Abstract
In the present study, we investigated the therapeutic effect of a classical TCM formula, Free Wanderer Powder (逍遙散 xiāo yáo sǎn), in a breast cancer mouse model induced with estrogen-insensitive breast cancer 4T1 cells. Ovariectomized Balb/c mice (6-8 weeks) or sham mice were injected into the fourth mammary fat pad with 4T1 cells in which tumors were palpable 7 days after injection. On the eighth day, the mice were divided into 4 groups and tubefed daily with vehicle, Free Wanderer Powder (逍遙散 xiāo yáo sǎn) formula or tamoxifen for 28 days. Tumor growth inhibition and the decrease of the average tumor mass were most evident in mice treated with Free Wanderer Powder (逍遙散 xiāo yáo sǎn). Free Wanderer Powder (逍遙散 xiāo yáo sǎn) treatment significantly reduced Bcl-2 and elevated Bax and p53 protein expressions in breast cancer tumor. These results were further confirmed by immunohistochemisty. Tamoxifen could decrease spleen mass and Bcl-2 protein expression, increase the Bax protein expression as well as exert uterotrophic effects by increasing uterus index and inducing the gene expressions in the uterus. Taken together, these results show that Free Wanderer Powder (逍遙散 xiāo yáo sǎn) treatment induced apoptosis at protein level and inhibited the tumor growth in 4T1-induced ovariectomized Balb/c female mice, indicating the possibility of its future use for treatment of estrogen-insensitive breast caner.
Keywords: Free Wanderer Powder (逍遙散 xiāo yáo sǎn, Xiaoyaosan); 4T1 cells; Breast cancer; Balb/c mice
Introduction
Breast cancer is a malignant growth that begins in the tissues of the breast. It is the most common cancer in women (Jemal et al., 2003), and has been increasing in men (Bagchi, 2007). Although advances in screening, surgery, adjuvant radiation, and systemic therapies are in practice, there is no effective cure for patient with advanced stages of the disease, especially in cases of hormone-insensitive cancer (Bange et al., 2001). The use of complementary and alternative medicine (CAM) is very popular amongst cancer patients. Traditional Chinese medicine (TCM) is considered as one type of CAM in western countries. Because of the holistic approach in diagnostics and therapies being employed by the TCM practitioners, prevention and treatment of cancer by TCM become increasingly popular (Dy et al., 2004; Frenkel et al., 2005).
According to traditional Chinese medicine, breast cancer was described as “Rock in the breast”. According to the Chinese medicine practitioners, nipple belongs to the foot reverting yin liver channel (足厥陰肝經 zú jué yīn gān jīng), and the breast belongs to the foot yang brightness stomach channel (足陽明胃經 zú yáng míng wèi jīng). The outer region of breast belongs to the foot lesser yang gallbladder channel (足少陽膽經 zú shào yáng dǎn jīng). They believed that the occurrence of breast cancer is due to the internal impairment of seven affects (七情 qī qíng), qi-blood vacuity (氣血兩 虛 qì xuè liǎng xū), liver depression and spleen vacuity (肝鬱脾虛 gān yù pí xū), obstruction of channels and network vessels (經絡阻滯 jīng luò zǔ zhì), phlegm and qi congestion (痰氣壅滯 tán qì yōng zhì), yang vacuity yin exuberance (陽虛陰盛 yáng xū yīn shèng). Therefore, the therapy prescribed by the Chinese medicine practitioners is to mainly course the liver (疏 肝 shū gān), supplement blood and boost qi (補血益氣 bǔ xuè yì qì), clear heat and transform phlegm (清熱化 痰 qīng rè huà tán), fortify the spleen and harmonize the stomach (健脾和胃 jiàn pí hé wèi).
Free Wanderer Powder (逍遙散 xiāo yáo sǎn) and modified Free Wanderer Powder (逍遙散 xiāo yáo sǎn), such as supplemented Free Wanderer Powder (加 味逍遙散 jiā wèi xiāo yáo sǎn), are among the most commonly-used Chinese formulas in the clinic. The basic functions of these formulas include coursing the liver (疏肝 shū gān), promoting Liver qi (肝氣 gān qì) circulation, nourishing Liver blood (肝血 gān xuè) and fortify the spleen (健脾 jiàn pí). In the clinic, Free Wanderer Powder (逍遙散 xiāo yáo sǎn) and modified Free Wanderer Powder (逍遙散 xiāo yáo sǎn) can be applied to treat anemia, functional uterine bleeding, menopausal syndrome, hepatitis, chronic gastritis, pelvic inflammatory disease (Shi, 2004; Wen et al., 2006; Nie and Wang, 2005). This formula is composed of Bupleuri Radix (柴胡 chái hú), Angelicae sinensis Radix (當歸 dāng guī), Paeoniae Radix Alba (白芍 bāi sháo), Atractylodis Macrocephale Rhizoma (白術 bāi shú), Poria (茯苓 fú líng), Glycyrrihizae Radix (甘 草 gān cǎo), mint (薄荷 bō hè) and roasted ginger (煨 薑 wēi jiāng). Bupleuri Radix (柴胡 chái hú) is the most common herb for coursing the liver (疏肝 shū gān) and resolve depression (解鬱 jiě yù), and mint can boost the former function further. Both Paeoniae Radix Alba (白芍 bāi sháo) and Angelicae sinensis Radix (當 歸 dāng guī) can supplement the blood (補血 bǔ xuè). Atractylodis Macrocephale Rhizoma (白術 bāi shú), Poria (茯苓 fú líng) and Glycyrrihizae Radix (甘草 gān cǎo) can supplement qi (補氣 bǔ qì) and harmonize the spleen and stomach (調和脾胃 tiáo hé pí wèi).
The 4T1 cells grow as adherent epithelial type in vitro, and are characterized as murine estrogen-nonresponsive mammary carcinoma cells (American Type Culture Collection (ATCC) catalogue no. CRL-2539). When injected into Balb/c mice, 4T1 cells rapidly multiply resulting in highly metastatic tumors and serves as an animal model for human stage IV breast cancer (Aslakson and Miller, 1992; Banka, et al., 2006). According to our knowledge, no study has reported on the effect of Free Wanderer Powder (逍遙 散 xiāo yáo sǎn) formula on the development of 4T1 cell induced breast cancer in Balb/c female mice. In the present study, we have employed mouse 4T1 breast caner cell to induce the cancer tumor in Balb/c mice that possesses intact immune system. The efficacies of Free Wanderer Powder (逍遙散 xiāo yáo sǎn) on breast cancer development were studied. Tamoxifen, a drug against hormone-dependent breast cancer and hormone insensitive breast cancer, was used as a control in our animal study. Furthermore, the uterotrophic effects of the formulae in vivo were evaluated by using uterus index and the expression of estrogen related gene and protein in the uterus.
Materials and Methods
Tumor development
Female Balb/c mice (6-8w) were purchased from the Laboratory Animal Services Center of the Chinese University of Hong Kong and pair-fed with phytoestrogen-free AIN-93 diet. Balb/c mice were subjected to ovariectomy to remove the influence of endogenous estrogen or sham operation. After surgery, mice were allowed to recover for 2 weeks. The 4T1 murine mammary carcinoma cell line was kindly provided by Dr. Fred Miller (Barbara Ann Karmanos Cancer Institute, Detroit, MI). The 4T1 cells were grown in EMEM supplemented with 10% FBS and penicillin 100 U/ml and streptomycin 100 g/ml (Invitrogen, Carlsbad, CA) at 37℃ in a humidified atmosphere of 95% air and 5% CO2. Following a 2-week post-surgical recovery period, the OVX or sham mice were injected into the mammary fat pad with 1×105 viable 4T1 cells in a 10 l volume. Palpable tumors appeared in 7-8 days (Lelekakis et al., 1999).
Preparation of Extracts of the XYS Decoction
Free Wanderer Powder (逍遙散 xiāo yáo sǎn) was composed of the following dried raw materials:
120g of Bupleuri Radix (柴胡 chái hú), 120g of Angelicae sinensis Radix (當 歸 dāng guī), 120 g of Paeoniae Radix Alba (白芍 bāi sháo), 120g of Atractylodis Macrocephale Rhizoma (白術 bāi shú), 120g of Poria (茯苓 fú líng), 60g of Glycyrrihizae Radix (甘草 gān cǎo), 4g of mint (薄荷 bō hè) and 4g of roasted ginger (煨薑 wēi jiāng). These herbs were authenticated by Dr. Sibao Chen of PearL Materia Development (Shenzhen) Ltd (Figure 1). Thin-layered chromatography (TLC) and high performance liquid chromatography (HPLC) analyses were conducted to confirm the compliance of the quality of the herbs with the China Pharmacopoeia (2010 version).
Figure 1.
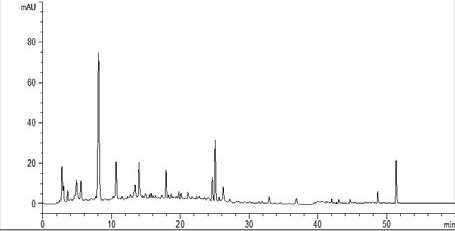
HPLC fingerprint of Free Wanderer Powder (逍遙散 xiāo yáo sǎn) decoction extract
The Agilent XDB C18 (250 × 4.6 mm, 5 μm) column was used. The mobile phase consisted of acetonitrile (A) and 0.1% trifluoroacetic acid (B) with a gradient programme as follows: 2% of A at 0 min, 5% of A at 6 min, 20% of A at 20 min, 29% of A at 35 min, 80% of A at 45 min. The flow rate was 1.0 ml/min and the DAD detector was set at 280 nm. Each peak represent the main active compounds in Free Wanderer Powder (逍遙散 xiāo yáo sǎn) decoction extract.
The herbs were soaked for 20min and extracted with boiling water, 10:1 (v/w) for 2h. The water extract was collected and the residue was then added with water and boiled for another 1h. The water extract was again collected and combined with the first one, and then dried by freeze drying. The yield of the extraction was 26%.
The HPLC profile of XYS decoction (Figure 1) was developed using the following conditions. The Agilent XDB C18 (250 × 4.6 mm, 5μm) column was used. The mobile phase consisted of acetonitrile (A) and 0.1% trifluoroacetic acid (B) with a gradient programme as follows: 2% of A at 0 min, 5% of A at 6 min, 20% of A at 20 min, 29% of A at 35 min, 80% of A at 45 min. The flow rate was 1.0 ml/min and the DAD detector was set at 280 nm.
In vivo administration of traditional Chinese medicine (TCM)
On the eighth day, primary tumor size was measured using calipers and the mice were randomly divided into 4 groups: (1) Vehicle, (2) Tamoxifen (100 g/mice), (3) Free Wanderer Powder (逍遙散 xiāo yáo sǎn) formula (13.92g/kg body weight, decoction extract) and (4) Sham according to tumor size and body weight. Tumor growth and body weight were measured every 3 days upon the start of treatment and the tumor volume were estimated by using the formula (length×width2×20.5). According to the preliminary study, the mice would die when the tumor reached a weight of 1-2g by 28-33 days treatment. Thus, all mice were sacrificed after TCM treatment for 28 days. Tumor and uterus were collected and deep frozen by liquid nitrogen for both gene and protein expression. Part of the tumor tissue was used for immunohistochemistry analysis.
Real time quantitative RT-PCR analysis
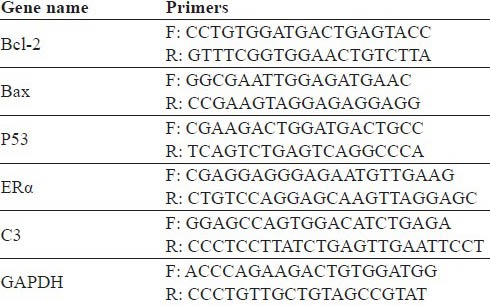
Total RNA was isolated from tumor and uterus using Trizol according to the manufacturer's protocol. Total RNA was reverse-transcribed in 20 µl of a reaction mixture that contained reverse transcription buffer, deoxynucleotide triphosphate mixture, random primers and MultiScribeTM reverse transcriptase, using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA), at 25℃ for 10 min, 37℃ for 2h and 85℃ for 5 sec. For the tumor, the target genes are Bcl-2, Bax, p53 and GAPDH. For the uterus, the target genes are ERa, C3 and GAPDH. The sequences of the PCR primers for these genes are listed in Table 1. PCR was carried out in 20 μl reaction mixture containing 10 μl iQTM SYBR Green Supermix (Bio Rad Laboratories, Hercules, CA) and 0.5 μl of cDNA template. The PCR was performed in an ABI 7900HT Fast Real-Time PCR system (Applied Biosystems). Standard curves were generated using serially diluted solutions of cDNA derived from control sample. The target gene transcripts in each sample were normalized on the basis of its GAPDH.
Table 1.
Primers used for real-time PCR
Immunoblotting
For the tumor, the target proteins are Bcl-2, Bax, p53 and β-actin. Part of tumor were homogenized and lysed with Nonidet P-40 buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, 10% glycerol, 1% Nonidet P-40) containing protease inhibitors (aprotinin 2 μg/ml, leupeptin 2 μg/ml, 1 mM PMSF) and phosphatase inhibitors (1 mM sodium orthovanadate, 10 mM NaF). Lysates were centrifuged at 14,000 rpm for 30 min at 4ºC and protein concentrations were analyzed by the method of Bradford (Bio-Rad, Hercules, CA, USA). Equal amounts of proteins (30 μg) were separated by SDS-PAGE on 10 % reducing gels at a constant voltage (150V), and transblotted onto PVDF membranes (Immobilin-P, Millipore Corp., MA, U.S.A.). Immuno-detection was performed after blocking non-specific binding sites on the membrane with 5%-skimmed milk. The blots were probed with primary antibody as follows: anti-Bcl-2, Bax and p53 (diluted 1:1000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) or mouse anti-beta-actin (diluted 1:2000; Abcam, Cambridge, UK). This was followed by incubation with the goat anti-rabbit or anti-mouse antibody conjugated with horseradish peroxidase (1:2000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) as the secondary antibody for 2h. The antigen-antibody complexes were then detected with enhanced chemiluminescence (ECL) reagent and visualized by the Lumi-Imager using Lumi Analyst version 3.10 software (Roche, Mannheim, Germany).
Immunohistochemistry
Part of the tumor tissue was used for immunohistochemistry analysis. Fresh tissues were fixed in buffered formalin, processed into paraffin block and were subjected to 3sm sections by rotary microtome. The specimen cytosection slides were deparaffinized, rehydrated in water, incubated with 0.3% H2O2 in methanol for 30min to block endogenous peroxidase activity and the antigen retrieval steps were followed. After blocking with 2-10% normal serum with 5% BSA in buffer for 10-20 min, the slides were incubated with primary antibody against Bcl-2, Bax and progesterone receptor (PR). The sections were then incubated with the appropriate biotinylated secondary antibody followed by peroxidase-conjugated ABC and 3, 3-diaminobenzidine (Dako, Carpinteria, CA). The sections were then counter-stained with Mayer haematoxylin. PR, Bcl-2 and Bax-2 immunoreactivity was visualized by diaminobenzidine (DAB). The positive cells were brown and three sections for each rat were used for counting in 400 fold × (40×objective) on an Olympus microscope. Negative control study was performed by the omission of the primary antibody.
Statistical analysis
The data were analyzed by the non-paired student's t-test between control group and each treatment group. Results were reported as Mean ± SEM. A p-value < 0.05 was considered statistically significant.
Results
Effect of TCM treatment on breast cancer tumor growth in ovariectomized Balb/c mice
Tumor growth and body weight were measured every 3 days upon the start of treatment and the tumor volume were estimated by using the formula (length×width2×0.5) (Figure 2A). Tumor growth inhibition was most evident in mice treated with Free Wanderer Powder (逍遙散 xiāo yáo sǎn) in which the percentage of inhibition was around 21~35% from day 10 to day 28 of the experiment. Tamoxifen treatment appeared to have no inhibitory effect for the tumor growth in ovariectomized Balb/c mice. After 28d of treatment, the tumor mass were calculated and the average tumor mass of Free Wanderer Powder (逍遙散 xiāo yáo sǎn) treated mice was found to be smaller than that of the untreated group. While the average tumor weight of tamoxifen treated mice and sham group were not significantly different from the ovariectomized mice treated with vehicle (Figure 2B). Since 4T1 cells possess high metastatic characteristic, the spleen of 4T1-inoculated mice were examined for evidence of secondary cancer development. In the control group, the spleen mass became larger on day 28 of the experiment. Tamoxifen treatment significantly reduced the spleen mass when compared with the control group. The inhibitory effect of Free Wanderer Powder (逍遙散 xiāo yáo sǎn) treatment was also discovered even though the difference was not significant (Figure 2C).
Figure 2.
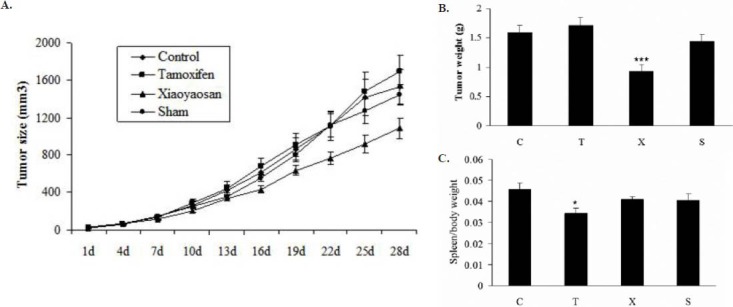
Effect of Free Wanderer Powder (逍遙散 xiāo yáo sǎn) treatment on tumor size, tumor weight and spleen mass in young ovariectomized Balb/c mice
A. Tumor growth and body weight will be measured every 3 days upon the start of treatment with vehicle (C), Tamoxifen (T), Free Wanderer Powder (逍遙散 xiāo yáo sǎn) (X), and sham (S). The tumor volume were estimated by using the formula (length×width2×0.5) (n=9-14).
B. Balb/c mice were sacrificed on day 28 and the mean tumor mass was calculated. C: ovariectomized (OVX) mice treated with vehicle, T: OVX mice treated with tamoxifen, X: OVX mice treated Free Wanderer Powder (逍遙散 xiāo yáo sǎn), S: sham mice treated with vehicle. *P<0.05, **P<0.01 vs control (n=9-14).
C. Spleen masses of Balb/c mice on day 28 of the experiment. C: ovariectomized (OVX) mice treated with vehicle, T: OVX mice treated with tamoxifen, X: OVX mice treated Free Wanderer Powder (逍遙散 xiāo yáo sǎn), S: sham mice treated with vehicle. *P<0.05, **P<0.01 vs control (n=9-14).
Effect of TCM on p53, Bax and Bcl-2 mRNA expression in breast cancer tumor obatined from young ovariecotmized Balb/c mice
To investigate if the mitochondrial apoptotic events were involved in inhibiting effect of TCM formula on breast cancer growth, we first evaluated the mRNA expression levels of pro-apoptotic protein p53 and Bax and anti-apoptotic protein Bcl-2 in breast cancer tumor obtained from young Balb/c mice. Figure 3A shows Free Wanderer Powder (逍遙散 xiāo yáo sǎn) treatment could slightly but not significantly induce p53 gene expression. No significant change was found for Bax gene expression in tumor tissue obtained from young Balb/c mice upon TCM treatment (Figure 3B). Tamoxifen, Free Wanderer Powder (逍遙散 xiāo yáo sǎn) treatment could slightly decrease the Bcl-2 gene expression in breast cancer tumor obtained from ovariectomized mice, but the changes were not significant due to large sample variation (Figure 3C). These results suggest that TCM did not inhibit the growth of breast cancer tumor via the alteration of mRNA expression of p53, Bax and Bcl-2 in tumor obtained from young ovariectomized Balb/c mice.
Figure 3.

Effect of Free Wanderer Powder (逍遙散 xiāo yáo sǎn) on p53, Bax and Bcl-2 mRNA expression in breast cancer tumor in young ovariectomized Balb/c mice
Young ovariectomized Balb/c mice were treated with either vehicle (C), Tamoxifen (T), Free Wanderer Powder (逍遙散 xiāo yáo sǎn) (X). Sham operated mice (S) were treated with vehicle. Upon sacrifice on day 28 of treatment, breast cancer tumors were obtained for study of p53 (A), Bax (B) and Bcl-2 (C) mRNA expressions. No significant changes were found for p53, Bax and Bcl-2 mRNA expressions between each treatment group (n=6-7).
Effect of TCM on p53, Bax and Bcl-2 protein expressions in breast cancer tumor obtained from young ovariectomized Balb/c mice
P53 protein expression was significant increased in ovariectomized mice treated with Free Wanderer Powder (逍遙散 xiāo yáo sǎn) as well as in Sham group (Figure 4A). Bax protein expressions were elevated in the tumor obtained from tamoxifen or Free Wanderer Powder (逍遙散 xiāo yáo sǎn) treated ovariectomized mice. Ovary intact mice (sham group) had a higher Bax protein expression than the ovariectomized mice (Figure 4B). Treatment of ovariectomized Balb/c mice with tamoxifen and Free Wanderer Powder (逍遙散 xiāo yáo sǎn) significantly reduced Bcl-2 protein expression in breast cancer tumor (Figure 4C). These results indicated that Free Wanderer Powder (逍遙散 xiāo yáo sǎn) might exert inhibitory effects on breast cancer tumor growth through its action on post-transcriptional regulation of proteins involved in the apoptotic pathways, including the upregulation of p53 and Bax protein and downregulation of Bcl-2 protein in the tumor.
Figure 4.
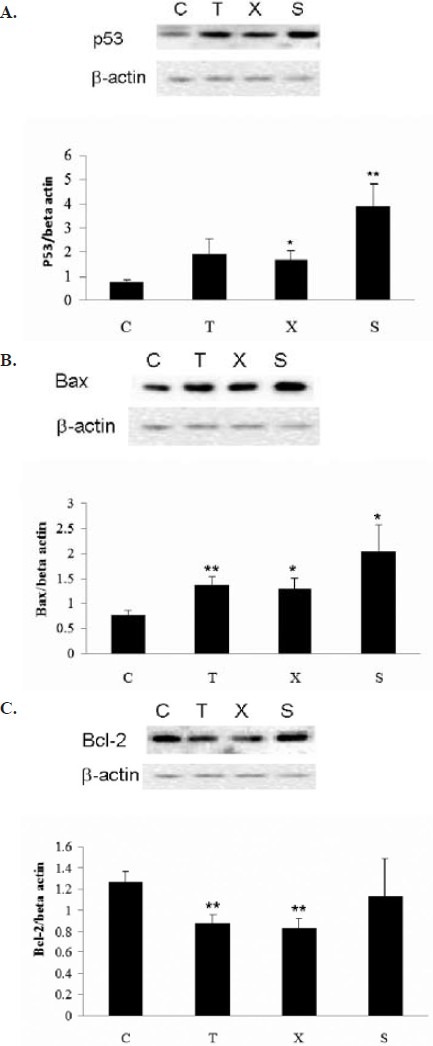
Effect of Free Wanderer Powder (逍遙散 xiāo yáo sǎn) on p53, Bax and Bcl-2 protein expression in breast cancer tumor in young ovariectomized Balb/c mice
Young ovariectomized Balb/c mice were treated with either vehicle (C), Tamoxifen (T), Free Wanderer Powder (逍遙散 xiāo yáo sǎn) (X). Sham operated mice (S) were treated with vehicle. Upon sacrifice on day 28 of treatment, breast cancer tumors were obtained for study of p53 (A), Bax (B) and Bcl-2 (A) protein expressions. *P<0.05, **P<0.01,*** P<0.001 vs control (n=8).
Immunohistochemistry assay for tumor tissue
The protein expression levels of progesterone receptor (PR), pro-apoptotic protein Bax and anti-apoptotic protein Bcl-2 in breast cancer tumor were detected by using immunohistochemistry. PR protein expressions in tumor were elevated in the tamoxifen treated ovariectomized mice (Figure 5A). Bax protein expressions were significantly increased in the tamoxifen tamoxifen and Free Wanderer Powder (逍遙散 xiāo yáo sǎn) treated ovariectomized mice and Sham mice (Figure 5B). In contrast, Bcl-2 protein expression were decreased significantly in tumor obtained from tamoxifen and Free Wanderer Powder (逍遙散 xiāo yáo sǎn) treated ovariectomized mice (Figure 5C). These results confirmed our western blotting analysis that these formulae exerted inhibitory effects on tumor growth through the alteration of protein expression of Bax and Bcl-2 in tumor. Moreover, it appeared that tamoxifen could upregulate PR protein expression in ovariectomized mice.
Figure 5.
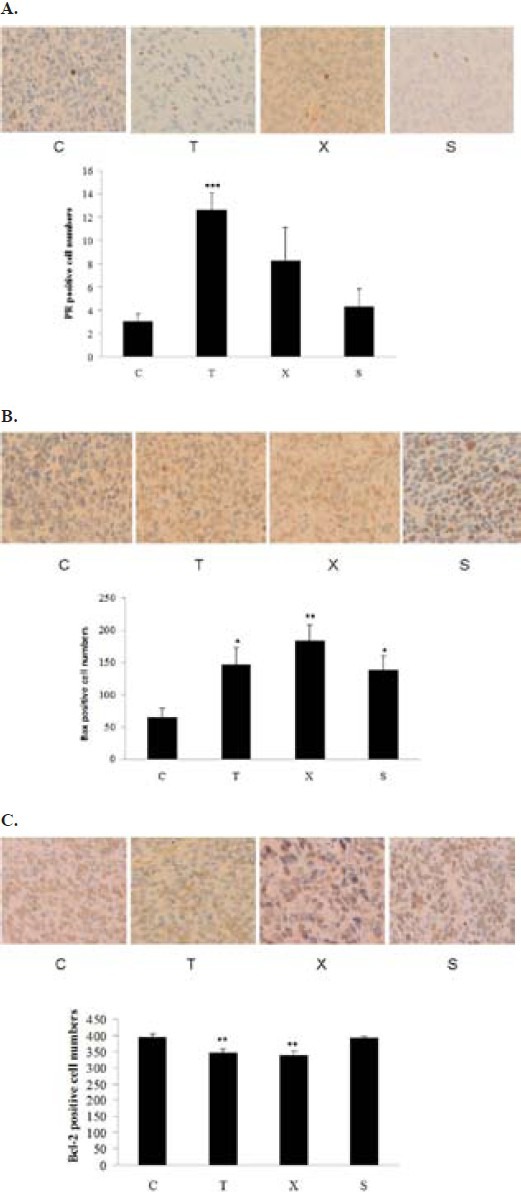
Immunohistochemical detection of progesterone receptor (PR) (A), Bax (B) and Bcl-2 (C) protein expression in breast cancer tumor obtained from young ovariectomized Balb/c mice. Young ovariectomized Balb/c mice were treated with either vehicle (C), Tamoxifen (T), Free Wanderer Powder (逍遙散 xiāo yáo sǎn) (X) for 28 days. Sham operated (Sham) Balb/c mice were treated with vehicle. *P<0.05, **P<0.01,*** P<0.001 vs control (n=5). The positive cells were brown and three sections for each rat were used for counting in 400 fold < (40 objective) on an Olympus microscope.
Uterotrophic effect of TCM
To study if tamoxifen and TCM exerted trophic effect on uterus, the uterus to body weight ratio was determined. The uterine index was significantly reduced in ovariectomized mice, suggesting that the surgery was successful. Tamoxifen significantly increased the uterus index compared with the control group. In contrast to tamoxifen, Free Wanderer Powder (逍遙散 xiāo yáo sǎn) did not increase uterus index in OVX mice (Figure 6).
Figure 6.
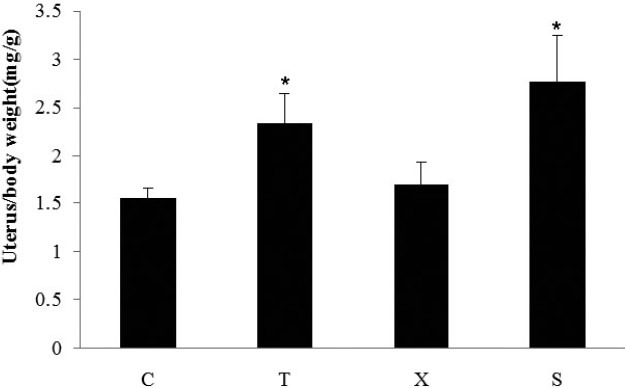
Effect of Free Wanderer Powder (逍遙散 xiāo yáo sǎn) on uterus index in young ovariectomized Balb/c mice
Young ovariectomized Balb/c mice were treated with either vehicle (C), Tamoxifen (T), Free Wanderer Powder (逍遙散 xiāo yáo sǎn) (X) for 28 days. Sham operated (Sham) Balb/c mice were treated with vehicle. Uterus was collected and the uterine index or uterus/body weight ratio was calculated by normalizing the weight of uterus with the final body weight of mice. *P<0.05 vs control (n=5).
Effect of TCM on ER alpha and C3 mRNA expressions in uterus
To investigate the estrogenic effect of TCM in uterus of young Balb/c mice, the expression of estrogen regulated genes, ER and C3, were determined. Tamoxifen treatment decreased ER gene expression and increased estrogen regulated gene C3 mRNA expression in young ovariectomized mice (Figure 7A & B). No significant changes were found in Free Wanderer Powder (逍遙散 xiāo yáo sǎn) treatment group.
Figure 7.
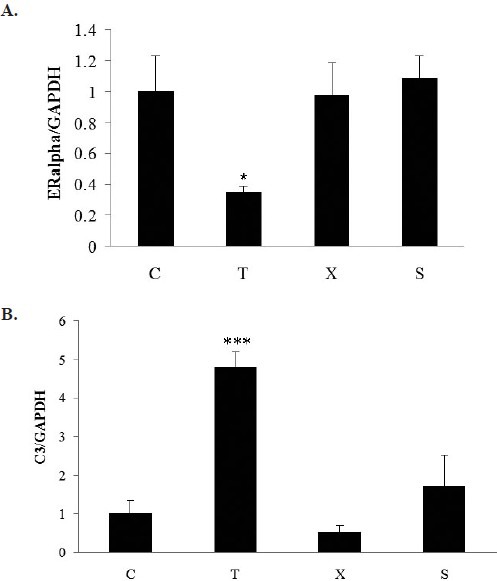
Effect of Free Wanderer Powder (逍遙散 xiāo yáo sǎn) on ER (A) and C3 (B) gene expressions in uterus of young ovariectomized Balb/c mice
Young ovariectomized Balb/c mice were treated with either vehicle (C), Tamoxifen (T), Free Wanderer Powder (逍遙散 xiāo yáo sǎn) (X), for 28 days. Sham operated (Sham) Balb/c mice were reated with vehicle *P<0.05, **P<0.01 vs control (n=6-7).
Discussion
In the present study, a murine breast cancer (4T1 cell line) model which stands for human stage IV breast cancer was established for the evaluation of the therapeutic actions of the classical TCM formula, namely Free Wanderer Powder (逍遙散 xiāo yáo sǎn) in vivo. The results clearly showed that Free Wanderer Powder (逍遙散 xiāo yáo sǎn) formula, but not tamoxifen, has inhibitory effect for 4T1-induced breast caner development in ovariectomized Balb/c mice. Free Wanderer Powder (逍遙散 xiāo yáo sǎn) could increase the expressions of pro-apoptotic protein p53 and Bax protein and decrease the expression of anti-apoptotic protein Bcl-2 protein in breast cancer tumor. Tamoxifen could increase PR, Bax and decrease Bcl-2 protein expression and reduce the spleen mass. Moreover, tamoxifen, but not Free Wanderer Powder (逍遙散 xiāo yáo sǎn) formula, exerted the uterotrophic effects by increasing uterus index and inducing the gene expressions in the uterus. These results suggest the apoptosis is involved in inhibitory effects of Free Wanderer Powder (逍遙散 xiāo yáo sǎn) treatment on 4T1 cell induced breast cancer tumor.
The efficacy of Free Wanderer Powder (逍遙散 xiāo yáo sǎn) formula against the 4T1 cell induced breast cancer was firstly evaluated by monitoring tumor growth and tumor weight. Tumor growth and body weight were measured every 3 days upon the start of treatment and the tumor volume were estimated by using the formula (length×width2×0.5). The tumor growth was significantly inhibited by Free Wanderer Powder (逍 遙散 xiāo yáo sǎn) formula in which the percentage of inhibition was around 21~35% from day 10 to day 28 of the experiment. The mean tumor mass was found to be smaller than that of the untreated group. These results suggest that the Free Wanderer Powder (逍遙散 xiāo yáo sǎn) formula is effective in inhibiting the 4T1 cell induced breast cancer tumor in ovariectomized Balb/c mice.
Tamoxifen has been used to treat hormone-sensitive breast cancers since the 1971 (Wyld, et al., 1999). Recently, studies have demonstrated the tamoxifen is also effective in the treatment of hormone-insensitive breast cancer which in advanced stages and exerts inhibitory effect on tumor growth (Jordan, 1994; Ercoli et al., 1998). This anti-breast cancer drug could limit breast cancer growth and reduce the risk of breast cancer of women who are at increased risk of developing breast cancer. However, continuous use of the drug could stimulate breast cancer progression and induce strokes, uterine cancer, blood clots and cataracts (Fisher, 1998; Fisher, 2005). In the present study, our data has shown that treatment with tamoxifen has no inhibitory effect on tumor growth and mean tumor mass. In a similar study conduced by Xanthopoulos et al (Xanthopoulos et al., 2005), tamoxifen treatment had no inhibitory effect on average tumor volume. The different degrees of 4T1 metastasis occurring across the treatment groups may be monitored by the spleen mass (Xanthopoulos et al., 2005). Tamoxifen treatment significantly reduced the spleen mass when compared with the untreated group, indicating the inhibitory effect of tamoxifen on tumor metastasis.
A variety of anti-cancer agent s can induce programmed cell death or apoptosis in tumor cells which leading to the regression of a cancerous tumor. The apoptosis process is modulated by various proto-oncogenes. P53 was the first identified tumor suppressor gene to functionally promote cell cycle arrest and inhibit abnormal cell proliferation. Studies have demonstrated that p53 plays an important role in hormone-induced protection in mammary tumorigenesis (Sivaraman et al., 2001; Jerry et al., 2000). P53 knockout will cause spontaneous development of mammary tumor (Pati et al., 2004). Bax is a pro-apoptotic gene that acts as a critical downstream effector of the p53-mediated apoptotic pathway. Bax can accelerate cell death and inhibit the protective function of Bcl-2 (Cory and Adams, 2002; Emdadual, et al., 2003). Bax forms dimmers with Bcl-2 and plays an important role in regulating the nature of the apoptotic response (Zhao et al., 2007). The inhibitory effects of Free Wanderer Powder (逍遙散 xiāo yáo sǎn) were accompanied by stronger induction of p53 and Bax as well as suppression of Bcl-2 protein expression in breast cancer tumor. The induction of Bax protein expression and suppression of Bcl-2 protein expression was also revealed by immunohistochemistry analysis. These results indicated that Free Wanderer Powder (逍遙散 xiāo yáo sǎn) could trigger the apoptosis by enhancing the p53 and Bax protein expressions and decreasing the Bcl-2 protein expression. Free Wanderer Powder (逍遙 散 xiāo yáo sǎn) formula might be useful for treatment of hormone insensitive breast cancer in postmenopausal women.
Our study indicated that Free Wanderer Powder (逍 遙散 xiāo yáo sǎn) were superior to tamoxifen for treatment of hormone insensitive breast cancer. Our results showed that Free Wanderer Powder (逍遙散 xiāo yáo sǎn) was effective in inhibiting the growth of breast cancer in ovariectomized Balb/c mice. Whereas, tamoxifen could not suppress 4T1 induced breast cancer growth even though the induction of Bax protein and suppression of Bcl-2 protein expression by tamoxifen was also observed in ovariectomized animals. Moreover, Free Wanderer Powder (逍遙散 xiāo yáo sǎn) exerted no estrogenic activities in uterus of ovariectomized mice. In contrast, tamoxifen exert undesirable uterotrophic effects in ovariectomized mice. The latter consistently increased uterus index, induced ER dependent C3 gene expression as well as suppressed ER mRNA expression in uterus of ovariectomized mice. Progesterone receptor (PR) status in the tumor was studied by immunohistochemistry as it is well accepted that PR positive tumor is slightly slower growing and has a better chance of responding to hormone-suppression treatment compared with PR negative tumor (Anderson, 2002). Our results showed that tamoxifen treatment significantly increased PR protein expression in breast cancer tumor.
The inability of tamoxifen to inhibit tumor growth in OVX mice triggered us to study if the inhibitory actions of tamoxifen on breast cancer depend on estrogen status of the animals. Further study will be performed to study the effect of tamoxifen on 4T1 cell induced breast cancer in ovary intact mice.
Taken together, these results show that Free Wanderer Powder (逍遙散 xiāo yáo sǎn) induced apoptosis and inhibited the 4T1-induced tumor growth in ovariectomized Balb/c mice. Tamoxifen had no inhibitory effect for the 4T1-induced tumor growth. Tamoxifen exerts uterotrophic effects in ovariectomized mice. Our study demonstrates that the established breast cancer animal model could be employed to determine the efficacy as well as mechanism of actions of different TCM formulae. These studies provide new information for designing novel breast cancer treatment based on Chinese medicine, in particular for postmenopausal women who are suffering from hormone insensitive breast cancer.
Acknowledgement
This work was supported by The Innovation and Technology Fund of Hong Kong (ZP17).
References
- 1.Anderson E. The role of oestrogen and progesterone receptors in human mammary development and tumorigenesis. Breast Cancer Research. 2002;4:197–201. doi: 10.1186/bcr452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aslakson C.J., Miller F.R. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Research. 1992;52:1399–1405. [PubMed] [Google Scholar]
- 3.Bagchi S. Men with breast cancer have high risk of second cancer. The Lancet Oncology. 2007;8:198. doi: 10.1016/s1470-2045(07)70067-0. [DOI] [PubMed] [Google Scholar]
- 4.Bange J., Zwick E., Ullrich A. Molecular targets for breast cancer therapy and prevention. Nature Medicine. 2001;7:548–552. doi: 10.1038/87872. [DOI] [PubMed] [Google Scholar]
- 5.Banka C.L., Lung C.V., Nguyen M.T., Pakchoian A.J., Mueller B.M., Eliceiri B.P. Estrogen Induces Lung Metastasis through a Host Compartment–Specific Response. Cancer Research. 2006;66:3667–3672. doi: 10.1158/0008-5472.CAN-05-4416. [DOI] [PubMed] [Google Scholar]
- 6.Cory S., Adams L.M. The Bcl-2 family, regulators of cellular life-or-death switch. Nature Reviews Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 7.Dy G.K., Bekele L., Hanson L.J., Furth A., Mandrekar S., Sloan J.A., Adjei A.A. Complementary and alternative medicine use by patients enrolled onto phase I clinical trials. Journal of Clinical Oncology. 2004;22:4810–4815. doi: 10.1200/JCO.2004.03.121. [DOI] [PubMed] [Google Scholar]
- 8.Emdadual H.M., Masato A., Youichirou H., Ikuko M., Ken- ichi T., Norio O. Apoptosis-inducing neurotoxicity of dopamine and its metabolites viareactive quinone generation in neuroblastoma cells. Biochimica et Biophysica Acta. 2003;1619:39–52. doi: 10.1016/s0304-4165(02)00440-3. [DOI] [PubMed] [Google Scholar]
- 9.Ercoli A., Scambia G., Fattorossi A., Raspaglio G., Battaglia A., Cicchillitti L., Malorni W., Rainaldi G., Benedetti Panici P, Mancuso S. Comparative study on the induction of cytostasis and apoptosis by ICI 182,780 and tamoxifen in an estrogen receptor-negative ovarian cancer cell line. International Journal of Cancer. 1998;76:47–54. doi: 10.1002/(sici)1097-0215(19980330)76:1<47::aid-ijc9>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 10.Fisher B., Costantino J.P., Wickerham D.L., Cecchini R.S., Cronin W.M., Robidoux A., Bevers T.B., Kavanah M.T., Atkins J.N., Margolese R.G., Runowicz C.D., James J.M., Ford L.G., Wolmark N. Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. Journal of the National Cancer Institute. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 11.Fisher B., Costantino J.P., Wickerham D.L., Cecchini R.S., Cronin W.M., Robidoux A., Bevers T.B., Kavanah M.T., Atkins J.N., Margolese R.G., Runowicz C.D., James J.M., Ford L.G., Wolmark N. Tamoxifen for the prevention of breast cancer: Current status of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. Journal of the National Cancer Institute. 2005;97:1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 12.Frenkel M., Ben-Arye E., Baldwin C., Sierpina V. Approach to communicating with patients about the use of nutritional supplements in cancer care. Southern Medical Journal. 2005;98:289–294. doi: 10.1097/01.SMJ.0000154776.71057.E8. [DOI] [PubMed] [Google Scholar]
- 13.Jemal A., Murray T., Samuels A., Ghafoor A., Ward E., Thun M.J. Cancer Statistics CA. A Cancer Journal for Clinicians. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 14.Jerry D.J., Kittrell F.S., Kuperwasser C., Laucirica R., Dickinson E.S., Bonilla P.J., Butel J.S., Medina D. A mammary- specific model demonstrates the role of the p53 tumor suppressor gene in tumor development. Oncogene. 2000;19:1052–1058. doi: 10.1038/sj.onc.1203270. [DOI] [PubMed] [Google Scholar]
- 15.Jordan V.C. Molecular mechanisms of antiestrogen action in breast cancer. Breast Cancer Research Treat. 1994;31:41–52. doi: 10.1007/BF00689675. [DOI] [PubMed] [Google Scholar]
- 16.Lelekakis M., Moseley J.M., Martin T.J., Hards D., Williams E., Ho P., Lowen D., Javni J., Miller F.R., Slavin J., Anderson R.L. A novel orthotopic model of breast cancer metastasis to bone. Clin Exp Metastasis. 1999;17:163–170. doi: 10.1023/a:1006689719505. [DOI] [PubMed] [Google Scholar]
- 17.Nie Y.Z., Wang C.Y. Progress of clinical application of Free Wanderer Powder. Journal of Shandong University of Traditional Chinese Medicine. 2005;29:327–330. [Google Scholar]
- 18.Pati D., Haddad B.R., Haegele A., Thompson H., Kittrell F.S., Shepard A., Montagna C., Zhang N., Ge G., Otta S.K., Mc Carthy M., Ullrich R.L., Medina D. Hormone-induced chromosomal instability in p53-null mammary epithelium. Cancer Research. 2004;64:5608–5616. doi: 10.1158/0008-5472.CAN-03-0629. [DOI] [PubMed] [Google Scholar]
- 19.Shi Y.P. The application of modified xiao yao san in the treatment of gynaecological disease. Journal of Chinese Medicine. 2004;74:25–29. [Google Scholar]
- 20.Sivaraman L., Conneely O.M., Medina D., O’Malley B.W. Vol. 98. Proceedings of the National Academy of Sciences of the United States of America; 2001. p53 is a potential mediator of pregnancy and hormone-induced resistance to mammary carcinogenesis; pp. 12379–12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wen L.B., Yang L.P., Huang H.S. Clinical observation of the effect of modified Free Wanderer Powder on the adverse effect of chemotherapy after the breast cancer surgery. Journal of Traditional Chinese Medicine University of Hunan. 2006;26:38–40. [Google Scholar]
- 22.Wyld D.K., Chester J.D., Perren T.J. Endocrine aspect of the clinical management of the breast cancer- current issues. Endocrine- Related Cancer. 1998;5:97–110. [Google Scholar]
- 23.Xanthopoulos J.M., Romano A.E., Majumdar S.K. Response of Mouse Breast Cancer Cells to Anastrozole, Tamoxifen, and the Combination. Journal of Biomedicine and Biotechnology. 2005;2005:10–19. doi: 10.1155/S111072430440504X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao D.L., Zou L.B., Lin S., Shi J.G., Zhu H.B. Anti- apoptosis effect of esculin on dopamine-induced cytotoxicity in the human neuroblastoma SH-SY5Y cell line. Neuropharmacology. 2007;53:724–732. doi: 10.1016/j.neuropharm.2007.07.017. [DOI] [PubMed] [Google Scholar]


