Abstract
Accumulating epidemiological and clinical evidence shows that inflammation is an important risk factor for various human diseases. Thus, suppressing chronic inflammation has the potential to delay, prevent, and control various chronic diseases, including cerebrovascular, cardiovascular, joint, skin, pulmonary, blood, lymph, liver, pancreatic, and intestinal diseases. Various natural products from traditional Chinese medicine (TCM) have been shown to safely suppress proinflammatory pathways and control inflammation-associated disease. In vivo and/or in vitro studies have demonstrated that anti-inflammatory effects of TCM occur by inhibition of the expression of master transcription factors (for example, nuclear factor-κB (NF-κB)), pro-inflammatory cytokines (for example, tumor necrosis factor-α (TNF-α), chemokines (for example, chemokine (C-C motif) ligand (CCL)-24), intercellular adhesion molecule expression and pro-inflammatory mediators (for example, inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX2)). However, a handful of review articles have focused on the anti-inflammatory activities of TCM and explore their possible mechanisms of action. In this review, we summarize recent research attempting to identify the anti-inflammatory constituents of TCM and their molecular targets that may create new opportunities for innovation in modern pharmacology.
Keywords: Anti-inflammatory activity, Traditional Chinese medicinal herbs, Pro-inflammatory cytokines
Inflammation and chronic disease
Inflammation is known to contribute to physiological and pathological processes by the activation of the immune system, local vascular system, and various cells within the damaged tissue (Coussens and Werb, 2002). Prolonged inflammation, known as chronic inflammation, is caused by a variety of factors, including microbial pathogen infection, physical, chemical, and surgical irritation, and/or wounding. The classical characteristics of inflammation are pain, swelling, edema, redness and heat (Mantovani, 2010). There is now growing evidence supporting the concept that chronic inflammation may affect many organ systems including skin, brain, colon, blood vessels, pancreas, joints, lung, and heart (Khatami, 2009).
Epidemiological studies have also revealed that chronic inflammation is causally linked to various human diseases, including cerebrovascular, cardiovascular, joint, cutaneous, pulmonary, blood, liver, and intestinal diseases as well as diabetes (Figure 1). The inflammatory process leads to the up-regulation of a series of pro-inflammatory enzymes, cytokines, reactive oxygen/nitrogen species (RO/NS) and signaling proteins in infected tissues and cells. Elevation in both the tissue and the serum levels of pro-inflammatory mediators predict an increased health risk at all stages of these diseases (Forrester and Bick-Forrester, 2005). Thus, blocking of inflammatory signaling is usually recognized as a potential therapeutic modality for chemoprevention.
Figure 1.
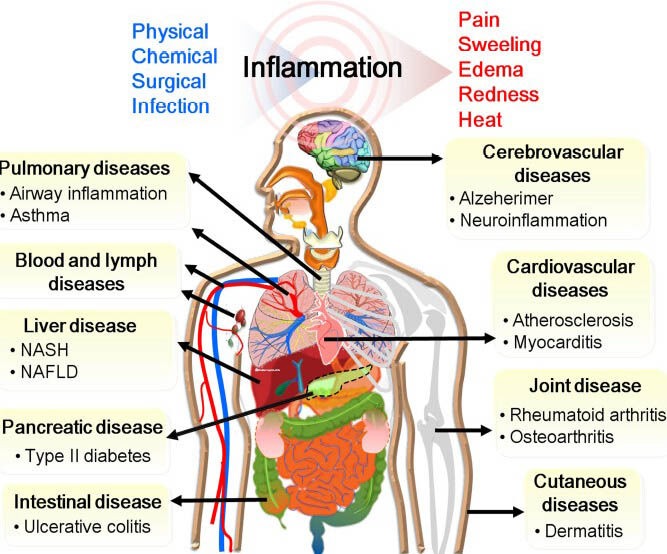
Human diseases linked with chronic inflammation
TCM herbs as promising anti-inflammatory agents
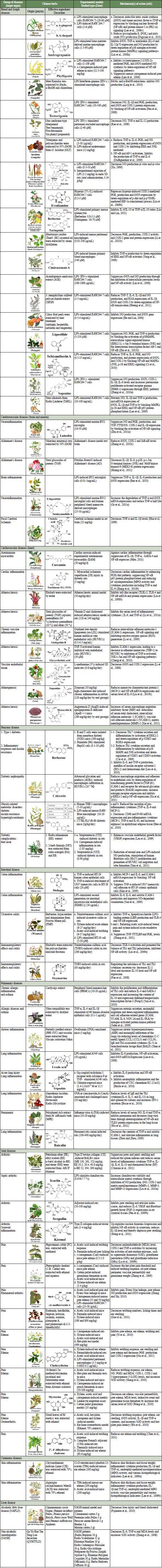
TCM has evolved over the past 5,000 years to prevent and manage human disease. The clinical recognition and diagnosis of disease in TCM are mainly based on the yin-yang and five elements theories (Lu et al., 2009a). Traditionally, the two most common methods of applying herb treatments are to make a decoction (a strong tea that must be simmered for an hour or more) and to make large pills containing honey as a binding agent. However, modern herbs, developed to replace the standard Chinese preparations, come in two popular forms, namely, extract powders (or granules) and smooth (Wang et al., 2009). Herbs used in TCM and their active components have been demonstrated in many animal or cell culture models to inhibit inflammatory responses in different organs including the lung, esophagus, cerebrum, colon, skin, prostate, mammary glands, liver, pancreas, and lung (Pan et al., 2011; Ichikawa et al., 2003; Yarosh et al., 2006). Table 1 summarizes the various natural products derived from TCM herbs, which have been shown to safely suppress proinflammatory signaling pathways and to control inflammation- associated disease.
Table 1.
Anti-inflammatory effects of the active components from TCM herbs
Anti-inflammatory properties in chronic diseases and its possible mechanisms
Blood and lymph diseases
The human's immune system is composed of organs such as the spleen and thymus along with lymph nodes and bone marrow that also contribute to the prevention of infection and disease by producing and storing specific immune cells (Schmid-Hempel, 2005). Indeed, inflammation is an integral part of the immune system, but sometimes chronic inflammation becomes a pathophysiological process leading to disease development and progression (Handschin and Spiegelman, 2008).
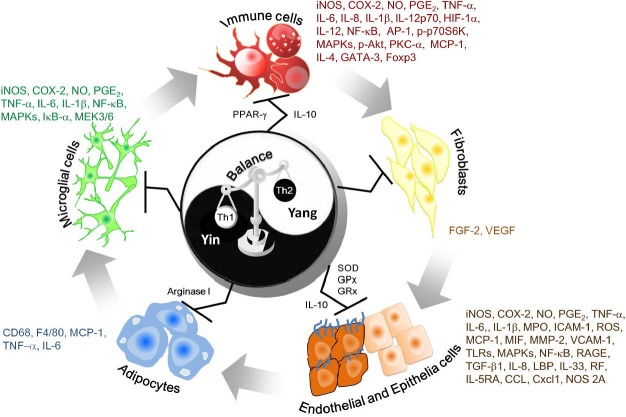
Macrophages play a central role in chronic inflammation by mechanisms such as the overproduction of pro-inflammatory cytokines (tumor necrosis factor-α (TNF-α and interleukins (IL-6 and IL-1β) and generation of inflammatory mediators in response to microbial products (LPS, lipopolysaccharide), such as reactive oxygen species (ROS), prostaglandin E2 (PGE2), nitric oxide (NO) and interferon-γ (IFN-γ). These mediators are potent activators of components of the pro-inflammatory signal transduction cascade, including NF-κB-inducing kinase, mitogen-activated protein kinase (MAPK), and protein kinase C (PKC) (Pan et al., 2009a). 6-gingerol, 6-shogaol, andrograpanin, phylligenin, tectorigenin, rhein, baicalin, berberine, naringenin, cimiracemate A, ligustilide and schicantherin A are bioactive substances in medicinal plants that have been reported to decrease LPS and/or IFN-γ-induced production of pro-inflammatory cytokines and mediators in macrophages and primary mouse splenocytes by down-regulation of MAPK and inhibition of PKC- mediated activation of downstream transcription factors NF-κB and activator protein 1 (AP-1) (Lee et al., 2009c; Lim et al., 2008; Dugasani et al., 2010; Liu et al., 2008a; Ling et al., 2010; Zhang et al., 2010a; Pan et al., 2008; Luo et al., 2009; Li et al., 2011b; Lin et al., 2008; Ho and Lin, 2008; Lin and Lin, 2011; Hwang et al., 2011; Yang et al., 2009; Su et al., 2011). Recently, evodiamine extracted from Evodiae Fructus (吳茱萸 wú zhū yú; the fruits of Evodia rutaecarpa), was also demonstrated to be effective in inhibiting the production of COX-2-mediated PGE2 and expression of iNOS through inhibition of PI3K/Akt/p70S6K signaling and inhibition of hypoxia-inducible factor-1α (HIF-1α) accumulation in hypoxia-stimulated RAW 264.7 macrophages (Liu et al., 2009b).
Cerebrovascular diseases
Recent studies have shown that the severity of cerebrovascular disease, including Alzheimer's disease, Parkinson's disease and cerebral ischemia, correlates with inflammation-mediated responses in neural cells (Britschgi and Wyss-Coray, 2007). Inflammation of the brain and central nervous system (CNS) are also mediated by the generation of various pro-inflammatory cytokines, including TNF-α, IL-1β and IL-6 in microglia, astrocytes, ependymal cells, macrophages and mast cells (Rivest, 2009). Moreover, microglia activation is one of the causative factors in neuroinflammation, which results in brain damage during neurodegenerative disease. Previous studies have shown that luteolin, senkyunolide A, Z-ligustilide and Agrimoniae Herba possess anti-inflammatory properties by decreasing LPS-induced NO and PGE2 production, by suppressing TNF-α, IL-1β, iNOS and COX-2 expression, and by blocking NF-κB activation in murine BV-2 microglial cells (Zhu et al., 2011b;Or et al., 2011b;Bae et al., 2010). In vivo studies have shown that cholalic acid, hyodeoxycholalic acid and total glucosides of peony (TGP) can prevent cerebral ischemia and Alzheimer's disease by inhibiting inflammatory cytokine (TNF-α, IL-1β and IL-6) production and down-regulating c-Jun N-terminal kinases (JNK), p-38 and MAPK extracellular signal-regulated kinases (ERK) kinase3/6 (MEK3/6) phosphorylation (Huang et al., 2011; Hua et al., 2009).
Cardiovascular diseases
Recent studies have demonstrated that inflammatory responses may cause myocardial damage and atherosclerosis, leading causes of cardiovascular disease (CVD) (Libby, 2006).
Tanshinone IIA and curcumin, substances with strong anti-inflammatory activity, have been shown to be effective in protecting against cardiac inflammation in in vitro and in vivo models (Ren et al., 2010; Pari et al., 2008). Mito et al. (Mito, 2011) reported that the cardioprotective effects of curcumin are elicited through the inhibition of IL-1I, TNF-α, GATA-4 and NF-κB expression and may provide a novel therapeutic strategy for the treatment of autoimmune myocarditis. In addition, Salviae Miltiorrhizae Radix (丹參 dān shēn; the roots of Salvia miltiorrhiza Bunge) preparations rich in tanshinone IIA were shown to reduce infarct size and improve cardiac apoptosis and inflammation by significantly enhancing Akt phosphorylation and suppressing NF-κB phosphorylation, myeloperoxidase (MPO) activity and production of inflammatory cytokines, such as TNF-α, and IL-6 (Zhang et al., 2010b). Clinical studies have shown that vascular inflammation is the earliest event in the development of atherosclerosis (Izumimoto and Kawakami, 2011). The process involves stimulation of cholesterol and oxidized low density lipoprotein (ox-LDL) accumulation within the vessel wall and generation of oxidative free radicals, which activate vascular endothelial cells and enhance the adhesion of monocytes to them by promoting expression of endothelial adhesion molecules, including selectins, vascular cell adhesion molecule-1 (VCAM-1) and intracellular adhesion molecule-1 (ICAM-1) (Libby and Theroux, 2005). Once monocytes firmly attach to the surface of the endothelium under the influence of chemoattractants such as monocyte chemoattractant protein-1 (MCP-1), they transmigrate into the arterial intima and differentiate into macrophages. These macrophages proliferate and amplify the inflammatory response through the secretion of numerous growth factors, adhesion molecules, pro-inflammatory cytokines (IL-6, IL-1β and TNF-α) and matrix metalloproteinases (MMPs) (Packard and Libby, 2008). In addition, the toll-like receptors (TLRs) TLR-2 and TLR-4 also play an important role in innate immune and inflammatory responses, and several reports have demonstrated the expression of TLR-2 and TLR-4 in atherosclerotic lesions (Schoneveld et al., 2008). TGP, ginsenoside, ginkgolide B, monacolin K and glycyrrhetinic acid have been shown in experimental animal studies and in vitro studies of human umbilical vein endothelial cells (HUVEC) to significantly attenuate the development of atherosclerotic disease by decreasing ROS generation, reducing expression of adhesion molecules, MMP-2 and pro-inflammatory mediators and increasing macrophage migration inhibitory factor (MIF) levels (Li et al., 2009; Chang et al., 2010; Xie et al., 2011; Li et al., 2008; Li et al., 2011a; Liu et al., 2008b; Liu et al., 2010b).
Pancreatic disease
Mounting evidence suggests that oxidative stress and chronic inflammation play an important role in obesity-related metabolic disorders such as type 2 diabetes (Hotamisligil, 2006). However, type 1 diabetes, one of the most common autoimmune diseases, is caused by T cell- mediated destruction of pancreatic beta cells (Kalousova et al., 2004). More and more evidence indicates that the anti-inflammatory effects of TCM may contribute to their antidiabetic action (Xie and Du, 2011).
Recent studies have shown that berberine can ameliorate type 1 diabetes and decrease the expression of Th17 cytokines in nonobese diabetic (NOD) mice via suppression of Th17 and Th1 differentiation. Berberine inhibited Th1 differentiation by decreasing the activity of STAT1 and STAT4 through suppression of p38 MAPK/JNK activity, but down-regulated Th17 differentiation through activation of ERK1/2 and reduction in the levels of STAT3 phosphorylation and retinoic acid-related orphan receptor γt (RORγt) expression (Cui et al., 2009).
Recently, the active principles in Astragali Radix (黃耆 huáng qí) (calycosin, calycosin-7-β-D-glucoside, ononin, calycosin and formononetin) that inhibit pro-inflammatory cytokine production were identified. Xu et al. (Xu et al., 2011) have reported that calycosin can inhibit advanced glycation end products (AGEs)-induced macrophage migration and adhesion to endothelial cells; calycosin also can relieve local inflammation by reducing expression of transforming growth factor-β1(TGF-β1), ICAM-1, p-ERK 1/2, p-NF-κB and receptor for advanced glycation end products (RAGE) and by increasing expression of the estrogen receptor in HUVECs (Xu et al., 2011). In another study, it was demonstrated that four natural compounds from Astragali Radix (黃耆 huáng qí) with anti-diabetic and insulin sensitizing effects can reduce the secretion of pro-inflammatory cytokines (TNF-α, IL-6 and MCP-1) and expression levels of inflammatory cell markers (CD68 and F4/80) and increase the level of agrinase I (Hoo et al., 2010).
This major increase in morbidity and mortality of diabetes is due to the development of both macro- and micro-vascular complications such as are commonly found in diabetic patients with foot ulcers (Levin, 2002). Previous scientific studies reported that Rehmanniae Radix (地黃 dì huáng) was effective in promoting diabetic foot ulcer healing, angiogenesis, and tissue regeneration and in inhibiting inflammation through induction of vascular endothelial growth factor (VEGF) expression, reduction of LPS-induced NO production, stimulation of human fibroblast cell (Hs27) proliferation and promotion of HUVEC cell migration and tube formation (Lau et al., 2009; Tam et al., 2011).
Intestinal disease
The major forms of inflammatory bowel disease (IBD), i.e., Crohn's disease and ulcerative colitis (UC), are chronic relapsing inflammatory conditions of the gastrointestinal tract, resulting from impairment of intestinal epithelial barrier function and subsequent defects in adaptive immunity (Tsianos and Katsanos, 2009). Increasing evidence demonstrates that infiltration and migration of innate immune cells depends on production of pro-inflammatory cytokines, chemokines and adhesion molecules (Jose et al., 2006). In addition, the inflammatory reaction involves complex interactions between immune cells and endothelial cells (ECs), the monolayer between blood and tissue (Bouguen et al., 2011). Therefore, restoration of the balance between pro- and anti-inflammatory cytokines may be a promising strategy for the treatment of IBD. Recent studies have shown that mollugin inhibits TNF-α-induced inflammatory responses and chemotaxis in HT-29 cells and U937 cells through inhibition of NF-κB activation and decreased MCP-1, IL-8 and ICAM-1 expression (Kim et al., 2009). In another model it has also been found that matrine, berberine, hypaconitine and skimmianine could inhibit the LPS-stimulated inflammatory reaction by improving NO-dependent vasomotion and inhibiting expression of inflammatory mediator (IL-6, IL-8, soluble ICAM-1, TNF-α LBP, and PGE2) (Zhang et al., 2011;Suo et al., 2009). The colitis model in rats has indicated that berberine, hypaconitine, skimmianine, oxymatrine and rhubarbs can ameliorate acetic acid- and 2, 4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis and bowel pain via decreases in TNFα, LBP, IL-12, TLR-4 and NF-κB activation and increases in the IL-10 level, resulting in an improved balance of Th1 and Th2 cells (Zhang et al., 2011; Fan et al., 2008; Liu et al., 2009a).
Pulmonary diseases
Respiratory epithelium plays a key role in airway inflammatory disease, including asthma, acute and chronic microbial infections, and obstructive pulmonary disease by the production of numerous cytokines, chemokines, inflammatory enzymes, and adhesion molecules (Iwamoto, 2003). Importantly, balance of Th1-Th2 cytokine secretion has been suggested as necessary to maintain healthy immune homeostasis. Imbalance has been hypothesized to underlie allergic asthma through a shift in immune responses from a Th1 (IFN-γ) pattern toward a Th2 (IL-4, IL-5, and IL-13) profile, which promotes IgE production, eosinophilic inflammation, activation and survival, and enhanced airway smooth muscle contractility (Busse and Rosenwasser, 2003). Previous studies also suggest that lung epithelial cells are involved in inflammatory processes by recruiting immune cells and producing pro-inflammatory cytokines, resulting in amplification of the inflammatory signal (Lee et al., 2007). Recent studies showed that ursolic acid, triptolide and Viticis Fructus (蔓荊子 màn jīng zǐ; the fruits of Vitex rotundifolia) extract could suppress Th2 cell proliferation, eosinophil migration and neutrophilic inflammation by down-regulation of cytokines, chemokines and cell adhesion molecules (Lee et al., 2007; Lee et al., 2008; Hoyle et al., 2010; Sohn et al., 2009). We found that Visci Ramus (槲寄生 hú jì shēng; the dried stem, with leaf Viscum coloratum), Ganoderma (靈芝 líng zhī; Ganoderma lucidum), Sophorae Flavescentis Radix (苦參 kǔ shēn) and Glycyrrhizae Radix (甘草 gān cǎo) extract potently inhibited airway hyperresponsiveness (AHR) and reduced eosinophil infiltration of the lungs in ovalbumin (OVA)-sensitized mice by reducing levels of IgE, CCLs (CCL11 and CCL24) and Th2-associated cytokines (IL-5, IL-4 and IL-13), and by increasing IFN-IIsecretion (Busse et al., 2010; Shen et al., 2011). Polyphenol rich extracts from officinal magnolia bark (厚朴 hòu pò) and baicalin were also shown to alleviate pneumonia by decreasing the level of NO, IL-6, TNF-α and ICAM-1 and reducing NF-κB and Toll like receptor (TLR)-3 expression in Pneumocystis carinii and influenza virus A (IVA)-infected lung tissue (Wu et al., 2011; Zhou and Zhou, 2009).
Joint diseases
Rheumatoid arthritis (RA) is a systemic and chronic inflammatory autoimmune disorder characterized by synovial hyperplasia, inflammatory cell infiltration and angiogenesis, which ultimately lead to cartilage erosion and articular destruction (Leff, 2006). Several inflammatory cytokines, such as TNF-α, IL-1β, IL-6, IL-33 and rheumatoid factor (RF) not only play important roles in the chronic inflammation of human RA, but are also associated with various manifestations of inflammation-related angiogenesis (Marrelli et al., 2011).
In the rat model of arthritis, the extract of Arisaematis Rhizoma (天南星 tiān nán xīng; the root of Arisaema rhizomatum), scopolin and kirenol inhibited paw and joint swelling by suppressing inflammatory cytokines and NO production, inhibiting VEGF and fibroblast growth factor (FGF-2) expression and up-regulating Annexin-1 which interacts with NF-κB to inhibit NF-κB activity (Chunxia et al., 2011; Wang et al., 2011; Pan et al., 2009b). Liu et al. (Liu et al., 2010a) also demonstrated that icariin can protect chondrocytes from LPS-induced inflammation and extracellular matrix degradation through inhibition of NO, MMP, iNOS and COX-2 expression. The direct anti-inflammatory and analgesic effects of TCM were observed in animal models of both acute and subacute inflammation, such as formalin-induced paw licking, carrageenan-induced paw edema, cotton pellet-induced granuloma, acetic acid-induced permeability, xylene-induced ear edema, collagen-induced arthritis, complete Freund's adjuvant (CFA)-induced joint inflammation and thermally induced pain.
Skin diseases
As the primary interface between the body and the external environment, the skin provides the first line of defense against traumatic injury and infection by microbial pathogens. Besides its properties as a physical barrier, the skin has many active defense mechanisms and regulation of these mechanisms is important because inappropriate or misdirected immune activity is implicated in the pathogenesis of a large variety of inflammatory skin diseases. High levels of pro-inflammatory cytokines and ROS are proposed to contribute to the pathophysiological mechanisms (Numerof et al., 2005). Lee et al. (Lee et al., 2009a; Lee et al., 2009b) found anti-inflammatory effects of the extract of wild chrysanthemum (野菊 yě jú; Chrysanthemum indicum, CIE) and Asparagi Radix (天門冬 tiān mén dōng; Asparagus cochinchinensis Merrill, ACE) in 12-O-tetradecanoyl-phorbol-13-acetate (TPA)-induced mouse ear edema and acetic acid-induced vascular permeability models. CIE and ACE showed potent inhibitory activity against topical edema of the mouse ear, leading to substantial reductions in skin thickness and tissue weight, inflammatory cytokine production (TNF-α and IL-1β, neutrophil-mediated MPO activity, and various histopathological indicators.
Liver disease
Most acute and chronic liver diseases are characterized by the presence of inflammatory and oxidative stress processes with enhanced expression of various pro-inflammatory cytokines and lipid mediators (Ferre and Claria, 2006; Tilg et al., 2006). In the non-alcoholic steatohepatitis (NASH) model, keishi-bukuryo-gan and Qu Yu Hua Tan Tong Luo decoctions have been found to relieve lipid peroxidation and inflammation of the liver by down-regulation of TNF-α, IL-8, cholesterol, triglycerides (TGs) and MDA levels and up-regulation of superoxide dismutase (SOD) activity (Fujimoto et al., 2010; Zhang et al., 2008).
Conclusion
Strong direct evidence suggests that chronic inflammation promotes development of numerous human diseases such as Alzheimer's, atherosclerosis, arthritis, asthma, diabetes and IBD. The Chinese herbs investigated are mostly qi supplementation (補氣 bǔ qì), heat-clearing (清熱 qīng rè) and toxin-resolving (解毒 jiě dú) drugs, as described previously in the theory of TCM (Xie and Du, 2011). TCM has a long history of human use, and the main active components recorded and identified, in heat-clearing and detoxifying Chinese herbs usually have widespread pharmacological effects including anti-inflammatory actions (Ren et al., 1994). Clinical trials have also demonstrated the effectiveness of TCM for the prevention and therapy of many chronic inflammatory diseases, and the related mechanisms have also been identified. Therefore, in this article, we systemically reviewed the evidence for the efficacy of anti-inflammatory products used in TCM in the treatment of inflammatory processes associated with various chronic diseases and shared their known mechanisms of action.
It is clear that natural bioactive compounds from herbs used in TCM can interfere with multiple cell signaling pathways and have multiple targets within the cells. These mechanisms include (a) modulation of inflammatory signal transduction pathways linked to NF-κB, AP-1, PI3K/Akt, MAPKs, STATs, and TLRs, (b) induction of antioxidant enzymes such as SOD, glutathione peroxidase (GPx) and glutathione reductase (GRx), (c) reduction of inflammatory molecule production including iNOS, COX-2, NO and PGE2 (d) diminished recruitment and activation of inflammatory cells, (e) altered regulation of cellular functions and (f) changes in the balance of Th1 and Th2 cell-derived cytokines (Figure 1). Besides their influence on the regulation of intracellular signaling pathways, the active components from TCM may also inhibit expression of growth factors (VEGF, FGF-2 and TGF-β1) and MMPs, which are important cofactors for angiogenesis, wound repair and tissue regeneration.
This information adds to the body of evidence indicating that the products of TCM, because of their safety and anti-inflammatory efficacy, may have a potential role in the prevention and treatment of chronic inflammatory disease (Figure 2). Furthermore, extensive research is needed concerning the influence of active herbal products on the pathological, immunological, biochemical and molecular biology-related aspects of disease processes, which may ultimately lead to enhanced formulations for chemoprevention.
Figure 2.
Effects of traditional Chinese medicine (TCM) mediated by the molecular mechanisms of anti-inflammatory action
Acknowledgements
This study was supported by the National Science Council NSC 98-2313-B-022-002-MY3 and 099-2811- B-022-002
Abbreviations
References
- 1.Bae H, Kim H.J, Shin M, Lee H, Yin C.S, Ra J, Kim J. Inhibitory effect of Agrimoniae Herba on lipopolysaccharide-induced nitric oxide and proinflammatory cytokine production in BV2 microglial cells. Neurological Research. 2010;32:53–57. doi: 10.1179/016164109X12537002794002. [DOI] [PubMed] [Google Scholar]
- 2.Bouguen G, Chevaux J.B, Peyrin-Biroulet L. Recent advances in cytokines: therapeutic implications for inflammatory bowel diseases. World Journal of Gastroenterology. 2011;17:547–556. doi: 10.3748/wjg.v17.i5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Britschgi M, Wyss-Coray T. Immune cells may fend off Alzheimer disease. Nature Medicine. 2007;13:408–409. doi: 10.1038/nm0407-408. [DOI] [PubMed] [Google Scholar]
- 4.Busse P.J, Schofield B, Birmingham N, Yang N, Wen M.C, Zhang T, Srivastava K, Li X.M. The traditional Chinese herbal formula ASHMI inhibits allergic lung inflammation in antigen-sensitized and antigen-challenged aged mice. Annals of Allergy Asthma and Immunology. 2010;104:236–246. doi: 10.1016/j.anai.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Busse W.W, Rosenwasser L.J. Mechanisms of asthma. Journal of Allergy and Clinical Immunology. 2003;111:S799–S804. doi: 10.1067/mai.2003.158. [DOI] [PubMed] [Google Scholar]
- 6.Chang Y.L, Chen C.L, Kuo C.L, Chen B.C, You J.S. Glycyrrhetinic acid inhibits ICAM-1 expression via blocking JNK and NF-kappaB pathways in TNF-alpha-activated endothelial cells. Acta Pharmacologica Sinica. 2010;31:546–553. doi: 10.1038/aps.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Z.P, Qu M.M, Chen H.X, Liu D, Xiao Y.Y, Chen J, Lu T.L, Cai B.C. The studies of anti-inflammatory and analgesic activities and pharmacokinetics of Oxytropis falcate Bunge extraction after transdermal administration in rats. Fitoterapia. 2011;82:426–433. doi: 10.1016/j.fitote.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 8.Chunxia C, Peng Z, Huifang P, Hanli R, Zehua H, Jizhou W. Extracts of Arisaema rhizomatum CEC Fischer attenuate inflammatory response on collagen-induced arthritis in BALB/c mice. Journal of Ethnopharmacology. 2011;133:573–582. doi: 10.1016/j.jep.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 9.Ci X, Ren R, Xu K, Li H, Yu Q, Song Y, Wang D, Li R, Deng X. Schisantherin A exhibits anti-inflammatory properties by down-regulating NF-kappaB and MAPK signaling pathways in lipopolysaccharide-treated RAW 264.7 cells. Inflammation. 2010;33:126–136. doi: 10.1007/s10753-009-9166-7. [DOI] [PubMed] [Google Scholar]
- 10.Coussens L.M, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui G, Qin X, Zhang Y, Gong Z, Ge B, Zang Y.Q. Berberine differentially modulates the activities of ERK p38 MAPK and JNK to suppress Th17 and Th1 T cell differentiation in type 1 diabetic mice. Journal of Biological Chemistry. 2009;284:28420–28429. doi: 10.1074/jbc.M109.012674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudhgaonkar S, Thyagarajan A, Sliva D. Suppression of the inflammatory response by triterpenes isolated from the mushroom Ganoderma lucidum. International Immunopharmacology. 2009;9:1272–1280. doi: 10.1016/j.intimp.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Dugasani S, Pichika M.R, Nadarajah V.D, Balijepalli M.K, Tandra S, Korlakunta J.N. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. Journal of Ethnopharmacology. 2010;127:515–520. doi: 10.1016/j.jep.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Fan H, Chen R, Shen L, Lv J, Xiong P, Shou Z, Zhuang X. Oxymatrine improves TNBS-induced colitis in rats by inhibiting the expression of NF-kappaB p65. Journal of Huazhong University of Science and Technology [Medical Sciences] 2008;28:415–420. doi: 10.1007/s11596-008-0409-x. [DOI] [PubMed] [Google Scholar]
- 15.Ferre N, Claria J. New insights into the regulation of liver inflammation and oxidative stress. Mini reviews in medicinal chemistry. 2006;6:1321–1330. doi: 10.2174/138955706778993049. [DOI] [PubMed] [Google Scholar]
- 16.Forrester J.S, Bick-Forrester J. Persistence of inflammatory cytokines cause a spectrum of chronic progressive diseases: implications for therapy. Medical Hypotheses. 2005;65:227–231. doi: 10.1016/j.mehy.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Fujimoto M, Tsuneyama K, Kinoshita H, Goto H, Takano Y, Selmi C, Keen C.L, Gershwin M.E, Shimada Y. The traditional Japanese formula keishibukuryogan reduces liver injury and inflammation in patients with nonalcoholic fatty liver disease. Annals of the NEW YORK Academy of Sciences. 2010;1190:151–158. doi: 10.1111/j.1749-6632.2009.05265.x. [DOI] [PubMed] [Google Scholar]
- 18.Guo T, Deng Y.X, Xie H, Yao C.Y, Cai C.C, Pan S.L, Wang Y.L. Antinociceptive and anti-inflammatory activities of ethyl acetate fraction from Zanthoxylum armatum in mice. Fitoterapia. 2011;82:347–351. doi: 10.1016/j.fitote.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Handschin C, Spiegelman B.M. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454:463–469. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho S.C, Lin C.C. Investigation of heat treating conditions for enhancing the anti-inflammatory activity of citrus fruit (Citrus reticulata) peels. Journal of Agricultural and Food Chemistry. 2008;56:7976–7982. doi: 10.1021/jf801434c. [DOI] [PubMed] [Google Scholar]
- 21.Hoo R.L, Wong J.Y, Qiao C, Xu A, Xu H, Lam K.S. The effective fraction isolated from Radix Astragali alleviates glucose intolerance, insulin resistance and hypertriglyceridemia in db/db diabetic mice through its anti-inflammatory activity. Nutrition and Metabolism (Lond) 2010;7:67. doi: 10.1186/1743-7075-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 23.Hoyle G.W, Hoyle C.I, Chen J, Chang W, Williams R.W, Rando R.J. Identification of triptolide, a natural diterpenoid compound, as an inhibitor of lung inflammation. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2010;298:830–836. doi: 10.1152/ajplung.00014.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hua Q, Zhu X.L, Li P.T, Liu Y, Zhang N, Xu Y, Jia X. The inhibitory effects of cholalic acid and hyodeoxycholalic acid on the expression of TNFalpha and IL-1beta after cerebral ischemia in rats. Archives of Pharmacal Research. 2009;32:65–73. doi: 10.1007/s12272-009-1119-z. [DOI] [PubMed] [Google Scholar]
- 25.Huang D, Liu M, Yan X. Effects of total glucosides of peony on expression of inflammatory cytokines and phosphorylated MAPK signal molecules in hippocampus induced by fibrillar Abeta42. Zhongguo ZhongYao Za Zhi. 2011;36:795–800. [PubMed] [Google Scholar]
- 26.Hwang P.A, Chien S.Y, Chan Y.L, Lu M.K, Wu C.H, Kong Z.L, Wu C.J. Inhibition of Lipopolysaccharide (LPS)-induced inflammatory responses by Sargassum hemiphyllum sulfated polysaccharide extract in RAW 264.7 macrophage cells. Journal of Agricultural and Food Chemistry. 2011;59:2062–2068. doi: 10.1021/jf1043647. [DOI] [PubMed] [Google Scholar]
- 27.Ichikawa H, Wang X, Konishi T. Role of component herbs in antioxidant activity of shengmai san--a traditional Chinese medicine formula preventing cerebral oxidative damage in rat. The American Journal of Chinese Medicine. 2003;31:509–521. doi: 10.1142/S0192415X03001193. [DOI] [PubMed] [Google Scholar]
- 28.Iwamoto I. Molecular mechanisms of allergic airway inflammation in asthma. Nihon Kokyuki.Gakkai Zasshi. 2003;41:600–605. [PubMed] [Google Scholar]
- 29.Izumimoto N, Kawakami A. Inflammation in atherosclerosis. Nihon Rinsho. 2011;69:2–5. [PubMed] [Google Scholar]
- 30.Jose L.A, Garrote J.A, Arranz E. Cytokines in the pathogenesis of inflammatory bowel diseases. Medicina Clinica (Barc.) 2006;127:145–152. doi: 10.1157/13090382. [DOI] [PubMed] [Google Scholar]
- 31.Kalousova M, Fialova L, Skrha J, Zima T, Soukupova J, Malbohan I.M, Stipek S. Oxidative stress, inflammation and autoimmune reaction in type 1 and type 2 diabetes mellitus. Prague Medical Report. 2004;105:21–28. [PubMed] [Google Scholar]
- 32.Khatami M. Inflammation aging, and cancer: tumoricidal versus tumorigenesis of immunity: a common denominator mapping chronic diseases. Cell Biochemistry and Biophysics. 2009;55:55–79. doi: 10.1007/s12013-009-9059-2. [DOI] [PubMed] [Google Scholar]
- 33.Kim K.J, Lee J.S, Kwak M.K, Choi H.G, Yong C.S, Kim J.A, Lee Y.R, Lyoo W.S, Park Y.J. Anti-inflammatory action of mollugin and its synthetic derivatives in HT-29 human colonic epithelial cells is mediated through inhibition of NF-kappaB activation. European Journal of Pharmacology. 2009;622:52–57. doi: 10.1016/j.ejphar.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Lau T.W, Lam F.F, Lau K.M, Chan Y.W, Lee K.M, Sahota D.S, Ho Y.Y, Fung K.P, Leung P.C, Lau C.B. Pharmacological investigation on the wound healing effects of Radix Rehmanniae in an animal model of diabetic foot ulcer. Journal of Ethnopharmacology. 2009;123:155–162. doi: 10.1016/j.jep.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Lee C.H, Chen J.C, Hsiang C.Y, Wu S.L, Wu H.C, Ho T.Y. Berberine suppresses inflammatory agents-induced interleukin-1beta and tumor necrosis factor-alpha productions via the inhibition of IkappaB degradation in human lung cells. Pharmacological Research. 2007;56:193–201. doi: 10.1016/j.phrs.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Lee C.H, Wu S.L, Chen J.C, Li C.C, Lo H.Y, Cheng W.Y, Lin J.G, Chang Y.H, Hsiang C.Y, Ho T.Y. Eriobotrya japonica leaf and its triterpenes inhibited lipopolysaccharide-induced cytokines and inducible enzyme production via the nuclear factor-kappaB signaling pathway in lung epithelial cells. The American Journal of Chinese Medicine. 2008;36:1185–1198. doi: 10.1142/S0192415X0800651X. [DOI] [PubMed] [Google Scholar]
- 37.Lee D.Y, Choi G, Yoon T, Cheon M.S, Choo B.K, Kim H.K. Anti-inflammatory activity of Chrysanthemum indicum extract in acute and chronic cutaneous inflammation. Journal of Ethnopharmacology. 2009a;123:149–154. doi: 10.1016/j.jep.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 38.Lee D.Y, Choo B.K, Yoon T, Cheon M.S, Lee H.W, Lee A.Y, Kim H.K. Anti-inflammatory effects of Asparagus cochinchinensis extract in acute and chronic cutaneous inflammation. Journal of Ethnopharmacology. 2009b;121:28–34. doi: 10.1016/j.jep.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Lee T.Y, Lee K.C, Chen S.Y, Chang H.H. 6-Gingerol inhibits ROS and iNOS through the suppression of PKC-alpha and NF-kappaB pathways in lipopolysaccharide-stimulated mouse macrophages. Biochemical and Biophysical Research Communications. 2009c;382:134–139. doi: 10.1016/j.bbrc.2009.02.160. [DOI] [PubMed] [Google Scholar]
- 40.Leff L. Emerging new therapies in rheumatoid arthritis: what's next for the patient? Journal of infusion nursing. 2006;29:326–337. doi: 10.1097/00129804-200611000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Levin M.E. Management of the diabetic foot: preventing amputation. Southern Medical Journal. 2002;95:10–20. [PubMed] [Google Scholar]
- 42.Li J, Chen C.X, Shen Y.H. Effects of total glucosides from paeony (Paeonia lactiflora Pall) roots on experimental atherosclerosis in rats. Journal of Ethnopharmacology. 2011a;135:469–475. doi: 10.1016/j.jep.2011.03.045. [DOI] [PubMed] [Google Scholar]
- 43.Li R, Chen B, Wu W, Bao L, Li J, Qi R. Ginkgolide B suppresses intercellular adhesion molecule-1 expression via blocking nuclear factor-kappaB activation in human vascular endothelial cells stimulated by oxidized low-density lipoprotein. Journal of Pharmacological Sciences. 2009;110:362–369. doi: 10.1254/jphs.08275fp. [DOI] [PubMed] [Google Scholar]
- 44.Li W, Zhou P, Zhang Y, He L. Houttuynia cordata, a novel and selective COX-2 inhibitor with anti-inflammatory activity. Journal of Ethnopharmacology. 2011b;133:922–927. doi: 10.1016/j.jep.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Y.N, Wu Y.L, Jia Z.H, Qi J.S. Interaction between COX-2 and iNOS aggravates vascular lesion and antagonistic effect of ginsenoside. Journal of Ethnopharmacology. 2008;119:305–311. doi: 10.1016/j.jep.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 46.Libby P. Inflammation and cardiovascular disease mechanisms. The American Journal of Clinical Nutrition. 2006;83:456–460. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- 47.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111:3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 48.Lim H, Lee S.H, Kim Y.S, Kim H.P. Anti-inflammatory activity of phylligenin, a lignan from the fruits of Forsythia koreana, and its cellular mechanism of action. Journal of Ethnopharmacology. 2008;118:113–117. doi: 10.1016/j.jep.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 49.Lin Q.Y, Jin L.J, Cao Z.H, Xu Y.P. Inhibition of inducible nitric oxide synthase by Acanthopanax senticosus extract in RAW264.7 macrophages. Journal of Ethnopharmacology. 2008;118:231–236. doi: 10.1016/j.jep.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 50.Lin W.C, Lin J.Y. Five bitter compounds display different anti-inflammatory effects through modulating cytokine secretion using mouse primary splenocytes in vitro. Journal of Agricultural and Food Chemistry. 2011;59:184–192. doi: 10.1021/jf103581r. [DOI] [PubMed] [Google Scholar]
- 51.Ling S, Zhang H, Zhang D, Zhang L, Bian K. Characterizing effects of solvent specific morus alba components on rat platelet aggregation, vascular tension and macrophage nitrite production. Zhongguo Zhong.Yao Za Zhi. 2010;35:3024–3028. [PubMed] [Google Scholar]
- 52.Liu J, Wang Z.T, Ge B.X. Andrograpanin isolated from Andrographis paniculata, exhibits anti-inflammatory property in lipopolysaccharide-induced macrophage cells through down-regulating the p38 MAPKs signaling pathways. International Immunopharmacology. 2008a;8:951–958. doi: 10.1016/j.intimp.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 53.Liu L, Yuan S, Long Y, Guo Z, Sun Y, Li Y, Niu Y, Li C, Mei Q. Immunomodulation of Rheum tanguticum polysaccharide (RTP) on the immunosuppressive effects of dexamethasone (DEX) on the treatment of colitis in rats induced by 2,4,6-trinitrobenzene sulfonic acid. International Immunopharmacology. 2009a;9:1568–1577. doi: 10.1016/j.intimp.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 54.Liu M.H, Sun J.S, Tsai S.W, Sheu S.Y, Chen M.H. Icariin protects murine chondrocytes from lipopolysaccharide- induced inflammatory responses and extracellular matrix degradation. Nutrition Research. 2010a;30:57–65. doi: 10.1016/j.nutres.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 55.Liu Y, Yan F, Liu Y, Zhang C, Yu H, Zhang Y, Zhao Y. Aqueous extract of rhubarb stabilizes vulnerable atherosclerotic plaques due to depression of inflammation and lipid accumulation. phytotherapy research. 2008b;22:935–942. doi: 10.1002/ptr.2429. [DOI] [PubMed] [Google Scholar]
- 56.Liu Y, Zhang H.G, Jia Y, Li X.H. Panax notoginseng saponins attenuate atherogenesis accelerated by zymosan in rabbits. Biological & Pharmaceutical Bulletin. 2010b;33:1324–1330. doi: 10.1248/bpb.33.1324. [DOI] [PubMed] [Google Scholar]
- 57.Liu Y.N, Pan S.L, Liao C.H, Huang D.Y, Guh J.H, Peng C.Y, Chang Y.L, Teng C.M. Evodiamine represses hypoxia-induced inflammatory proteins expression and hypoxia-inducible factor 1alpha accumulation in RAW264.7. Shock. 2009b;32:263–269. doi: 10.1097/SHK.0b013e31819940cb. [DOI] [PubMed] [Google Scholar]
- 58.Lou T, Zhang Z, Xi Z, Liu K, Li L, Liu B, Huang F. Berberine Inhibits Inflammatory Response and Ameliorates Insulin Resistance in Hepatocytes Inflammation. 26. 2010 doi: 10.1007/s10753-010-9276-2. [DOI] [PubMed] [Google Scholar]
- 59.Lu L.M, Chen X, Xu J.T. Determination methods for inspection of the complexion in traditional Chinese medicine: a review. Zhong.Xi.Yi.Jie.He.Xue.Bao. 2009a;7:701–705. doi: 10.3736/jcim20090801. [DOI] [PubMed] [Google Scholar]
- 60.Lu T.C, Liao J.C, Huang T.H, Lin Y.C, Liu C.Y, Chiu Y.J, Peng W.H. Analgesic and Anti-Inflammatory Activities of the Methanol Extract from Pogostemon cablin. Evidence-based Complementary and Alternative Medicine. 2009b;2011:1–9. doi: 10.1093/ecam/nep183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo Y, Liu M, Yao X, Xia Y, Dai Y, Chou G, Wang Z. Total alkaloids from Radix Linderae prevent the production of inflammatory mediators in lipopolysaccharide-stimulated RAW 264.7 cells by suppressing NF-kappaB and MAPKs activation. Cytokine. 2009;46:104–110. doi: 10.1016/j.cyto.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 62.Mantovani A. Molecular pathways linking inflammation and cancer. Current Molecular Medicine. 2010;10:369–373. doi: 10.2174/156652410791316968. [DOI] [PubMed] [Google Scholar]
- 63.Marrelli A, Cipriani P, Liakouli V, Carubbi F, Perricone C, Perricone R, Giacomelli R. Angiogenesis in rheumatoid arthritis: A disease specific process or a common response to chronic inflammation? Autoimmunity Reviews. 2011;10:595–598. doi: 10.1016/j.autrev.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 64.Mito S. Curcumin ameliorates cardiac inflammation in rats with autoimmune myocarditis. 2011;34:974–9. doi: 10.1248/bpb.34.974. [DOI] [PubMed] [Google Scholar]
- 65.Niu X.F, Zhou P, Li W.F, Xu H.B. Effects of chelerythrine, a specific inhibitor of cyclooxygenase-2, on acute inflammation in mice. Fitoterapia. 2011;82:620–625. doi: 10.1016/j.fitote.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 66.Numerof R.P, Dinarello C.A, Asadullah K. Cytokines as potential therapeutic targets for inflammatory skin diseases. European Cytokine Network. 2005;16:101–103. [PubMed] [Google Scholar]
- 67.Or T.C, Yang C.L, Law A.H, Li J.C, Lau A.S. Isolation and identification of anti-inflammatory constituents from Ligusticum chuanxiong and their underlying mechanisms of action on microglia. Neuropharmacology. 2011b;60:823–831. doi: 10.1016/j.neuropharm.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 68.Or T.C, Yang C.L, Law A.H, Li J.C, Lau A.S. Isolation and identification of anti-inflammatory constituents from Ligusticum chuanxiong and their underlying mechanisms of action on microglia. Neuropharmacology. 2011a;60:823–831. doi: 10.1016/j.neuropharm.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 69.Packard R.R, Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clinical Chemistry. 2008;54:24–38. doi: 10.1373/clinchem.2007.097360. [DOI] [PubMed] [Google Scholar]
- 70.Pan C.H, Kim E.S, Jung S.H, Nho C.W, Lee J.K. Tectorigenin inhibits IFN-gamma/LPS-induced inflammatory responses in murine macrophage RAW 264.7 cells. Archives of Pharmacal Research. 2008;31:1447–1456. doi: 10.1007/s12272-001-2129-7. [DOI] [PubMed] [Google Scholar]
- 71.Pan M.H, Lai C.S, Dushenkov S, Ho C.T. Modulation of inflammatory genes by natural dietary bioactive compounds. Journal of Agricultural and Food Chemistry. 2009a;57:4467–4477. doi: 10.1021/jf900612n. [DOI] [PubMed] [Google Scholar]
- 72.Pan R, Dai Y, Gao X, Xia Y. Scopolin isolated from Erycibe obtusifolia Benth stems suppresses adjuvant-induced rat arthritis by inhibiting inflammation and angiogenesis. International Immunopharmacology. 2009b;9:859–869. doi: 10.1016/j.intimp.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 73.Pan W, Liu Y, Fang Z, Zhu X, Pan W, Kwak S, Yamamoto Y. A compound belonging to traditional Chinese medicine improves nocturnal activity in Parkinson's disease. Sleep Medicine. 2011;12:307–308. doi: 10.1016/j.sleep.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 74.Pari L, Tewas D, Eckel J. Role of curcumin in health and disease. Archives of Physiology and Biochemistry. 2008;114:127–149. doi: 10.1080/13813450802033958. [DOI] [PubMed] [Google Scholar]
- 75.Rao Y.K, Fang S.H, Hsieh S.C, Yeh T.H, Tzeng Y.M. The constituents of Anisomeles indica and their anti-inflammatory activities. Journal of Ethnopharmacology. 2009;121:292–296. doi: 10.1016/j.jep.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 76.Ren Y, Song C.S, Liu X.H, Shi Y, Gao J.F, He X.D. Experimental study on compatible application of heat-clearing and detoxifying drugs with blood circulation improving drugs. Zhongguo Zhong.Yao Za Zhi. 1994;19:626–8. 640. [PubMed] [Google Scholar]
- 77.Ren Z.H, Tong Y.H, Xu W, Ma J, Chen Y. Tanshinone II A attenuates inflammatory responses of rats with myocardial infarction by reducing MCP-1 expression. Phytomedicine. 2010;17:212–218. doi: 10.1016/j.phymed.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 78.Rivest S. Regulation of innate immune responses in the brain. Nature Reviews Immunology. 2009;9:429–439. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- 79.Schmid-Hempel P. Natural insect host-parasite systems show immune priming and specificity: puzzles to be solved. Bioessays. 2005;27:1026–1034. doi: 10.1002/bies.20282. [DOI] [PubMed] [Google Scholar]
- 80.Schoneveld A.H, Hoefer I, Sluijter J.P, Laman J.D, de Kleijn D.P, Pasterkamp G. Atherosclerotic lesion development and Toll like receptor 2 and 4 responsiveness. Atherosclerosis. 2008;197:95–104. doi: 10.1016/j.atherosclerosis.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 81.Shang J.H, Cai X.H, Feng T, Zhao Y.L, Wang J.K, Zhang L.Y, Yan M, Luo X.D. Pharmacological evaluation of Alstonia scholaris: anti-inflammatory and analgesic effects. Journal of Ethnopharmacology. 2010;129:174–181. doi: 10.1016/j.jep.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 82.Shen J.J, Chiang M.S, Kuo M.L, Leu Y.L, Hwang T.L, Liou C.J, Huang W.C. Partially purified extract and viscolin from Viscum coloratum attenuate airway inflammation and eosinophil infiltration in ovalbumin-sensitized mice. Journal of Ethnopharmacology. 2011;135:646–653. doi: 10.1016/j.jep.2011.03.065. [DOI] [PubMed] [Google Scholar]
- 83.Sohn S.H, Ko E, Oh B.G, Kim S.H, Kim Y, Shin M, Hong M, Bae H. Inhibition effects of Vitex rotundifolia on inflammatory gene expression in A549 human epithelial cells. Annals of Allergy Asthma and Immunology. 2009;103:152–159. doi: 10.1016/S1081-1206(10)60169-X. [DOI] [PubMed] [Google Scholar]
- 84.Su Y.W, Chiou W.F, Chao S.H, Lee M.H, Chen C.C, Tsai Y.C. Ligustilide prevents LPS-induced iNOS expression in RAW 264.7 macrophages by preventing ROS production and down-regulating the MAPK. NF-kappaB and AP-1 signaling pathways. International Immunopharmacology. 2011;11:1166–1172. doi: 10.1016/j.intimp.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 85.Sun W, Yu J, Shi Y.M, Zhang H, Wang Y, Wu B.B. Effects of Cordyceps extract on cytokines and transcription factors in peripheral blood mononuclear cells of asthmatic children during remission stage. Zhong.Xi.Yi.Jie.He.Xue.Bao. 2010;8:341–346. doi: 10.3736/jcim20100407. [DOI] [PubMed] [Google Scholar]
- 86.Suo Z, Liu Y, Ferreri M, Zhang T, Liu Z, Mu X, Han B. Impact of matrine on inflammation related factors in rat intestinal microvascular endothelial cells. Journal of Ethnopharmacology. 2009;125:404–409. doi: 10.1016/j.jep.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 87.Tam J.C, Lau K.M, Liu C.L, To M.H, Kwok H.F, Lai K.K, Lau C.P, Ko C.H, Leung P.C, Fung K.P, Lau C.B. The in vivo and in vitro diabetic wound healing effects of a 2-herb formula and its mechanisms of action. Journal of Ethnopharmacology. 2011;134:831–838. doi: 10.1016/j.jep.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 88.Tilg H, Kaser A, Moschen A.R. How to modulate inflammatory cytokines in liver diseases. Liver International. 2006;26:1029–1039. doi: 10.1111/j.1478-3231.2006.01339.x. [DOI] [PubMed] [Google Scholar]
- 89.Tsianos E.V, Katsanos K. Do we really understand what the immunological disturbances in inflammatory bowel disease mean? World Journal of Gastroenterology. 2009;15:521–525. doi: 10.3748/wjg.15.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang S.J, Tong Y, Lu S, Yang R, Liao X, Xu Y.F, Li X. Anti-inflammatory activity of myricetin isolated from Myrica rubra. Sieb et Zucc leaves Planta Medica. 2010;76:1492–1496. doi: 10.1055/s-0030-1249780. [DOI] [PubMed] [Google Scholar]
- 91.Wang Y, Kong L, Lei X, Hu L, Zou H, Welbeck E, Bligh S.W, Wang Z. Comprehensive two-dimensional high-performance liquid chromatography system with immobilized liposome chromatography column and reversed-phase column for separation of complex traditional Chinese medicine Longdan Xiegan Decoction. Journal of Chromatography A. 2009;1216:2185–2191. doi: 10.1016/j.chroma.2008.05.074. [DOI] [PubMed] [Google Scholar]
- 92.Wang Z.M, Zhu S.G, Wu Z.W, Lu Y, Fu H.Z, Qian R.Q. Kirenol upregulates nuclear Annexin-1 which interacts with NF-kappaB to attenuate synovial inflammation of collagen-induced arthritis in rats. Journal of Ethnopharmacology. 2011;137:774–782. doi: 10.1016/j.jep.2011.06.037. [DOI] [PubMed] [Google Scholar]
- 93.Wu L, Li P, Wang X, Zhuang Z, Farzaneh F, Xu R. Evaluation of anti-inflammatory and antinociceptive activities of Murraya exotica. Pharmaceutical Biology. 2010;48:1344–1353. doi: 10.3109/13880201003793723. [DOI] [PubMed] [Google Scholar]
- 94.Wu X.N, Yu C.H, Cai W, Hua J, Li S.Q, Wang W. Protective effect of a polyphenolic rich extract from Magnolia officinalis bark on influenza virus-induced pneumonia in mice. Journal of Ethnopharmacology. 2011;134:191–194. doi: 10.1016/j.jep.2010.11.074. [DOI] [PubMed] [Google Scholar]
- 95.Xie W, Du L. Diabetes is an inflammatory disease: evidence from traditional Chinese medicines. Diabetes, Obesity and Metabolism. 2011;13:289–301. doi: 10.1111/j.1463-1326.2010.01336.x. [DOI] [PubMed] [Google Scholar]
- 96.Xie X, Wang Y, Zhang S, Zhang G, Xu Y, Bi H, Daugherty A, Wang J.A. Chinese red yeast rice attenuates the development of angiotensin II-induced abdominal aortic aneurysm and atherosclerosis. The Journal of Nutritional Biochemistry. 2011 doi: 10.1016/j.jnutbio.2011.02.011. doi:10.1016/j.jnutbio.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 97.Xu G. Treatment of reflux laryngopharyngitis with modified banxia xiexin tang (Pinellia decoction for draining the heart)--a report of 40 cases. Home - Journal of Traditional Chinese Medicine. 2006;26:127–131. [PubMed] [Google Scholar]
- 98.Xu Y, Feng L, Wang S, Zhu Q, Zheng Z, Xiang P, He B, Tang D. Calycosin protects HUVECs from advanced glycation end products-induced macrophage infiltration. Journal of Ethnopharmacology. 2011;137:359–370. doi: 10.1016/j.jep.2011.05.041. [DOI] [PubMed] [Google Scholar]
- 99.Yang C.L, Chik S.C, Li J.C, Cheung B.K, Lau A.S. Identification of the bioactive constituent and its mechanisms of action in mediating the anti-inflammatory effects of black cohosh and related Cimicifuga species on human primary blood macrophages. Journal of Medicinal Chemistry. 2009;52:6707–6715. doi: 10.1021/jm9006164. [DOI] [PubMed] [Google Scholar]
- 100.Yarosh D.B, Galvin J.W, Nay S.L, Pena A.V, Canning M.T, Brown D.A. Anti-inflammatory activity in skin by biomimetic of Evodia rutaecarpa extract from traditional Chinese medicine. Journal of Dermatological Science. 2006;42:13–21. doi: 10.1016/j.jdermsci.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 101.Yi T, Zhao Z.Z, Yu Z.L, Chen H.B. Comparison of the anti-inflammatory and anti-nociceptive effects of three medicinal plants known as “Snow Lotus” herb in traditional Uighur and Tibetan medicines. Journal of Ethnopharmacology. 2010;128:405–411. doi: 10.1016/j.jep.2010.01.037. [DOI] [PubMed] [Google Scholar]
- 102.Zhang D, Nie X, Pan H, Yu L, Yang X, Xu J, Bian K. Study on effect of total saponins from Semen Nigellae on inflammatory mediators and ERK/MAPK pathway in stimulated macrophage. Zhongguo ZhongYao Za Zhi. 2010a;35:2594–2598. [PubMed] [Google Scholar]
- 103.Zhang L, Hu J.J, Lin J.W, Fang W.S, Du G.H. Anti-inflammatory and analgesic effects of ethanol and aqueous extracts of Pterocephalus hookeri (C.B. Clarke) Hoeck Journal of Ethnopharmacology. 2009;123:510–514. doi: 10.1016/j.jep.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 104.Zhang M, Long Y, Sun Y, Wang Y, Li Q, Wu H, Guo Z, Li Y, Niu Y, Li C, Liu L, Mei Q. Evidence for the complementary and synergistic effects of the three-alkaloid combination regimen containing berberine, hypaconitine and skimmianine on the ulcerative colitis rats induced by trinitrobenzene-sulfonic acid. European Journal of Pharmacology. 2011;651:187–196. doi: 10.1016/j.ejphar.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 105.Zhang S.J, Chen Z.X, Jiang K.P, Cheng Y.H, Gu Y.L. The effect of QuYuHuaTanTongLuo Decoction on the non-alcoholic steatohepatitis. Complementary Therapies in Medicine. 2008;16:192–198. doi: 10.1016/j.ctim.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 106.Zhang Y, Wei L, Sun D, Cao F, Gao H, Zhao L, Du J, Li Y, Wang H. Tanshinone IIA pretreatment protects myocardium against ischaemia/reperfusion injury through the phosphatidylinositol 3-kinase/Akt-dependent pathway in diabetic rats. Diabetes Obesity and Metabolism. 2010b;12:316–322. doi: 10.1111/j.1463-1326.2009.01166.x. [DOI] [PubMed] [Google Scholar]
- 107.Zhang Z, Shi L. Anti-inflammatory and analgesic properties of cis-mulberroside A from Ramulus mori. Fitoterapia. 2010;81:214–218. doi: 10.1016/j.fitote.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 108.Zhang Z.L, Zuo Y.M, Wang Q.H, Xiao H.B, Kuang H.X. Effects of Valeriana amurensis on the expressions of iNOS, COX-2 and IkappaCB-alpha in Alzheimer's disease model rat's brain. Zhong Yao Cai. 2010c;33:581–583. [PubMed] [Google Scholar]
- 109.Zhou L, Zhou B.Y. Influence of baicalin on TNF-alpha and soluble intercellular adhesion molecule-1 in rats infected with Pneumocystis carinii. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2009;27:144–147. [PubMed] [Google Scholar]
- 110.Zhu L.H, Bi W, Qi R.B, Wang H.D, Lu D.X. Luteolin inhibits microglial inflammation and improves neuron survival against inflammation. International Journal of Neuroscience. 2011b;121:329–336. doi: 10.3109/00207454.2011.569040. [DOI] [PubMed] [Google Scholar]
- 111.Zhu L.H, Bi W, Qi R.B, Wang H.D, Lu D.X. Luteolin inhibits microglial inflammation and improves neuron survival against inflammation. International Journal of Neuroscience. 2011a;121:329–336. doi: 10.3109/00207454.2011.569040. [DOI] [PubMed] [Google Scholar]