Abstract
The aim of this study is to elucidate how the Isatidis Radix (板藍根 bǎn lán gēn) tonic, as an aqueous mixture of hundreds of compositions, interrupts the infection of influenza viruses to their host cells. The efficacy of the tonic was evaluated and expressed as cell proliferation rate and plaque reduction rate in Madin-Darby Canine Kidney (MDCK) cells, against 3 strains of influenza A and B viruses. This boiling water (at 100°C) extract of Isatidis Radix (RIE) showed antiviral activity against influenza virus A and B. The concentration for 50% inhibition of influenza virus A replication (IC50) in MDCK cell was 12.6 mg/mL with a therapeutic index >8. When cells were incubated with RIE prior to virus adsorption, the numbers of viable cell were at least doubled compared to the numbers of virus control, RIE incubation after virus adsorption and RIE incubation with virus prior to adsorption, in both influenza virus A and B. Moreover, much less virus particles were spotted by scanning electron microscope (SEM) in the RIE pre-treated cells than the cells without RIE treatment. These results indicate the antiviral activity of RIE is mainly attributed to its host cell protection effect but not actions on virus or post-virus-adsorption interruption. Cell, but not virus, is more likely to be the action target of RIE.
Keywords: Isatidis Radix (板藍根 bǎn lán gēn), Antiviral effects, Cell binding, Influenza virus, Cell protection
Introduction
Isatidis Radix (板藍根 bǎn lán gēn) is the dried roots of the plant Isatis indigotica Fort. or Isatis tinctoria L. (Fam. Brassicaceae), and a widely-used antiviral traditional Chinese medicine. As an officially approved medicinal material (Chinese Pharmacopoeia Commission, 2005), Isatidis Radix and its decoction (RIE) have been employed as a major herbal tonic in prevention and treatment against a wide range of viral infections, including seasonal flu and the deadly Severe Acute Respiratory Syndrome (SARS) (Lin et al., 2005). Regardless of the different subtypes of viruses and their constant mutations, RIE was found to be clinically effective against infections caused by various subtypes and strains of influenza viruses. The antiviral mechanism of RIE remains unclear, with no indication of the target of the antiviral action of RIE, an essential prerequisite to elucidation of the mechanism. There is, as yet, no explanation of the wide spectrum of activity against all strains of the influenza virus.
In our previous studies (Chen et al., 2006), RIE was demonstrated to prevent the influenza virus induced hemaegglutination, and remarkably change the surface charge of erythrocytes, measuring with capillary electrophoresis of erythrocytes (Lu et al., 2003). It implied that the anti-hemaegglutination activity of RIE might rely on the action upon erythrocytes rather than virus.
In this paper we sought to determined the antiviral activity of RIE using Madin-Darby Canine Kidney (MDCK) cells and 3 strains of influenza virus A and B. Attempts were made to identify either cell or virus the target of RIE's action, by comparison of the antiviral efficacy of RIE interference introduced before or after virus adsorption on MDCK cells.
Materials and Methods
Preparation of the aqueous extracts of Isatidis Radix (RIE)
Fresh roots of Isatis indigotica Fort. were harvested and sun-dried in Fuyang City, Anhui Province, P.R. China. Roots were dried at 20~25℃ (day), 5~10℃ (night), for 2 ~4 weeks. The yield of sun drying was 1 kg sun-dried roots from 2.5 kg fresh roots. The preparation of aqueous extracts of Isatidis Radix (RIE) was following the instructions of China Pharmacopeia (Chinese Pharmacopoeia Commission, 2005). 1400 g sun-dried roots were extracted twice with 3 L boiling water at 100℃, for 2 h and 1 h, respectively. The solution was filtered and vacuum concentrated at 50℃ until the relative density reached 1.20. After the stock was precipitated by 60% ethanol at 4℃, the precipitates were removed, then the suspension was vacuum concentrated at 50℃ to produce the RIE as brown powder.
Cells and virus
Continuous MDCK cells (China Center for Type Culture Collection) were maintained in DMEM (Invitrogen Corporation) with 10% calf serum (GIBCO), penicillin G (100 mg/L), streptomycin (80 mg/L), and sodium bicarbonate (3.7 g/L).
Influenza strains A/Beijing/95-262(H1N1/C2E3, 1:640 8/2/99) and B/Shanghai/93-1(C3C2, 1:320 9/2/99) were purchased from the National Influenza Center of China. Influenza A virus FM1 (H1N1, mouse-adapted strain) was generously provided by Fujian Centre of Disease Control (Fuzhou, Fujian, P.R.China). All viruses were propagated in the allantoic cavity of 11-day-old chick embryos at 33℃ for 48 h. Isolated virus stocks were stored in aliquots of phosphate buffered saline at -80℃.
Determination of Cytotoxicity
Cytotoxic effects of the decoction (1-100 mg/mL) were determined by a dye uptake assay with crystal violet (0.03%, w/v) using MDCK cells (Schmidtke et al., 2001). Six duplicates were use for each concentration. The cell viability was presented as cell survival rate, which is calculated with Equation 1.
Cell survival rate = (mean O.D.sample/ mean O.D.control) × 100% Equation 1
Determination of antiviral activities upon 3 strains of influenza virus A and B
Following the established protocol (Levi et al., 1995), MDCK cell monolayers (105 cells/well) in 96-well plates were washed with PBS and infected with 50 PFU (plaque forming units) of influenza virus A/Beijing/95-262, FM1, B/Shanghai/93-1, for 60 min at 33℃ in the presence or absence of RIE. The infected cells were then incubated with 200 µL maintenance medium containing RIE (1-100 mg/mL) for 3 d. Antiviral activity was determined using MTT assay (van de Loosdrecht et al., 1994) and plaque reduction assay (Gaush and Smith, 1968; Burleson et al., 1992). Cell control with/without the extracts and virus controls were included. Antiviral activity was calculated as a percentage of protection from virus-induced cell proliferation inhibition and cell destruction, in relation to infected cell without RIE and mock-infected control (Equation 2&3). The concentration for 50% inhibition of virus (IC50) were calculated by Reed-Muench method (Reed and Muench, 1938).
Cells protection rate (%) =(O.D. VR − O.D. V)/(O.D. R − O.D. V)×100 Equation 2
(O.D.VR = infected cell with RIE; O.D.V = infected cell without RIE; O.D.R = cell with RIE)
Plaque inhibition rate (%) = [(control -sample)/ control] ×100 Equation 3
Determination of antiviral activities on different stages of viral replication cycle
A single concentration of RIE (50 mg/mL) was introduced to influenza virus A/Beijing/95-262, A FM1 and B/Shanghai/93-1, in three different modes: 1) cell protecting mode: cells were pre-treated with RIE at 33℃ for 180 min before virus adsorption; 2) cell repairing mode: after virus adsorption, infected cells were incubated with RIE at 33℃ for 180 min, and were washed twice with PBS; 3) virus diminishing mode: virus was pre-incubated with RIE at 33℃ for 180 min before being introduced to cells. Antiviral activity of different mode was determined with MTT assay and presented as cell viability, calculated with Equation 1.
Scanning Electron Microscopy (SEM)
Scanning electron microscope was used to directly observe the morphological cytopathogenicity induced by 50 PFU influenza A FM1. MDCK cells were grown to confluence on polyethyleneimine-coated cover slips (10 mm diameter) in 24-well plastic tissue culture plates (16 mm well diameter). RIE (15 mg/mL) was administrated to cells in 3 modes described above. In addition, for investigating RIE's effects on virus adsorption, the cells with/without RIE pre-treatment were incubated with viruses at 4℃ for 1 h to allow the occurrence of virus attachment to cell membrane. The cells were then prepared for SEM observation with an established protocol (Watanabe et al., 2004). After being washed with PBS buffer for 3 times, the cells were fixed with 2.5% glutaraldehyde-2.0% paraformaldehyde in 0.1 M cacodylate buffer (pH 7.4) for 1 h at room temperature, and post-fixed with 1% OsO4 in the same buffer for 45 min, followed by thoroughly washing and dehydrating in ethanol, being treated with isoamyl acetate and dried to a critical point with HCP-2. The cells were mounted on stubs, coated with gold and observed with a scanning electron microscope (JSM5310/LV, JEOL).
Results and Discussion
Cytotoxicity of RIE
Cell survival rates decreased significantly when the concentration of RIE risen from 40 to 100 mg/mL, whereas no significant difference was observed at concentrations lower than 10 mg/mL. The lowest cell survival rate of all assayed RIE concentrations was 53% (at 100 mg/mL), indicating the 50% cytotoxic concentration (CC50) of RIE on MDCK cells is estimative >100 mg/mL.
Antiviral activity against influenza virus A and B
As shown in Table 1, two strains of influenza virus A appeared to be more susceptive to RIE than did the influenza virus B. IC50 of RIE on virus A was under 20 mg/mL, whereas it was around 50 mg/mL on virus B. The therapeutic index (TI) of RIE was >8 on virus A while only >2 on virus B. The different impacts of RIE on influenza A and B viruses warrant further study. Moreover, in this test, the incubation of 100 mg/mL RIE with cells and influenza A virus together raise the cells survival rate up to 91%, and the plaque inhibition rate up to 96%. The survival rate here is higher than its counterpart in the cytotoxicity test (53%) in the presence of high concentration of RIE. It is because the impacts of RIE treatment on the viability of normal cells have been subtracted for the evaluation of RIE's influence on the viral infection. This result indicates that RIE efficiently inhibited viral infection even at its cytotoxic concentrations.
Table 1.
Antiviral activity of extract of Isatidis Radix against 3 strains of influenza virus A and B using MTT assay and plaque reduction assay.
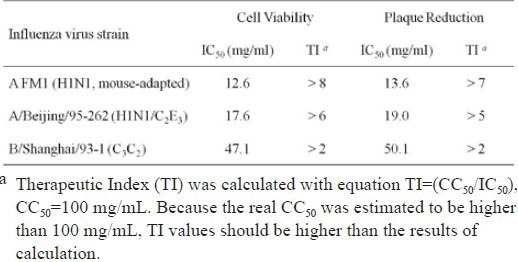
Moreover, the IC50 of RIE ranged from 12.6 to 50.1 mg/mL, which seems too high to be an efficient antiviral agent. However, two factors have to be taken into account. Firstly, during IC50 evaluation, RIE was introduced to cells with the virus together instead of prior to virus incubation, which might provide an undervalued IC50. Secondly, as a mixture, RIE contains hundreds of compositions, of which a small proportion would be responsible for the efficacy. For example, only one fifth of total protein of Isatidis Radix is aqueous soluble. Nagai et al. have reported the anti-influenza virus activity of a glycoprotein from roots of Isatis tinctoria L. (Nagai et al., 1998). Therefore, aqueous soluble proteins may possibly contribute to the antiviral effects of Isatidis Radix. Purification of the effective components of RIE is in progress.
Antiviral activities on different stages of viral replication cycle
As enveloped viruses, the infectious cycle of influenza virus primarily involves four steps: binding to a receptor on the membrane, membrane fusion or entry, replication and assembly, and budding (Whittaker and Digard, 2006). Together these provide several opportunities for antiviral agents to inhibit the viral infectious cycle, by acting on either the host cells or the viruses. For example, some antibodies inhibit the attachment of viruses to cells (Reading and Dimmock, 2007). Peptidyl chloroalkyl ketone inhibitors prevent the fusion event by inhibiting cleavage of the precursor of HA (Tashiro and Rott, 1996); the approved drugs amantadine and rimantadine stop viral particles from releasing into the cytoplasm by the inhibition of viral particles uncoating (Pinto and Lamb, 2007); neuraminidase inhibitor, i.e. Tamiflu, prevents the budding of progeny virus from the cell membrane (Moscona, 2005).
Prior to the antiviral mechanism of RIE and its components are illuminated, the action targets of RIE have to be firstly narrowed down to either cells or viruses. The anti-hemaegglutination activity of RIE attributed to the action upon erythrocytes rather than virus (Chen et al., 2006). Likewise, another traditional Chinese herbal tonic, Momordicae Charantiae Fructus (苦瓜 kǔ guā) extracts, has shown its cytoprotective effects on pancreatic islet cells (Ke et al., 2011). Therefore, RIE is hypothesised to conduct cytoprotective effects on virus host cells, i.e. MDCK cells in this study.
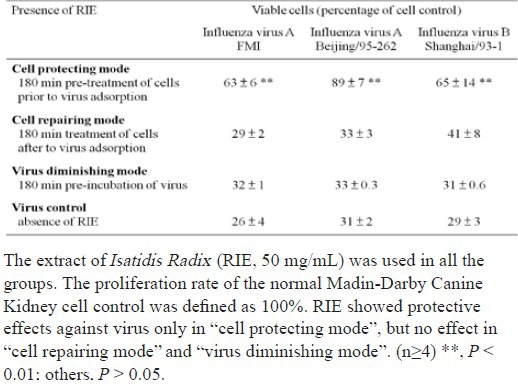
It is important to note that the efficacy of RIE is corresponding to with which stage of virus infection RIE interfered. In contrast with virus control, only the “cell protecting mode” achieved a significant improvement (P < 0.01) on cell survival rates among the three modes, as shown in Table 2, whereas neither “cell repairing mode” nor “virus diminishing mode” did the same. Corresponding with cell survival rates, the virus titres in suspensions measured with the hemaegglutination assay only decreased in the “cell protecting mode” of cells.
Table 2.
Effects of extract of Isatidis Radix (RIE) on viruses, host cells and early steps of infectious cycle of influenza virus A and B using MTT assay.
Consistent with the cell viability tests above, no cytopathogenic effect (CPE) of MDCK cells was observed with SEM in the “cell protecting mode”, in which cells were incubated with RIE prior to virus infection. Much fewer virus particles appeared on the surface of RIE pre-treated cells (Figure 1a) than the cells without RIE treatment (Figure 1b). To ensure the proposed inhibitory effects, the concentration of RIE used in the SEM study was 15 mg/mL, a middle value of the effective concentrations against influenza virus A.
Figure 1.
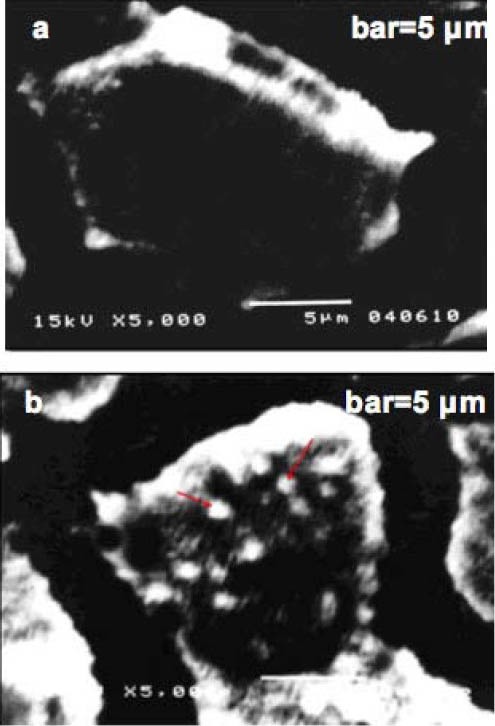
Scanning electron micrographs of Madin-Darby Canine Kidney cells inoculated with 50 PFU influenza A FM1 (H1N1, mouse-adapted) virus, with/without extract of Isatidis Radix (RIE, 15 mg/mL) pre-treatment.
(a) “Protecting mode: with RIE pre-treatment”, cells were incubated with viruses at 4°C , magnified ×5000;
(b) “Control: without RIE pre-treatment”, cells were incubated with viruses at 4°C , magnified ×5000;
Cells were fixed with Karnovsky's fixative, post-fixed with 1% OsO4 for 45 min, dehydrated in ethanol, treated with isoamyl acetate and dried to a critical point with HCP-2. The cells were mounted on stubs, coated with gold and observed with a scanning electron microscope (JSM5310/LV, JEOL).
Arrows: virus particles bound to the microvilli and other parts of plasma membrane.
Two assumptions are drawn from the results. Firstly, as RIE failed to prevent viral infection by direct actions on viruses and neutralising them, RIE is assumed to act on MDCK cells rather than influenza viruses. Secondly, RIE can bind to cell plasma membrane or enter cell plasma and affect the early events of viral infection, i.e. binding and fusion of membrane, and thereafter uncoating of virus particles in cytoplasm. These assumptions are based on following observations. As any free RIE was thoroughly washed off after incubation with cells in all three groups, only those RIE who bound to the cell plasma membrane or internalised into the cell plasma would contribute to the rise of cell survival rates. Furthermore, because of the stronger effects of “protecting mode” than “repairing mode”, it is crucial for RIE to participate cell-virus interaction in an early stage, particular prior to the presence of viruses.
Hence, the inhibitory effects of RIE on viral infection may be confined to the adsorption and fusion stage of infection. This assumption is supported by SEM studies assayed, in which very few virus particles were seen to bind to the RIE pre-treated cells.
Conclusions
The present study indicates that the decoction of Isatidis Radix inhibits the attachment of influenza virus to MDCK cells by binding to and subsequently modifying the cell plasma membrane. This is the first time the target of an antiviral traditional Chinese medicine has been located in the host cells. Potentially, a new family of antiviral agents may be developed from RIE, using its unique antiviral approach of preventing the virus from attaching to its host cell plasma membrane. Furthermore, as it targets on an universal substance, the cell plasma membrane, this approach may function upon a wide range of enveloped viruses, regardless of their families, types, strains or year of epidemic. More beautifully, due to the high conservativeness of membrane lipids, this approach is expected to have a low risk of developing virus resistance. Further studies are requested to purify the effective components of RIE and identify their binding sites on cell membrane.
Acknowledgements
This research was supported by the National Basic Research Program of China (Grant No.2010CB530605); National Natural Science Foundation of China (Grant N o. No.30973871). Fujian Centre of Disease Control (Fuzhou, Fujian, P.R.China) is acknowledged for generous providing the influenza virus strains and part of virological study facilities. Hutchison Whampoa Guangzhou Baiyunshan Chinese Medicine Co. Ltd. (Guangzhou, P.R.China) is acknowledged for providing the raw Isatidis Radix materials.
References
- 1.Burleson F.G., Chambers M.T., Wiedbrauk L.D. Techniques for assessing antiviral agents. In: Burleson F.G., editor. Virology A Laboratory Manual. California: Academic Press, Inc; 1992. pp. 74–85. [Google Scholar]
- 2.Chen Z.W., Liu S.T., Cai C.P., Rao P.F., Ke L.J. Mechanism study of anti-influenza effects of radix Isatidis water extract by red blood cells capillary electrophoresis. China Journal of Chinese Material Medica. 2006;31:1715–1719. [PubMed] [Google Scholar]
- 3.China Pharmacopeia Committee. China Pharmacopeia. 2005 ed. Section One. Chemical Industry Press; 2005. [Google Scholar]
- 4.Gaush C.R., Smith T.F. Replication and plaque assay of influenza virus in an established line of canine kidney cells. Applied Microbiology. 1968;16:588–594. doi: 10.1128/am.16.4.588-594.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ke L.J., Zhou J.W., Lu W., Gao G.Z., Rao P.F. The power of soups: super-hero or team-work? Trends in Food Science and Technology. 2011;22:492–497. [Google Scholar]
- 6.Levi R., Beeor-Tzahar T., Arnon R. Microculture virus titration-a simple colourimetric assay for influenza virus titration. Journal of Virological Methods. 1995;52:55–64. doi: 10.1016/0166-0934(94)00137-6. [DOI] [PubMed] [Google Scholar]
- 7.Lin C.W., Tsai F.J., Tsai C.H., Lai C.C., Wan L., Ho T.Y., Hsieh C.C., Chao P.L. Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and Plant-derived Phenolic Compounds. Antiviral Research. 2005;68:36–42. doi: 10.1016/j.antiviral.2005.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu W.H., Deng W.H., Liu S.T., Chen T.B., Rao P.F. Capillary electrophoresis of erythrocytes. Analytical Biochemistry. 2003;314:194–198. doi: 10.1016/s0003-2697(02)00533-x. [DOI] [PubMed] [Google Scholar]
- 9.Moscona A. Neuraminidase Inhibitors for Influenza. New England Journal of Medicine. 2005;353:1363–1373. doi: 10.1056/NEJMra050740. [DOI] [PubMed] [Google Scholar]
- 10.Nagai T., Yang J.W., Sakurai M., Yamada H. Anti-influenza viral glycoprotein from the roots of Isatis tinctoria L. Antivira Research. 1998;37:88. [Google Scholar]
- 11.Pinto L.H., Lamb R.A. The M2 Proton Channels of Influenza A and B Viruses. Journal of Biological Chemistry. 2007;281:8997–9000. doi: 10.1074/jbc.R500020200. [DOI] [PubMed] [Google Scholar]
- 12.Reading S.A., Dimmock N.J. Neutralization of animal virus infectivity by antibody. Archives of Virology. 2007;152:1047–1059. doi: 10.1007/s00705-006-0923-8. [DOI] [PubMed] [Google Scholar]
- 13.Reed L., Muench H. A simple method of estimating fifty percent endpoints. The American Journal of Hygiene. 1938;27:493–497. [Google Scholar]
- 14.Schmidtke M., Schnittler U., Jahn B., Dahse H., Stelzner A. A rapid assay for evaluation of antiviral activity against coxsackie virus B3, Influenza virus A, and herpes simplex virus type 1. Journal of Virological Methods. 2001;95:133–143. doi: 10.1016/s0166-0934(01)00305-6. [DOI] [PubMed] [Google Scholar]
- 15.Tashiro M., Rott R. The role of proteolytic cleavage of viral glycoproteins in the pathogenesis of influenza virus infections. Seminars in Virology. 1996;7:237–243. [Google Scholar]
- 16.van de Loosdrecht A., Beelen RHJ, Ossenkoppele G.J., Broekhoven M.G., Langenhuijsen MMAC. A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. Journal of Immunological Methods. 1994;174:311–320. doi: 10.1016/0022-1759(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe S., Watanabe T., Noda T., Takada A., Feldmann H., Jasenosky L.D., Kawaoka Y. Production of novel Ebola virus-like particles from cDNAs: an alternative to Ebola virus generation by reverse genetics. Journal of Virology. 2004;78:999–1005. doi: 10.1128/JVI.78.2.999-1005.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whittaker G.R., Digard P. England: Caister Academic Press; 2006. Entry and intracellular transport of influenza virus, in: Kawaoka Y (Eds), Influenza virology: current topics; pp. 37–64. [Google Scholar]


