eyes of 15 dogs were used. The remaining eyes were used to investigate changes in CCT according to a fixed increase in IOP (30, 45, 60 and 75 mmHg).
Before induction of anesthesia, atropine eye drops (Ocutropine, Samil, Gyeonggi, South Korea), a combination of phenylephrine and tropicamide eye drops (Mydrin-P, Saten Pharmaceutical, Osaka, Japan), a combination of dexamethasone, polymyxin B and neomycin eye drops (Maxitrol, S.A. Alcon-Couvreur N.V., Puurs, Belgium) and flurbiprofen eye drops (OcufenTM, Allergan Sales LLC, Waco, TX, U.S.A.) were all applied twice to achieve mydriasis and reduce inflammation induced by anterior chamber paracentesis. Acepromazine 0.03 mg/kg (Sedaject, Samwoo Medical, Chungnam, South Korea), cefazoline 30 mg/kg (CKD Cefazolin Inj., Chong Kun Dang, Gyeonggi, South Korea) and dexamethasone 0.3 mg/kg (Je Il Dexamethasone Inj., Je Il Pharmaceutical, Daegu, South Korea) were administered intravenously 5 min before anesthesia induction using propofol (ProviveTM 1%, Claris Lifesciences, Ahmedabad, India). Anesthesia was maintained with isoflurane (Ifran, Hana Pharm. Co., Ltd., Gyeonggi, South Korea) and oxygen. During anesthesia, ECG, pulse oxymetry, noninvasive blood pressure, end tidal CO2, capnography and temperature (with a digital thermometer) were monitored.
After induction of anesthesia, the dog was laid in a dorsoventral position during the experiment. To induce extraocular muscle akinesia and prevent pain, retrobulbar injection of 2 ml of 2% lidocaine (Je Il lidocaine injection (2%), Je Il Pharmaceutical) was performed with a 23-gauge retrobulbar needle in each eye using the inferior-temporal palpebral technique [1]. In cases with inadequate extraocular muscle akinesia, a second injection of 1 ml of 2% lidocaine was performed 10 min after the first injection. An eye speculum was placed to expose the cornea after induction of extraocular muscle akinesia. The exposed cornea was frequently lubricated with 0.5% sodium carboxymethyl cellulose (Refresh PlusTM, Allergan Sales LLC) to prevent corneal desiccation during the experiment.
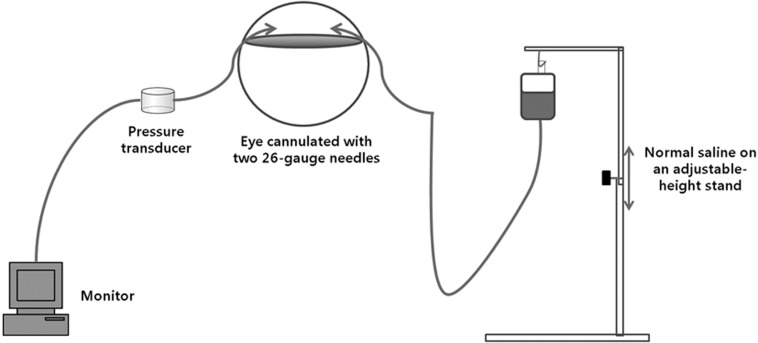
The anterior chamber was entered with two 26-gauge needles through the limbus (2 and 10 o’clock positions) for each eye. Cyanoacrylate glue (3M VetbondTM, 3M Animal Care Products, St. Paul, MN, U.S.A.) was used to prevent leakage from the entry site after needle insertion. One needle was connected to a normal saline reservoir containing 5 IU per milliliter of heparin through a polypropylene line. The IOP was adjusted to different levels by changing the reservoir. The other needle was connected to a pressure transducer system for IOP measurement through a polypropylene line and contained normal saline with 5 IU per milliliter of heparin. The system comprised a pressure monitoring kit (Transpac IV Monitoring Kit, ICU Medical, Inc., San Clemente, CA, U.S.A.) with a transducer, polyethylene tubes, monitoring cable (Transpac Reusable Cable, Hospira, Inc., Lake Forest, IL, U.S.A.) and a monitor (Datex-Ohmeda S/5, Helsinki, Finland) for continuous monitoring of IOP (Fig. 1). The transducer was positioned at eye level. Before each experiment, the transducer was calibrated to a mercury manometer, and a zero balance was set using the manufacturer’s instructions.
Fig. 1.
Schematic of the experiment after cannulation in an eye.
Procedures for stepwise increase of IOP and fixed normal IOP: Thirty eyes of 15 dogs were randomly assigned to groups T and C15. In group T, the IOP of 1 eye per dog was adjusted to 10 mmHg immediately after cannulation and was then raised in increments of 10 mmHg until a maximum IOP of 70 mmHg. CCT was measured with an ultrasonic pachymeter (Pachmate DGH 55, DGH Technology, Exton, PA, U.S.A.) 3 times at each IOP after each IOP maintenance for 10 min. The IOP in the other eye was adjusted to 15 mmHg (group C15) immediately after cannulation, which was maintained throughout the experiment. An ultrasonic pachymeter was also applied every 10 min in the same manner as for group T.
Procedures for fixed increase of IOP: Twenty eyes of 10 dogs were randomly assigned to groups C30, C45, C60 and C75. Each group consisted of 5 eyes from different dogs. The IOP was respectively adjusted to 30, 45, 60 and 75 mmHg in these groups immediately after cannulation and was maintained throughout the experiment. An ultrasonic pachymeter was also applied every 10 min in the same manner as for groups T and C15.
Post-experimental care: Triamcinolone 4 mg/kg (Rheudenolone Inj., Kukje Pharma. Ind., Gyeonggi, South Korea) and gentamicin 8 mg/kg (Kukje Gentamicin Inj., Kukje Pharma. Ind.) were injected into the subconjunctiva to reduce inflammation and infection after the experiment. A combination eye drop comprising dexamethasone, polymyxin B and neomycin was then applied 2 times per day for 1 week.
Statistical analysis: Statistical analysis was performed using PASW Statistics 18 for Windows (SPSS Inc., Chicago, IL, U.S.A.). CCT data were expressed as the mean ± SE (standard error), and the level of significance used was P<0.05. In one-way repeated measures ANOVA following pairwise comparison, Bonferroni adjustment was performed to evaluate the variation in CCT values against time in the same group and to evaluate variation in the difference in CCT (dCCT) from baseline (10 min after cannulation) against time, which was compared with dCCT at the same time point between groups.
RESULTS
Tables 1 and 2 show the results of one-way repeated measures ANOVA following pairwise comparison with Bonferroni adjustment of the CCT and dCCT, respectively. There was a significant difference in the effect of time on CCT (P<0.001) and dCCT (P<0.001) between groups. There was no significant difference in the effect of time on CCT in group C15 (P=0.729). The CCT in group C15 remained constant over the course of 70 min. However, there was a significant difference in the effect of time on CCT in group T (P<0.001). The CCT in group T initially decreased until 40 min and then began to increase. The CCT in group T was unchanged at 20 min compared with at 10 min. However, the CCTs at 30 (P=0.006) and 40 min (P=0.028) were significantly thinner than that at 10 min in group T. Furthermore, the CCTs at 50, 60 and 70 min were significantly thicker than those at 30 and 40 min in group T. There was a significant difference in the effect of time on CCT in group C30 (P=0.032). The CCT in group C30 decreased until 30 min and was then maintained. The CCT in group C30 was unchanged at 20 min compared with at 10 min. However, the CCTs at 30 (P=0.003) and 40 min (P=0.045) were significantly thinner than that at 10 min in group C30. There was a significant difference in the effect of time on CCT in group C45 (P<0.001). The CCT in group C45 was unchanged at 40 min compared with at 10 min and then showed a significant increase. The CCTs in group C45 at 20, 30 and 40 min were significantly thinner than those at 50, 60 and 70 min. There was a significant difference in the effect of time on CCT in group C60 (P<0.001) and group C75 (P<0.001). The CCT in group C60 was unchanged at 20 min compared with at 10 min and then showed a significant increase, except at 50 min to 60 min. The CCT in group C75 showed a steady significant increase after cannulation, except at 30 min to 40 min.
Table 1. Central corneal thickness (CCT; microns) changes according to experimental adjustment of IOP.
| The time after cannulation (min) |
IOP of T group (mmHg) |
T | C15* | C30* | C45* | C60* | C75* |
|---|---|---|---|---|---|---|---|
| 10 | 10 | 519.8 ± 15.0 | 513.9 ± 15.0 | 508.1 ± 23.3 | 487.7 ± 23.3 | 513.3 ± 23.3 | 480.2 ± 23.3 |
| 20 | 20 | 516.3 ± 15.1 | 513.6 ± 15.1 | 500.4 ± 23.1 | 479.5 ± 23.1 | 519.4 ± 23.1 | 490.8 ± 23.1a) |
| 30 | 30 | 512.2 ± 14.9a) | 514.2 ± 14.9 | 494.2 ± 22.9a) | 482.5 ± 22.9 | 531.5 ± 22.9a),b) | 504.7 ± 22.9a),b) |
| 40 | 40 | 511.7 ± 15.1a) | 513.9 ± 15.1 | 494.7 ± 23.0a) | 486.8 ± 23.0 | 544.9 ± 23.0a),b),c) | 512.2 ± 23.0a),b) |
| 50 | 50 | 519.1 ± 15.6c),d) | 513.6 ± 15.6 | 494.3 ± 23.4 | 498.5 ± 23.4b),c),d) | 557.3 ± 23.4a),b),c),d) | 524.1 ± 23.4a),b),c),d) |
| 60 | 60 | 529.8 ± 15.4b),c),d),e) | 512.3 ± 15.4 | 494.3 ± 23.4 | 504.1 ± 23.4b),c),d) | 561.9 ± 23.4a),b),c),d) | 532.7 ± 23.4a),b),c),d),e) |
| 70 | 70 | 535.3 ± 15.2a),b),c),d),e) | 515.3 ± 15.2 | 495.3 ± 23.4 | 513.3 ± 23.4a),b),c),d),e) | 581.1 ± 23.4a),b),c),d),e),f) | 545.5 ± 23.4a),b),c),d),e),f) |
Data for CCT are expressed as the mean ± SE (standard error). a) Values in the same column with this superscript are significantly different (P<0.05) compared with the CCT at 10 min. b) Values in the same column with this superscript are significantly different (P<0.05) compared with the CCT at 20 min from 30 min onward. c) Values in the same column with this superscript are significantly different (P<0.05) compared with the CCT at 30 min from 40 min onward. d) Values in the same column with this superscript are significantly different (P<0.05) compared with the CCT at 40 min from 50 min onward. e) Values in the same column with this superscript are significantly different (P<0.05) compared with the CCT at 50 min from 60 min onward. f) Values in the same column with this superscript are significantly different (P<0.05) in CCT at 70 min compared with that at 60 min. *The IOPs of groups C15, C30, C45, C60 and C 75 were maintained at 15, 30, 45, 60 and 75 mmHg, respectively, during the experiment.
There was a statistically significant difference in dCCT between groups C15 and C70 at 20 min (P=0.040). The dCCTs in groups C60 (P=0.031) and C75 (P=0.001) were significantly thicker than that in group C30 at 20 min. The dCCTs in groups C60 and C75 were also significantly thicker than those in groups T, C15, C30 and C45 at 30, 40, 50, 60 and 70 min. There was a statistically significant difference in dCCT between groups C15 and C30 at 30 min (P=0.010). There were also statistically significant differences in dCCT between groups T and C30 at 60 (P=0.012) and 70 min (P=0.016). There were statistically significant differences in dCCT between groups C30 and C45 at 50 (P=0.024), 60 (P=0.008) and 70 min (P=0.005; Table 2).
Table 2. Difference in CCT (dCCT; microns) from baseline value according to experimental adjustment of IOP.
| The time after cannulation (min) |
IOP of T group (mmHg) |
T | C15* | C30* | C45* | C60* | C75* |
|---|---|---|---|---|---|---|---|
| 10(baseline) | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| 20 | 20 | –3.5 ± 1.7f) | –0.3 ± 1.7f) | –7.7 ± 3.0e),f) | –8.2 ± 3.0e),f) | 6.1 ± 3.0c),d) | 10.6 ± 3.0a),b),c),d) |
| 30 | 30 | –7.6 ± 1.9e),f) | 0.3 ± 1.9c),e),f) | –13.9 ± 3.4b),e),f) | –5.1 ± 3.4e),f) | 18.1 ± 3.4a),b),c),d) | 24.4 ± 3.4a),b),c),d) |
| 40 | 40 | –8.1 ± 2.4e),f) | 0.0 ± 2.4e),f) | –13.4 ± 4.1e),f) | –0.9 ± 4.1e),f) | 31.5 ± 4.1a),b),c),d) | 32.0 ± 4.1a),b),c),d) |
| 50 | 50 | –0.7 ± 3.0e),f) | –0.3 ± 3.0e),f) | –13.8 ± 5.1d),e),f) | 10.8 ± 5.2c),e),f) | 44.0 ± 5.2a),b),c),d) | 43.8 ± 5.2a),b),c),d) |
| 60 | 60 | 10.0 ± 3.3c),e),f) | –1.5 ± 3.3e),f) | –13.8 ± 5.7a),d),e),f) | 16.4 ± 5.7c),e),f) | 48.6 ± 5.7a),b),c),d) | 52.5 ± 5.7a),b),c),d) |
| 70 | 70 | 15.5 ± 4.0e),f) | 1.4 ± 4.0e),f) | –12.9 ± 7.0a),d),e),f) | 25.6 ± 7.0c),e),f) | 67.7 ± 7.0a),b),c),d) | 65.2 ± 7.0a),b),c),d) |
Data for dCCT are expressed as the mean ± SE (standard error). a) Values in the same row with this superscript are significantly different (P<0.05) compared with the group T. b) Values in the same row with this superscript are significantly different (P<0.05) compared with the group C15. c) Values in the same row with this superscript are significantly different (P<0.05) compared with the group C30. d) Values in the same row with this superscript are significantly different (P<0.05) compared with the group C45. e) Values in the same row with this superscript are significantly different (P<0.05) compared with the group C60. f) Values in the same row with this superscript are significantly different (P<0.05) compared with the group C75. *The IOPs of groups C15, C30, C45, C60 and C75 were maintained at 15, 30, 45, 60 and 75 mmHg, respectively, during the experiment.
DISCUSSION
Although IOP can be a factor in CCT variation, there are few experimental studies that have investigated this relationship in either animals or humans [12, 24]. The current study showed that CCT varied according to different experimental adjustments of IOP. The CCT showed two core changes according to increased IOP. CCT initially decreased in response to increased IOP in groups T and C30. Also, a significant decrease in dCCT in group C30 compared with C15 was identified at 30 min. However, in groups T, C45, C60 and C75, it appears that the CCT ultimately increased after passing the critical pressure. Comparing groups C45, C60 and C75, there is evidence to say the duration and degree of increase in IOP was a factor contributing to the increase in CCT.
In previous human studies, there was no obvious change in CCT when the IOP was increased by approximately 10 mmHg, although the duration of the increase in IOP was only 5 min [12]. A similar result was found in the current study with no statistical difference in CCT values between 10 and 20 mmHg in group T. However, a significant difference in CCT was shown in this group when the IOP was elevated above 30 mmHg, which is adjacent to the upper limit of the physiologically normal IOP [6].
Olsen [18] suggested that IOP showed a dual effect on corneal thickness according to corneal endothelium conditions in human patients with acute glaucoma. First, CCT decreases in response to an increase in IOP in the intact corneal endothelium, and second, it increases in the acutely damaged corneal endothelium. Ehlers et al. documented that CCT in eyes with a low IOP was thicker than that in contralateral normal eyes in human patients with retinal detachment [4]. The results of this study also indicated a similar conclusion. Initially, CCT gradually decreased with an increase in IOP until 40 min (40 mmHg) in group T. The CCTs at 30 and 40 min were significantly different from that at 10 min in group T. However, CCT was increased from 50 min (50 mmHg) to 70 min (70 mmHg) in group T. The CCTs at 30 and 40 min were significantly different from those at 50, 60 and 70 min in group T.
The initial decrease in CCT according to elevated IOP is associated with microsturctural changes in the corneal anterior stroma. The anterior stroma is more resistant to corneal hydration, and the transverse collagen lamellae of the anterior stroma are short [3, 23]. An initial decrease in CCT can be caused by immediate loss of anterior stromal interlamellar gaps with increasing IOP in rabbits [23].
Abnormal thickening of the cornea presents as corneal edema, which can appear when the IOP is above 40 mmHg in human glaucoma patients [25]. In this study, CCT increased after IOP passed the lowest point of 40 mmHg (40 min) in group T, and significant differences in CCT were also observed between CCTs in group C45 at 20 min and 50 min. Therefore, it can be proposed that corneal endothelial decompensation was initiated at an IOP of 40 to 45 mmHg in normal canine eyes. The duration and degree of increased IOP should also be considered when assessing the initiation of thickening of the CCT. In group C45, there was a significant increase in CCT beginning at 50 min. Also, there were significant increases in CCT beginning at 30 min and 20 min in groups C60 and C75, respectively. Furthermore, there were significant increases in dCCT compared with group C15 beginning at 30 min and 20 min in groups C60 and C75, respectively. Therefore, the initiation of thickening of the CCT was influenced by the duration and degree of IOP increase. A high degree of IOP may cause thickening of the CCT to occur rapidly.
In humans, a thin CCT was considered a risk factor for glaucoma in the Ocular Hypertension Treatment Study [8]. This relationship might be partially explained when considering the effect of CCT on IOP measurement [15]. Although the effect of CCT was different with each type of tonometer used, the measured IOP can be lower than the real IOP in both humans and dogs with a thin CCT [2, 10, 19]. Therefore, glaucoma patients with a thin CCT can be measured as having a normal IOP when using tonometry. CCT is also correlated with the development of glaucomatous visual field loss in humans [9, 13, 16]. Although this study was performed over a short duration (70 min), the results support that thin CCT can be a promising source for diagnosis and monitoring of glaucoma in dogs. Thinning of the cornea was not correlated with tonometer readings in this study, because manometric values were used. Although the reductions in CCT were quite small, there were significant differences in CCT between 10 min and 30 min in groups T and C30. CCT itself changed with an increased IOP, exhibiting an initial decrease and then a subsequent increase.
In this study, changes in CCT according to experimental adjustment of IOP were documented, and the possibility of using CCT as a diagnostic and monitoring factor for canine glaucoma was suggested. This study provides basic data to enable further investigation of the relationship between CCT and IOP in dogs.
ACKNOWLEDGMENTS
This study was supported by the College of Veterinary Medicine, the Research Institute for Veterinary Science and the BK21 Program for Veterinary Science of Seoul National University, Korea.
REFERENCES
- 1.Accola P. J., Bentley E., Smith L. J., Forrest L. J., Baumel C. A., Murphy C. J. 2006. Development of a retrobulbar injection technique for ocular surgery and analgesia in dogs. J. Am. Vet. Med. Assoc. 229: 220–225. doi: 10.2460/javma.229.2.220 [DOI] [PubMed] [Google Scholar]
- 2.Bhan A., Browning A. C., Shah S., Hamilton R., Dave D., Dua H. S. 2002. Effect of corneal thickness on intraocular pressure measurements with the pneumotonometer, Goldmann applanation tonometer, and Tono-Pen. Invest. Ophthalmol. Vis. Sci. 43: 1389–1392 [PubMed] [Google Scholar]
- 3.Bron A. J. 2001. The architecture of the corneal stroma. Br. J. Ophthalmol. 85: 379–381. doi: 10.1136/bjo.85.4.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehlers N., Riise D. 1967. On corneal thickness and intraocular pressure: a clinical study on thickness of cornea in eyes with retinal detachment. Acta Ophthalmol. 45: 809–813 [DOI] [PubMed] [Google Scholar]
- 5.Fischbarg J., Maurice D. M. 2004. An update on corneal hydration control. Exp. Eye. Res. 78: 537–541. doi: 10.1016/j.exer.2003.09.010 [DOI] [PubMed] [Google Scholar]
- 6.Gelatt K. N., MacKay E. O. 1998. Distribution of intraocular pressure in dogs. Vet. Ophthalmol. 1: 109–114. doi: 10.1046/j.1463-5224.1998.00024.x [DOI] [PubMed] [Google Scholar]
- 7.Gilger B. C., Whitley D., Mclaughlin S. A., Wright J. C., Drane J. W. 1991. Canine corneal thickness measured by ultrasonic pachymetry. Am. J. Vet. Res. 52: 1570–1572 [PubMed] [Google Scholar]
- 8.Gordon M. O., Beiser J. A., Brandt J. D., Heuer D. K., Higginbotham E. J., Johnson C. A., Keltner J. L., Miller J. P., Parrish R. K., Wilson M. R., Kass M. A. 2002. The Ocular Hypertension treatment study: baseline factors that predict the onset of primary open-angle glaucoma. Arch. Ophthalmol. 120: 714–720. doi: 10.1001/archopht.120.6.714 [DOI] [PubMed] [Google Scholar]
- 9.Gunvant P., Porsia L., Watkins R. J., Bayliss-Brown H., Broadway D. C. 2008. Relationships between central corneal thickness and optic disc topography in eyes with glaucoma, suspicion of glaucoma, or ocular hypertension. Clin. Ophthalmol. 2: 591–599. doi: 10.2147/OPTH.S2814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iliev M. E., Goldblum D., Katsoulis K., Amstutz C., Frueh B. 2006. Comparison of rebound tonometry with Goldmann applanation tonometry and correlation with central corneal thickness. Br. J. Ophthalmol. 90: 833–835. doi: 10.1136/bjo.2005.089870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawana K., Tokunaga T., Miyata K., Okamoto F., Kiuchi T., Oshika T. 2004. Comparison of corneal thickness measurements using Orbscan II, non-contact specular microscopy, and ultrasonic pachymetry in eyes after laser in situ keratomileusis. Br. J. Ophthalmol. 88: 466–468. doi: 10.1136/bjo.2003.030361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lam A. K., Douthwaite A. 1997. The effect of an artificially-elevated intraocular pressure on corneal thickness in Chinese eye. Ophthalmic. Physiol. Opt. 17: 414–420. doi: 10.1111/j.1475-1313.1997.tb00074.x [DOI] [PubMed] [Google Scholar]
- 13.Lin W., Aoyama Y., Kawase K., Yamamoto T. 2009. Relationship between central corneal thickness and visual field defect in open-angle glaucoma. Jpn. J. Ophthalmol. 53: 477–481. doi: 10.1007/s10384-009-0702-7 [DOI] [PubMed] [Google Scholar]
- 14.Macdonald J. M., Geroski D. H., Edelhauser H. F. 1987. Effect of inflammation on the corneal endothelial pump and barrier. Curr. Eye Res. 6: 1125–1132. doi: 10.3109/02713688709034885 [DOI] [PubMed] [Google Scholar]
- 15.Manni G., Oddone F., Parisi V., Tosto A., Centofanti M. 2008. Intraocular pressure and central corneal thickness. Prog. Brain Res. 173: 25–30. doi: 10.1016/S0079-6123(08)01103-5 [DOI] [PubMed] [Google Scholar]
- 16.Medeiros F. A., Sample P. A., Zangwill L. M., Bowd C., Aihara M., Weinreb R. N. 2003. Corneal thickness as a risk factor for visual field loss in patients with preperimetric glaucomatous optic neuropathy. Am. J. Ophthalmol. 136: 805–813. doi: 10.1016/S0002-9394(03)00484-7 [DOI] [PubMed] [Google Scholar]
- 17.Miglior S., Albe E., Guareschi M., Mandelli G., Gomarasca S., Orzalesi N. 2004. Intraobserver and interobserver reproducibility in the evaluation of ultrasonic pachymetry measurements of central corneal thickness. Br. J. Ophthalmol. 88: 174–177. doi: 10.1136/bjo.2003.023416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olsen T. 1980. The endothelial cell damage in acute glaucoma. On the corneal thickness response to intraocular-pressure. Acta ophthalmologica. 58: 257–266. doi: 10.1111/j.1755-3768.1980.tb05719.x [DOI] [PubMed] [Google Scholar]
- 19.Park Y. W., Jeong M. B., Kim T. H., Ahn J. S., Ahn J. T., Park S. A., Kim S. E., Seo K. 2011. Effect of central corneal thickness on intraocular pressure with the rebound tonometer and the applanation tonometer in normal dogs. Vet. Ophthalmol. 14: 169–173. doi: 10.1111/j.1463-5224.2010.00859.x [DOI] [PubMed] [Google Scholar]
- 20.Riley M. V. 1971. The role of the epithelium in control of corneal hydration. Exp. Eye. Res. 12: 128–137. doi: 10.1016/0014-4835(71)90137-0 [DOI] [PubMed] [Google Scholar]
- 21.Samuelson D. A. 2007. Ophthalmic anatomy. pp. 49–60. In: Veterinary Ophthalmology. 4th ed. (Gelatt, K., N. ed.), Blackwell Publishing, Oxford. [Google Scholar]
- 22.Solomon O. D. 1999. Corneal indentation during ultrasonic pachymetry. Cornea 18: 214–215. doi: 10.1097/00003226-199903000-00012 [DOI] [PubMed] [Google Scholar]
- 23.Wu Q., Yeh A. T. 2008. Rabbit cornea microstructure response to changes in intraocular pressure visualized by using nonlinear optical microscopy. Cornea 27: 202–208. doi: 10.1097/ICO.0b013e318159221e [DOI] [PubMed] [Google Scholar]
- 24.Ytteborg J., Dohlman C. 1965. Corneal edema and intraocular pressure. I. Animal experiments. Arch. Ophthalmol. 74: 375–381. doi: 10.1001/archopht.1965.00970040377018 [DOI] [PubMed] [Google Scholar]
- 25.Ytteborg J., Dohlman C. 1965. Corneal edema and intraocular pressure. II. Clinical results. Arch. Ophthalmol. 74: 477–484. doi: 10.1001/archopht.1965.00970040479008 [DOI] [PubMed] [Google Scholar]