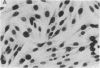
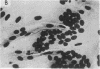
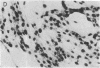
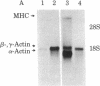
Abstract
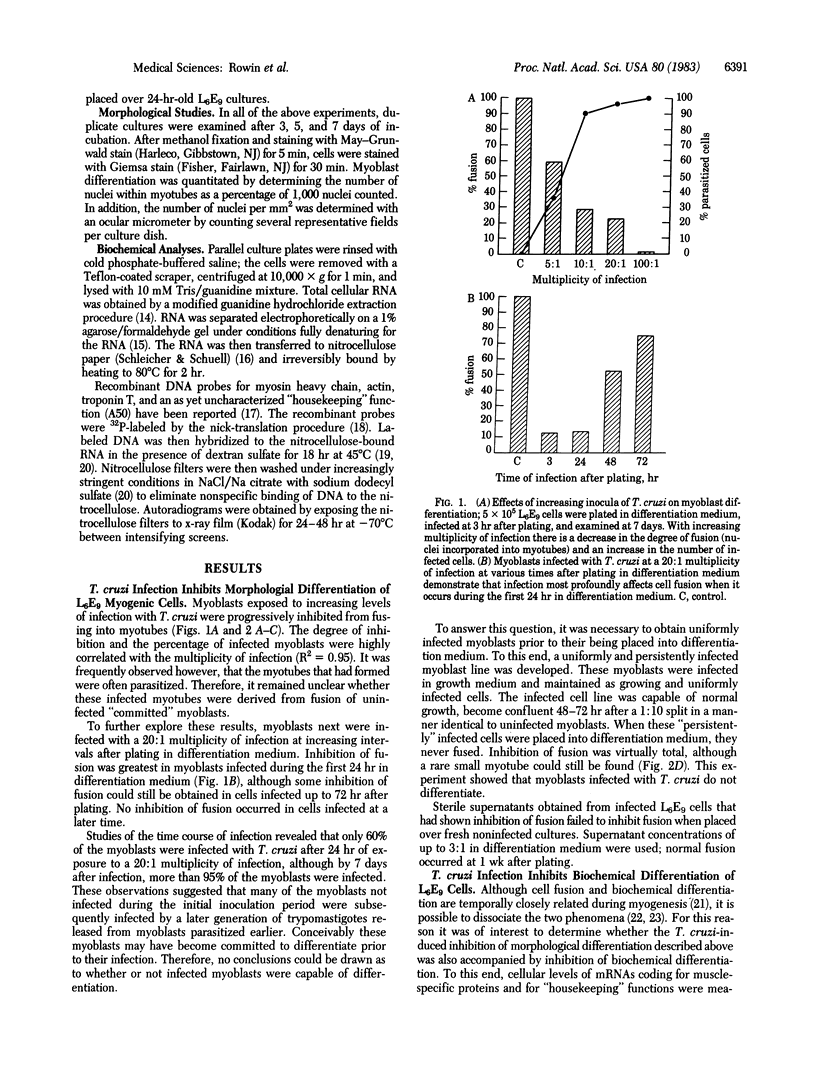
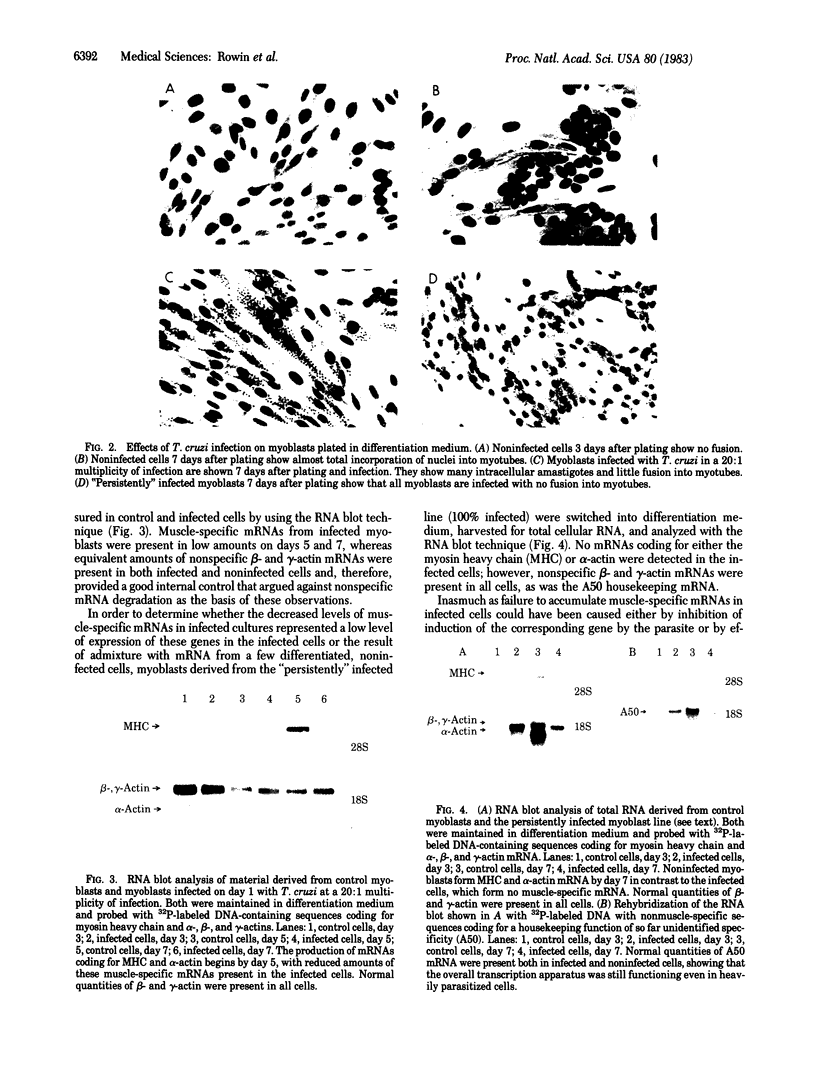
L6E9 rat myoblasts were infected in tissue culture with the myotropic Brazil strain of Trypanosoma cruzi. The effect of parasite infection on the ability of myoblasts to differentiate into myotubes was studied. Both morphological and biochemical differentiation were found to be profoundly affected by parasitic infection in a dose-related fashion. Evidence is presented to suggest that infected myoblasts can no longer differentiate. Differentiation, once underway, seemed unaffected by the parasitic infection; biochemical markers of differentiation remained intact.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoff S., Nadal-Ginard B. N. Cell-free translation of mammalian myosin heavy-chain messenger ribonucleic acid from growing and fused-L6E9 myoblasts. Biochemistry. 1979 Feb 6;18(3):494–500. doi: 10.1021/bi00570a019. [DOI] [PubMed] [Google Scholar]
- Benoff S., Nadal-Ginard B. Transient induction of poly(A)-short myosin heavy chain messenger RNA during terminal differentiation of L6E9 myoblasts. J Mol Biol. 1980 Jun 25;140(2):283–298. doi: 10.1016/0022-2836(80)90106-0. [DOI] [PubMed] [Google Scholar]
- Bioul-Marchand M., Jadin J. M., Steiger R. F., Boon T. Multiplication of Trypanosoma cruzi in a mouse myocardial cell line. J Parasitol. 1980 Dec;66(6):1050–1052. [PubMed] [Google Scholar]
- Cossermelli W., Friedman H., Pastor E. H., Nobre M. R., Manzione A., Camargo M. E., Shiroma M. Polymyositis in Chagas's disease. Ann Rheum Dis. 1978 Jun;37(3):277–280. doi: 10.1136/ard.37.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossio P. M., Diez C., Szarfman A., Kreutzer E., Candiolo B., Arana R. M. Chagasic cardiopathy. Demonstration of a serum gamma globulin factor which reacts with endocardium and vascular structures. Circulation. 1974 Jan;49(1):13–21. doi: 10.1161/01.cir.49.1.13. [DOI] [PubMed] [Google Scholar]
- Delain D., Wahrmann J. P. Is fusion a trigger for myoblast differentiation? Exp Cell Res. 1975 Jul;93(2):495–498. doi: 10.1016/0014-4827(75)90480-2. [DOI] [PubMed] [Google Scholar]
- Dvorak J. A., Hyde T. P. Trypanosoma cruzi: interaction with vertebrate cells in vitro. 1. Individual interactions at the cellular and subcellular levels. Exp Parasitol. 1973 Oct;34(2):268–283. doi: 10.1016/0014-4894(73)90087-8. [DOI] [PubMed] [Google Scholar]
- Fiszman M. Y., Fuchs P. Temperature-sensitive expression of differentiation in transformed myoblasts. Nature. 1975 Apr 3;254(5499):429–431. doi: 10.1038/254429a0. [DOI] [PubMed] [Google Scholar]
- Fiszman M. Y., Montarras D., Wright W., Gros F. Expression of myogenic differentiation and myotube formation by chick embryo myoblasts in the presence of sodium butyrate. Exp Cell Res. 1980 Mar;126(1):31–37. doi: 10.1016/0014-4827(80)90467-x. [DOI] [PubMed] [Google Scholar]
- Holtzer H., Biehl J., Yeoh G., Meganathan R., Kaji A. Effect of oncogenic virus on muscle differentiation. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4051–4055. doi: 10.1073/pnas.72.10.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Martin G. S., Shearer M., Critchley D. R., Epstein C. J. Viral transformation of rat myoblasts: effects on fusion and surface properties. Dev Biol. 1976 Jan;48(1):35–46. doi: 10.1016/0012-1606(76)90043-9. [DOI] [PubMed] [Google Scholar]
- Laguens R. P., Cossio P. M., Diez C., Segal A., Vasquez C., Kreutzer E., Khoury E., Arana R. M. Immunopathologic and morphologic studies of skeletal muscle in Chagas' disease. Am J Pathol. 1975 Jul;80(1):153–162. [PMC free article] [PubMed] [Google Scholar]
- Medford R. M., Wydro R. M., Nguyen H. T., Nadal-Ginard B. Cytoplasmic processing of myosin heavy chain messenger RNA: evidence provided by using a recombinant DNA plasmid. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5749–5753. doi: 10.1073/pnas.77.10.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskin R., Easton T. G., Maelicke A., Reich E. Metabolism of acetylcholine receptor in chick embryo muscle cells: effects of RSV and PMA. Cell. 1978 Dec;15(4):1287–1300. doi: 10.1016/0092-8674(78)90054-5. [DOI] [PubMed] [Google Scholar]
- Nadal-Ginard B. Commitment, fusion and biochemical differentiation of a myogenic cell line in the absence of DNA synthesis. Cell. 1978 Nov;15(3):855–864. doi: 10.1016/0092-8674(78)90270-2. [DOI] [PubMed] [Google Scholar]
- ROSENBAUM M. B. CHAGASIC MYOCARDIOPATHY. Prog Cardiovasc Dis. 1964 Nov;7:199–225. doi: 10.1016/s0033-0620(64)80020-7. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schmatz D. M., Murray P. K. Trypanosoma cruzi: selective isolation of pure trypomastigotes from cultured muscle cells. J Parasitol. 1981 Aug;67(4):517–521. [PubMed] [Google Scholar]
- Sterin-Borda L., Cossio P. M., Gimeno M. F., Gimeno A. L., Diez C., Laguens R. P., Meckert P. C., Arana R. M. Effect of chagasic sera on the rat isolated atrial preparation: immunological, morphological and function aspects. Cardiovasc Res. 1976 Nov;10(6):613–622. doi: 10.1093/cvr/10.6.613. [DOI] [PubMed] [Google Scholar]
- Tanowitz H., Wittner M., Kress Y., Bloom B. Studies of in vitro infection by Trypanosoma cruzi. I. Ultrastructural studies on the invasion of macrophages and L-cells. Am J Trop Med Hyg. 1975 Jan;24(1):25–33. doi: 10.4269/ajtmh.1975.24.25. [DOI] [PubMed] [Google Scholar]
- Taratuto A., Pagano M. A., Fumo T., Sanz O. P., Sica R. E. Histological and histochemical changes of the skeletal muscle in human chronic Chagas' disease. Arq Neuropsiquiatr. 1978 Dec;36(4):327–331. doi: 10.1590/s0004-282x1978000400006. [DOI] [PubMed] [Google Scholar]
- Teixeira A. R., Teixeira M. L., Santos-Buch C. A. The immunology of experimental Chagas' disease. IV. Production of lesions in rabbits similar to those of chronic Chagas' disease in man. Am J Pathol. 1975 Jul;80(1):163–180. [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]