Abstract
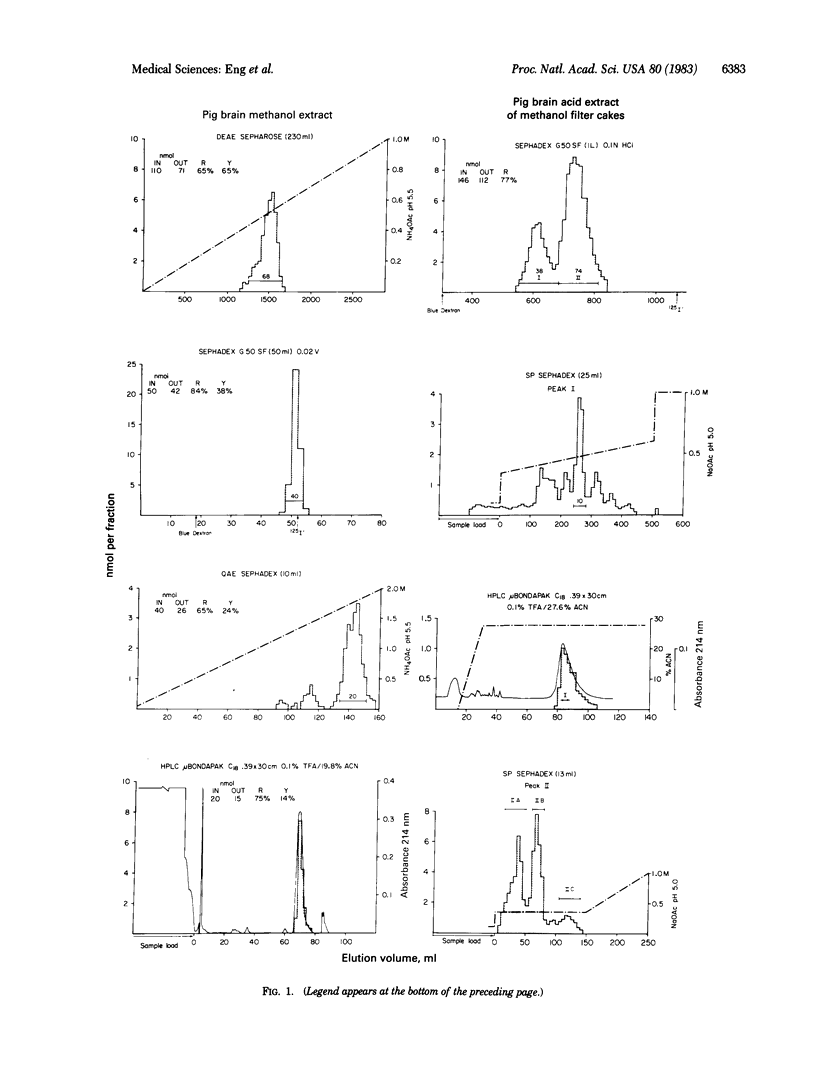
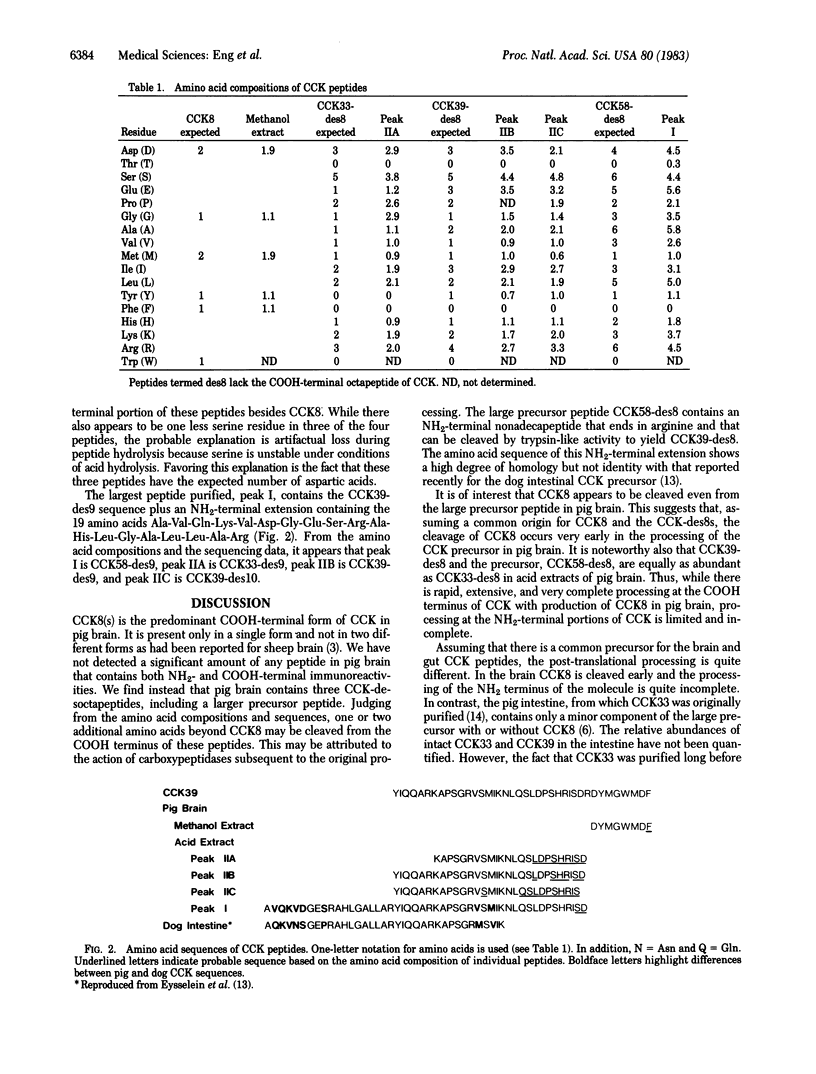
A sequential method employing methanol extraction of the COOH-terminal fragment of cholecystokinin (CCK) from pig brain followed by HCl extraction of the more basic CCK peptides was used as the first step in purification of these peptides. Recovery was monitored with two different assays, one directed to the COOH terminus of CCK and the other to the NH2 terminus. The amino acid content and sequence were determined for each of five peptides after purification. The only peptide containing COOH-terminal immunoreactivity was CCK-octapeptide (CCK8). The other four peptides did not contain CCK8 and had lost one or two additional amino acids, perhaps as a consequence of the action of carboxypeptidases. These peptides were shown to be CCK33-desnonapeptide, CCK39-desnonapeptide and -desdecapeptide, and a large molecular weight precursor, CCK58-desnonapeptide, containing 19 amino acids (Ala-Val-Gln-Lys-Val-Asp-Gly-Glu-Ser-Arg-Ala-His-Leu-Gly-Ala-Leu-Leu-Ala-Arg) NH2-terminal to CCK39. The three NH2-terminal fragments of CCK58, CCK39, and CCK33 were about equally prominent. The brain, unlike the gut, appears to cleave CCK8 rapidly from a precursor peptide but to process the NH2-terminal portions of the molecule more slowly and incompletely.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dockray G. J., Gregory R. A., Hutchison J. B., Harris J. I., Runswick M. J. Isolation, structure and biological activity of two cholecystokinin octapeptides from sheep brain. Nature. 1978 Aug 17;274(5672):711–713. doi: 10.1038/274711a0. [DOI] [PubMed] [Google Scholar]
- Dockray G. J. Immunochemical evidence of cholecystokinin-like peptides in brain. Nature. 1976 Dec 9;264(5586):568–570. doi: 10.1038/264568a0. [DOI] [PubMed] [Google Scholar]
- Eng J., Shiina Y., Straus E., Yalow R. S. Post-translational processing of cholecystokinin in pig brain and gut. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6060–6064. doi: 10.1073/pnas.79.19.6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysselein V. E., Reeve J. R., Jr, Shively J. E., Hawke D., Walsh J. H. Partial structure of a large canine cholecystokinin (CCK58): amino acid sequence. Peptides. 1982 Jul-Aug;3(4):687–691. doi: 10.1016/0196-9781(82)90171-1. [DOI] [PubMed] [Google Scholar]
- Hawke D., Yuan P. M., Shively J. E. Microsequence analysis of peptides and proteins. II. Separation of amino acid phenylthiohydantoin derivatives by high-performance liquid chromatography on octadecylsilane supports. Anal Biochem. 1982 Mar 1;120(2):302–311. doi: 10.1016/0003-2697(82)90351-7. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Hunkapiller M. W., Hood L. E. Direct microsequence analysis of polypeptides using an improved sequenator, a nonprotein carrier (polybrene), and high pressure liquid chromatography. Biochemistry. 1978 May 30;17(11):2124–2133. doi: 10.1021/bi00604a016. [DOI] [PubMed] [Google Scholar]
- Lewis R. V., Stern A. S., Kimura S., Rossier J., Stein S., Udenfriend S. An about 50,000-dalton protein in adrenal medulla: a common precursor of [Met]- and [Leu]enkephalin. Science. 1980 Jun 27;208(4451):1459–1461. doi: 10.1126/science.7384787. [DOI] [PubMed] [Google Scholar]
- Muller J. E., Straus E., Yalow R. S. Cholecystokinin and its COOH-terminal octapeptide in the pig brain. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3035–3037. doi: 10.1073/pnas.74.7.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S., Inoue A., Kita T., Nakamura M., Chang A. C., Cohen S. N., Numa S. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature. 1979 Mar 29;278(5703):423–427. doi: 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- Pinget M., Straus E., Yalow R. S. Localization of cholecystokinin-like immunoreactivity in isolated nerve terminals. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6324–6326. doi: 10.1073/pnas.75.12.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinget M., Straus E., Yalow R. S. Release of cholecystokinin peptides from a synaptosome-enriched fraction of rat cerebral cortex. Life Sci. 1979 Jul 23;25(4):339–342. doi: 10.1016/0024-3205(79)90264-9. [DOI] [PubMed] [Google Scholar]
- Stein S., Böhlen P., Stone J., Dairman W., Udenfriend S. Amino acid analysis with fluorescamine at the picomole level. Arch Biochem Biophys. 1973 Mar;155(1):202–212. doi: 10.1016/s0003-9861(73)80022-0. [DOI] [PubMed] [Google Scholar]
- Straus E., Ryder S., Eng J., Yalow R. S. Nature of immunoreactive CCK in rat and pig brain. Peptides. 1981;2 (Suppl 2):89–92. doi: 10.1016/0196-9781(81)90017-6. [DOI] [PubMed] [Google Scholar]
- Straus E., Yalow R. S. Species specificity of cholecystokinin in gut and brain of several mammalian species. Proc Natl Acad Sci U S A. 1978 Jan;75(1):486–489. doi: 10.1073/pnas.75.1.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhaeghen J. J., Signeau J. C., Gepts W. New peptide in the vertebrate CNS reacting with antigastrin antibodies. Nature. 1975 Oct 16;257(5527):604–605. doi: 10.1038/257604a0. [DOI] [PubMed] [Google Scholar]