Abstract
Fatigue is a noticeable and highly prevalent symptom in tense, industriously, and economically affluent modern society. Therefore, new antifatigue agents to smooth the fatigue feature are an energetic topic. The total ethanol extract (ESI) of Saussurea involucrata Kar et Kir., known as Tian-Shan snow lotus, was evaluated for antifatigue activity in ICR mice with mice forced swimming test and the determination of the contents of blood lactic acid and serum urea nitrogen. ESI (0.05, 0.15, 0.25 g/kg) was administered orally to mice for 4 weeks. The average swimming times to exhaustion of the ESI-treated ICR mice (0.15, 0.25 g/kg) were prolonged by 132% and 180% (p<0.001) with a lessening of fatigue compared with that of the control group. Analysis of biochemical parameters showed that levels of serum urea nitrogen and blood lactic acid of experimental groups were also decreased significantly (p<0.001) compared with that of the control group. The antioxidant activity of ESI was investigated by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical-scavenging assay and the hydrogen peroxide-induced luminol chemiluminescence assay and the results indicated that ESI exerts DPPH scavenging ability and reducing power. These results provide scientific evidence that S. involucrata may have been potential as an antifatigue agent.
Keywords: blood lactic acid, Chinese medicinal herb, forced swimming test, Saussurea involucrate, serum urea nitrogen
Introduction
Physiological fatigue, an incapacitating or disabling illness, means a reduction in the force output and energy generating capacity of a body after chronic exposure to work or usual activities at the same intensity (Shevchuk, 2007). Fatigue frequently associated with multiple physiological, biological and immunological aberrations may have miscellaneous manifestations (Fukuda et al., 1994; Cleare, 2003), such as physical or mental tiredness, loss of attention, concentration or motivation (Del Fabbro et al., 2006). In busily, strained and economically affluent modern societies, fatigue have become an important and highly prevalent symptom. To evaluate physiological and general fatigue, the muscle strength and exercise endurance tests were constructive and significant predictors. Exhausting exercise would associate with increased the reactive oxygen species (ROS) production, which induced an imbalance between ROS and antioxidant defenses mechanisms, then may lead to the mutilation of biological and cellular constituent (Clanton, 2007; Pacher et al., 2007). For search new antifatigue drugs, a variety of studies focused on the character of reactive oxygen species. Extensive evidence has accumulated demonstrating the beneficial effects of antioxidants in chronic fatigue. Antioxidants expressed various defensive pathways against the deleterious action of free radical-induced oxidative stress, of which can contribute to reduce the degree of exhaustion caused by continuous physical activity (Valko et al., 2007). Simultaneously, many plant products, including medicinal herbs and some functional botanical foods, have been increasingly discovered to possess antioxidant activity, and to perform protective and beneficial effects to combat fatigue (Achike and Kwan, 2003).
Saussurea involucrata KAR. et KIR. is a rare and beneficial traditional Chinese medicinal herb, grows in the mountains at heights of 4000–4300 m in the Tianshan and A’er Tai areas in China. Because of its very slow growth and the exhaustive collection, the wild population of this plant has been threatened with depletion in recent years due to excessive harvesting. It has been listed as a protected plant by Chinese government. With a reputation for diminishing inflammation and facilitating blood circulation, Saussureae Involucratae Herba (天山雪蓮 tiān shān xuě lián; dried aerial parts of S. involucrata) have long been used under the name “Snow Lotus” for the treatment of rheumatoid arthritis, impotence, irregular menses, cough with cold, stomachache, and altitude sickness, among others (Li and Cai, 1998). Modern pharmacological studies showed that it has possession of several biological functions such as free radical scavenging, cardiotonic, anti-inflammation, abortifacient, and immunomodulation (Li and Cai, 1998; Zheng et al., 1993.). In our recent work, we first described that S. involucrata could inhibit the EGF receptor signaling in human hormone-resistant prostate cancer PC-3 cells, as an antineoplastic agent (Way et al., 2010).
The major constituents with biological activity isolated from wild S. involucrata include flavonoids, alkaloids, steroids, polysaccharides and guaianolides (Li et al., 2007). Although polysaccharides, isolated from wild S. involucrata, have previously been studied for their antifatigue function by i.p. administration in mice (Zheng et al., 1993), neither the beneficial and valuable roles of S. involucrata as traditional herbal medicines, nor the biological mechanisms yet have not been fully revealed. Flavonoids have also been reported for other antioxidant-rich plants, represented non-enzymatic antioxidant defense mechanism against fatigue in over-stimulated muscle (Valko et al., 2007). To obtain further insights into the underlying mechanisms involved in antioxidative protection, we assessed the effects of a flavonoid extract of S. involucrata on physical exercise in rats. This study used ethanol to isolate fractions to further investigate the antifatigue function and biological mechanism of S. involucrata for its potential to combat fatigue and health promoting. The present study was undertaken to explore the effectiveness of S. involucrata by using mice exhausting swimming test. The level of blood lactic acid and the content of serum urea nitrogen were important fatigue relevance factors. Therefore, biochemical estimates were also carried out to determine the antifatigue capability of S. involucrata.
Materials and methods
Chemicals and Reagents
DPPH (2,2-diphenyl-1-picrylhydrazyl), vitamin C, vitamin E (α-tocopherol), were purchased from Sigma Chemical Co. (St. Louis, MO, USA). DMSO (dimethyl sulfoxide) and ethanol were purchased from Tedia Co. (St. Fairfield, OH, USA). All other chemicals were of analytical reagent grade.
Vegetal material and preparation of extracts
The wild plant of S. involucrata used in this study was a kind gift from Biopure Biotechnology (Changhua, Taiwan). Dried and powdered aerial parts, including flower, of S. involucrate (1 kg) was extracted with 70% ethanol three times under reflux for 2 h, respectively. The ethanol extracts were combined, and dried under vacuum evaporation to afford deep-brown syrup (yield 7.49%). These syrupy extracts, then, were suspended with twice-distilled water to obtain the contents being 0.3 g/mL before use.
Animals
Adult male ICR mice (20-25 g; 8-10 weeks of age) were purchased from The National Laboratory Animal Center, Taipei, Taiwan. Animals had access to food, water and maintained in a pressurized ventilated cage according to institutional regulations. 40 ICR mice were randomly divided into 4 groups for treatment with distilled water, administered with the ethanol extracts of S. involucrate (ESI) by intragastric twice a day, 5 days per week for four consecutive weeks at doses of 0.05, 0.15, and 0.25 g/kg body weight. Animal body weights were measured twice a week before, during and after treatments.
Weight-loaded swimming test
One week before the weight-loaded swimming test, 30 min after feeding, the mice were trained to swim for 20 minutes per day to accustom of swimming. The mice were fasted 24 h before forced swimming studies. Briefly, 30 min after the last intragastric administration, the mice were placed individually in a glass jar with 30 cm diameter, 25 cm depth of water maintained at 27.0 ± 1.0 ºC. A lead wire (5% of body weight) was loaded on the dorsum of the mice. The swimming period was regarded the time spent by the mice floating in the water with struggling and making necessary movements until exhausting its strength and drowning. Exhaustion was determined by observing failure to swim, when the mice failed to rise to the surface of water to breathe within 8 seconds period. The swimming time to exhaustion was used as the index of the forced swimming capacity.
Detection of level of Serum urea nitrogen
Another 40 mice were used with the purpose of collecting enough blood for assay. 30 minutes after the last intragastric administration of ESI, the mice (weight-unloaded) were forced to swim in the glass jar of water maintained at 30 ºC for 90 min, the mice were allowed to rest for 60 min. Whole blood was respectively collected in heparinized tubes and free-anticoagulant tubes by extirpating the eyeball of mice, after anesthetization with ether. The serum sample was kept at 4 ºC for 3 h, then was prepared by centrifugation at 2000 rpm for 15 min. Serum urea nitrogen was determined by using the automatic biochemical analyzer (COBAS MIRAplus, Roche), and commercial kits (RAICHEM, BUN Slow Rate Reagent, Lot. U6159).
Detection of level of whole blood lactic acid
To determine the concentration of whole blood lactic acid, thirty minutes after the last dose was administered, the mice (weight-unloaded) were forced to swim in water at 30 ºC for 10 min, whole blood samples were collected in heparinized tubes and free-anticoagulant tubes by extirpating the eyeball of mice, after anesthetization with ether. The mice were allowed to rest for 20 min, and a whole blood sample collected again from each animal. The concentration of blood lactic acid was determined according to the Barker-Summerson method (Pryce, 1969) by using the automatic biochemical analyzer (COBAS MIRAplus, Roche), and commercial kits (Randox, LAC, Lot. 114859).
DPPH free radical-scavenging assay
Quantitative measurement of radical scavenging properties was carried out in a 96-well plate assay. The free radical-scavenging activity of the ESI on the DPPH radical was assessed using the method described by Fenglin et al., with some modifications (Fenglin et al., 2004). A stock solution (1 mg/mL) of each extract was prepared and diluted with methanol to various concentrations. An aliquot of 50 μL of each dilution was transferred into a 96-well microplate (NUNC, Roskilde, Denmark). A working solution of DPPH (250 μM) in methanol was freshly prepared and then an aliquot of 150 μL was added to each well. After incubation for 30 min, the quenching at an absorbance of 517 nm was measured on an ELISA reader (ThermoLabsystems, Cheshire, UK). Each dilution was performed at least in triplicate. Free radical-scavenging activities of test samples and the positive control (Vitamin E) were expressed in terms of IC50 values, which is the concentration of a sample required to decrease the absorbance at 517 nm by 50% compared to the control response.
Hydrogen peroxide-induced luminol chemiluminescence assay
The hydrogen peroxide-induced luminol chemiluminescence (CL) assay was performed as described by Sun et al. (Sun et al.,1998). CL measurements were carried out using a CL analyzer (CLA-2100, Tohoku, Japan). A luminol solution (5 lg/ mL, in PBS buffer at pH 7.4) and a hydrogen peroxide solution (30 mM, in distilled water) were freshly prepared before the experiment. For each measurement, a mixture of 0.1 mL of the luminol solution and 0.2 mL of the sample was added to a special chamber unit (Model TLU-21) including a stainless steel cell with a magnetic stirrer and stirrer bar, in an absolutely dark chamber of the CL analyzing system (model CLA-2100, Tohoku Electronic Industrial, Sendai, Japan). This system contains a photon detector (model CLA-2100), a CL counter (model CLC-10), a water circulator (model CH-20), and a Pentium 4 HP personal computer system. The cooler circulator was connected to the model CLA-2100 photon detector to maintain the temperature at 5 ºC. After 120 s, an aliquot (0.1 mL) of the hydrogen peroxide solution was injected into the special chamber unit of the CL analyzer. Total CL counts were continuously recorded for 300 s. Each measurement was performed at least in triplicate.
Statistical analysis
The results are expressed as the mean ± SD values. Comparisons between groups were made using Student's t-test with P<0.05 considered significant.
Results
Antifatigue activity of alcoholic extract from Saussurea involucrata
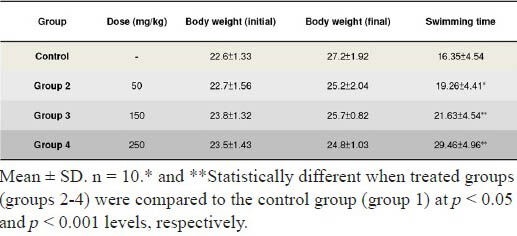
The results of weight-loaded swimming test demonstrated that the experimental groups enhanced the swimming capacity and extended the swimming time on the dose-dependent manner, compared with that of the control group (Table 1). The average swimming times to exhaustion of the ESI-treatment ICR mice at doses of 0.05, 0.15 and 0.25 g/kg body weight for four consecutive weeks, was extended by 117%, 132% and 180%, respectively. During the course of antifatigue evaluation, no significant body weight changes were detected and no deaths occurred in either treatment or control mice (Table 1). This reveals that ESI has no apparent toxicity to the mice.
Table 1.
Effects of the ESI on swimming endurance (min) and body weight (g) of mice.
The alcoholic extract from Saussurea involucrata decreased the serum urea nitrogen and blood lactic acid in experimental groups
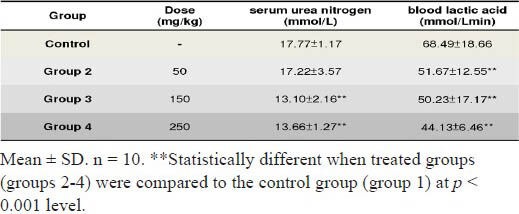
The blood lactic acid level and serum urea nitrogen content were important fatigue relevance factors. Serum urea nitrogen is a protein metabolic product which increases after physical activity. The ESI-treatment ICR mice at doses of 0.05, 0.15 and 0.25 g/kg body weight for four consecutive weeks, the accumulation levels of blood lactic acid and serum urea nitrogen were reduced by 23% to 35.5% after swimming test, compared with those of the control groups (Table 2).
Table 2.
Effects of ESI on the levels of serum urea nitrogen (mmol/l) and blood lactic acid (mmol/L min) of mice after swimming trials.
DPPH free radical-scavenging assay
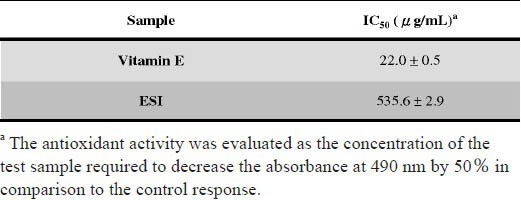
The DPPH method has been widely accepted as a measure for free radical-scavenging activities of antioxidants (Fenglin et al., 2004; Jin and Chen, 1998; Kim et al., 2002; Leong and Shui, 2002). The antioxidant activity, the IC50, which is the concentration of sample required to decrease the absorbance at 490 nm by 50% are shown in Table 3. The IC50 values of ESI and Vitamin E were 535.6 ± 2.9 μg/ml, 22.0 ± 0.5 μg/ml, respectively.
Table 3.
Antioxidant activities of the ESI were using the DPPH free radical-scavenging assay.
Hydrogen peroxide-induced luminol chemiluminescence assay
The hydrogen peroxide-induced luminol chemiluminescence assay is another widely used method to evaluate antioxidant activities (Desmarchelier et al., 1999; Murr et al., 1996; Navas and Jiménez, 1996). CL is the emission of light through an oxidizing reaction of luminol by hydrogen peroxide, a ROS and an intermediate during endogenous oxidative processes; CL formation in the presence of luminol can be applied to estimate the hydrogen peroxide elimination activity of antioxidants. The inhibition of hydrogen peroxide-induced CL in the presence of the ESI is shown in Figure 1. The ESI could effectively inhibit CL formation in a concentration-dependent manner, indicating that Saussurea involucrata possesses antioxidant activity.
Figure 1.
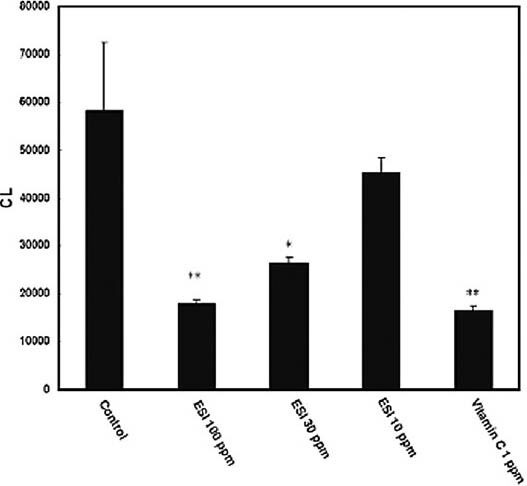
Inhibitory activity on hydrogen peroxide-induced chemiluminescence after addition of the ethanol extract (ESI) of Saussurea involucrata and Vitamin C. Data are presented as means ± standard deviation (n=3).
Discussion
Muscle strength and exercise tolerance were significant predictors for general and physical fatigue. This provides some explanation as to how therapies that improve exercise tolerance and depression can have an impact on fatigue. Our results clearly show that S. involucrata enhanced the swimming capacity of ICR mice in weight-loaded swimming test, by delaying the accumulation of lactic acid and decreasing serum urea nitrogen, performed potent antifatigue activity. We also performed the experiment of antioxidant activity of ESI and the results indicated that ESI exerts DPPH scavenging ability and reducing power. Therefore, the present study suggests that ESI could alleviate the oxidative stress and fatigue, which was induced by chronic fatigue syndrome and the antioxidant property of ESI, may be responsible for its anti-oxidative stress effect. In conclusion, based on its aforementioned excellent antifatigue and antioxidant activity profiles, S. involucrata is highly promising for further evaluation, and thus may have been potential as an antifatigue agent, which also provided a novel and effective strategy to combat fatigue and health promoting.
Acknowledgments
This study was supported in part by the China Medical University (CMU98-OC-04, CMU98-S-13, and CMU97-263).
References
- 1.Achike F.I, Kwan C.Y. Nitric oxide, human diseases and the herbal products that affect the nitric oxide signaling pathway. Clinical and Experimental Pharmacology & Physiology. 2003;30:605–615. doi: 10.1046/j.1440-1681.2003.03885.x. [DOI] [PubMed] [Google Scholar]
- 2.Clanton T.L. Hypoxia-induced reactive oxygen species formation in skeletal muscle. Journal of Applied Physiology. 2007;102:2379–2388. doi: 10.1152/japplphysiol.01298.2006. [DOI] [PubMed] [Google Scholar]
- 3.Cleare A.J. The neuroendocrinology of chronic fatigue syndrome. Endocrine Reviews. 2003;24:236–252. doi: 10.1210/er.2002-0014. [DOI] [PubMed] [Google Scholar]
- 4.Del Fabbro E, Dalal S, Bruera E. Symptom control in palliative care-Part II: Cachexia/anorexia and fatigue. Journal of Palliative Medicine. 2006;9:409–421. doi: 10.1089/jpm.2006.9.409. [DOI] [PubMed] [Google Scholar]
- 5.Desmarchelier C, Romão R.L, Coussio J, Ciccia G. Antioxidant and free radical scavenging activities in extracts from medicinal trees used in the ‘Caatinga’ region in northeastern Brazil. Journal of Ethnopharmacology. 1999;67:69–77. doi: 10.1016/s0378-8741(98)00200-1. [DOI] [PubMed] [Google Scholar]
- 6.Fenglin H, Ruili L, Bao H, Liang M. Freeradical scavenging activity of extracts prepared from fresh leaves of selected Chinese medicinal plants. Fitoterapia. 2004;75:14–23. doi: 10.1016/j.fitote.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Fukuda K, Straus S.E, Hickie I, Sharpe M.C, Dobbins J.G, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. Annals of Internal Medicine. 1994;121:953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 8.Jin Z.Q, Chen X. A simple reproducible model of free radical- injured isolated heart induced by 1,1-diphenyl-2-picrylhydrazyl (DPPH) Journal of Pharmacological and Toxicological Methods. 1998;39:63–70. doi: 10.1016/s1056-8719(97)00093-2. [DOI] [PubMed] [Google Scholar]
- 9.Kim Y.K, Guo Q, Packer L. Free radical scavenging activity of red ginseng aqueous extracts. Toxicology. 2002;172:149–156. doi: 10.1016/s0300-483x(01)00585-6. [DOI] [PubMed] [Google Scholar]
- 10.Leong L.P, Shui G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chemistry. 2002;76:69–75. [Google Scholar]
- 11.Li J.S, Cai S.Q. Chemical constituents and pharmcological activity of eight species herb Xuelianhua. Chinese Pharmaceutical Journal. 1998;33:449–452. [Google Scholar]
- 12.Li Y, Wang C, Guo S, Yang J, Xiao P. Three guaianolides from Saussurea involucrata and their contents determination by HPLC. Journal of Pharmaceutical and Biomedical Analysis. 2007;44:288–292. doi: 10.1016/j.jpba.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Murr C, Baier-Bitterlich G, Fuchs D, Werner E.R, Esterbauer H, Pfleiderer W, Wachter H. Effects of neopterin-derivatives on H2O2-induced luminol chemiluminescence: mechanistic aspects. Free Radical Biology and Medicine. 1996;21:449–456. doi: 10.1016/0891-5849(96)00036-6. [DOI] [PubMed] [Google Scholar]
- 14.Navas M.J, Jime´nez A.M. Review of chemiluminescent methods in food analysis. Food Chemistry. 1996;50:7–15. [Google Scholar]
- 15.Pacher P, Beckman J.S, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiological Reviews. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pryce J.D. A modification of the Barker-Summerson method for the determination of lactic acid. The Analyst. 1969;94:1151–1152. doi: 10.1039/an9699401151. [DOI] [PubMed] [Google Scholar]
- 17.Shevchuk N.A. Possible use of repeated cold stress for reducing fatigue in chronic fatigue syndrome: a hypothesis. Behavioral and Brain Functions. 2007;3:55–80. doi: 10.1186/1744-9081-3-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun J.S, Tsuang Y.W, Chen I.J, Huang W.C, Hang Y.S, Lu F.J. An ultra-weak chemiluminescence study on oxidative stress in rabbits following acute thermal injury. Burns. 1998;24:225–231. doi: 10.1016/s0305-4179(97)00115-0. [DOI] [PubMed] [Google Scholar]
- 19.Valko M, Leibfritz D, Moncol J, Cronin M.T, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. The lnternational Journal of Biochemistry & Cell Biology. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Way T.D, Lee J.C, Kuo D.H, Fan L.L, Huang C.H, Lin H.Y, Shieh P.C, Kuo P.T, Liao C.F, Liu H, Kao J.Y. Inhibition of epidermal growth factor receptor signaling by Saussurea involucrata, a rare traditional Chinese medicinal herb, in human hormone-resistant prostate cancer PC-3 cells. Journal of Agricultural and Food Chemistry. 2010;58:3356–3365. doi: 10.1021/jf903793p. [DOI] [PubMed] [Google Scholar]
- 21.Zheng R.L, Liu G.S, Xing G.X. Free radical scavenging and antifatigue activities of Saussurea involucrata polysaccharides. Acta Pharmacologica Sinica. 1993;14:47–49. [PubMed] [Google Scholar]


