Abstract
This article will review selected herbal products from Chinese Materia Medica that are used in Traditional Chinese Medicine. The herbs come from the upper, middle, and lower class medicines as listed in The Divine Husbandman's Herbal Foundation Canon (神農本草經 Shén Nóng Běn Cǎo Jīng). The review will focus on the active constituents of the herbs and their bioactivities, with emphasis on the most recent progress in research for the period of 2003 to 2011.
Keywords: Herbal products, Chinese Materia Medica (CMM) (中藥 zhōng yào), Traditional Chinese Medicine (TCM), Shén Nóng Běn Cǎo Jīng (神農本草經 The Divine Husbandman's Herbal Foundation Canon by Shen Nong)
Introduction
Herbal products, including Chinese Materia Medica (CMM) (中藥 zhōng yào), have been used in Traditional Chinese Medicine (TCM) for thousands of years by generations in China and other Asian countries as foods to promote good health and as drugs to treat disease. For the latter purpose, processed CMM, primarily medicinal herbs, are used mostly in multiple combinations as formulas based upon TCM theories of disease diagnosis.
In TCM, crude CMMs formerly were divided into three categories: upper, middle, and lower medicines, according to The Divine Husbandman's Herbal Foundation Canon (神農本草經 shén nóng běn cǎo jīng) by Divine Husbandman (神農 shén nóng). Top-grade (上品 shàng pǐn) herbs are generally nontoxic and form the basis of dietary functional foods. Low-grade (下品 xià pǐn) herbs can contain toxic substances, which can be used judiciously as medicines, and are generally invaluable as therapeutic agents and also as a source of drug discovery. Medium-grade (中品 zhōng pǐn) herbs fall in between these two categories.
The CMMs used in TCM from all three classes can have various pharmacological effects in the human body. Identification, characterization, and biological evaluations of the specific herbal components can not only validate the traditional use of the herb, but also provide leads for new “single-component” drug discovery and development.
This article will review selected CMM herbs from all three classes. Their bioactive constituents will be discussed with emphasis on research during the period of 2003-2011. The current review updates and expands upon a prior similar review published by the author in 2003 (Lee et al., 2003).
Upper Class Herbs
Ginseng (人參 rén shēn)
Asian Ginseng (亞洲人參 yǎ zhōu rén shēn; the root of Panax ginseng, Araliaceae)
Panax ginseng is a famous traditional herbal medicine, which has been used as a tonic, prophylactic, and restorative agent. The active constituents in ginseng include, but are not limited to, ginsenosides (steroid glycosides/triterpene saponins), polysaccharides, peptides, polyacetylenes, vitamins, phenols, and enzymes (Xiang et al., 2008). Recently, new polysaccharides, which have shown immunomodulation, antitumor, anti-adhesive, antioxidant, and hypoglycemic activities, have been isolated from the leaves, fruits, and roots of P. ginseng (Buettner et al., 2006; Hasegawa et al., 2002; Kaneko and Nakanishi, 2004; Keum et al., 2000; Kitt and Hu, 2000).
P. ginseng is well tolerated by most people, although some concerns have been noted about its use together with coumadin, oral hypoglycemic agents, insulin, and phenelzine (Kiefer and Pantuso, 2003). Various studies have found increasing evidence for the anti-inflammatory effects of extracts of P. ginseng and its constituent ginsenosides, including Rb1, Rd, Rg1, Rg3, Rh1, Rh2, Rh3, and Rp1 (Lee and Lau, 2011). Ultimately, the productions of cytokines and other inflammatory mediators are decreased due to inhibition of signaling pathways activated by inflammatory inducers, such as tumor necrosis factor-alpha (TNF-α) (Lee and Lau, 2011). P. ginseng and ginseng-specific ginsenosides have effects on various metabolic activities (Yin et al., 2008), with possible use for metabolic syndrome and diabetes. The ginsenoside Rg1 has pharmacological actions on the central nervous system and can improve learning and memory in normal rats (Chu and Zhang, 2009). A concise review of general information on P. ginseng plus clinical indications/trials for immune modulation, diabetes, and cancer prevention was published recently (Anonymous, 2009). Analytical methodologies related to this prominent herb have also been reviewed (Qi et al., 2011a).
Both Asian ginseng (P. ginseng C.A. Meyer) and American ginseng (P. quinquefolius L.), which is discussed separately below, are well known as restorative herbs, or adaptogens, and are widely used to enhance vitality, increase stamina, and strengthen resistance to stress, anxiety, and fatigue. The former plant has been used in China and other parts of Asia for thousands of years. However, the latter plant was introduced into southeastern Asia only within the last 400 years (Chen et al., 2008; Hsu et al., 1986), but since then has become one of the most important medicinal plants worldwide. The two ginsengs do have many differences, and their uses are not always interchangeable. Asian ginseng acts more as a stimulant, while the pharmacological profile of American ginseng is calming (Chen et al., 2008; Qi et al., 2011b; Schlag and McIntosh, 2006). American ginseng contains greater total ginsenosides; as a result, it is more useful as an anticancer agent than Asian ginseng (Qi et al., 2011b). Asian ginseng contains ginsenoside Rf, while American ginseng does not (Assinewe et al., 2003; Qi et al., 2010). American ginseng contains 24(R)-pseudoginsenoside F11, while Asian ginseng does not (Qi et al., 2010). Various assessments have also been made of the relative proportions of other ginsenosides in the two ginsengs (Chen et al., 2008; Qi et al., 2011b).
Notoginseng Radix (三七人參 sān qī rén shēn; the root of Panax notoginseng, Araliaceae)
Notoginseng Radix is the root of the Araliaceae plant Panax notoginseng (Burk.) F.H. Chen. This important herbal medicine has a long history of use in Asian countries, particularly to stop bleeding and relieve pain. It is distributed largely in southwestern China, Burma, and Nepal, and is also cultivated commercially in Yunnan and Guangxi provinces of China (Wang et al., 2006a). P. notoginseng is the major active herb in the traditional prescriptions “Yunnan Bai Yao”, which is a hemostatic topical medicine (Fan et al., 2005), and “Pien Tze Huang”, which is used to treat acute or chronic hepatitis and inflammation (Lee et al., 2002), and also showed significant inhibitory effects on human cancers (Lu et al., 2009). These two prescriptions are designated as the only two Class-1 protected TCM in China, and their exact formulas are closely guarded secrets. They are treasured and used by Chinese populations both in Asia and the US.
The bioactivities associated with P. notoginseng include antithrombotic, hepatoprotective, anti-inflammatory, analgesic, antitumor, antihypertensive, anti-atherosclerotic, and neuroprotective effects (Ng, 2006; Wang et al., 2006a). Crude extracts of P. notoginseng and some of its constituent triterpene saponins (ginsenosides Rb1 and Rg1) have shown antitumor activity. Co-administration of ginsenosides with chemotherapeutic drugs (Rd with doxorubicin, Re with cisplatin, Rg3 with capecitabine) was shown to reduce drug resistance and increase tumoricidal activity or anti-angiogenic effects (Aung et al., 2007; Pokharel et al., 2010; Zhang et al., 2008). However, the mechanisms for these actions remain unclear, suggesting that new efforts are certainly needed to provide in-depth scientific evidence to support the pharmacological effects.
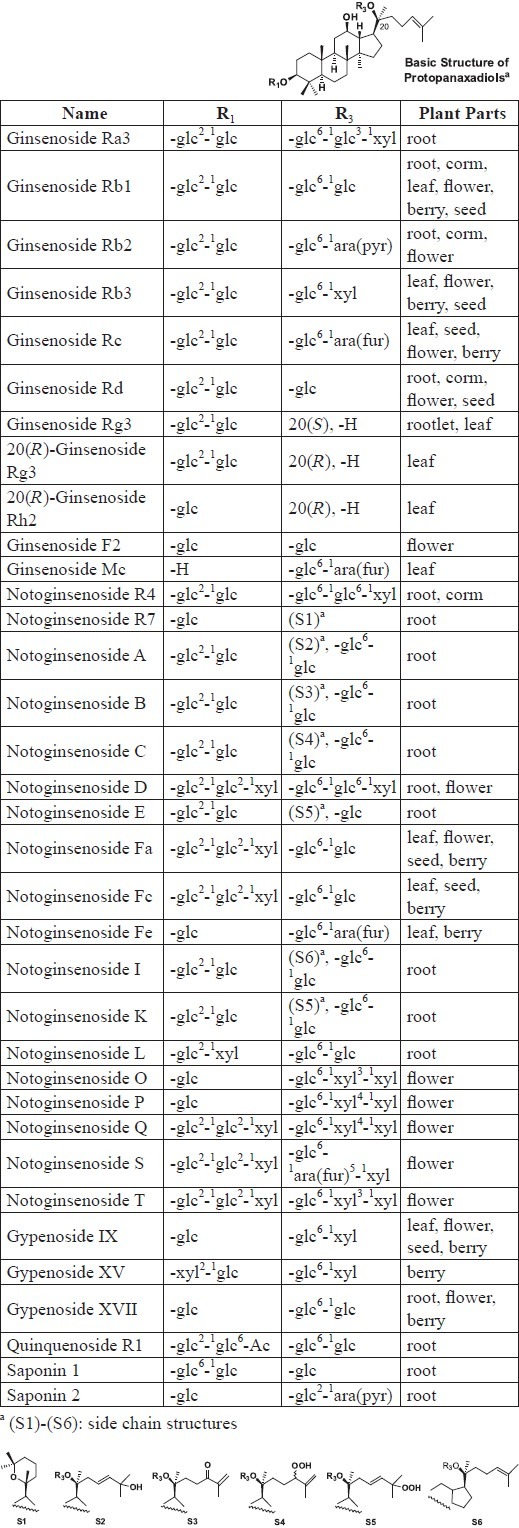
P. notoginseng contains a complex mixture of constituents, including triterpenoid saponins (ginsenosides, notoginsenosides), polysaccharides (e.g., sanchinan A), and amino acids (e.g., L-dencichin). Saponins are the main active constituents, although other components, such as polyacetylenes, phytosterols, and flavonoids, also show pharmacological effects. Currently, 56 saponins have been isolated from P. notoginseng. All 56 compounds are classified as dammarane saponins, with 35 in the protopanaxadiol (PPD) group (Table 1) and 21 in the protopanaxatriol (PPT) group (Table 2) (Wang et al., 2006a). The PPD notoginsenosides are largely unique to P. notoginseng, but their activities remain unclear. To date, no oleanane saponins have been found in P. notoginseng, although they are found in Asian and American ginsengs.
Protopanaxadiol (PPD) saponins isolated from different plant parts of P. notoginseng
Protopanaxatriol (PPT) saponins isolated from different plant parts of P. notoginseng
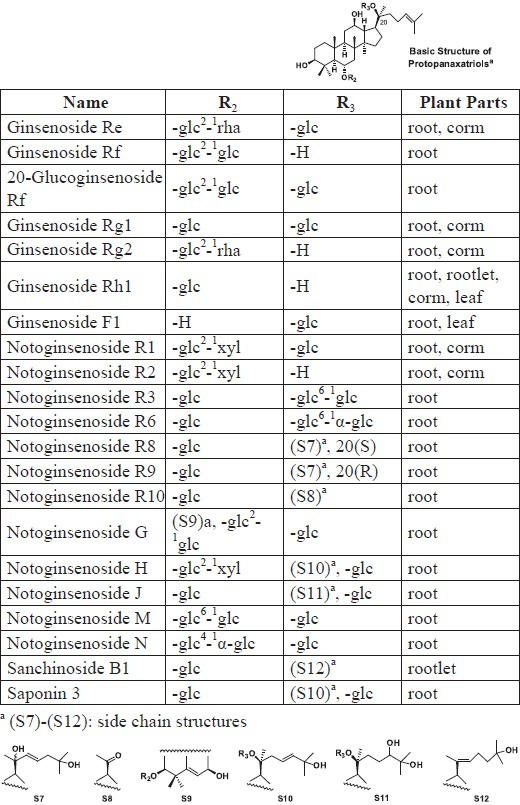
Ginsenosides Rg3, Rg5, and Rh2 can actively reduce cancer cell invasion and metastasis, inhibit cell cycle progression, and induce apoptosis (Jia and Qian, 2011). Thus, the presence of these ginsenosides in P. notoginseng may partially substantiate its anticancer activity. A prior report also suggested that different plant parts (root, rhizome, flower, berry) of P. notoginseng exerted varied antiproliferative effects against human SW480 colorectal cancer cell lines, with the flower extract possessing the most potent activity (Wang et al., 2009). However, because neither Rg3 nor Rh2 is present in the P. notoginseng flower, further investigation is undeniably needed to identify which specific constituent(s) in this plant part result in the enhanced bioactivity.
Overall, P. notoginseng is a very important herbal remedy used in TCM for treating acute/chronic hepatitis and inflammation, and thus, could be considered as an innovative complementary and alternative medicine (CAM) for these purposes. In addition, because inflammation and inflammatory mediators play critical roles in the tumorigenic pathways of breast and skin cancers, P. notoginseng could have potential use as a novel anticancer CAM.
American ginseng (西洋參 xī yáng shēn; the dried root of Panax quinquefolium, Araliaceae)
American ginseng refers to the dried root of Panax quinquefolium, sometimes called P. quinquefolius. It grows in eastern North American temperate forests ranging from southern Quebec to Georgia (Assinewe et al., 2003). In TCM, the dried root is mixed with other herbs and prepared as a decoction or, less frequently, ground into powder, formed into honey pills, or encapsulated as pills (Liu et al., 2005). However, bioactive components have also been found in the leaves and berries (Wang et al., 2006b), as well as the flowers and stems of the plant (Qi et al., 2011).
American ginseng is used in TCM to counter fatigue and weakness, strengthen the immune system (Assinewe et al., 2003; Liu et al., 2005), and treat certain kinds of cough (Liu et al., 2005). The plant root has been associated with immunopotentiating, antioxidant, anti-inflammatory, and antidiabetic properties, as well as cardiovascular, cancer chemoprevention, and cognition effects (Assinewe et al., 2003; Qi et al., 2010; Qi et al., 2011; Wang et al., 2006b; Wang et al., 2010a).
The primary bioactive constituents of American ginseng include ginsenosides (triterpenoid saponins), polysaccharides, and polyacetylenes (Assinewe et al., 2003). Peptides and fatty acids are also present (Qi et al., 2010). As with Asian and Sanqi ginsengs, the protopanaxadiol (PPD) and protopanaxatriol (PPT) groups predominate, although minor ginsenoside groups have been identified (Qi et al., 2010; Qi et al., 2011; Schlag and McIntosh, 2006).
In pharmacological studies on P. quinquefolium, steaming the root decreased the quantity of polar ginsenosides, and resulted in increased activity against human cancer cells (Qi et al., 2010). Sugar number, hydroxyl location, and stereochemistry were postulated to influence anticancer activity in ginsenosides (Qi et al., 2010).
In studies related to safety and quality control, Schlag and McIntosh (2006) investigated ginsenoside profiles of selected wild and cultivated American ginseng populations. They observed two distinct chemotypes among the populations studied, but within a defined chemotype, did not find significant differences between the ginsenoside profiles of wild and cultivated American ginseng. In contrast, Wang et al. (2010a) found significant differences between the chemical profiles of wild and cultivated American ginseng, with the Rg1/Rd ratio being one of several reported distinguishing metrics. Further work is needed in this area to more clearly characterize the ginsenoside profiles of the different sources of American ginseng.
Astragali Radix (黃耆 huáng qí; the root of Astragalus membranaceus, Leguminosae)
Astragali Radix is made from the root of Astragalus membranaceus or A. membranaceus var. mongholicus (Leguminosae). It is sometimes referred to by its Latin name, Radix Astragali, or its common English name, milk-vetch root. Within Asia, A. membranaceus is found in northern China, as well as Mongolia and Siberia (Zhang et al., 2009). In TCM, the root of the plant is dried, mixed with other herbs, and prepared as a decoction (Hsu et al., 1986; Liu et al., 2005). Occasionally, it is used as a powder or formed into honey pills (Liu et al., 2005). While TCM calls for use of the root of the plant only, recent studies have also identified bioactive constituents in the aerial parts of the plant (Yu et al., 2007).
Astragali Radix is used in TCM for fortifying qi, building strength and stamina, ameliorating sweating (including night sweats), reducing swelling, preventing frequent colds, healing abscesses, mitigating nephritis, and for treating spleen-associated deficiencies, including diarrhea (Anonymous, 2003; Hsu et al., 1986; Zhu, 1998). It has also been used to treat diabetes (Yu et al., 2007; Zhang et al., 2009), and as an adjunct therapy for cancer (Anonymous, 2003; Su et al., 2009). Other beneficial properties that have been attributed to the herb include immunopotentiating, antiviral/antibacterial, antioxidant/anti-aging, cardiotonic, and hepatoprotective effects (Hsu et al., 1986; Yu et al., 2007; Zhang et al., 2009).
Significant bioactive constituents of A. membranaceus include saponins (referred to as ASS or AST), flavonoids (AFS), and polysaccharides (APS) (Lu et al., 2011; Zhu, 1998). The triterpenoid saponin astragaloside IV (Figure 1) is the main active component of the herb (Lu et al., 2011; Su et al., 2009). Amino acids, inorganic elements (zinc, iron, copper), choline, betaine, sterols, folic acid, and linoleic acid are also found in this plant (Anonymous, 2003; Lu et al., 2011).
Figure 1.
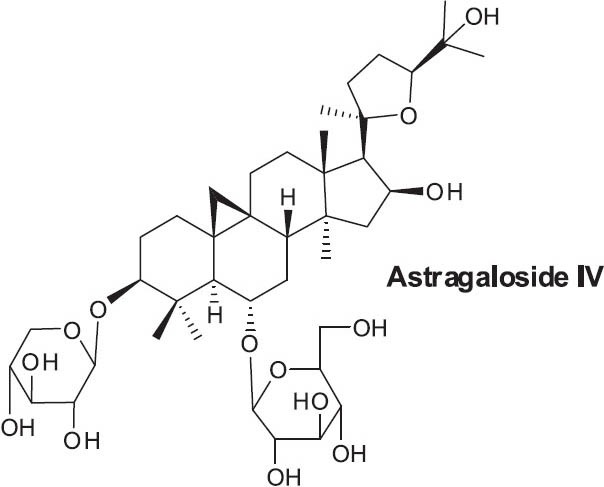
Structure of astragaloside IV from Astragalus membranaceus
Pharmacological studies have associated the immunopotentiating property of A. membranaceus with the polysaccharides and, to a lesser degree, the flavonoids (Zhang et al., 2009). Cardiotonic properties have been attributed to the saponins, including astragaloside IV (Lu et al., 2011). Antioxidant properties have been associated with the saponins, while protection against free radicals has been attributed to the saponins, flavonoids, and polysaccharides (Anonymous, 2003; Zhang et al., 2009).
Astragaloside IV has been studied for applications in treating diabetes and associated conditions (Zhang et al., 2009), as well as cardiovascular disease (Anonymous, 2003; Lu et al., 2011). While it is commonly used as an adjunct treatment for cancer, one study found that it might promote the recurrence of lung cancer (Su et al., 2009). Nevertheless, this compound likely presents the current best lead for new drug discovery from A. membranaceus.
Recently, Zhang et al. (2006) studied the mechanism of action of astragaloside IV on myocardial ischemia in dogs and rats, finding that astragaloside IV reduced the size of myocardial infarcts in dogs and improved multiple measures of heart function following ischemia/reperfusion in rats. The authors proposed that, in addition to providing antioxidant effects, astragaloside IV may enhance endothelium-derived nitric oxide, thereby increasing coronary flow during and following ischemic events. Cui et al. (2003) found that intragastric injection of A. membranaceus reduced the number and size of precancerous foci in rats with induced hepatocarcinogenesis. Cho and Leung (2007) demonstrated that a bioactive fraction of A. membranaceus root extract enhanced immune response in mice in vitro and in vivo. Additionally, the same fraction showed in vitro activity in human lymphocytes. Lee and Jeon (2005) found that APS stimulated production of NO (via inducible NO synthase, or iNOS) in murine macrophages, and that gene expression was mediated by nuclear factor-kappaB (NF-κB)/Rel.
In 2010, PG2, an IV injection of polysaccharides developed from extracts of Astragalus root (Astragali Radix) was approved as a botanical new drug by the Taiwan Food and Drug Administration to alleviate cancer-related fatigue (http://www.phytohealth.com.tw/en/). It is also currently undergoing clinical trials in Taiwan as a treatment for hemorrhagic stroke (http://clinicaltrials.gov/ct2/show/NCT01325233).
Angelicae Sinensis Radix (當歸 dāng guī; the root of Angelica sinensis, Umbelliferae)
Angelicae Sinensis Radix, the dried root of Angelica sinensis (Oliv.) Diels (Umbelliferae), has been used for thousands of years in traditional Chinese, Korean, and Japanese medicines. Angelicae Sinensis Radix is predominantly used in the treatment of various gynecological conditions (Upton, 2003). It has also been widely used to treat anemia, constipation, cardiovascular disease, and hepatic fibrosis (Hou et al., 2005). To date, over 70 compounds have been isolated and identified from Angelicae Sinensis Radix, including essential oils (mainly monomeric phthalides), phthalide dimers, coumarins, organic acids and their esters, polysaccharides, polyacetylenes, vitamins, amino acids, and others. Low-molecular weight compounds such as phthalides and organic acids have been considered to be the major bioactive components. A recent study found that Z-ligustilide, a monomeric phthalide isolated from Angelicae Sinensis Radix, could improve cognitive dysfunction and brain damage (Kuant et al., 2008). Z-Ligustilide and the dimeric neodiligustilide (Figure 2) were identified as the cytotoxic constituents of Angelicae Sinensis Radix extracts (Chen et al., 2007). Angelicae Sinensis Radix polysaccharides exhibit multiple biological activities, such as antitumor effects (Shang et al., 2003) and potent anticoagulant and hemostasis effects (Yang et al., 2002).
Figure 2.
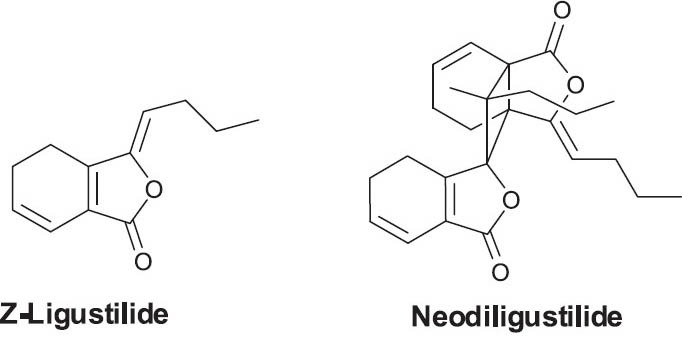
Structures of active phthalides in Angelica sinensis
Eucommiae Cortex (杜仲 dù zhòng; the dried bark of Eucommia ulmoides, Eucommiaceae)
The bark, cortex, and leaves of Eucommia ulmoides have been used since ancient times as analgesics, tonics, and hypotensives in China, Japan, and Korea. The natural products contained in this plant exhibit many pharmacological activities, including anti-oxidative, antifungal, anti-inflammatory, antihypertensive, anti-obesity, and anti-aging effects. (Hirata et al., 2011; Kim et al., 2009; Zhou et al., 2009; Zhu et al., 2009). Some specific constituents of Eucommiae Cortex and their pharmacological effects are given below (Luo et al., 2004a).
-
(1)
Flavonoids: rutin, quercetin, kaempferol, astragalin
-
(2)
Phenolic derivatives: pyrogallol, protocatechuic acid, coumaric acid, chlorogenic acid (Glc-6-P- translocase inhibitor)
-
(3)
Triterpenoids and lignans: betulinic acid, quercetin 3-O-glucopyranoside, pinoresinol-di-O-β-D-glucopyranoside (antihypertensive), quercetin 3-O-sambubioside (antioxidant and anticarcinogenic), kaempferol 3-O-rutinoside
-
(4)
Iridoids: asperuloside, asperulosidic acid, deacetyl asperulosidic acid, scandoside 10-O-acetate, geniposidic acid (prevents aging and stimulates collagen synthesis), geniposide (antithrombotic effect), aucubin (inhibits NF-κB activation)
Three new iridoids (named eucomosides A-C) (Figure 3) were isolated recently, two of which may be regarded as the first naturally occurring conjugates of an iridoid and an amino acid. However, their activity profiles were not yet available (Takamura et al., 2007).
Figure 3.
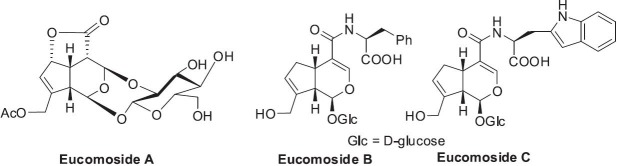
Structures of iridoids in Eucommia ulmoides
Jujubae Fructus (大棗 dà zǎo; the fruit of Ziziphus jujuba, Rhamnaceae) & Ziziphi Spinosi Semen (酸 棗仁 suān zǎo rén; the seeds of Ziziphus jujube var. spinosa, Rhamnaceae)
The Ziziphus jujuba species grows in the Chinese provinces of Gansu, Hebei, Henan, Ningxia, Shandong, Shaanxi, Shanxi, and Xinjiang (Guo et al., 2009). It is the source of two herbal products that have significance in TCM. Jujubae Fructus is prepared from the dried fruit (“Chinese Date” is the common English name) of Z. jujuba, while Suan Zao Ren, or “Sour Jujube Seed,” is made from the dried seed of Z. jujuba var. spinosa (Guo et al., 2011; Han et al., 2009; Liu et al., 2005; Zhao et al., 2006). Although the products come from plants of the same species, their pharmacological properties and applications in TCM are distinct from one another.
Jujubae Fructus
Jujubae Fructus is used in TCM for strengthening the spleen and stomach, fortifying the blood, and relaxing the mind (Hsu et al., 1986; Liu et al., 2005; Zhu, 1998). It is also used for “harmonizing” mixtures of medicinal herbs (Hsu et al., 1986; Liu et al., 2005). Anti-inflammatory, hepatoprotective, and anti-allergic properties have been attributed to Jujubae Fructus, and it has also been used in the management of diabetes (Goyal et al., 2011).
Chemical constituents of Jujubae Fructus include sugars, nucleotides, alkaloids, flavonoids (such as swertish and spinosin), terpenoids, triterpenes (such as betulonic acid, betulinic acid, oleanonic acid, oleanolic acid, and ursolic acid), ziziphus saponins (I, II, and III), and jujubosides A and B (Figure 4) (Goyal et al., 2011; Hsu et al., 1986; Zhu, 1998).
Figure 4.

Structures of selected compounds found in Ziziphus jujuba
While many recent pharmacological studies involving Z. jujuba have focused on Ziziphi Spinosi Semen (discussed further below), some work has been reported for Jujubae Fructus. Goyal et al. (2011) found that Z. jujuba fruit prevented inflammation in rat. Also, Hatano et al. (2005) reported that procyanidins B3 and B4 (Figure 4), two dimeric procyanidins isolated from Z. jujuba fruit, effectively lowered the antibiotic resistance of methicillin-resistant Staphylococcus aureus (MRSA). These compounds may present the most promising current leads for new drug discovery from the fruit of Z. jujuba.
Ziziphi Spinosi Semen
Ziziphi Spinosi Semen is used in TCM for treating insomnia and anxiety (Guo et al., 2011; Han et al., 2009; Liu et al., 2005; Ma et al., 2008; Zhao et al., 2006). Pain-relieving and anti-convulsant properties have also been attributed to the herb (Han et al., 2009; Ma et al., 2008; Zhu, 1998).
Chemical constituents of Ziziphi Spinosi Semen include alkaloids, flavonoids, saponins (including jujubosides A and B), and fatty acids (Cao et al., 2010; Ma et al., 2008; Zhao et al., 2006; Zhu, 1998).
Some pharmacological studies have associated sedative effects with swertisin, spinosin, and acylspinosins based on animal experiments (Zhu, 1998), while others have attributed the same effects to saponins and fatty acids (Zhao et al., 2006), or also the alkaloids (Ma et al., 2008). Clearly, further work is needed to resolve the principle bioactive components of Ziziphi Spinosi Semen and elucidate mechanisms of action.
In 2009, Han et al. found that sanjoinine A (Figure 4), an alkaloid from the seeds of Z. jujuba var. spinosa, improved measures of anxiety in mice, without locomotor or grip force effects, at low doses. Ma et al. (2008) demonstrated that sanjoinine A proffers anti-seizure effects in mice and rats. Cao et al. (2010) reported that jujubosides induced sleep in rats, dependent on time of day.
Lycii Fructus (枸杞子 gǒu qǐ zǐ; the fruit of Lycium barbarmu, Solanaceae)
Lycii Fructus, the fruit of Lycium barbarmu and L. chinenese, has historically been used in East Asia as both food and medicine. The traditional English name for L. barbarum is “wolfberry”. In the last 10 years, Lycii Fructus has become more common in Western markets, where it is sold as “Goji berries” in food products ranging from trail mix to yogurt to juice (Potterat, 2010). The plant is cultivated in the Ningxia and Xinjiang regions, as well as Inner Mongolia, Hebei province, and other areas of northern China (Zheng et al., 2010). While the berries and root bark (fructus Lycii and cortex Lycii radicis, respectively) are the parts of the plant most commonly used in TCM, the leaves also have medicinal properties (Yao et al., 2011). TCM calls for the berries to be prepared as a decoction or ground into a powder and mixed with other herbs (Liu et al., 2005).
Lycii Fructus is used in TCM to improve eyesight and to strengthen the liver and kidney (Liu et al., 2005). Other applications include infertility, cough, and fatigue (Potterat, 2010). Anti-aging, antioxidant, immunomodulating, hypotensive, antimicrobial/ antifungal/antiviral, anti-diabetic, neuroprotective, and anticancer properties have been associated with L. barbarum (Amagase and Farnsworth, 2011; Yao et al., 2011).
The berries of L. barbarum contain polysaccharides (known as LBP, or L. barbarum polysaccharides), carotenoids, including zeaxanthin (Figure 5), vitamins, and flavonoids (Amagase and Farnsworth, 2011; Potterat, 2010). The roots of L. barbarum or L. chinense have been reported to contain alkaloids, flavonoids, betaine, vitamin C, and the cyclic octapeptides lyciumins A-D (Figure 5) (Morita et al., 1996), among other components (Potterat, 2010).
Figure 5.
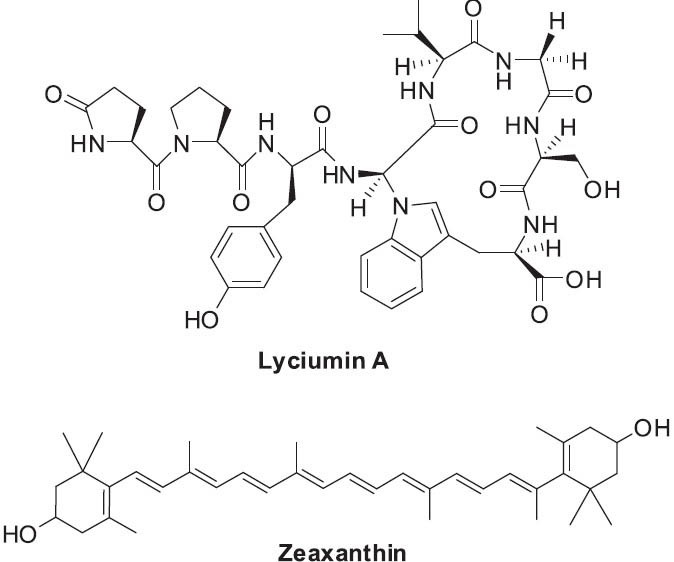
Structures of selected compounds found in Lycium barbarmu
The LBP polysaccharides found in Lycii Fructus are associated with the antioxidant, immunomodulatory, ant i tumor, hypotens ive, neuroprotect ive, and hepatoprotective effects (Amagase and Farnsworth, 2011; Yao et al., 2011). The beneficial effects on age-related eye diseases are attributed to the carotenoid zeaxanthin, which occurs in the fruit (Potterat, 2010). The anti-aging properties associated with Lycii Fructus appear to be due in large part, although not exclusively, to antioxidant effects (Chang and So, 2008; Yao et al., 2011). Antimicrobial activity has been reported for alcoholic extracts of L. barbarum root bark (Yao et al., 2011), and several components found in the root bark have been associated with hypotensive effects. Specifically, lyciumins A and B were found to inhibit angiotensin converting enzyme (ACE) and renin in vitro (Yahara et al., 1989). These and similar constituents are among various opportunities for new drug discovery with L. barbarum.
In 2003, Gan et al. showed that a L. barbarum polysaccharide-protein complex increased activity of interleukin-2 and tumor necrosis factor-α in human peripheral blood mononuclear cells in vitro. Their results indicate potential application of LBP to tumor immunotherapy. Also, Luo et al. (2004b) found that LBP caused reduction of blood glucose levels, total serum cholesterol, and serum triglyceride concentrations in alloxan-induced diabetic or hyperlipidemic rabbits, together with a concurrent increase in high density lipoprotein cholesterol (HDL-c). Finally, Li et al. (2007) observed decreased lipid peroxides (MDA), increased antioxidant enzymes (SOD, CAT, GSH-Px), increased total antioxidant activity (TAOC), and reduced lipofuscin (LPF) in tissues of aged mice treated with LBP compared with controls. These results support the use of L. barbarum to counter age-related oxidative stress.
Salviae Miltiorrhizae Radix (丹參 dān shēn; the root of Salvia miltiorrhiza, Labiatae)
Salviae Miltiorrhizae Radix (the roots of Salvia miltiorrhiza Bung, also called Chinese sage) is one of the most important ancient Chinese herbal drugs (Zhang et al., 1990). Danshen has been used extensively in TCM for the treatment of coronary heart diseases, particularly angina pectoris and myocardial infarction (Wang et al., 2007). It has also been used for centuries in the treatment of inflammatory diseases, such as edema, arthritis, endangitis, hemorrhage, dysmenorrhea, and miscarriage, as well as chronic hepatitis and liver fibrosis (Jang et al., 2003; Liu et al., 2000a; Ryu et al., 1997; Wu et al., 1991a).
Salviae Miltiorrhizae Radix contains two main groups of chemical constituents. The first group is phenolic acids, such as caffeic acid and salvinal (Ai and Li, 1988; Wang et al., 2007). Since the 1980s, over 20 phenolic acids have been isolated from this plant. The second group includes abietane-type diterpene quinone pigments such as tanshinone I, tanshinone IIA, and cryptotanshinone, which are more lipophilic (Dong et al., 2011a). Currently, more than 40 diterpenoids have been isolated from S. miltiorrhiza. The major chemical constituents from both groups and their biological activities are highlighted below.
Phenolic acids (Figure 6) are water soluble compounds with a phenolic ring and a β-carboxylic acid, including caffeic acid monomers and oligomers; the latter are also called depsides or salvianolic acids. Phenolic acids, such as salvianolic acid A, caffeic acid, and magnesium lithospermate B (Mg salt of lithospermic acid B), displayed significant antioxidant effects through anti-lipid-peroxidation and radical scavenging (Liu et al., 1992). Magnesium lithospermate B was also effective against renal failure in rats and significantly reduced blood pressure in hypertensive rats (Yokozawa et al., 1989). Moreover, lithospermic acid and lithospermic acid B inhibited HIV-1 integrase activity at micromolar concentrations (Abd-Elazem et al., 2002). These compounds show other biological effects, including antitumor (salvinal), liver protective (salvianolic acid A), and anticoagulant (salvianolic acid A) (Chang et al., 2004; Li et al., 1984; Liu et al., 2000b).
Figure 6.
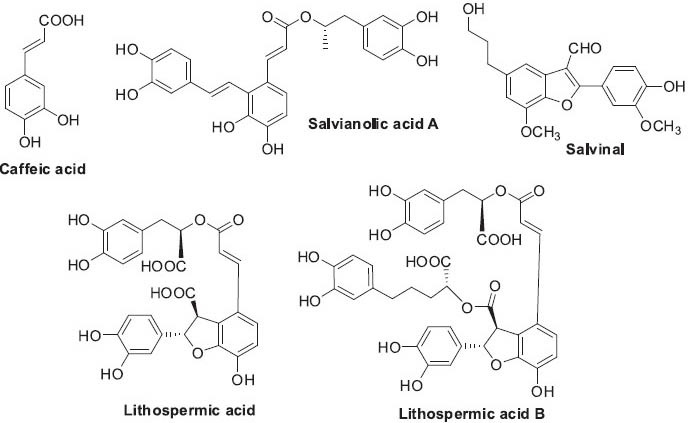
Structures of phenolic acids found in Salvia miltiorrhiza
Tanshinones (Figure 7) generally contain four rings, including naphthalene or tetrahydronaphthalene rings A and B, ortho- or para-quinone or lactone ring C, and furan or dihydrofuran ring D. Tanshinones and their analogs exhibit various pharmacological activities, including antibacterial, antioxidant, anti-inflammatory, anti-allergic, and particularly prominent cardiovascular and antitumor activities (Wang et al., 2007).
Figure 7.
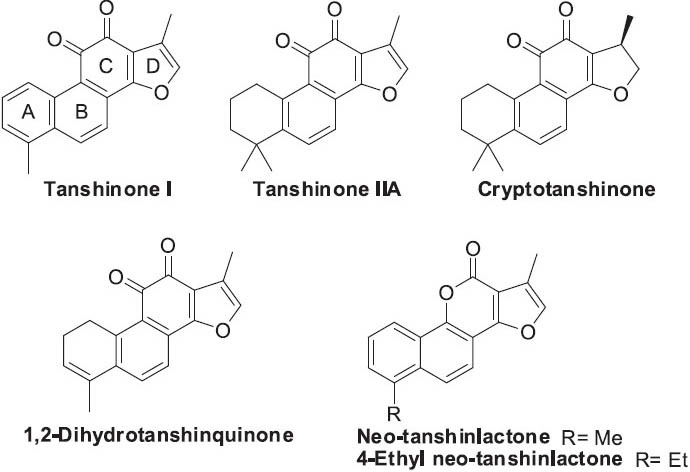
Structures of tanshinones found in Salvia miltiorrhiza and a synthetic analog
Regarding cardiovascular effects, tanshinone I, tanshinone IIA, cryptotanshinone, and 1,2-dihydrotan-shinquinone induced coronary artery dilation in early studies (Chen et al., 1986). Since then, tanshinone IIA has been particularly well studied and, in more recent studies, caused vasodilation in rat coronary arterioles (Wu et al., 2009), as well as inhibited the p38 MAPK signaling and calcineurin/NFATc3 pathways (Tan et al., 2011; Zhang et al., 2010). Sodium tanshinonate IIA sulfonate (STS), a water-soluble derivative of tanshinone IIA, has already been used pre-clinically and clinically in coronary heart disease. It reduced heart infarct sizes and protected hearts against ischemia-reperfusion injury (Yang et al., 2008). STS can also attenuate hypertrophy induced by angiotensin II (Takahashi et al., 2002). In addition, tanshinone I, tanshinone IIA, and 1,2-dihydrotanshinquinone can protect against myocardial ischemia (Yagi et al., 1994). Other tanshinones and their derivatives also exhibited beneficial effects on post-hypoxic recovery of cardiac function (Takeo et al., 1990).
Regarding antitumor effects, tanshinones and their analogs have shown antitumor activity in different cell lines and various animal models. For instance,tanshinone I can induce apoptosis in activated T-HSC/C1-6 hepatic stellate cells, regulate adhesion molecules in human MDA-MB-231 breast cancer cells, and reduce metastasis and tumorigenesis (Kim et al., 2003; Lee et al., 2008a; Nizamutdinova et al., 2008). In addition, tanshinone IIA can induce apoptosis, inhibit cell invasion and metastasis, inhibit angiogenesis, down-regulate epidermal growth factor receptors, and inhibit signal transducers and activators of transcription 3 (Stat 3) (Liu et al., 2006; Liu et al., 2009; Su and Lin, 2008; Tang et al., 2010; Yang et al., 2005a). In studies by the authors’ Natural Products Research Laboratories, neo-tanshinlactone (Figure 7), which has a lactone (NPRL) C-ring rather than an ortho-quinone, showed significant and selective inhibition of two ER+ human breast cancer cell lines and was 10-fold more potent and 20-fold more selective as compared with tamoxifen citrate, a widely used selective estrogen receptor modulator (Wang et al., 2004). Structural modification established structure-activity relationship (SAR) conclusions, and led to 4-ethyl neo-tanshinlactone (Figure 7), which was about twice as active as neo-tanshinlactone against MCF-7 and SK-BR-3 cell lines (Dong et al., 2010a; Wang et al., 2006c). In addition, 4-ethyl neo-tanshinlactone showed potent in vivo activity against a ZR-75-1 xenograft model, but not PC-3 and MDA-MB-231 xenografts. Furthermore, 4-ethyl neo-tanshinlactone was tested independently against cell lines derived from normal breast tissue (MCF10A and 184A1) versus SK-BR-3 as a positive breast cancer cell line control, showing that 4-ethyl neo-tanshinlactone was selective for a subset of breast cancer-derived cell lines and was significantly less active against normal breast-derived tissue. Kinase assays indicated that 4-ethyl neo tanshinlactone significantly suppressed several important protein kinases, including CK2R1, ABL, and AKT1. Lee et al. also designed and developed six series of neo-tanshinlactone analogs (Figure 8): 2-(furan-2-yl)naphthalen-1-ol (FNO) (Dong et al., 2009a), tetrahydronaphthalen-1-ol (TNO) (Dong et al., 2009b), 6-phenyl-4H-furo[3, 2-c]pyran-4-one (AFPO) (Dong et al., 2010b), 4-amino-2H-benzo[h]chromen-2-one (ABO) (Dong et al., 2010c), 4-amino-7, 8, 9, 10-tetrahydro-2H-benzo[h]chromen-2-one (ATBO) (Dong et al., 2011b), and 4-amino-2H-pyran-2-one (APO) (Dong et al., 2011c). Some analogs of FNO, TNO, and AFPO exhibited high anti-breast cancer selectivity, for example, being approximately 100–250-fold more potent against SK-BR-3 than other tested human tumor cell lines (Dong et al., 2009a,b; Dong et al., 2010b). In contrast, lead compounds with the ABO, ATBO, and APO scaffolds generally displayed potent antitumor activity against a broad range of cancer cell lines with ED50 values of 0.008-0.76 μM (Dong et al., 2010c; Dong et al., 2011b,c). More importantly, these lead ABO, ATBO, and APO compounds were seven-fold more potent than paclitaxel against KB-VIN, a vincristine-resistant MDR KB subline. Mechanistic studies are ongoing for the lead compounds. Overall, these novel analogs display significant antitumor activity, high selectivity compared with normal cell lines, and different tumor-tissue-type selectivity. Thus, they show significant promise for development as clinical trials candidates.
Figure 8.
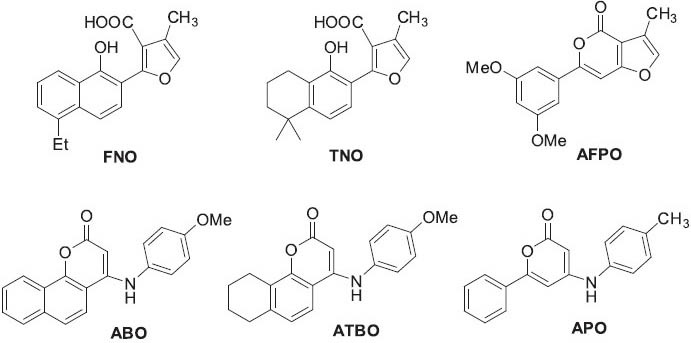
Structures of lead compounds developed based on the structure of neo-tanshinlactone
Coicis Semen (薏苡仁 yì yǐ rén; the kernel of Coix lachryma-jobi var. ma-yuen Stapf, Gramineae)
Coicis semen refers to the dried ripe kernels of Coix lachryma-jobi var. ma-yuen Stapf, which is rich in nutrients and compounds with various pharmacological activities. Recent studies showed that active ingredients in coicis semen could be used to treat flat wart, verruca vulgaris, and infectious condyloma. Coicis semen is also applied as an adjuvant to treat stomach, colon, and cervical cancers (Hu et al., 2009). The ingredients include lipids, polysaccharides, lignans, phenols, and adenosines. Kanglaite injection is an aqueous microemulsion of an oil extracted from the Chinese crude drug by using the latest and most complex modern technologies (Lu et al., 2008). It is a new diphasic broad-spectrum antitumor drug, which has a depressant effect on many kinds of tumor cells through inhibition of NF-κB-dependent transcription (Li, 2001; Wei et al., 2000; Woo et al., 2007). Coixenolide (Figure 9) is one component of the oil (Wei et al., 2000). Also, Ha et al. (2010) identified three polysaccharides that were effective in reducing blood sugar level.
Figure 9.
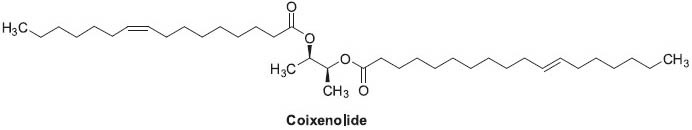
Structure of coixenolide found in Coix
Middle Class Herbs
Chuanxiong Rhizoma (川芎 chuān xiōng; the tuber of Ligusticum chuanxiong, Umbelliferae)
Chuanxiong Rhizoma is the dried root of Ligusticum chuanxiong Hort., Umbelliferae. Chuanxiong Rhizoma is widely used to treat headache, arthralgia, abdominal pain, tendon spasms, amenorrhea, menstrual disorders, and female genital inflammatory diseases. It is mainly distributed in the Chinese provinces of Sichuan, Yunan, Guizhou, Guanxi, and Hubei. Chuanxiong Rhizoma contains essential oils, alkaloids, and organic acids and lactones (Li et al., 2006). Representative compounds include chuanxiongzine, chrysophanic acid, ferulic acid, senkyunone (Figure 10), senkyunolide, ligustilide, neocindilide, and wallichilide (Jiang et al., 2008). A recent study found that sodium ferulate (Figure 10) could eliminate free radicals and protect liver organelles and enzymatic structure (Cai et al., 2007). Essential oils from the related species Cnidium officinale showed significant free radical scavenging ability, and thus, may exert inhibitory effects on DNA damage and apoptosis caused by ultraviolet B radiation (Jeong et al., 2009).
Figure 10.
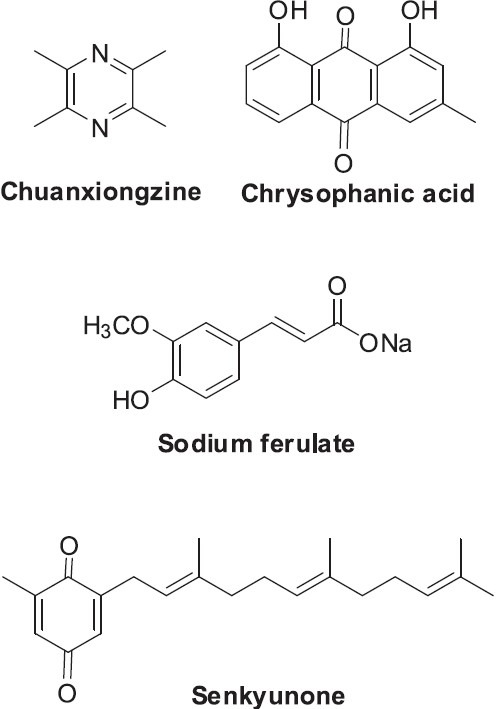
Structures of selected compounds found in Ligusticum chuanxiong
Schisandrae Fructus (五味子 wǔ wèi zǐ; the fruit of Schisandra chinensis, Schisandraceae)
Schisandrae Fructus is made from the berries of Schisandra chinensis, which is widely distributed in northeastern China, Russian, Korea, and Japan. The name means “five-flavour fruit” in Chinese, because it has all five basic flavors: sweet, salty, bitter, sour, and spicy. In TCM, Schisandrae Fructus is used to treat many ailments, such as infections, coughing, thirst, spontaneous diaphoresis, insomnia, and amnesia. Modern phytochemical and pharmacological studies have shown that this family is a rich source of lignans and lanostane- and cycloartane-type triterpenoids, which exhibit various beneficial pharmacological effects such as antihepatitis (Staudinger et al., 2006), antitumor, and anti-HIV-1 (Xiao et al., 2008). Various highly oxygenated, polycyclic nortriterpenoids have been isolated from the Schisandraceae family, including schintrilactones A and B (Figure 11) from S. chinensis (Huang et al., 2007). Both compounds showed weak anti-HIV activity. Two lignans, schisandrol B and schisandrin A (Figure 11), isolated from Schisandrae Fructus extracts, were recently reported to activate the pregnane X receptor and increase warfarin clearance in rats (Mu et al., 2006).
Figure 11.
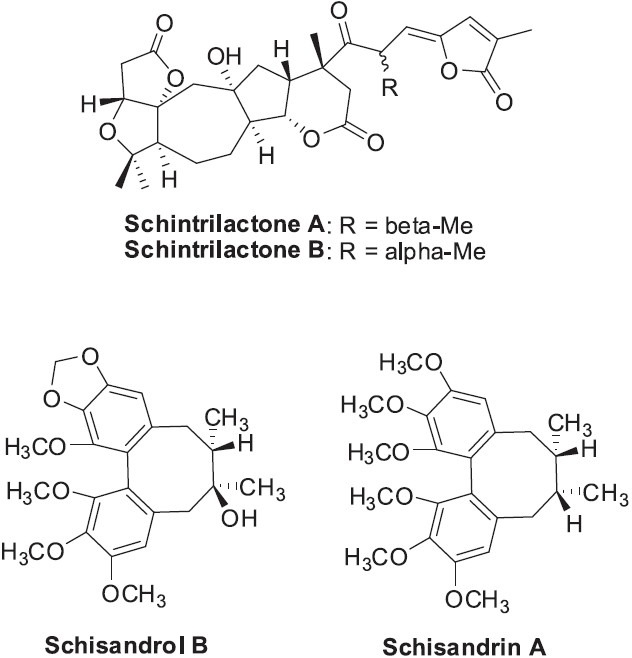
Structures of representative nortriterpenoids and lignans found in Schisandra chinensis
Dried Ginger (or Zingiberis Rhizoma) (乾薑 gān jiāng; the dried tuber of Zingiber officinale, Zingiberaceae)
Dried Ginger (Zingiber officinale) has long been used as a spice worldwide. It is also an important ingredient in Chinese, Ayurvedic, and Tibb-Unani herbal medicines for the treatment of inflammatory joint diseases, such as rheumatism and arthritis, nervous diseases, gingivitis, toothache, asthma, stroke, constipation, and diabetes (Awang, 1992; Tapsell et al., 2006; Wang and Wang, 2005). Ginger's antiemetic and gastroprotective effects are linked to its popular use to counter pregnancy-, motion sickness-, surgery-, and chemotherapy-related nausea (Hoffman, 2007).
Ginger's odor is mainly due to the volatile oil. Its pungency is due to different phenols, both gingerols and shogaols. Ginger and certain components show anti-inflammatory and antioxidant properties. In one study, 6-shogaol exhibited the greatest free radical scavenging potency, while 10-gingerol, with the longest carbon chain length, was more potent than 6- and 8-gingerols (Dugasani et al., 2010). Gingerols and shogaols also exhibit anticancer and anti-metastatic effects (Ling et al., 2010; Sang et al., 2009; Weng et al., 2010), as well as cancer chemopreventive properties (Baliga et al, 2011; Oyagbemi et al., 2010; Shukla and Singh, 2007). Various monoterpenoids and sesquiterpenoids are also found in the plant. Ali et al. reviewed the phytochemistry and pharmacology of ginger (Ali et al., 2008).
-
(1)
Gingerols: 6-gingerol (most abundant) (Figure 12)
-
(2)
Shogaols (dehydrated forms of gingerols): 6-shogaol (Figure 12)
-
(3)
Monoterpenoids: β-phellandrene, (+)-camphene, cineole
-
(4)
Sesquiterpenoids: α-zingiberene, β-sesquiphellandrene
Figure 12.
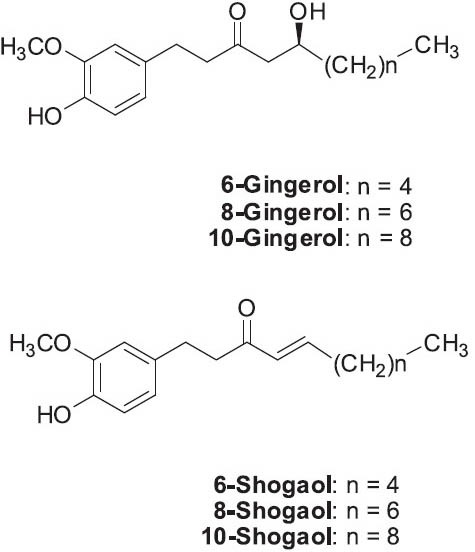
Structures of representative gingerols and shogaols found in Zingiber officinale
Puerariae Radix 葛根 gé gēn; the root of Puerariae lobata, Leguminosae)
Puerariae Radix is the dried root of Pueraria lobata (Willd.) Ohwi or P. thomsonii Benth. Puerariae Radix has been used for centuries in TCM in the form of Puerariae radix (0.02-2%), an isoflavone-rich extract, to treat various conditions including migraine, hypertension, pain, allergies, angina, and alcoholism (Thiem, 2003; Reppert et al., 2008). Daidzein and genistein and their C- and -O-glycosides, most notably the C-glycoside puerarin, are the main isoflavones in Puerariae Radix (Figure 13) (Prasain et al., 2007). These compounds possess pharmacological effects on the heart, blood vessels, brain, and liver, as well as antihyperglycemic and anti-inflammatory effects in diabetes mellitus (Li, 2008; Xie and Du, 2011). The isoflavone daidzein has been studied for such beneficial effects as protection against coronary heart disease, reduction of high blood pressure, decrease of blood fat, inhibition of hormone-related tumors, and prevention of osteoporosis (Wang and Lu, 2007).
Figure 13.

Structures of common isoflavones found in Puerariae lobata
Lower Class Herbs
Kansui Radix (甘遂 gān suì; the root of Euphorbia kansui, Euphorbiaceae)
The dried roots of the herb Euphorbia kansui are well known as “Kan Sui” or “Gan Sui” in TCM. They have been used to treat edema, ascites, and cancer (Chang et al., 2010). Various terpene compounds found in E. kansui have a broad range of pharmacologic properties such as antiviral, anticancer, antinematodal, anti-allergic, and pesticidal activities (Dang et al., 2010; Nunomura et al., 2006; Shi et al., 2007). Some specific constituents and their pharamacological properties are listed below.
Ingenols are the most abundant diterpene derivatives in E. kansui. Among them, kansuiphorins A-D have been linked to anticancer activity (Pan et al., 1991; Wu et al., 1991b). Kansuiphorin A also showed cytotoxic activity against various leukemia, melanoma, non-small cell lung, colon, and renal cancer cell lines, and kansuiphorins A and B (Figure 14) suppressed P-388 leukemia tumor growth in mice with T/C values of 176 and 177% at 0.1 and 0.5 mg/kg, respectively (Pan et al., 1991; Wu et al., 1991b). The inhibition of cancer cell proliferation is due, at least in part, to suppression of topoisomerase II (Miyata et al., 2006; Yoshida et al., 2010). E. kansui also contains jatrophane-type diterpenes, including kansuinines A-J (Chang et al., 2010; Guo et al. 2010a). Kansuinin E (Figure 14) specifically enhanced the survival of TrKA-expressing fibroblasts, which are high-affinity receptors for nerve growth factor (Pan et al., 2004). Kansuinone (Figure 14), a newly found euphane-type triterpene, was reported to exhibit inhibitory activity against 11β-hydroxysteroid dehydrogenase type 1 (Guo et al., 2010b).
Figure 14.
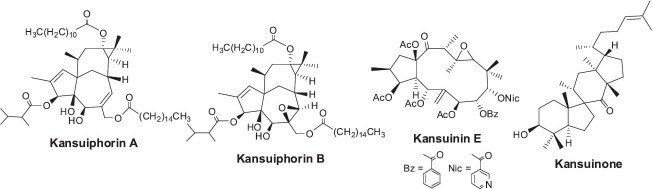
Structures of ingenols and a triterpene found in Euphorbia kansui
Aconiti Radix Lateralis Praeparata (附子 fù zǐ; the lateral root of Aconitum species, Ranunculaceae)
The roots of Aconitum plants have been used as “fù zǐ”, an herbal drug in TCM prescriptions for the treatment of hypometabolism, dysuria, cardiac weakness, chills, neuralgia, gout, and certain rheumatic diseases (Pelletier and Page, 1984; Wang and Chen, 2010; Wang et al., 2010b). Many diterpenoid alkaloids have been isolated from various Aconitum species, and are classified according to their chemical structure as C18-, C19- and C20-diterpenoid alkaloids (Amiya and Bando, 1988; Pelletier and Page, 1986; Wang and Chen, 2010). The first group includes lappaconitine (Figure 15) and ranaconitine; the second group includes aconitine (Figure 15), jesaconitine, and mesaconitine; and the third group includes lucidusculine (Figure 15), atisine, kobusine, and pseudokobusine. The former five compounds in the C18 and C19 groups are highly toxic, while the latter four compounds in the C20 group are far less toxic. Lappaconitine (C18-diterpenoid alkaloid), 3-acetylaconitine (C19-diterpenoid alkaloid), and crassicauline A (C19-diterpenoid alkaloid) have been used clinically in China as non-narcotic analgesic drugs. Lappaconitine and guanfu-base A (a C20-diterpenoid alkaloid) have been used in Russia and China for the therapeutic treatment of arrhythmia (Wang and Chen, 2010). Recently, some C19- and C20-diterpenoid alkaloids were investigated for cytotoxic properties in human tumor cells (Chodoeva et al., 2005; de Ines et al., 2006; Hazawa et al., 2009; Hazawa et al., 2011; Wada et al., 2007; Wada et al., 2011), and three novel C20-diterpenoid alkaloid derivatives, 11, 15-dianisoylpseudokobusine, 11-anisoylpseudokobusine and 11,15-di-p-nitrobenzoylpseudokobusine, showed significant suppressive effects, with IC50 values of 1.7, 2.2, and 2.7 µM, respectively, against A549 human lung carcinoma cells (Wada et al., 2011).
Figure 15.

Structures of diterpenoids alkaloids found in Aconitum
Stellerae seu Euphorbiae Radix (狼毒 láng dú; the root of Stellera chamaejasme, Euphorbia fischeriana, E. ebracteolata, Euphorbiaceae)
The TCM “láng dú” has been used for more than 5000 years. In Chinese characters, “láng” means wolf and “dú” means poison, probably a reference to the toxicity of the TCM, which is classified as a lower class medicine. The most characteristic constituents of “láng dú” are structurally unique tri- or tetra-cyclic diterpenoids, especially with daphnane-, ingenane-, and tigliane-type skeletons. Most of these diterpenoids are tumor-promoting and pro-inflammatory agents and are responsible for the skin irritant and toxic effects of the herb. The original sources of this crude drug were mainly the dried roots of three species, Stellera chamaejasme L. (Thymelaeaceae), Euphorbia fischeriana Steud. (Syn. E. pallasii Turcz.) and E. ebracteolata Hayata (Euphorbiaceae); the first two species are discussed individually below.
Stellera chamaejasme L. (Thymelaeaceae) is a toxic perennial herb widespread in northern and southern China and Nepal. The dried root (ruì xiāng láng dú) is used as an emulgent and dermatological agent. Studies on the chemical constituents have identified biflavanoids and lignans with antitumor, antimalarial, and antibacterial activities (Xu et al., 2001; Yang et al., 2005b). Daphnane-type diterpenes, such as huratoxin, simplexin, and pimelea factor P2 (Figure 16), were also isolated from the roots using piscicidal activity on killifish as a guide (Niwa et al., 1983). Very recently, three new 1-alkyldaphnane-type diterpenes, stelleralides A, B, and C, and two known compounds gnidimacrin and wirkstroelide F (Figure 16) were isolated from this plant. Stelleralide A showed extremely potent anti-HIV activity (EC90: 0.4 nM) with low cytotoxicity (IC50: 4.3 μM) and appears to be a promising compound for development as an anti-AIDS clinical trial candidate (Asada et al., 2011).
Figure 16.

Structures of diterpenes found in Stellera chamaejasme
Euphorbia fischeriana Steud. (Syn. E. pallasii Turcz.) (Euphorbiaceae) is a perennial herbaceous plant distributed mainly in northern China. The dried root is used as the TCM “Bai Lang Du” to treat edema and indigestion and as an expectorant. The plant contains various unique diterpenoids, including pimarane-, abietane-, modified abietane-, and tigliane-type diterpenoids. Many of these diterpenes exhibited significant cytotoxic activities against several tumor cell lines (Wang et al., 2006d). Interestingly, prostratin (Figure 17) has also been isolated from this herb (Ma et al., 1997). This tigliane type diterpene was originally isolated from a Samoan folk medicinal plant [Homalanthus nutans (Foster) Pax (Euphorbiaceae)]. It exhibited efficient anti-HIV activity, as well as a unique ability to expose hidden virus to the action of other drugs (Gustafson et al, 1992).
Figure 17.
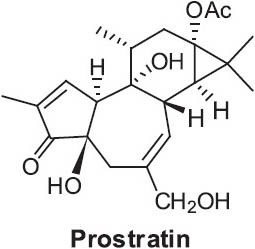
Structure of prostratin found in Euphorbia fischeriana
Dysosmae Versipellis Rhizoma (鬼臼 guǐ jiù; the tuber of Podophyllum emodi, Berberidaceae)
Podophyllum emodi Wall. (syn. P. hexandrum) is a traditional herbal medicine (Kuei-chiu) used for treating rheumatism, cough, stomach ache, and fractures in China. It is a primary source of podophyllum lignans (Figure 18), of which podophyllotoxin is the main component. The podophyllum lignans show various biological activities such as significant antitumor, mitotic spindle inhibition, antiviral, and insecticidal activities (Bedows and Hatfield, 1982; Inamori et al., 1986). While podophyllotoxin is too toxic to use as a cancer drug in humans, it has been an important precursor for significant semisynthetic antineoplastic drugs, such as etoposide, etopophos, and teniposide.
Figure 18.
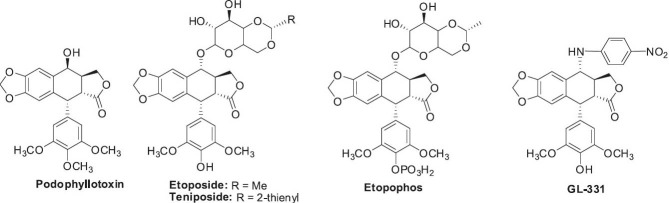
Structures of podophyllotoxin found in Podophyllum emodi and semisynthetic analogs used as anticancer agents
Much research has been and continues to be performed to optimize the structures of podophyllotoxin-related derivatives and improve the pharmacological profiles of this compound class (Botta et al., 2001; Canel et al., 2000; Damayanthi and Lown, 1998; You, 2005), including the development of the arylamino analog GL-331 by the authors’ NPRL (Lee, 2010). In addition, research efforts are also focused on developing alternative and renewable sources of podophyllotoxin from plants and in vitro cultures to facilitate sufficient production of the semisynthetic anticancer drugs (Farkya et al., 2004; Lamblin et al., 2008).
A review chapter on podophyllotoxins and their analogs was published by Lee and Xiao in 2005, and then updated in 2011.
Prunellae Spica (夏枯草 xià kū cǎo; the dried fruit bunch of Prunella vulgaris, Labiatae)
P. vulgaris L. (xià kū cǎo) has been used as an herbal medicine in China for thousands of years to treat high blood pressure, headaches, lymphatic system disorders, goiter, tuberculosis, and tumors. The plant exhibits a wide spectrum of biological effects, including antimicrobial, anti-inflammatory, antioxidant, antiestrogenic, and immunomodulatory actions, in addition to significant activity against malignant tumors, such as human gingival fibroblast and human thyroid cancer SW579 (Collins et al., 2009; Yoon et al., 2010; Zhang et al., 2007). Other specific compounds and their associated activities are as follows.
Polysaccharides P31 and P32 have shown anti-lung adenocarcinoma activity (Feng et al., 2010a). Various terpenoids found in P. vulgaris have been linked to the indicated activities: oleanolic acid (anti-HIV), ursolic acid (cancer inhibition by STAT-3 pathway), and maslinic acid (anti-colon cancer) (Figure 19) (Gu et al., 2007; Lee et al., 2008a). Phenolics, including caffeic acid, rosmarinic acid, quercetin, and rutin, have been associated with strong antitumor activities via different mechanisms (Feng et al, 2010b). In addition, rosmarinic acid (Figure 19) displayed antitumor and anti-metastatic activity, and decreased migration of Ls174-T cells by 83.33% at a concentration of 80 μg/mL (Xu et al., 2010).
Figure 19.
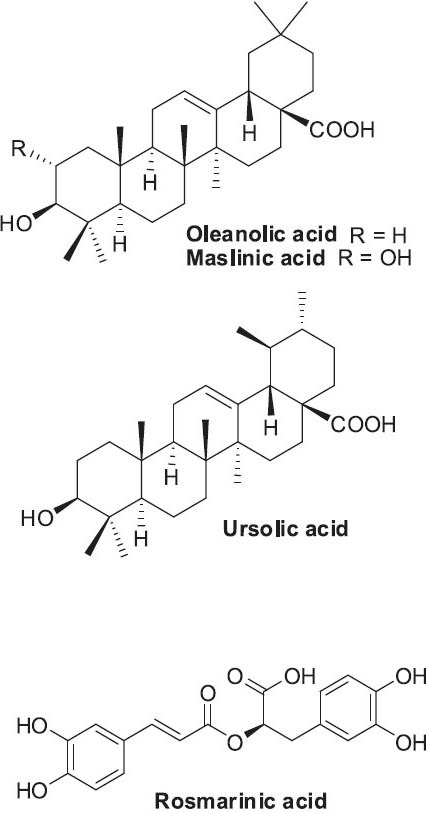
Structures of selected triterpenoids and rosmarinic acid found in Prunella vulgaris
Conclusion
CMM herbal products are used frequently either as single crude drugs or as components in TCM formulas to prevent or treat many diseases. Many of these herb are also used as foods, as well as dietary supplements, to promote good health. Accordingly, special attention must be paid to apply stringent quality control to ensure that these products are free from contamination with heavy metals and pesticides, as well as bacteria or fungi. In addition, the authenticity and origin of these products must be clearly specified and certified, so that the best quality can be maintained. For developing CMM as dietary supplements, toxicological studies must be performed so that the products will be assured as safe. TCM is still the best source for new drug discovery research in the post-genomic era, because its efficacy has been documented by centuries of practical use. Application of the principles and practice of medicinal chemistry is a very efficient and effective method to find new leads from the CMM used in TCM to develop as modern medicines.
Acknowledgement
We would like to thank the Taiwan Department of Health Clinical Trial and Research Center of Excellence (DOH100-TD-B-111-0004) for partial support.
References
- 1.Abd-Elazem I.S., Chen H.S., Bates R.B., Huang RCC. Isolation of two highly potent and non-toxic inhibitors of human immunodeficiency virus type 1 (HIV-1) integrase from Salvia miltiorrhiza. Antiviral Research. 2002;55:91–106. doi: 10.1016/s0166-3542(02)00011-6. [DOI] [PubMed] [Google Scholar]
- 2.Ai C., Li L. Stereostructure of salvianolic acid B and isolation of salvianolic acid C from Salvia miltiorrhiza. Journal of Natural Products. 1988;51:145–149. [Google Scholar]
- 3.Ali B.H., Blunden B., Tanira M.O., Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food and Chemical Toxicology. 2008;46:409–420. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 4.Amagase H., Farnsworth N.R. A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (Goji) Food Research International. 2011;44:1702–1717. [Google Scholar]
- 5.Amiya Tin, Bando H. In: The Alkaloids. Brossi A., editor. Vol. 34. San Diego: Academic Press; 1988. pp. 95–179. [Google Scholar]
- 6.Anonymous. Astragalus membranaceus (Monograph) Alternative Medicine Review. 2003;8:72–77. [PubMed] [Google Scholar]
- 7.Anonymous. Panax ginseng (Monograph) Alternative Medicine Review. 2009;14:172–176. [PubMed] [Google Scholar]
- 8.Asada Y., Sukemori A., Watanabe T., Malla K.J., Yoshikawa T., Li W., Koike K., Chen C.H., Akiyama T., Qian K., Nakagawa-Goto K., Morris-Natschke S.L., Lee K.H. Stelleralides A-C, novel potent anti-HIV daphnane-type diterpenoids from Stellera chamaejasme L. Organic Letters. 2011;13:2904–2907. doi: 10.1021/ol200889s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Assinewe V.A., Baum B.R., Gagnon D., Arnason J.T. Phytochemistry of wild populations of Panax quinquefolius L. (North American ginseng) Journal of Agricultural and Food Chemistry. 2003;51:4549–4553. doi: 10.1021/jf030042h. [DOI] [PubMed] [Google Scholar]
- 10.Aung H.H., Mehendale S.R., Wang C.Z., Xie J.T., Mc Entee E., Yuan C.S. Cisplatin's tumoricidal effect on human breast carcinoma MCF-7 cells was not attenuated by American ginseng. Cancer Chemotherapy and Pharmacology. 2007;59:369–374. doi: 10.1007/s00280-006-0278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Awang DVC. Ginger. Canadian Pharmaceutical Journal. 1992;125:309–311. [Google Scholar]
- 12.Baliga M.S., Haniadka R., Pereira M.M., D’Souza J.J., Pallaty P.L., Bhat H.P., Popuri S. Update on the chemopreventive effects of ginger and its phytochemicals. Critical Reviews in Food Science and Nutrition. 2011;51:499–523. doi: 10.1080/10408391003698669. [DOI] [PubMed] [Google Scholar]
- 13.Bedows E., Hatfield G.M. An investigation of the antiviral activity of Podophyllum peltatum. Journal of Natural Products. 1982;45:725–729. doi: 10.1021/np50024a015. [DOI] [PubMed] [Google Scholar]
- 14.Botta B., Delle Monache G., Misiti D., Vitali A., Zappia G. Arylte tralinlignans: chemistry, pharmacology and biotransformations. Current Medicinal Chemistry. 2001;8:1363–1681. doi: 10.2174/0929867013372292. [DOI] [PubMed] [Google Scholar]
- 15.Buettner C., Yeh G.Y., Phillips R.S., Mittleman M.A., Kaptchuk T.J. Systematic review of the effects of ginsengon cardiovascular risk factors. Annals of Pharmacotherapy. 2006;40:83–95. doi: 10.1345/aph.1G216. [DOI] [PubMed] [Google Scholar]
- 16.Cai D.W., Rao W.T., Yin X.F., Li H. Anti-hepatotoxic activity of sodium ferulate on experimental liver injury. Zhongguo Yaoshi (Wuhan China) 2007;10:975–977. [Google Scholar]
- 17.Canel C., Morses R.M., Dayan R.E., Ferreira D. Podophyllotoxin Phytochemistry. 2000;54:115–120. doi: 10.1016/s0031-9422(00)00094-7. [DOI] [PubMed] [Google Scholar]
- 18.Cao J.X., Zhang Q.Y., Cui S.Y., Cui X.Y., Zhang J., Zhang Y.H., Bai Y.J., Zhao Y.Y. Hypnotic effect of jujubosides from Semen Ziziphi Spinosae. Journal of Ethnopharmacology. 2010;130:163–166. doi: 10.1016/j.jep.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 19.Chang J., Lee S., Park M., Kim M., Hudson B., Park S., Lee W., Rho M. Kansuinine A and kansuinine B from Euphorbia kansui L. inhibit IL-6-induced stat3 activation. Planta Medica. 2010;76:1544–1549. doi: 10.1055/s-0030-1249805. [DOI] [PubMed] [Google Scholar]
- 20.Chang J.Y., Chang C.Y., Kuo C.C., Chen L.T., Wein Y.S., Kuo Y.H. Salvinal a novel microtubule inhibitor isolated from Salvia miltiorrhizae Bunge (Danshen), with antimitotic activity in multidrug-sensitive and -resistant human tumor cells. Molecular Pharmacology. 2004;65:77–84. doi: 10.1124/mol.65.1.77. [DOI] [PubMed] [Google Scholar]
- 21.Chang RCC, So K.F. Use of anti-aging lerbal medicine, Lycium barbarum, against aging-associated diseases What do we know so far? Cellular and Molecular Neurobiology. 2008;28:643–652. doi: 10.1007/s10571-007-9181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen C.C., Chen H.T., Chen Y.P., Hsu H.Y., Hsieh T.C. Isolation of the components of Salviae miltiorrhizae radix and their coronary dilator activities. Taiwan Yaoxue Zazhi. 1986;38:226–230. [Google Scholar]
- 23.Chen C.F., Chiou W.F., Zhang J.T. Comparison of the pharmacological effects of Panax ginseng and Panax quinquefolium. Acta Pharmacologica Sinica. 2008;29:1103–1108. doi: 10.1111/j.1745-7254.2008.00868.x. [DOI] [PubMed] [Google Scholar]
- 24.Chen Q.C., Lee J., Jin W., Youn U., Kim H., Lee I.S., Zhang X., Song K., Seong Y., Bae K. Cytotoxic constituents from Angelicae sinensis radix. Archives of Pharmacal Research. 2007;30:565–569. doi: 10.1007/BF02977650. [DOI] [PubMed] [Google Scholar]
- 25.Cho W.C.S., Leung K.N. Invitro and invivo immunomodulating and immunorestorative effects of Astragalus membranaceus. Journal of Ethnopharmacology. 2007;113:132–141. doi: 10.1016/j.jep.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 26.Chodoeva A., Bosc J.J., Guillon J., Decendit A., Petraud M., Absalon C., Vitry C., Jarry C., Robert J. 8-O-Azeloyl-14-benzoylaconine: a new alkaloid from the roots of Aconitum karacolicum Rapcs and its antiproliferative activities. Bioorganic & Medicinal Chemistry. 2005;13:6493–6501. doi: 10.1016/j.bmc.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Chu S.F., Zhang J.T. New achievements in ginseng research and its future prospects. Chinese Journal of Integrative Medicine. 2009;15:403–408. doi: 10.1007/s11655-009-0403-6. [DOI] [PubMed] [Google Scholar]
- 28.Collins N.H., Lessey E.C., Du Sell C.D., Mc Donnell D.P., Fowler L., Palomino W.A., Illera M.J., Yu X., Mo B., Houwing A.M., Lessey B.A. Characterization of antiestrogenic activity of the Chinese herb, Prunella vulgaris, using in vitro and in vivo (mouse xenograft) models. Biology of Reproduction. 2009;80:375–383. doi: 10.1095/biolreprod.107.065375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui R., He J., Wang B., Zhang F., Chen G., Yin S., Shen H. Suppressive effect of Astragalus membranaceus Bunge on chemical hepatocarcinogenesis in rats. Cancer Chemotherapy and Pharmacology. 2003;51:75–80. doi: 10.1007/s00280-002-0532-5. [DOI] [PubMed] [Google Scholar]
- 30.Damayanthi Y., Lown J.W. Podophyllotoxins: current status and recent developments. Current Medicinal Chemistry. 1998;5:205–252. [PubMed] [Google Scholar]
- 31.Dang Q., Choi Y., Choi G., Jang K., Park M., Park N., Lim C., Kim H., Ngoc L., Kim J. Pesticidal activity of ingenane diterpenes isolated from Euphorbia kansui against Nilaparvata lugens and Tetranychus urticae. Journal of Asia-Pacific Entomology. 2010;13:51–54. [Google Scholar]
- 32.de Ines C., Reina M., Gavin J.A., Gonzalez-Coloma A. In vitro cytotoxicity of norditerpenoid alkaloids. Zeitschrift für Naturforschung C. 2006;61:11–18. doi: 10.1515/znc-2006-1-203. [DOI] [PubMed] [Google Scholar]
- 33.Dong Y., Morris-Natschke S.L., Lee K.H. Biosynthesis total syntheses, and antitumor activity of tanshinones and their analogs as potential therapeutic agents. Natural Product Reports. 2011a;28:529–542. doi: 10.1039/c0np00035c. [DOI] [PubMed] [Google Scholar]
- 34.Dong Y., Nakagawa-Goto K., Lai C.Y., Morris-Natschke S.L., Bastow K.F., Lee K.H. Antitumor agents 278 4-Amino- 2H-benzo[h]chromen-2-one (ABO) analogs as potent in vitro anti- cancer agents. Bioorganic & Medicinal Chemistry Letters. 2010c;20:4085–4087. doi: 10.1016/j.bmcl.2010.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong Y., Nakagawa-Goto K., Lai C.Y., Morris-Natschke S.L., Bastow K.F., Lee K.H. Antitumor agents 281 Design synthesis, and biological activity of substituted 4-amino-7,8,9,10- tetrahydro-2H-benzo[h]chromen-2-one analogs (ATBO) as potent in vitro anticancer agents. Bioorganic & Medicinal Chemistry Letters. 2011b;21:546–549. doi: 10.1016/j.bmcl.2010.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong Y., Nakagawa-Goto K., Lai C.Y., Morris-Natschke S.L., Bastow K.F., Lee K.H. Antitumor agents 287 Substituted 4-amino-2H-pyran-2-one (APO) analogs reveal a new scaffold from neo-tanshinlactone with in vitro anticancer activity. Bioorganic & Medicinal Chemistry Letters. 2011c;21:2341–2344. doi: 10.1016/j.bmcl.2011.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong Y., Shi Q., Liu Y.N., Wang X., Bastow K.F., Lee K.H. Antitumor agents 266 Design synthesis, and biological evaluation of novel 2-(furan-2-yl)naphthalen-1-ol derivatives as potent and selective antibreast cancer agents. Journal of Medicnal Chemistry. 2009a;52:3586–3590. doi: 10.1021/jm9001567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong Y., Shi Q., Nakagawa-Goto K., Wu P.C., Bastow K.F., Morris-Natschke S.L., Lee K.H. Antitumor agents 269 Non-aromatic ring-A neotanshinlactone analog, TNO, as a new class of potent antitumor agents. Bioorganic & Medicinal Chemistry Letters. 2009b;19:6289–6292. doi: 10.1016/j.bmcl.2009.09.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong Y., Shi Q., Nakagawa-Goto K., Wu P.C., Morris-Natschke S.L., Brossi A., Bastow K.F., Lang J.Y., Hung M.C., Lee K.H. Antitumor agents 270 Novel substituted 6-phenyl-4H- furo[3,2-c]pyran-4-one derivatives as potent and highly selective anti-breast cancer agents. Bioorganic & Medicinal Chemistry. 2010b;18:803–808. doi: 10.1016/j.bmc.2009.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong Y., Shi Q., Pai H.C., Peng C.Y., Pan S.L., Teng C.M., Nakagawa-Goto K., Yu D., Liu Y.N., Wu P.C., Bastow K.F., Morris-Natschke S.L., Brossi A., Lang J.Y., Hsu J.L., Hung M.C., Lee E.Y., Lee K.H. Antitumor agents 272 Structure-activity relationships and in vivo selective anti-breast cancer activity of novel neo-tanshinlactone analogues. Journal of Medicinal Chemistry. 2010a;53:2299–2308. doi: 10.1021/jm1000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dugasani S., Pichika M.R., Nadarajah V.D., Balijepalli M.K., Tandra S., Korlakunta J.N. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. Journal of Ethnopharmacology. 2010;127:515–520. doi: 10.1016/j.jep.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Fan C., Song J., White C.M. A comparision of the hemostatic effects of notoginseng and yun nan bai yao to placebo control. Journal of Herbal Pharmacotherapy. 2005;5:1–5. [PubMed] [Google Scholar]
- 43.Farkya S., Bisaria V.S., Srivastava A.K. Biotechnological aspects of the production of the anticancer drug podophyllotoxin. Applied Microbiology and Biotechnology. 2004;65:504–519. doi: 10.1007/s00253-004-1680-9. [DOI] [PubMed] [Google Scholar]
- 44.Feng L., Jia X., Shi F., Chen Y. Identification of two polysaccharides from Prunella vulgaris L and evaluation of their anti-lung adenocarcinoma activity. Molecules. 2010a;15:5093–5103. doi: 10.3390/molecules15085093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng L., Jia X., Zhu M., Chen Y., Shi F. Antioxidant activities of total phenols of Prunella vulgaris L in vitro and in tumor-bearing mice. Molecules. 2010b;15:9145–9156. doi: 10.3390/molecules15129145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gan L., Zhang S.H., Liu Q., Xu H.B. A polysaccharide- protein complex from Lycium barbarum upregulates cytokine expression in human peripheral blood mononuclear cells. European Journal of Pharmacology. 2003;471:217–222. doi: 10.1016/s0014-2999(03)01827-2. [DOI] [PubMed] [Google Scholar]
- 47.Goyal R., Sharma P.L., Singh M. Possible attenuation of nitric oxide expression in anti-inflammatory effect of Ziziphus jujuba in rat. Journal of Natural Medicines. 2011;65:514–518. doi: 10.1007/s11418-011-0531-0. [DOI] [PubMed] [Google Scholar]
- 48.Gu X., Li Y., Li P., Qian S., Zhang J. Triterpenoid saponins from the spikes of Prunella vulgaris. Helvetica Chimica Acta. 2007;90:72–78. [Google Scholar]
- 49.Guo J., Fang X., Di Y., Hua H., Hao X. Kansuinine J, a new macrocyclic diterpenoid from the roots of Euphorbia kansui. Chinese Chemical Letters. 2010a;21:943–946. [Google Scholar]
- 50.Guo J., He H., Fang X., Di Y., Li X., Zhang Z., Leng Y., Hua H., Hao X. Kansuinone a novel euphane-type triterpene from Euphorbia kansui. Tetrahedron Letters. 2010b;51:6286–6289. [Google Scholar]
- 51.Guo S., Duan J., Tang Y., Qian D., Zhu Z., Qian Y., Shang E., Su S. UHPLC-TOFMS coupled with chemometric method as a powerful technique for rapid exploring of differentiating components between two Ziziphus species. Journal of Separation Science. 2011;34:659–666. doi: 10.1002/jssc.201000788. [DOI] [PubMed] [Google Scholar]
- 52.Guo S., Duan J., Tang Y., Su S., Shang E., Ni S., Qian D. High-performance liquid chromatography – Two wavelength detection of triterpenoid acids from the fruits of Ziziphus jujuba containing various cultivars in different regions and classification using chemometric analysis. Journal of Pharmaceutical and Biomedical Analysis. 2009;49:1296–1302. doi: 10.1016/j.jpba.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 53.Gustafson K.R., Cardelina J.H., II, Mc Mahon J.B., Gulakowski R.J., Ishitoya J., Szllasi Z., Lewin N.E., Blumberg P.M., Weislow O.S., Beutler J.A., Buckheit R.W., Jr, Cragg G.M., Cox P.A., Bader J.P., Boyd M.R. A nonpromoting phorbol from the Samoan medicinal plant Homalanthus nutans inhibits cell killing by HIV-1. Journal of Medicinal Chemistry. 1992;35:1978–1986. doi: 10.1021/jm00089a006. [DOI] [PubMed] [Google Scholar]
- 54.Ha do T., Nam Trung T., Bich Thu N., Van On T., Hai Nam N., Van Men C., Thi Phuong T., Bae K. Adlay seed extract (Coix lachryma-jobi L) decreased adipocyte differentiation and increased glucose uptake in 3T3-L1 cells. Journal of Medicinal Food. 2010;13:1331–1339. doi: 10.1089/jmf.2010.1155. [DOI] [PubMed] [Google Scholar]
- 55.Han H., Ma Y., Eun J.S., Li R., Hong J.T., Lee M.K., Oh K.W. Anxiolytic-like effects of sanjoinine A isolated from Zizyphi spinosi Semen: possible involvement of GABAergic transmission. Pharmacology, Biochemistry and Behavior. 2009;92:206–213. doi: 10.1016/j.pbb.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 56.Hasegawa H., Suzuki R., Nagaoka T., Tezuka Y., Kadota S., Saiki I. Prevention of growth and metastasis of murine melanoma through enhanced natural-killer cytotoxicity by fatty acid-conjugate of protopanaxatriol. Biological and Pharmaceutical Bulletin. 2002;25:861–866. doi: 10.1248/bpb.25.861. [DOI] [PubMed] [Google Scholar]
- 57.Hatano T., Kusuda M., Inada K., Ogawa T., Shiota S., Tsuchiya T., Yoshida T. Effect of tannins and related polyphenols on methicillin-resistant Staphylococcus aureus. Phytochemistry. 2005;66:2047–2055. doi: 10.1016/j.phytochem.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 58.Hazawa M., Takahashi K., Wada K., Mori T., Kawahara N., Kashiwakura I. Structure-activity relationships between the Aconitum C20-diterpenoid alkaloid derivatives and the growth suppressive activities of non-Hodgkin's lymphoma Raji cells and human hematopoietic stem/progenitor cells. Investigational New Drugs. 2011;29:1–8. doi: 10.1007/s10637-009-9327-4. [DOI] [PubMed] [Google Scholar]
- 59.Hazawa M., Wada K., Takahashi K., Mori T., Kawahara N., Kashiwakura I. Suppressive effects of novel derivatives prepared from Aconitum alkaloids on tumor growth. Investigational New Drugs. 2009;27:111–119. doi: 10.1007/s10637-008-9141-4. [DOI] [PubMed] [Google Scholar]
- 60.Hirata T., Kobayashi T., Wada A., Ueda T., Fujikawa T., Miyashita H., Ikeda T., Tsukamoto S., Nohara T. Anti-obesity compounds in green leaves of Eucommia ulmoides. Bioorganic & Medicinal Chemistry Letters. 2011;21:1786–1791. doi: 10.1016/j.bmcl.2011.01.060. [DOI] [PubMed] [Google Scholar]
- 61.Hoffman T. Ginger: an ancient remedy and modern miracle drug. Hawaii Medical Journal. 2007;66:326–327. [PubMed] [Google Scholar]
- 62.Hou Y.Z., Zhao G.R., Yuan Y.J., Zhu G.G., Hiltunen R. Inhibition of rat vascular smooth muscle cell proliferation by extract of Ligusticum chuanxiong and Angelica sinensis. Journal of Ethnopharmacology. 2005;100:140–144. doi: 10.1016/j.jep.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 63.Hsu H.Y., Chen Y.P., Shen S.J., Hsu C.S., Chen C.C., Chang H.C. Long Beach: Oriental Healing Arts Institute; 1986. Oriental Materia Medica: A Concise Guide. [Google Scholar]
- 64.Hu S.H., Xiao X.N., Yi X., JP Z. Study progress of coix seed. Shizhen Guoyi Guoyao. 2009;20:1059–1060. [Google Scholar]
- 65.Huang S.X., Yang J., Huang H., Li L.M., Xiao W.L., Li R.T., Sun H.D. Structural characterization of schintrilactone, a new class of nortriterpenoids from Schisandra chinensis. Organic Letters. 2007;9:4175–4178. doi: 10.1021/ol701679n. [DOI] [PubMed] [Google Scholar]
- 66.Inamori Y., Kubo M., Tsujibo H., Ogawa M., Baba K., Kozawa M., Fujitaet E. The biological activities of podophyllotoxin compounds. Chemical & Pharmaceutical Bulletin. 1986;34:3928–3932. doi: 10.1248/cpb.34.3928. [DOI] [PubMed] [Google Scholar]
- 67.Jang S.I., Jeong S.I., Kim K.J., Kim H.J., Yu H.H., Park R., Kim H.M., You Y.O. Tanshinone IIA from Salvia miltiorrhiza inhibits inducible nitric oxide synthase expression and production of TNF-alpha, IL-1beta and IL-6 in activated RAW 264.7 cells. Planta Medica. 2003;69:1057–1059. doi: 10.1055/s-2003-45157. [DOI] [PubMed] [Google Scholar]
- 68.Jeong J.B., Ju S.Y., Park J.H., Lee J.R., Yun K.W., Kwon S.T., Lim J.H., Chung G.Y., Jeong H.J. Antioxidant activity in essential oils of Cnidium officinale makino and Ligusticum chuanxiong Hort and their inhibitory effects on DNA damage and apoptosis induced by ultraviolet B in mammalian cell. Cancer Epidemiology. 2009;33:41–46. doi: 10.1016/j.canep.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 69.Jia L., Qian K.D. An evidence-based perspective of Panax ginseng (Asian ginseng) and Panax quinquefolius (American ginseng) as a preventing or supplementary therapy for cancer patients. In: Cho WCS, editor. Evidence-Based Anticancer Complementary and Alternative Medicine. New York: Springer; 2011. pp. 85–96. [Google Scholar]
- 70.Jiang G., Chen S., Wu Y., Ma Y. Application and quality control on the compound prescription of rhizoma. Chuanxiong Shizhen Guoyi Guoyao. 2008;19:615–618. [Google Scholar]
- 71.Kaneko H., Nakanishi K. Proof of the mysterious efficacy of ginseng: Basic and clinical trials: Clinical effects of medical ginseng, Korean red ginseng: specifically, its anti-stress action for prevention of disease. Journal of Pharmacological Sciences. 2004;95:158–162. doi: 10.1254/jphs.fmj04001x5. [DOI] [PubMed] [Google Scholar]
- 72.Keum Y.S., Park K.K., Lee J.M., Chun K.S., Park J.H., Lee S.K. Antioxidant and anti-tumor promoting activities of the methanol extract of heat-processed ginseng. Cancer Letters. 2000;150:41–48. doi: 10.1016/s0304-3835(99)00369-9. [DOI] [PubMed] [Google Scholar]
- 73.Kiefer D., Pantuso T. Panax ginseng. American Family Physician. 2003;68:1539–1542. [PubMed] [Google Scholar]
- 74.Kim B.H., Park K.S., Chang I.M. Elucidation of anti- inflammatory potencies of Eucommia ulmoides bark and Plantago asiatica seeds. Journal of Medicinal Food. 2009;12:764–769. doi: 10.1089/jmf.2008.1239. [DOI] [PubMed] [Google Scholar]
- 75.Kim J.Y., Kim K.M., Nan J.X., Zhao Y.Z., Park P.H., Lee S.J., Sohn D.H. Induction of apoptosis by tanshinone I via cytochrome c release in activated hepatic stellate cells. Pharmacology & Toxicology. 2003;92:195–200. doi: 10.1034/j.1600-0773.2003.920410.x. [DOI] [PubMed] [Google Scholar]
- 76.Kitt D., Hu C. Efficacy and safety of ginseng. Public Health Nutrition. 2000;3:473–485. doi: 10.1017/s1368980000000550. [DOI] [PubMed] [Google Scholar]
- 77.Kuang X., Du J.R., Liu Y.X., Zhang G.Y., Peng H.Y. Postischemic administration of Z-ligustilide ameliorates cognitive dysfunction and brain damage induced by permanent forebrain ischemia in rats. Pharmacology Biochemistry and Behavior. 2008;88:213–221. doi: 10.1016/j.pbb.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 78.Lamblin F., Hano C., Fliniaux O., Mesnard F., Fliniaux M.A., Lainé E. Interest of lignans in prevention and treatment of cancers. Médecine Sciences (Paris) 2008;24:511–519. doi: 10.1051/medsci/2008245511. [DOI] [PubMed] [Google Scholar]
- 79.Lee C.Y., Sher H.F., Chen H.W., Liu C.C., Chen C.H., Lin C.S., Yan P.C., Tsay H.S., Chen J.J. Anticancer effects of tanshinone I in human non-small cell lung cancer. Molecular Cancer Therapeutics. 2008a;7:3527–3538. doi: 10.1158/1535-7163.MCT-07-2288. [DOI] [PubMed] [Google Scholar]
- 80.Lee D.C., Lau A.S. Effects of Panax ginseng on tumor necrosis factor-α-mediated inflammation: a mini-review. Molecules. 2011;16:2802–2818. doi: 10.3390/molecules16042802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee I.K., Kim D.H., Lee S.Y., Kim K.R., Choi S.U., Hong J.K., Lee J.H., Park Y.H., Lee K.R. Triterpenoic acids of Prunella vulgaris var. Iilacina and their cytotoxic activities in vitro. Archives of Pharmacal Research. 2008b;31:1578–1583. doi: 10.1007/s12272-001-2154-6. [DOI] [PubMed] [Google Scholar]
- 82.Lee K.H. Discovery and development of natural product- derived chemotherapeutic agents based on a medicinal chemistry approach. Journal of Natural Products. 73:500–516. doi: 10.1021/np900821e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee K.H., Xiao Z. Antitumor agents 240 Podophyllotoxins and analogs. In: Cragg G.M., Kingston DGI, Newman D.J., editors. Antitumor Agents from Natural Products. New York: Taylor & Francis; 2005. pp. 71–87. [Google Scholar]
- 84.Lee K., Xiao Z. Antitumor Agent 277. Podophyllotoxins and analogs. In: Cragg G.M, Kingston D.G.I, Newman D.J, editors. Antitumor Agents from Natural Products. Second Edition. Chapter 5. New York: Taylor & Francis; 2011. pp. 95–122. [Google Scholar]
- 85.Lee K.H, Itokawa H., Kozuka M. Oriental herbal products: the basis for development of dietary supplements and new medicines in the 21st century. In: Ho C.T, Lin J.K, Zheng Q.Y, editors. Oriental Foods and Herbs – Chemistry and Health Effects. Washington, D.C: American Chemical Society Press; 2003. pp. 2–31. [Google Scholar]
- 86.Lee K.K., Kwong W.H., Chau F.T., Yew D.T., Chan W.Y. Pien Tze Huang protects the liver against carbon tetrachloride- induced damage. Pharmacology and Toxicology. 2002;91:185–192. doi: 10.1034/j.1600-0773.2002.910406.x. [DOI] [PubMed] [Google Scholar]
- 87.Lee K.Y., Jeon Y.J. Macrophage activation by polysaccharide isolated from Astragalus membranaceus. International Immunopharmacology. 2005;5:1225–1233. doi: 10.1016/j.intimp.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 88.Li D.P. Progress on mechanism of KLT injection antitumour effect. Traditional Chinese Drug Research & Clinical Pharmacology. 2001;2:122–124. [Google Scholar]
- 89.Li J. Research development in flavonoids of Radix Puerariae. Anhui Yiyao. 2008;12:1117–1118. [Google Scholar]
- 90.Li L., Tan R., Chen W. Salvianolic acid A a new depside from roots of Salvia miltiorrhiza. Planta Medica. 1984;50:227–228. doi: 10.1055/s-2007-969684. [DOI] [PubMed] [Google Scholar]
- 91.Li Q., Gan G., Liu M. Chemical composition and pharmacological progress of rhizoma Chuanxiong. Shizhen Guoyi Guoyao. 2006;17:1298–1299. [Google Scholar]
- 92.Li X.M., Ma Y.L., Liu X.J. Effect of the Lycium barbarum polysaccharides on age-related oxidative stress in aged mice. Journal of Ethnopharmacology. 2007;111:504–511. doi: 10.1016/j.jep.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 93.Ling H., Yang H., Tan S.H., Chui W.K., Chew E.H. 6-Shogaol an active constituent of ginger, inhibits breast cancer cell invasion by reducing matrix metalloproteinase-9 expression via blockade of nuclear factor-κB activation. British Journal of Pharmacology. 2010;161:1763–1777. doi: 10.1111/j.1476-5381.2010.00991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu C., Tseng A., Yang S. Boca Raton: CRC Press; 2005. Chinese Herbal Medicine: Modern Applications of Traditional Formulas. [Google Scholar]
- 95.Liu C.H., Liu P., Hu Y.Y., Xu L.M., Tan Y.Z., Wang Z.N., Liu C. Effects of salvianolic acid-A on rat hepatic stellate cell proliferation and collagen productionin culture. Acta Pharmacologica Sinica. 2000b;21:721–726. [PubMed] [Google Scholar]
- 96.Liu G.T., Zhang T.M., Wang B.E., Wang Y.W. Protective action of seven natural phenolic compounds against peroxidative damage to biomembranes. Biochemical Pharmacology. 1992;43:147–152. doi: 10.1016/0006-2952(92)90271-j. [DOI] [PubMed] [Google Scholar]
- 97.Liu J., Shen H.M., Ong C.N. Salvia miltiorrhiza inhibits cell growth and induces apoptosis in human hepatoma HepG2 cells. Cancer Letters. 2000a;153:85–93. doi: 10.1016/s0304-3835(00)00391-8. [DOI] [PubMed] [Google Scholar]
- 98.Liu J.J., Lin D.J., Liu P.Q., Huang M., Li X.D., Huang R.W. Induction of apoptosis and inhibition of cell adhesive and invasive effects by tanshinone IIA in acute promyelocytic leukemia cells in vitro. Journal of Biomedical Science. 2006;13:813–823. doi: 10.1007/s11373-006-9110-x. [DOI] [PubMed] [Google Scholar]
- 99.Liu J.J., Zhang Y., Lin D.J., Xiao R.Z. Tanshinone IIA inhibits leukemia THP-1 cell growth by induction of apoptosis. Oncology Reports. 2009;21:1075–1081. doi: 10.3892/or_00000326. [DOI] [PubMed] [Google Scholar]
- 100.Lu L., Wai M.S., Yew D.T., Mak Y.T. Pien Tze Huang a composite Chinese traditional herbal extract, affects survival of neuroblastoma cells. International Journal of Neuroscience. 2009;119:255–262. doi: 10.1080/00207450802324770. [DOI] [PubMed] [Google Scholar]
- 101.Lu S., Chen K.J., Yang Q.Y., Sun H.R. Progress in the research of Radix Astragali in treating chronic heart failure: effective ingredients, dose-effect relationship and adverse reaction. Chinese Journal of Integrative Medicine. 2011;17:473–477. doi: 10.1007/s11655-011-0756-5. [DOI] [PubMed] [Google Scholar]
- 102.Lu Y., Li C.S., Dong Q. Chinese herb related molecules of cancer-cell-apoptosis: a minireview of progress between Kanglaite injection and related genes. Journal of Experimental & Clinical Cancer Research. 2008;27:31–35. doi: 10.1186/1756-9966-27-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Luo Q., Cai Y., Yan J., Sun M., Corke H. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sciences. 2004b;76:137–149. doi: 10.1016/j.lfs.2004.04.056. [DOI] [PubMed] [Google Scholar]
- 104.Luo X., Ma M., Chen B., Yao S., Wan Z., Yang D., Hang H. Analysis of nine bioactive compounds in Eucommia ulmoides Oliv. and their preparation by HPLC - photo diodearray detection and mass spectrometry. Journal of Liquid Chromatography & Related Technologies. 2004a;27:63–81. [Google Scholar]
- 105.Ma Q.G., Liu W.Z., Wu X.Y., Zhou T.X., Qin G.W. Diterpenoids from Euphorbia fischeriana. Phytochemistry. 1997;44:663–666. [Google Scholar]
- 106.Ma Y., Yun S.R., Nam S.Y., Kim Y.B., Hong J.T., Kim Y., Choi H., Lee K., Oh K.W. Protective effects of sanjoinine A against N-methyl-D-aspartate-Induced seizure. Biological & Pharmaceutical Bulletin. 2008;31:1749–1754. doi: 10.1248/bpb.31.1749. [DOI] [PubMed] [Google Scholar]
- 107.Miyata S., Wang L., Yoshida C., Kitanaka S. Inhibition of cellular proliferation by diterpenes, topoisomerase II inhibitor. Bioorganic & Medicinal Chemistry. 2006;14:2048–2051. doi: 10.1016/j.bmc.2005.10.059. [DOI] [PubMed] [Google Scholar]
- 108.Morita H., Yoshida N., Takeya K., Itokawa H., Shirota O. Configurational and conformational analyses of a cyclic octapeptide, lyciumin A, from Lycium chinense Mill. Tetrahedron. 1996;52:2795–2802. [Google Scholar]
- 109.Mu Y., Zhang J., Zhang S., Zhou H.H., Toma D., Ren S., Huang L., Yaramus M., Baum A., Venkataramanan R., Xie W. Traditional Chinese medicines Wu Wei Zi (Schisandra chinensis Baill) and Gan Cao (Glycyrrhiza uralensis Fisch) activate pregnane X receptor and increase warfarin clearance in rats. Journal of Pharmacology and Experimental Therapeutics. 2006;316:1369–1377. doi: 10.1124/jpet.105.094342. [DOI] [PubMed] [Google Scholar]
- 110.Ng T.B. Pharmacological activity of sanchi ginseng (Panax notoginseng) Journal of Pharmacy and Pharmacology. 2006;58:1007–1019. doi: 10.1211/jpp.58.8.0001. [DOI] [PubMed] [Google Scholar]
- 111.Niwa M., Takamizawa H., Tatematsu H., Hirata Y. Piscicidal constituents of Stellera chamaejasme L. Chemical & Pharmaceutical Bulletin. 1982;30:4518–4520. [Google Scholar]
- 112.Nizamutdinova L.T., Lee G.W., Lee J.S., Cho M.K., Son K.H., Jeon S.J., Kang S.S., Kim Y.S., Lee J.H., Seo H.G., Chang K.C., Kim H.J. Tanshinone I suppresses growth and invasion of human breast cancer cells, MDA-MB-231, through regulation of adhesion molecules. Carcinogenesis. 2008;29:1885–1892. doi: 10.1093/carcin/bgn151. [DOI] [PubMed] [Google Scholar]
- 113.Nunomura S., Kitanakak S., Ra C. 3-O- (2, 3 - Dimethylbutanoyl)-13-O-decanoylingenol from Euphorbia kansui suppresses IgE-mediated mast cell activation. Biological & Pharmaceutical Bulletin. 2006;29:286–290. doi: 10.1248/bpb.29.286. [DOI] [PubMed] [Google Scholar]
- 114.Oyagbemi A.A., Saba A.B., Azeez O.I. Molecular targets of [6]-gingerol: Its potential roles in cancer chemoprevention. Biofactors. 2010;36:169–178. doi: 10.1002/biof.78. [DOI] [PubMed] [Google Scholar]
- 115.Pan D.J., Hu C.Q., Chang J.J., Lee TTY, Chen Y.P., Hsu H.Y., Mc Phail D.R., Mc Phail A.T., Lee K.H. Kansuiphorin-C and - D: cytotoxic diterpenes from Euphorbia kansui. Phytochemistry. 1991;30:1020–1023. [Google Scholar]
- 116.Pan Q., Ip FCF, Ip N.Y., Zhu H., Min Z. Activity of macrocyclic jatrophane diterpenes from Euphorbia kansui in a TrkA fibroblast survival assay. Journal of Natural Products. 2004;67:1548–1551. doi: 10.1021/np030541c. [DOI] [PubMed] [Google Scholar]
- 117.Pelletier S.W., Page S.W. Diterpenoid alkaloids. Natural Product Reports. 1984;1:375–386. doi: 10.1039/np9860300451. [DOI] [PubMed] [Google Scholar]
- 118.Pelletier S.W., Page S.W. Diterpenoid alkaloids. Natural Product Reports. 1986;3:451–464. doi: 10.1039/np9860300451. [DOI] [PubMed] [Google Scholar]
- 119.Pokharel Y.R., Kim N.D., Han H.K., Oh W.K., Kang K.W. Increased ubiquitination of multidrug resistance 1 by ginsenoside Rd. Nutrition and Cancer. 2010;62:252–259. doi: 10.1080/01635580903407171. [DOI] [PubMed] [Google Scholar]
- 120.Potterat O. Goji (Lyciumbar barum and L. chinense): phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Medica. 2010;76:7–19. doi: 10.1055/s-0029-1186218. [DOI] [PubMed] [Google Scholar]
- 121.Prasain J.K., Reppert A., Jones K., Moore R., Barnes S., Lilia M.A. Identification of isoflavone glycosides in Pueraria lobata cultures by tandem mass spectrometry. Phytochemical Analysis. 2007;18:50–59. doi: 10.1002/pca.951. [DOI] [PubMed] [Google Scholar]
- 122.Qi L.W., Wang C.Z., Yuan C.S. Isolation and analysis of ginseng: advance and challenges. Natural Product Reports. 2011a;28:467–495. doi: 10.1039/c0np00057d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Qi L.W., Wang C.Z., Yuan C.S. American ginseng: potential structure-function relationship in cancer chemoprevention. Biochemical Pharmacology. 2010;80:947–954. doi: 10.1016/j.bcp.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 124.Qi L.W., Wang C.Z., Yuan C.S. Ginsenosides from American ginseng: chemical and pharmacological diversity. Phytochemistry. 2011b;72:689–699. doi: 10.1016/j.phytochem.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Reppert A., Yousef G.G., Rogers R.B., Lila M.A. Isolation of radiolabeled isoflavones from kudzu (Pueraria lobata) root cultures. Journal of Agriculture and Food Chemistry. 2008;56:7860–7865. doi: 10.1021/jf801413z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ryu S.Y., Lee C.O., Choi S.U. In vitro cytotoxicity of tanshinones from Salvia miltiorrhiza. Planta Medica. 1997;63:339–342. doi: 10.1055/s-2006-957696. [DOI] [PubMed] [Google Scholar]
- 127.Sang S., Hong J., Wu H., Liu J., Yang C.S., Pan M.H., Badmaev V., Ho C.T. Increased growth inhibitory effects on human cancer cells and anti-inflammatory potency of shogaols from Zingiber officinale relative to gingerols. Journal of Agriculture and Food Chemistry. 2009;57:10645–10650. doi: 10.1021/jf9027443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schlag E.M., McIntosh M.S. Ginseno side content and variation among and within Americang inseng (Panax quinquefolius L) populations. Phytochemistry. 2006;67:1510–1519. doi: 10.1016/j.phytochem.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 129.Shang P., Qian A.R., Yang T.H., Jia M., Mei Q.B., Cho C.H., Zhao W.M., Chen Z.N. Experimental study of anti-tumor effects of polysaccharides from Angelica sinensis. World Journal of Gastroenterology. 2003;9:1963–1967. doi: 10.3748/wjg.v9.i9.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shi J., Li Z., Nitoda T., Izumi M., Kanzaki H., Baba N., Kawazu K., Nakajima S. Three antinematodal diterpenes from Euphorbia kansui. Bioscience Biotechnology and Biochemistry. 2007;71:1086–1089. doi: 10.1271/bbb.60677. [DOI] [PubMed] [Google Scholar]
- 131.Shukla Y., Singh M. Cancer preventive properties of ginger: a brief review. Food and Chemical Toxicology. 2007;45:683–690. doi: 10.1016/j.fct.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 132.Staudinger J.L., Ding X., Lichti K. Pregnane X receptor and natural products: beyond drug-drug interactions. Expert Opinion on Drug Metabolism and Toxicology. 2006;2:847–857. doi: 10.1517/17425255.2.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Su C.C., Chiou T.L., Chan M.H., Lin J.G. Astragaloside IV increases MMP-2 mRNA and protein expression in human lung cancer A549 cells. Molecular Medicine Reports. 2009;2:107–113. doi: 10.3892/mmr_00000070. [DOI] [PubMed] [Google Scholar]
- 134.Su C.C., Lin Y.H. Tanshinone IIA down-regulates the protein expression of ErbB-2 and up-regulates TNF-alpha in colon cancer cells in vitro and in vivo. International Journal of Molecular Medicine. 2008;22:847–851. [PubMed] [Google Scholar]
- 135.Takahashi K., Ouyang X., Komatsu K., Nakamura N., Hattori M., Baba A., Azuma J. Sodium tanshinone IIA sulfonate derived from Danshen (Salvia miltiorrhiza) attenuates hypertrophy induced by angiotensin II in cultured neonatal rat cardiac cells. Biochemical Pharmacology. 2002;64:745–750. doi: 10.1016/s0006-2952(02)01250-9. [DOI] [PubMed] [Google Scholar]
- 136.Takamura C., Hirata T., Ueda T., Ono M., Miyashita H., Ikeda T., Nohara T. Iridoids from the green leaves of Eucommia ulmoides. Journal of Natural Products. 2007;70:1312–1316. doi: 10.1021/np0780046. [DOI] [PubMed] [Google Scholar]
- 137.Takeo S., Tanonaka K., Hirai K., Kawaguchi K., Ogawa M., Yagi A., Fujimoto K. Beneficial effect of Tan-Shen an extract from the root of Salvia, on post-hypoxic recovery of cardiac contractile force. Biochemical Pharmacology. 1990;40:1137–1143. doi: 10.1016/0006-2952(90)90504-e. [DOI] [PubMed] [Google Scholar]
- 138.Tan X., Li J., Wang X., Chen N., Cai B., Wang G., Shan H., Dong D., Liu Y., Li X., Yang F., Li X., Zhang P., Li X., Yang B., Lu Y. Tanshinone IIA protects against cardiac hypertrophy via inhibiting calcineurin/NFATc3 pathway. International Journal of Biological Sciences. 2011;7:383–389. doi: 10.7150/ijbs.7.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tang C., Xue H.L., Huang H.B., Wang X.G. Tanshinone IIA inhibits constitutive STAT3 activation, suppresses proliferation, and induces apoptosis in rat C6 glioma cells. Neuroscience Letters. 2010;470:126–129. doi: 10.1016/j.neulet.2009.12.069. [DOI] [PubMed] [Google Scholar]
- 140.Tapsell L.C., Hemphill I., Cobiac L., Patch C.S., Sullivan D.R., Fenech M., Roodenrys S., Keogh J.B., Clifton P.M., Williams P.G., Fazio V.A., Inge K.E. Health benefits of herbs and spices: the past, the present, the future. The Medical Journal of Australia. 2006;185:S4–S24. doi: 10.5694/j.1326-5377.2006.tb00548.x. [DOI] [PubMed] [Google Scholar]
- 141.Thiem B. In vitro propagation of isoflavone-producing Pueraria lobata (Willd.) Ohwi Plant Science. 2003;165:1123–1128. [Google Scholar]
- 142.Upton R. CA: Diels Scotts Valley; 2003. American herbal pharmacopoeia and therapeutic compendium: Dang Gui root-Angelica sinensis (Oliv.) [Google Scholar]
- 143.Wada K., Hazawa M., Takahashi K., Mori T., Kawahara N., Kashiwakura I. Inhibitory effects of diterpenoid alkaloids on the growth of A172 human malignant cells. Journal of Natural Products. 2007;70:1854–1858. doi: 10.1021/np070270w. [DOI] [PubMed] [Google Scholar]
- 144.Wada K., Hazawa M., Takahashi K., Mori T., Kawahara N., Kashiwakura I. Structure-activity relationships and the cytotoxic effects of novel diterpenoid alkaloid derivatives against A549 human lung carcinoma cells. Journal of Natural Medicines. 2011;65:43–49. doi: 10.1007/s11418-010-0452-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang C.Z., Mc Entee E., Wicks S., Wu J.A., Yuan C.S. Phytochemical and analytical studies of Panax notoginseng (Burk). FH. Chen. Journal of Natural Medicines. 2006a;60:97–106. [Google Scholar]
- 146.Wang C.Z., Wu J.A., Mc Entee E., Yuan C.S. Saponins composition in American ginseng leaf and berry assayed by high- performance liquid chromatography. Journal of Agricultural and Food Chemistry. 2006b;54:2261–2266. doi: 10.1021/jf052993w. [DOI] [PubMed] [Google Scholar]
- 147.Wang C.Z., Xie J.T., Fishbein A., Aung H.H., He H., Mehendale S.R., He T.C., Du W., Yuan C.S. Antiproliferative effects of different plant parts of Panax notoginseng on SW480 human colorectal cancer cells. Phytotherapy Research. 2009;23:6–13. doi: 10.1002/ptr.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang F.P., Chen Q.H., Liu X.Y. Diterpenoid alkaloids. Natural Product Reports. 2010b;27:529–570. doi: 10.1039/b916679c. [DOI] [PubMed] [Google Scholar]
- 149.Wang F.P., Chen Q.H. In: The Alkaloids. Cordell G.A, editor. Vol. 69. New York: Elsevier Science; 2010. pp. 1–623. [Google Scholar]
- 150.Wang J.R., Leung C.Y., Ho H.M., Chai S., Yau L.F., Zhao Z.Z., Jiang Z.H. Quantitative comparison of ginsenosides and polyacetylenes in wild and cultivated American ginseng. Chemistry & Biodiversity. 2010a;7:975–983. doi: 10.1002/cbdv.200900264. [DOI] [PubMed] [Google Scholar]
- 151.Wang W.H., Wang Z.M. Studies of commonly used traditional medicine-ginger. Zhongguo Zhong Yao Za Zhi. 2005;30:1569–1573. [PubMed] [Google Scholar]
- 152.Wang X., Bastow K.F., Sun C.M., Lin Y.L., Yu H.J., Don M.J., Wu T.S., Nakamura S., Lee K.H. Antitumor Agents, 239, Isolation, structure elucidation, total synthesis, and anti-breast cancer activity of neo-tanshinlactone from Salvia miltiorrhiza. Journal of Medicinal Chemistry. 2004;47:5816–5819. doi: 10.1021/jm040112r. [DOI] [PubMed] [Google Scholar]
- 153.Wang X., Morris-Natschke S.L., Lee K.H. New developments in the chemistry and biology of the bioactive constituents of Tanshen. Medicinal Research Reviews. 2007;27:133–148. doi: 10.1002/med.20077. [DOI] [PubMed] [Google Scholar]
- 154.Wang X., Nakagawa-Goto K., Bastow K.F., Don M.J., Lin Y.L., Wu T.S., Lee K.H. Antitumor agents. 254, Synthesis and biological evaluation of novel neo-tanshinlactone analogues as potent anti-breast cancer agents. Journal of Medicinal Chemistry. 2006c;49:5631–5634. doi: 10.1021/jm060184d. [DOI] [PubMed] [Google Scholar]
- 155.Wang Y., Lu L. Pharmacological effect and clinical application of daidzein. Zhongguo Yaoshi (Wuhan China) 2007;10:910–912. [Google Scholar]
- 156.Wang Y.B., Huang R., Wang H.B., Jin H.Z., Lou L.G., Qin G.W. Diterpenoids from the roots of Euphorbia fischeriana. Journal of Natural Products. 2006d;69:967–970. doi: 10.1021/np0600088. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 157.Wei C.Y., Tang Z.P., Tang K. The experimental study on cytotoxicity of primary liver cancer which caused by extract of coixenolide. Journal of Cancer Prevention & Treatment. 2000;7:610–611. [Google Scholar]
- 158.Weng C.J., Wu C.F., Huang H.W., Ho C.T., Yen G.C. Anti-invasion effects of 6-shogaol and 6-gingerol, two active components in ginger, on human hepatocarcinoma cells. Molecular Nutrition and Food Research. 2010;54:1618–1627. doi: 10.1002/mnfr.201000108. [DOI] [PubMed] [Google Scholar]
- 159.Woo J.H., Li D., Wilsbach K., Orita H., Coulter J., Tully E., Kwon T.K., Xu S., Gabrielson E. Coix seed extract, a commonly used treatment for cancer in China inhibits NF-κB and protein kinase C signaling. Cancer Biology & Therapy. 2007;6:2005–2011. doi: 10.4161/cbt.6.12.5168. [DOI] [PubMed] [Google Scholar]
- 160.Wu G.B., Zhou E.X., Qing D.X. Tanshinone II(A) elicited vasodilation in rat coronary arteriole: roles of nitric oxide and potassium channels. European Journal of Pharmacology. 2009;617:102–107. doi: 10.1016/j.ejphar.2009.06.046. [DOI] [PubMed] [Google Scholar]
- 161.Wu T.S., Lin Y.M., Haruna M., Pan D.J., Shingu T., Chen Y.P., Hsu H.Y., Nakano T., Lee K.H. Antitumor agents, 119 Kansuiphorins A and B: two novel antileukemia diterpene esters from Euphobia kansui. Journal of Natural Products. 1991b;54:823–829. doi: 10.1021/np50075a011. [DOI] [PubMed] [Google Scholar]
- 162.Wu W.L., Chang W.L., Chen C.F. Cytotoxic activities of tanshinones against human carcinoma cell lines. The American Journal of Chinese Medicine. 1991a;19:207–216. doi: 10.1142/S0192415X91000284. [DOI] [PubMed] [Google Scholar]
- 163.Xiang Y.Z., Shang H.C., Gao X.M., Zhang B.L. A comparison of the ancient use of ginseng in traditional Chinese medicine with modern pharmacological experiments and clinical trials. Phytotherapy Research. 2008;22:851–858. doi: 10.1002/ptr.2384. [DOI] [PubMed] [Google Scholar]
- 164.Xiao W.L., Li R.T., Huang S.X., Pu J.X., Sun H.D. Triterpenoids from the Schisandraceae family. Natural Product Reports. 2008;25:871–891. doi: 10.1039/b719905h. [DOI] [PubMed] [Google Scholar]
- 165.Xie W., Du L. Diabetes is an inflammatory disease: evidence from traditional Chinese medicines. Diabetes Obesity and Metabolism. 2011;13:289–301. doi: 10.1111/j.1463-1326.2010.01336.x. [DOI] [PubMed] [Google Scholar]
- 166.Xu Y., Xu G., Liu L., Xu D., Liu J. Anti-invasion effect of rosmarinic acid via the extracellular signal-regulated kinase and oxidation-reduction pathway in Ls174-T cells. Journal of Cellular Biochemistry. 2010;111:370–379. doi: 10.1002/jcb.22708. [DOI] [PubMed] [Google Scholar]
- 167.Xu Z.H., Qin G.W., Li X.Y., Xu R.S. New biflavanones and bioactive compounds from Stellera chamaejasme L. Yao Xue Xue Bao. 2001;36:669–671. [PubMed] [Google Scholar]
- 168.Yagi A., Okamura N., Tanonaka K., Takeo S. Effects of tanshinone VI derivatives on post-hypoxic contractile dysfunction of perfused rat hearts. Planta Medica. 1994;60:405–409. doi: 10.1055/s-2006-959519. [DOI] [PubMed] [Google Scholar]
- 169.Yahara S., Shigeyama C., Nohara T. Structures of anti-ACE and rennin peptides from Lycii radicis cortex. Tetrahedron Letters. 1989;30:6041–6042. [Google Scholar]
- 170.Yang G., Liao Z., Xu Z., Zhang H., Chen D.F. Antimitotic and antifungal C-3/C-3″-biflavanones from Stellera chamaejasme. Chemical & Pharmaceutical Bulletin. 2005b;53:776–779. doi: 10.1248/cpb.53.776. [DOI] [PubMed] [Google Scholar]
- 171.Yang L.J., Jeng C.J., Kung H.N., Chang C.C., Wang A.G., Chau G.Y., Don M.J., Chau Y.P. Tanshinone IIA isolated from Salvia miltiorrhiza elicits the cell death of human endothelial cells. Journal of Biomedical Science. 2005a;12:347–361. doi: 10.1007/s11373-005-0973-z. [DOI] [PubMed] [Google Scholar]
- 172.Yang R., Liu A., Ma X., Li L., Su D., Liu J. Sodium tanshinone IIA sulfonate protects cardiomyocytes against oxidative stress-mediated apoptosis through inhibiting JNK activation. Journal of Cardiovascular Pharmacology. 2008;51:396–401. doi: 10.1097/FJC.0b013e3181671439. [DOI] [PubMed] [Google Scholar]
- 173.Yang T., Jia M., Mei Q., Shang P. Effects of Angelica polysaccharide on blood coagulation and platelet aggregation. Zhong Yao Cai. 2002;25:344–345. [PubMed] [Google Scholar]
- 174.Yao X., Peng Y., Xu L.J., Li L., Wu Q.L., Xiao P.G. Phytochemical and biological studies of Lycium medicinal plants. Chemistry & Biodiversity. 2011;8:976–1010. doi: 10.1002/cbdv.201000018. [DOI] [PubMed] [Google Scholar]
- 175.Yin J., Zhang H., Ye J. Traditional Chinese medicine in treatment of metabolic syndrome. Endocrine, Metabolic & Immune System Disorders Drug Targets. 2008;8:99–111. doi: 10.2174/187153008784534330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yokozawa T., Chung H.Y., Lee T.W., Oura H., Tanaka T., Nonaka G., Nishioka I. Effect of magnesium lithospermate B on urinary excretion of arachidonate metabolites in rats with renal failure. Chemical & Pharmaceutical Bulletin. 1989;37:2766–2769. doi: 10.1248/cpb.37.2766. [DOI] [PubMed] [Google Scholar]
- 177.Yoon M., Choi G., Choi Y., Jang K., Park M., Cha B., Kim J. Effect of polyacetylenic acids from Prunella vulgaris on various plant pathogens. Letters in Applied Microbiology. 2010;51:511–517. doi: 10.1111/j.1472-765X.2010.02922.x. [DOI] [PubMed] [Google Scholar]
- 178.Yoshida C., Hishiyama K., Miyazaki K., Watanabe M., Kanbe M., Yamada Y., Matsuzaki K., Miyashita K., Kitanaka S., Miyata S. Analysis of inhibition of topoisomerase IIa and cancer cell proliferation by ingenolEZ. Cancer Science. 2010;101:374–378. doi: 10.1111/j.1349-7006.2009.01408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.You Y. Podophyllotoxin derivatives: current synthetic approaches for new antitumor agents. Current Pharmaceutical Design. 2005;11:1695–1717. doi: 10.2174/1381612053764724. [DOI] [PubMed] [Google Scholar]
- 180.Yu Q.T., Qi L.W., Li P., Yi L., Zhao J., Bi Z. Determination of seventeen main flavonoids and saponins in the medicinal plant Huang-qi (Radix Astragali) by HPLC-DAD-ELSD. Journal of Separation Science. 2007;30:1292–1299. doi: 10.1002/jssc.200600422. [DOI] [PubMed] [Google Scholar]
- 181.Zhang J., Xie X., Li C., Fu P. Systematic review of the renal protective effect of Astragalus membranaceus (root) on diabetic nephropathy in animal models. Journal of Ethnopharmacology. 2009;126:189–196. doi: 10.1016/j.jep.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 182.Zhang K.Q., Bao Y., Wu P., Rosen R.T., Ho C.T. Antioxidative components of tanshen (Salvia miltiorhiza Bung) Journal of Agriculture and Food Chemistry. 1990;38:1194–1197. [Google Scholar]
- 183.Zhang Q., Kang X., Yang B., Wang J., Yang F. Antiangiogenic effect of capecitabine combined with ginsenoside Rg3 on breast cancer in mice. Cancer Biotherapy and Radiopharmaceuticals. 2008;23:647–653. doi: 10.1089/cbr.2008.0532. [DOI] [PubMed] [Google Scholar]
- 184.Zhang W.D., Chen H., Zhang C., Liu R.H., Li H.L., Chen H.Z. Astragaloside IV from Astragalus membranaceus shows cardioprotection during myocardial ischemia in vivo and in vitro. Planta Medica. 2006;72:4–8. doi: 10.1055/s-2005-873126. [DOI] [PubMed] [Google Scholar]
- 185.Zhang Y., But P., Ooi V., Xua H., Delaney G.D., Lee SHS, Lee S. Chemical properties, mode of action, and in vivo anti- herpes activities of alignin-carbohydrate complex from Prunella vulgaris. Antiviral Research. 2007;75:242–249. doi: 10.1016/j.antiviral.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 186.Zhang Y., Zhang L., Chu W., Wang B., Zhang J., Zhao M., Li X., Lu Y., Yang B., Shan H. Tanshinone IIA inhibits miR-1 expression through p38 MAPK signal pathway in post-infarction rat cardiomyocytes. Cellular Physiology and Biochemistry. 2010;26:991–998. doi: 10.1159/000324012. [DOI] [PubMed] [Google Scholar]
- 187.Zhao J., Li S.P., Yang F.Q., Li P., Wang Y.T. Simultaneous determination of saponins and fatty acids in Ziziphus jujuba (Suanzaoren) by high performance liquid chromatography- evaporative light scattering detection and pressurized liquid extraction. Journal of Chromatography A. 2006;1108:188–194. doi: 10.1016/j.chroma.2005.12.104. [DOI] [PubMed] [Google Scholar]
- 188.Zheng G.Q., Zheng Z.Y., Xu X., Hu Z.H. Variation in fruit sugar composition of Lycium barbarum L. and Lycium chinense Mill of different regions and varieties. Biochemical Systematics and Ecology. 2010;38:275–284. [Google Scholar]
- 189.Zhou Y., Liang M., Li W., Li K., Li P., Hu Y., Yang Z. Protective effects of Eucommia ulmoides Oliv bark and leaf on amyloid-induced cytotoxicity. Environmental Toxicology and Pharmacology. 2009;28:342–349. doi: 10.1016/j.etap.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 190.Zhu H., Di H., Zhang Y., Zhang J., Chen D. A protein- bound polysaccharide from the stem bark of Eucommia ulmoides and its anti-complementary effect. Carbohydrate Research. 2009;344:1319–1324. doi: 10.1016/j.carres.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 191.Zhu Y.P. Australia: Harwood Academic Publishers; 1998. Chinese Materia Medica: Chemistry, Pharmacology and Applications. [Google Scholar]


