Abstract
Objective
Mirror movements (MM) have been described in several pathological conditions. Their association with neural tube defects is rare, and only 5 cases have been reported in literature to date. We report on a case of MM associated with cervical myelomeningocele, and we discuss the diffusion tensor imaging findings and the underlying mechanism.
Key Words: Mirror movements, Cervical myelomeningocel, Diffusion tensor imaging
Case report
An 11-year-old right-handed boy was born to non consanguineous parents, with no family history. At birth, he was diagnosed with cervical myelomeningocele (Fig1) and operated at the age of 7 months with no immediate postoperative complication. Five months later, his parents noticed that voluntary movements of one hand was unintentionally reproduced by the other hand. Developmental milestones were normal.
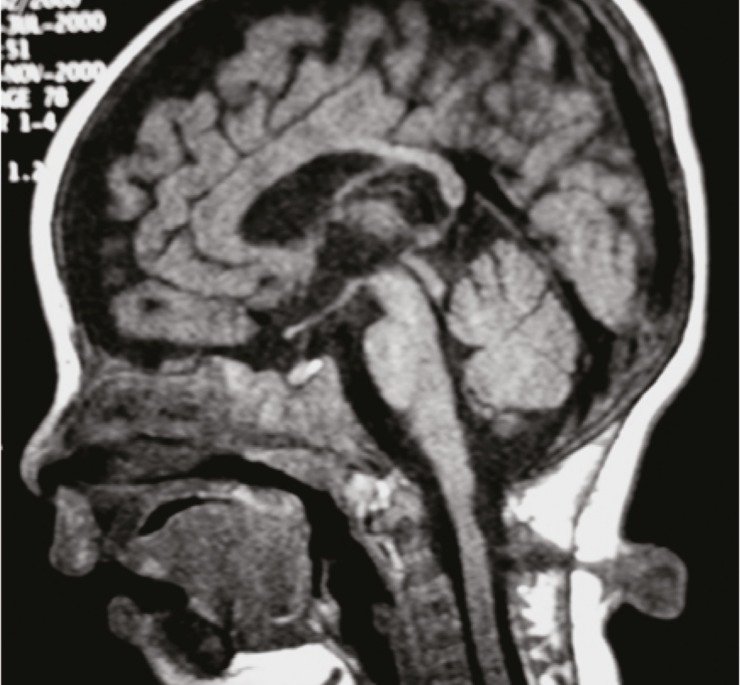
Fig 1.
Cervical sagittal T1 weighted MRI shows cervical myelomeningocele at the C2-C3 level
At the age of 6 years, he complained of difficulties in bimanual activities. He was referred to our “Movement Disorders and Botulinum Toxin” consultation for evaluation of movement disorders in hands. Neurological examination showed bilateral involuntary synkinetic imitative movements in one hand, occurring with all voluntary movements of the opposite hand, concluding to hand’s MM. Amplitude of the intended movement was higher than that of the mirroring hand. He had also a right trapezium muscle atrophy and pyramidal syndrome in all four limbs. Sensory examination was normal.
Cerebral and spinal MRI at the age of 7 years was normal. EMG and NCV revealed mild neurogene changes in trapezium, supraspinatus and infraspinatus nerves, bilaterally.
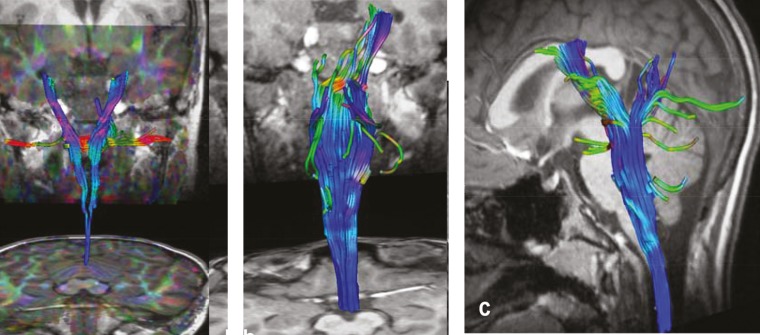
Diffusion tensor imaging (DTI) of the brainstem and the cervical spine was performed. No obvious cortico-spinal tract decussation in lower medulla oblongata was observed. Cervical DTI was normal (Fig. 2 a,b,c,).
Fig 2.
Diffusion tensor imaging of brainstem and cervical spine
Coronal (a,b) and sagittal (c) sections of the brainstem. No evidence of cortico-spinal tract decussation in lower medulla oblongata is noticed. Cervical DTI is normal (c)
Discussion
This case report illustrates a rare association of MM and cervical myelomeningocele. The association of MM with congenital neural tube defects is rare. Only five cases have been reported in literature (Table 1) (3-7). Our patient showed clinical and paraclinical similarities with the reported cases. In fact, MM were described to have a childhood onset (4,6,7). They were usually seen in upper limbs (distal parts of hand) and reproducing all movements of the opposite side (3,4,6,7). Other clinical features (pyramidal syndrom, sensory impairment) were reported and were related to congenital neural tube defects (3,4,6). All patients had neuroimaging investigations (CTscan, cerebral and spinal MRI) in order to determine the type of the neural tube defect and the eventual spinal cord involvement (3-7). However, DTI was not performed in these cases. Some of them underwent an electromyographic study or a transcranial magnetic stimulation (TMS) to better understand the underlying mechanisms (3-5,7). Pathophysiological mechanisms of MM vary according to the pathological condition. Overactivation of ipsilateral corticospinal tract and abnormal transcallosal inhibitory connexions are the major hypotheses. In cases of neural tube defects, the underlying mechanism corresponds to damage in the corticospinal tract decussation in lower medulla oblongata, which results in alternative less specific bilateral spinal pathways (2). Activation of the uncrossed fibers will generate ipsilateral movements reproducing the intentional movements in the opposite side. Closeness of lower medulla oblongata to the cervical myelomeningocele in the reported cases is highly suggestive of a more complicated defect with uncrossed corticospinal tract. This hypothesis is supported by pathological findings in cases of Klippel Feil syndrome presenting with cervical myelomeningocele, mirror movements, and uncrossed corticospinal fibers (8,9). Nevertheless, Klippel Feil syndrome was not reported in these five cases and excluded in our patient. Actually, using advanced radiological functional techniques, such as DTI may be helpful in detecting corticospinal tract pathways in vivo, without resorting to pathological examinations. In this case report, no evidence of corticospinal tract decussation was noted on DTI of lower medulla. This could be a corroborating evidence of the presumed pathophysiological mechanism. However, DTI with high angular resolution may be more efficient to demonstrate uncrossed pathways (10,11). Unfortunately, no effective treatment is currently available (12).
Table 1.
Reported Cases of Mirror Movements Associated with Cervical Neural Tube Defects
| Authors, year | Number of cases | Gender | Age (years) |
Age of MM onset |
Clinical features | Neural tube defect nature |
|---|---|---|---|---|---|---|
| Forget et al., 1986 | 1 | M | _ | Adulthood (25 years) |
MM, deep sensation impairment |
Cervicodorsal meningocele |
| Odabasi et al., 1998 | 1 | M | _ | Childhood | MM | Cervical meningocele, anomaly at the posterior to the cervical spinal cord-medulla junction |
| Erdincler et al., 2002 | 1 | F | 18 | Childhood | MM, broad-based unsteady gait, pyramidal syndrome | Cervical meningocele, spinal cord tethering |
| Erol et al., 2004 | 1 | F | 14 | Childhood | MM, brisk tendon reflexes, recurrent meningitis | Cervical meningocele and dermoid sinus tract |
| Andrabi et al., 2008 | 1 | M | 3 | Childhood | MM | Cervical myelomeningocele |
| Our case | 1 | M | 11 | Childhood (1 year) |
MM, pyramidal syndrome, right trapezium hypotrophy | Cervical myelomeningocele |
MM: mirror movements; M: male, F: female
In conclusion, our observation is exceptional based on the rare association of MM to cervical myelomeningocele. MM should be searched in every child with cervical myelomeningocele. DTI with high- resolution line may be a helpful tool to better understand the underlying mechanisms.
Disclosures
Funding was provided by Ministry of Public Health and Ministry of Scientific Research in Tunisia
References
- 1.Cohen LG, Meer J, Tarkka I, Bierner S, Leiderman DB, Dubinsky RM, et al. Congenital Mirror MovementsAbnormal organization of motor pathways in two patients. Brain. 1991;114(Pt 1B):381–403. doi: 10.1093/brain/114.1.381. [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen P. Persistent mirror movements: a clinical study of 17 children, adolescents and young adults. Dev Med Child Neurol. 1993;35(8):699–707. doi: 10.1111/j.1469-8749.1993.tb11715.x. [DOI] [PubMed] [Google Scholar]
- 3.ForgetR , BoghenD , AttigE , LamarreY Electromyographic studies of congenital mirror movements. Neurology. 1986;36(10):1316–22. doi: 10.1212/wnl.36.10.1316. [DOI] [PubMed] [Google Scholar]
- 4.Erdincler P. Cervical cord tethering and congenital mirror movements: is it an association rather than a coincidence? Br J Neurosurg. 2002;16(5):519–22. doi: 10.1080/026886902320909196. [DOI] [PubMed] [Google Scholar]
- 5.Odabasi Z, Gökçil Z, Kütükçü Y, Vural O, Yardim M. Mirror movements associated with cervical meningocele: case report. Minim Invas Neurosurg. 1998;41(2):99–100. doi: 10.1055/s-2008-1052026. [DOI] [PubMed] [Google Scholar]
- 6.Erol FS, Topsakal C, Ozveren MF, Akdemir I, Cobanoglu B. Meningocele with cervical dermoid sinus tract presenting with congenital mirror movement and recurrent meningitis. Yonsei Med J. 2004;45(3):568–72. doi: 10.3349/ymj.2004.45.3.568. [DOI] [PubMed] [Google Scholar]
- 7.Andrabi Y, Nejat F, El Khashab, Ashrafi MR. Mirror movementassociated with neural tube defects. Neuropsychiatr Dis Treat. 2008;4(6):1273–76. doi: 10.2147/ndt.s3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avery LW, Rentfro CC. The Klippel–Feil syndrome. A pathological report. Arch Neurol Psychiat. 1936;36:1068–76. [Google Scholar]
- 9.Gunderson CH, Solitaire GB. Mirror movements in patients with Klippel–Feil syndrome. Arch Neurol. 1968;18(6):675–9. doi: 10.1001/archneur.1968.00470360097009. [DOI] [PubMed] [Google Scholar]
- 10.Tuch DS, Reese TG, Wiegell MR, Makris N, Belliveau JW, Wedeen VJ. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med. 2002;48(4):577–82. doi: 10.1002/mrm.10268. [DOI] [PubMed] [Google Scholar]
- 11.Mamata H, Mamata Y, Westin CF, Shenton ME, Kikinis R, Jolesz FA, et al. High-resolution line scan diffusion tensor MR imaging of white matter fiber tract anatomy. AJNR Am J Neuroradiol. 2002;23(1):67–75. [PMC free article] [PubMed] [Google Scholar]
- 12.Galléa C, Popa T, Billot S, Méneret A, Depienne C, Roze E. Congenital mirror movements: a clue to understanding bimanual motor control. J Neurol. 2011;258(11):1911–9. doi: 10.1007/s00415-011-6107-9. [DOI] [PubMed] [Google Scholar]