Abstract
An antiserum to alpha-melanocyte-stimulating hormone (alpha-MSH) was found to contain antibodies to at least two types of determinants on the alpha-MSH peptide: one is present only on the free peptide, the other is shared with neurofilaments. Immunoblots from mouse brain showed the neurofilament crossreactivity to be located on proteins in the Mr 140,000 range. The neurofilament-crossreactive portion of the antiserum could be selectively absorbed out with a cytoskeletal preparation, which abolished all affinity of the antiserum to the retina but did not affect the labeling pattern in the pituitary. Absorptions with desacetyl-alpha-MSH and corticotropin seemed to indicate that the determinant shared with neurofilaments is not located at either end of the alpha-MSH peptide, but somewhere in between. The immunohistochemical labeling of the retina with the alpha-MSH antiserum was compared to the labeling with monoclonal antibodies against Mr 200,000 neurofilaments. In the adult retina the alpha-MSH-like immunoreactivity was found to be slightly more widespread; most consistently it was detectable in cell bodies of large ganglion cells, whereas the heavy neurofilament subunit was absent from somata and proximal axons of these cells. In the developing mouse brain, expression of the heavy subunit was found to lag 2-3 wk behind expression of the Mr 140,000 proteins. This confirms previous reports of a more restricted distribution and late expression of high molecular weight neurofilaments as compared to the lower subunits.
Full text
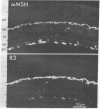
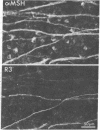
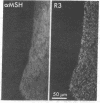
PDF

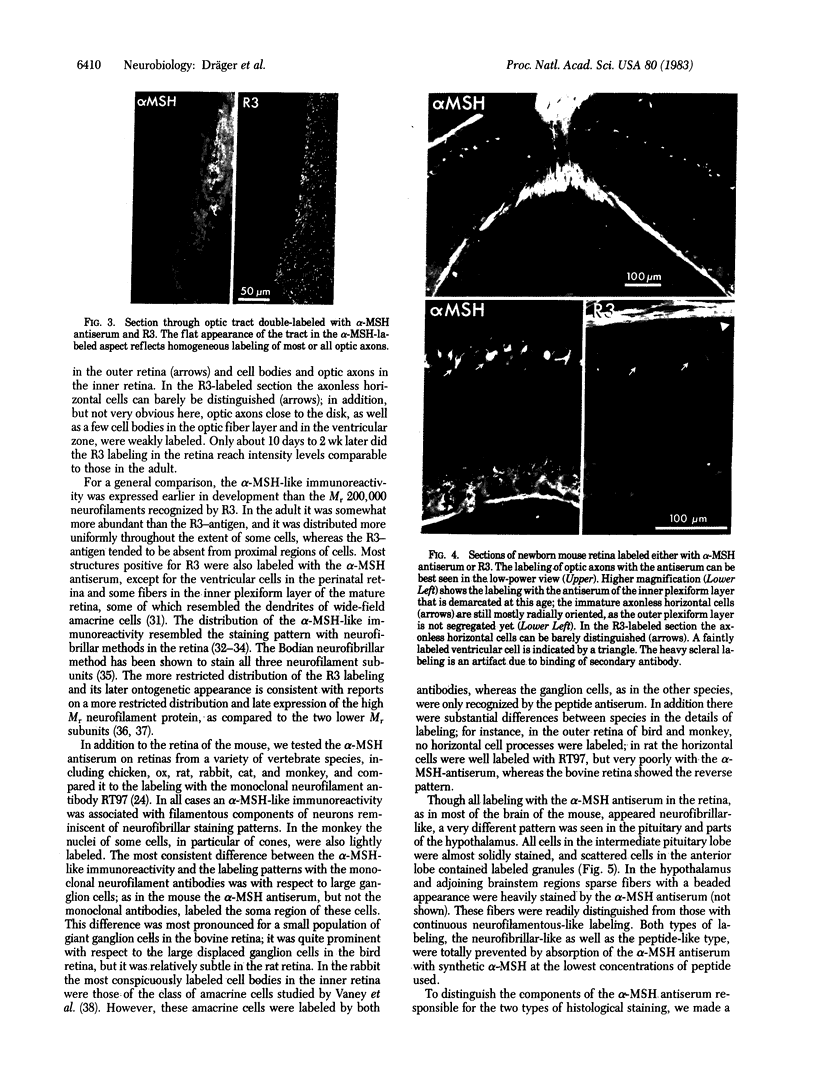


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderton B. H., Breinburg D., Downes M. J., Green P. J., Tomlinson B. E., Ulrich J., Wood J. N., Kahn J. Monoclonal antibodies show that neurofibrillary tangles and neurofilaments share antigenic determinants. Nature. 1982 Jul 1;298(5869):84–86. doi: 10.1038/298084a0. [DOI] [PubMed] [Google Scholar]
- Bauer B., Ehinger B. Action of alpha-MSH on the release of neurotransmitters from the retina. Acta Physiol Scand. 1980 Jan;108(1):105–107. doi: 10.1111/j.1748-1716.1980.tb06506.x. [DOI] [PubMed] [Google Scholar]
- Brown B. A., Majocha R. E., Staton D. M., Marotta C. A. Axonal polypeptides cross-reactive with antibodies to neurofilament proteins. J Neurochem. 1983 Feb;40(2):299–308. doi: 10.1111/j.1471-4159.1983.tb11283.x. [DOI] [PubMed] [Google Scholar]
- Chiu F. C., Norton W. T., Fields K. L. The cytoskeleton of primary astrocytes in culture contains actin, glial fibrillary acidic protein, and the fibroblast-type filament protein, vimentin. J Neurochem. 1981 Jul;37(1):147–155. doi: 10.1111/j.1471-4159.1981.tb05302.x. [DOI] [PubMed] [Google Scholar]
- Chrétien M., Seidah N. G. Chemistry and biosynthesis of pro-opiomelanocortin. ACTH, MSH's, endorphins and their related peptides. Mol Cell Biochem. 1981 Jan 28;34(2):101–127. doi: 10.1007/BF02354864. [DOI] [PubMed] [Google Scholar]
- De Wied D., Jolles J. Neuropeptides derived from pro-opiocortin: behavioral, physiological, and neurochemical effects. Physiol Rev. 1982 Jul;62(3):976–1059. doi: 10.1152/physrev.1982.62.3.976. [DOI] [PubMed] [Google Scholar]
- Dräger U. C. Coexistence of neurofilaments and vimentin in a neurone of adult mouse retina. Nature. 1983 May 12;303(5913):169–172. doi: 10.1038/303169a0. [DOI] [PubMed] [Google Scholar]
- Dräger U. C., Olsen J. F. Origins of crossed and uncrossed retinal projections in pigmented and albino mice. J Comp Neurol. 1980 Jun;191(3):383–412. doi: 10.1002/cne.901910306. [DOI] [PubMed] [Google Scholar]
- Dubé D., Lissitzky J. C., Leclerc R., Pelletier G. Localization of alpha-melanocyte-stimulating hormone in rat brain and pituitary. Endocrinology. 1978 Apr;102(4):1283–1291. doi: 10.1210/endo-102-4-1283. [DOI] [PubMed] [Google Scholar]
- Gambetti P., Autilio Gambetti L., Papasozomenos S. C. Bodian's silver method stains neurofilament polypeptides. Science. 1981 Sep 25;213(4515):1521–1522. doi: 10.1126/science.6169146. [DOI] [PubMed] [Google Scholar]
- Glembotski C. C. Acetylation of alpha-melanotropin and beta-endorphin in the rat intermediate pituitary. Subcellular localization. J Biol Chem. 1982 Sep 10;257(17):10493–10500. [PubMed] [Google Scholar]
- Goldberg S., Galin M. A. Response of retinal ganglion cell axons to lesions in the adult mouse retina. Invest Ophthalmol. 1973 May;12(5):382–385. [PubMed] [Google Scholar]
- Hoffman P. N., Lasek R. J. The slow component of axonal transport. Identification of major structural polypeptides of the axon and their generality among mammalian neurons. J Cell Biol. 1975 Aug;66(2):351–366. doi: 10.1083/jcb.66.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loh Y. P., Eskay R. L., Brownstein M. alpha MSH-like peptides in rat brain: identification and changes in level during development. Biochem Biophys Res Commun. 1980 Jun 16;94(3):916–923. doi: 10.1016/0006-291x(80)91322-4. [DOI] [PubMed] [Google Scholar]
- Loh Y. P., Gainer H. Heterogeneity of melanotropic peptides in the pars intermedia and brain. Brain Res. 1977 Jul 8;130(1):169–175. doi: 10.1016/0006-8993(77)90854-x. [DOI] [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A., Ling N. Common precursor to corticotropins and endorphins. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3014–3018. doi: 10.1073/pnas.74.7.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maley B., Elde R. Immunohistochemical localization of putative neurotransmitters within the feline nucleus tractus solitarii. Neuroscience. 1982 Oct;7(10):2469–2490. doi: 10.1016/0306-4522(82)90208-1. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Inoue A., Kita T., Nakamura M., Chang A. C., Cohen S. N., Numa S. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature. 1979 Mar 29;278(5703):423–427. doi: 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- Nixon R. A., Brown B. A., Marotta C. A. Posttranslational modification of a neurofilament protein during axoplasmic transport: implications for regional specialization of CNS axons. J Cell Biol. 1982 Jul;94(1):150–158. doi: 10.1083/jcb.94.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donohue T. L., Dorsa D. M. The opiomelanotropinergic neuronal and endocrine systems. Peptides. 1982 May-Jun;3(3):353–395. doi: 10.1016/0196-9781(82)90098-5. [DOI] [PubMed] [Google Scholar]
- O'Donohue T. L., Miller R. L., Jacobowitz D. M. Identification, characterization and stereotaxic mapping of intraneuronal alpha-melanocyte stimulating hormone-like immunoreactive peptides in discrete regions of the rat brain. Brain Res. 1979 Oct 26;176(1):101–123. doi: 10.1016/0006-8993(79)90873-4. [DOI] [PubMed] [Google Scholar]
- O'Donohye T. L., Handelmann G. E., Miller R. L., Jacobowitz D. M. N-acetylation regulates the behavioral activity of alpha-melanotropin in a multineurotransmitter neuron. Science. 1982 Feb 26;215(4536):1125–1127. doi: 10.1126/science.7063845. [DOI] [PubMed] [Google Scholar]
- Pawelek J. M., Körner A. M. The biosynthesis of mammalian melanin. Am Sci. 1982 Mar-Apr;70(2):136–145. [PubMed] [Google Scholar]
- Perry V. H. Evidence for an amacrine cell system in the ganglion cell layer of the rat retina. Neuroscience. 1981;6(5):931–944. doi: 10.1016/0306-4522(81)90174-3. [DOI] [PubMed] [Google Scholar]
- Pruss R. M., Mirsky R., Raff M. C., Thorpe R., Dowding A. J., Anderton B. H. All classes of intermediate filaments share a common antigenic determinant defined by a monoclonal antibody. Cell. 1981 Dec;27(3 Pt 2):419–428. doi: 10.1016/0092-8674(81)90383-4. [DOI] [PubMed] [Google Scholar]
- Rudman D., Chawla R. K., Hollins B. M. N,O-Diacetylserine1 alpha-melanocyte-stimulating hormone, a naturally occurring melanotropic peptide. J Biol Chem. 1979 Oct 25;254(20):10102–10108. [PubMed] [Google Scholar]
- Sharp G. A., Shaw G., Weber K. Immunoelectronmicroscopical localization of the three neurofilament triplet proteins along neurofilaments of cultured dorsal root ganglion neurones. Exp Cell Res. 1982 Feb;137(2):403–413. doi: 10.1016/0014-4827(82)90042-8. [DOI] [PubMed] [Google Scholar]
- Shaw G., Osborn M., Weber K. An immunofluorescence microscopical study of the neurofilament triplet proteins, vimentin and glial fibrillary acidic protein within the adult rat brain. Eur J Cell Biol. 1981 Dec;26(1):68–82. [PubMed] [Google Scholar]
- Shaw G., Weber K. Differential expression of neurofilament triplet proteins in brain development. Nature. 1982 Jul 15;298(5871):277–279. doi: 10.1038/298277a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberger L. A., Harwell L. W., Sternberger N. H. Neurotypy: regional individuality in rat brain detected by immunocytochemistry with monoclonal antibodies. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1326–1330. doi: 10.1073/pnas.79.4.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaab D. F., Visser M., Tilders F. J. Stimulation of intra-uterine growth in rat by alpha-melanocyte-stimulating hormone. J Endocrinol. 1976 Sep;70(3):445–455. doi: 10.1677/joe.0.0700445. [DOI] [PubMed] [Google Scholar]
- Tilders F. J., van Delft A. M., Smelik P. G. Re-introduction and evaluation of an accurate, high capacity bioassay for melanocyte-stimulating hormone using the skin of Anolis carolinensis in vitro. J Endocrinol. 1975 Aug;66(2):165–175. doi: 10.1677/joe.0.0660165. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhler M., Herbert E. Complete amino acid sequence of mouse pro-opiomelanocortin derived from the nucleotide sequence of pro-opiomelanocortin cDNA. J Biol Chem. 1983 Jan 10;258(1):257–261. [PubMed] [Google Scholar]
- Vaney D. I., Peichi L., Boycott B. B. Matching populations of amacrine cells in the inner nuclear and ganglion cell layers of the rabbit retina. J Comp Neurol. 1981 Jul 1;199(3):373–391. doi: 10.1002/cne.901990305. [DOI] [PubMed] [Google Scholar]
- Watson S. J., Akil H. alpha-MSH in rat brain: occurrence within and outside of beta-endorphin neurons. Brain Res. 1980 Jan 20;182(1):217–223. doi: 10.1016/0006-8993(80)90849-5. [DOI] [PubMed] [Google Scholar]
- Willard M., Simon C. Antibody decoration of neurofilaments. J Cell Biol. 1981 May;89(2):198–205. doi: 10.1083/jcb.89.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H., Peichl L., Boycott B. B. Morphology and topography of on- and off-alpha cells in the cat retina. Proc R Soc Lond B Biol Sci. 1981 May 22;212(1187):157–175. doi: 10.1098/rspb.1981.0032. [DOI] [PubMed] [Google Scholar]
- Wässle H., Peichl L., Boycott B. B. Topography of horizontal cells in the retina of the domestic cat. Proc R Soc Lond B Biol Sci. 1978 Dec 18;203(1152):269–291. doi: 10.1098/rspb.1978.0105. [DOI] [PubMed] [Google Scholar]
- Yen S. H., Fields K. L. Antibodies to neurofilament, glial filament, and fibroblast intermediate filament proteins bind to different cell types of the nervous system. J Cell Biol. 1981 Jan;88(1):115–126. doi: 10.1083/jcb.88.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]