Abstract
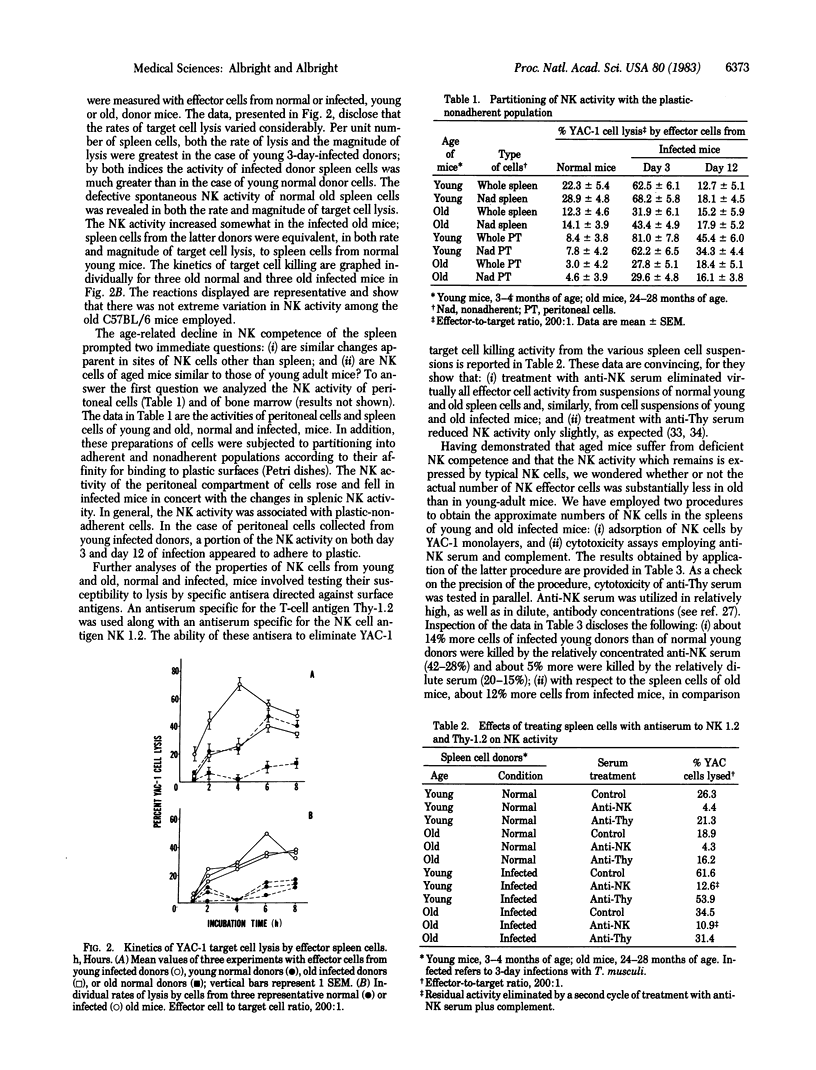
Natural cell-mediated cytotoxicity may be critical in the resistance displayed by animals and humans to tumors and various pathogenic microorganisms. Because the frequency of tumors and infections increases markedly in aging populations, we have compared the natural killer (NK) competence of lymphoid tissues (spleen, bone marrow) and of peritoneal cells of young adult and aged mice. Spontaneous NK activity was much lower, and the rate of target cell lysis was much less, in aged mice. The level of NK activity was only modestly increased in old, compared to young, mice when they were exposed to Trypanosoma musculi, an organism that provides strong stimulation of NK activity. Restricted NK activity of aged mice was not attributable to suppressor cells. The NK effector cells in old mice were characterized as being nonadherent to plastic, completely susceptible to lysis by complement plus an antiserum against specificity NK 1.2, and only slightly affected by treatment with antiserum against specificity Thy-1.2. Two indirect methods were employed to assess the relative frequency of splenic NK cells at the time of maximal stimulation by T. musculi: a cytotoxicity assay with antiserum against NK 1.2 and a binding assay involving monolayers of YAC-1 tumor target cells. Similar results were obtained in both assays, indicating that at maximal stimulation about 10% of the total spleen cells of both young and old mice were NK cells. We conclude (cautiously) that the functional efficiency of aged NK cells is impaired and that this defect may account, in part, for reduced ability of aged individuals to resist certain types of cancer and certain pathogenic microorganisms.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright J. F., Deitchman J. W., Hassell S. A., Ozato K. Differential antibody production of adherent and nonadherent spleen cells transferred to irradiated and cyclophosphamide-treated recipient mice. J Reticuloendothel Soc. 1975 Apr;17(4):195–209. [PubMed] [Google Scholar]
- Albright J. W., Albright J. F. Differences in resistance to Trypanosoma musculi infection among strains of inbred mice. Infect Immun. 1981 Aug;33(2):364–371. doi: 10.1128/iai.33.2.364-371.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright J. W., Albright J. F., Dusanic D. G. Mechanisms of trypanosome-mediated suppression of humoral immunity in mice. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3923–3927. doi: 10.1073/pnas.75.8.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright J. W., Albright J. F. The decline of immunological resistance of aging mice to Trypanosoma musculi. Mech Ageing Dev. 1982 Dec;20(4):315–330. doi: 10.1016/0047-6374(82)90099-9. [DOI] [PubMed] [Google Scholar]
- Albright J. W., Albright J. F. Trypanosome-mediated suppression of murine humoral immunity independent of typical suppressor cells. J Immunol. 1980 May;124(5):2481–2484. [PubMed] [Google Scholar]
- Albright J. W., Huang K. Y., Albright J. F. Natural killer activity in mice infected with Trypanosoma musculi. Infect Immun. 1983 Jun;40(3):869–875. doi: 10.1128/iai.40.3.869-875.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright J. W., Makinodan T. Decline in the growth potential of spleen-colonizing bone marrow stem cells of long-lived aging mice. J Exp Med. 1976 Nov 2;144(5):1204–1213. doi: 10.1084/jem.144.5.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton R. C., Winn H. J. Studies on natural killer (NK) cells. I. NK cell specific antibodies in CE anti-CBA serum. J Immunol. 1981 May;126(5):1985–1989. [PubMed] [Google Scholar]
- Callard R. E., Basten A. Immune function in aged mice. I. T-cell responsiveness using phytohaemagglutinin as a functional probe. Cell Immunol. 1977 Jun 1;31(1):13–25. doi: 10.1016/0008-8749(77)90002-8. [DOI] [PubMed] [Google Scholar]
- Callard R. E., Basten A., Waters L. K. Immune function in aged mice. II. B-cell function. Cell Immunol. 1977 Jun 1;31(1):26–36. doi: 10.1016/0008-8749(77)90003-x. [DOI] [PubMed] [Google Scholar]
- Chino F., Makinodan T., Lever W. E., Peterson W. J. The immune systems of mice reared in clean and in dirty conventional laboratory farms. I. Life expectancy and pathology of mice with long life-spans. J Gerontol. 1971 Oct;26(4):497–507. doi: 10.1093/geronj/26.4.497. [DOI] [PubMed] [Google Scholar]
- Djeu J. Y., Heinbaugh J. A., Holden H. T., Herberman R. B. Augmentation of mouse natural killer cell activity by interferon and interferon inducers. J Immunol. 1979 Jan;122(1):175–181. [PubMed] [Google Scholar]
- Eugui E. M., Allison A. C. Differences in susceptibility of various mouse strains to haemoprotozoan infections: possible correlation with natural killer activity. Parasite Immunol. 1980 Winter;2(4):277–292. doi: 10.1111/j.1365-3024.1980.tb00059.x. [DOI] [PubMed] [Google Scholar]
- Friedman D., Globerson A. Immune reactivity during aging. II. Analysis of the cellular mechanisms involved in the deficient antibody response in old mice. Mech Ageing Dev. 1978 Apr;7(4):299–307. doi: 10.1016/0047-6374(78)90073-8. [DOI] [PubMed] [Google Scholar]
- Grimm E., Bonavida B. Mechanism of cell-mediated cytotoxicity at the single cell level. I. Estimation of cytotoxic T lymphocyte frequency and relative lytic efficiency. J Immunol. 1979 Dec;123(6):2861–2869. [PubMed] [Google Scholar]
- Haller O., Hansson M., Kiessling R., Wigzell H. Role of non-conventional natural killer cells in resistance against syngeneic tumour cells in vivo. Nature. 1977 Dec 15;270(5638):609–611. doi: 10.1038/270609a0. [DOI] [PubMed] [Google Scholar]
- Hanna N., Burton R. C. Definitive evidence that natural killer (NK) cells inhibit experimental tumor metastases in vivo. J Immunol. 1981 Nov;127(5):1754–1758. [PubMed] [Google Scholar]
- Hatcher F. M., Kuhn R. E., Cerrone M. C., Burton R. C. Increased natural killer cell activity in experimental American trypanosomiasis. J Immunol. 1981 Sep;127(3):1126–1130. [PubMed] [Google Scholar]
- Hatcher F. M., Kuhn R. E. Destruction of Trypanosoma cruzi by Natural killer cells. Science. 1982 Oct 15;218(4569):295–296. doi: 10.1126/science.6812218. [DOI] [PubMed] [Google Scholar]
- Hauser W. E., Jr, Sharma S. D., Remington J. S. Natural killer cells induced by acute and chronic toxoplasma infection. Cell Immunol. 1982 May 15;69(2):330–346. doi: 10.1016/0008-8749(82)90076-4. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Holden H. T. Natural cell-mediated immunity. Adv Cancer Res. 1978;27:305–377. doi: 10.1016/s0065-230x(08)60936-7. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Holden H. T. Low density of Thy 1 antigen on mouse effector cells mediating natural cytotoxicity against tumor cells. J Immunol. 1978 Jul;121(1):304–309. [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Lavrin D. H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 1975 Aug 15;16(2):216–229. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- Itoh K., Suzuki R., Umezu Y., Hanaumi K., Kumagai K. Studies of murine large granular lymphocytes. II. Tissue, strain, and age distributions of LGL and LAL. J Immunol. 1982 Jul;129(1):395–405. [PubMed] [Google Scholar]
- Jensen P. J., Koren H. S. Depletion of NK by cellular immunoadsorption. J Immunol. 1979 Sep;123(3):1127–1132. [PubMed] [Google Scholar]
- Kiessling R., Klein E., Pross H., Wigzell H. "Natural" killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975 Feb;5(2):117–121. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Wigzell H. An analysis of the murine NK cell as to structure, function and biological relevance. Immunol Rev. 1979;44:165–208. doi: 10.1111/j.1600-065x.1979.tb00270.x. [DOI] [PubMed] [Google Scholar]
- Melder R. J., Ho M. Modulation of natural killer cell activity in mice after interferon induction: depression of activity and depression of in vitro enhancement by interferon. Infect Immun. 1982 Jun;36(3):990–995. doi: 10.1128/iai.36.3.990-995.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., McDaniel D. O. In vitro reactivity of natural killer (NK) cells against Cryptococcus neoformans. J Immunol. 1982 Apr;128(4):1577–1583. [PubMed] [Google Scholar]
- Phillips W. H., Ortaldo J. R., Herberman R. B. Selective depletion of human natural killer cells on monolayers of target cells. J Immunol. 1980 Nov;125(5):2322–2327. [PubMed] [Google Scholar]
- Price G. B., Makinodan T. Immunologic deficiencies in senescence. I. Characterization of intrinsic deficiencies. J Immunol. 1972 Feb;108(2):403–412. [PubMed] [Google Scholar]
- Quan P. C., Kolb J. P., Lespinats G. NK activity in carrageenan-treated mice. Immunology. 1980 Aug;40(4):495–503. [PMC free article] [PubMed] [Google Scholar]
- Reynolds C. W., Timonen T., Herberman R. B. Natural killer (NK) cell activity in the rat. I. Isolation and characterization of the effector cells. J Immunol. 1981 Jul;127(1):282–287. [PubMed] [Google Scholar]
- Roder J. C. Target-effector interaction in the natural killer (NK) cell system. VI. The influence of age and genotype on NK binding characteristics. Immunology. 1980 Oct;41(2):483–489. [PMC free article] [PubMed] [Google Scholar]
- Ross M. H., Bras G. Influence of protein under- and overnutrition on spontaneous tumor prevalence in the rat. J Nutr. 1973 Jul;103(7):944–963. doi: 10.1093/jn/103.7.944. [DOI] [PubMed] [Google Scholar]
- Timonen T., Ortaldo J. R., Herberman R. B. Analysis by a single cell cytotoxicity assay of natural killer (NK) cells frequencies among human large granular lymphocytes and of the effects of interferon on their activity. J Immunol. 1982 Jun;128(6):2514–2521. [PubMed] [Google Scholar]
- Weindruch R., Devens B. H., Raff H. V., Walford R. L. Influence of dietary restriction and aging on natural killer cell activity in mice. J Immunol. 1983 Feb;130(2):993–996. [PubMed] [Google Scholar]


