Abstract
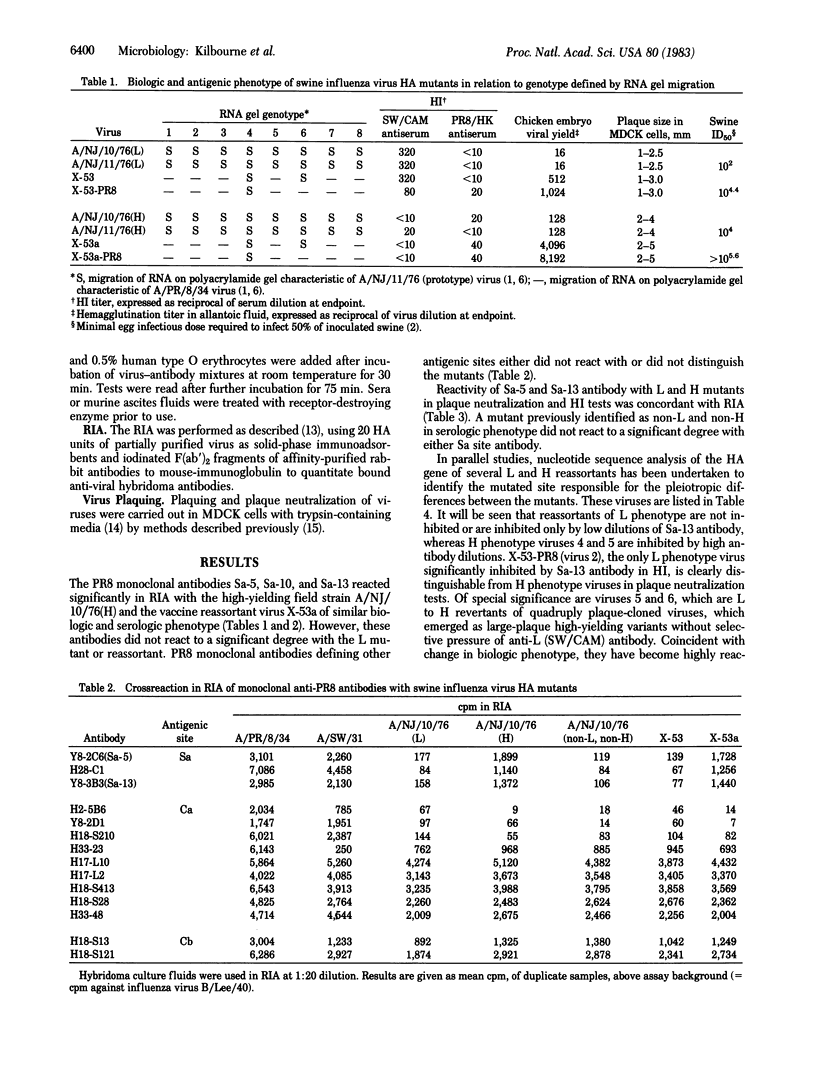
The dimorphic L and H hemagglutinin mutants of A/NJ/11/76(H1N1) (swine) influenza virus differ pleiotropically in their replication and virulence characteristics and in their antigenicity. L mutants replicate less well in chicken embryos and Madin-Darby canine kidney cells and are more infective for swine than are H mutants. L and H mutants are not antigenically distinguishable in cross-neutralization tests with homotypic antisera, but they can be identified with certain heterotypic heterogeneous antisera. The present studies demonstrate that two monoclonal antibodies (Sa-5 and Sa-13) to the Sa antigenic site of the hemagglutinin of A/PR/8/34H1N1 influenza virus react with mutants and viral reassortants containing the H hemagglutinin in radioimmunoassay, neutralization, and hemagglutination-inhibition tests but to a lesser degree or not at all with L mutants and reassortants. Conversely, monoclonal antibody (9C8) to the L mutant does not react with H mutants. L to H and H to L revertants, whether or not selected with monoclonal antibody, demonstrate concomitant change in biological and antigenic phenotype. Reactivity of H mutants with Sa monoclonal antibodies localizes the mutational site to a position on the hemagglutinin near the receptor binding site--a position in which single amino acid changes could readily influence both antigenic and biologic activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caton A. J., Brownlee G. G., Yewdell J. W., Gerhard W. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype). Cell. 1982 Dec;31(2 Pt 1):417–427. doi: 10.1016/0092-8674(82)90135-0. [DOI] [PubMed] [Google Scholar]
- Gerhard W., Yewdell J., Frankel M. E., Webster R. Antigenic structure of influenza virus haemagglutinin defined by hybridoma antibodies. Nature. 1981 Apr 23;290(5808):713–717. doi: 10.1038/290713a0. [DOI] [PubMed] [Google Scholar]
- Jahiel R. I., Kilbourne E. D. Reduction in plaque size and reduction in plaque number as differing indices of influenza virus-antibody reactions. J Bacteriol. 1966 Nov;92(5):1521–1534. doi: 10.1128/jb.92.5.1521-1534.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbourne E. D. Genetic dimorphism in influenza viruses: characterization of stably associated hemagglutinin mutants differing in antigenicity and biological properties. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6258–6262. doi: 10.1073/pnas.75.12.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbourne E. D., McGregor S., Easterday B. C. Hemagglutinin mutants of swine influenza virus differing in replication characteristics in their natural host. Infect Immun. 1979 Oct;26(1):197–201. doi: 10.1128/iai.26.1.197-201.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski H., Gerhard W., Croce C. M. Production of antibodies against influenza virus by somatic cell hybrids between mouse myeloma and primed spleen cells. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2985–2988. doi: 10.1073/pnas.74.7.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Air G. M., Dopheide T. A., Ward C. W. Amino acid sequence changes in the haemagglutinin of A/Hong Kong (H3N2) influenza virus during the period 1968--77. Nature. 1980 Jan 31;283(5746):454–457. doi: 10.1038/283454a0. [DOI] [PubMed] [Google Scholar]
- Lubeck M. D., Gerhard W. Topological mapping antigenic sites on the influenza A/PR/8/34 virus hemagglutinin using monoclonal antibodies. Virology. 1981 Aug;113(1):64–72. doi: 10.1016/0042-6822(81)90136-7. [DOI] [PubMed] [Google Scholar]
- Lubeck M. D., Schulman J. L., Palese P. Antigenic variants of influenza viruses: marked differences in the frequencies of variants selected with different monoclonal antibodies. Virology. 1980 Apr 30;102(2):458–462. doi: 10.1016/0042-6822(80)90114-2. [DOI] [PubMed] [Google Scholar]
- Palese P., Ritchey M. B., Schulman J. L., Kilbourne E. D. Genetic composition of a high-yielding influenza A virus recombinant: a vaccine strain against "Swine" influenza. Science. 1976 Oct 15;194(4262):334–335. doi: 10.1126/science.968486. [DOI] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Schulman J. L., Palese P. Selection and identification of influenza virus recombinants of defined genetic composition. J Virol. 1976 Oct;20(1):248–254. doi: 10.1128/jvi.20.1.248-254.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh M. J., Both G. W., Underwood P. A., Bender V. J. Antigenic drift in the hemagglutinin of the Hong Kong influenza subtype: correlation of amino acid changes with alterations in viral antigenicity. J Virol. 1981 Mar;37(3):845–853. doi: 10.1128/jvi.37.3.845-853.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobita K., Sugiura A., Enomote C., Furuyama M. Plaque assay and primary isolation of influenza A viruses in an established line of canine kidney cells (MDCK) in the presence of trypsin. Med Microbiol Immunol. 1975 Dec 30;162(1):9–14. doi: 10.1007/BF02123572. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Wilson I. A., Skehel J. J. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981 Jan 29;289(5796):373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]
- Yewdell J., Gerhard W. Delineation of four antigenic sites on a paramyxovirus glycoprotein via which monoclonal antibodies mediate distinct antiviral activities. J Immunol. 1982 Jun;128(6):2670–2675. [PubMed] [Google Scholar]


