Abstract
Dementia in children or young adults is most frequently caused by neuronal ceroidlipofuscinoses (NCL), a group of incurable lysosomal storage disorders linked by the accumulation of a characteristic intracellular storage material and progressive clinical deterioration, usually in combination with visual loss, epilepsy, and motor decline. The clinical characteristics can vary and the age at disease onset ranges from birth to over 30 years. Diagnosis of an NCL is difficult because of genetic heterogeneity with14 NCL forms (CLN1-CLN14) identified and a high phenotype variability. A new classification of the disorders is based on the affected gene and the age at disease onset and allows a precise and practicable delineation of every NCL disease. We present a clear diagnostic algorithm to identify each NCL form. A precise diagnosis is essential for genetic counseling of affected families and for optimizing palliative care. As patient management profits from recognizing characteristic complications, care supported by a specialized team of NCL clinicians is recommended.
The development of curative therapies remains difficult as the underlying pathophysiological mechanism remains unclear for all NCL forms.
Key Words: NCL, Causes, Dementia, Childhood
Introduction
Unexpected loss of cognitive abilities (dementia) in young patients presents a diagnostic challenge. It is most frequently caused by neuronal ceroidlipofuscinoses (NCL), a group of lysosomal storage disorders linked by the accumulation of a characteristic storage material in neurons and other cell types, and a progressive clinical deterioration, usually in combination with visual loss, epilepsy, and motor decline (1). Diagnosis of an NCL is difficult because of a high number of different NCL-causing genes and significant genotype phenotype variability. Nevertheless, the specific diagnosis of an NCL disease can be made in a time- and cost-effective manner using very few diagnostic steps.
New nomenclature of NCL diseases
Traditionally, NCL diseases were classified according to the age at disease onset into congenital, infantile, late infantile, juvenile, and adult forms and sometimes also according to the respective authors (Haltia-Santavuori, Jansky-Bielschowsky, Batten, Spielmeyer-Vogt, Kufs) (2). More recently it became clear that NCL diseases are much more heterogeneous than previously thought and that mutations in the same gene can lead to very different clinical courses (3, 4). Designations such as “Finish” or “Turkish” NCL variants are also not useful any longer, as mutations in the respective genes have been shown to occur worldwide (5).The traditional nomenclature has therefore become obsolete and has recently been replaced by an internationally developed combined genetic and clinical classification of NCL disorders (table 1) (2). It classifies both the defective gene as well as the age at disease onset (congenital, infantile, late infantile, juvenile or adult). An exact diagnosis is essential for genetic counseling, sharing information regarding prognosis and optimal palliative care.
Table 1.
Classification of NCL Disorders According to Genetic and Clinical Characteristics
| Disease |
MIM
Number / reference |
Gene designation |
|---|---|---|
| CLN1 disease, infantile CLN1 disease, late-nfantile CLN1 disease, juvenile CLN1 disease, adult |
256730 | CLN1 (PPT1) |
| CLN2 disease, late-infantile CLN2 disease, juvenile |
204500 | CLN2 (TPP1) |
| CLN3 disease, juvenile | 204200 | CLN3 |
| CLN4 disease, adult (autosomal dominant) | 162350 | CLN4 (DNAJC5) |
| CLN5 disease, late-infantile CLN5 disease, juvenile CLN5 disease, adult |
256731 | CLN5 |
| CLN6 disease, late-infantile CLN6 disease, adult (Kufs type A) |
601780 | CLN6 |
| CLN7 disease, infantile | 610951 | CLN7 (MFSD8) |
| CLN8 disease, late-infantile CLN8 disease, EPMR |
600143 | CLN8 |
| CLN10 disease, congenital CLN10 disease, late- infantile CLN10 disease, juvenile CLN10 disease, adult |
610127 | CLN10 (CTSD) |
| CLN11 disease, adult | 138945 | CLN11 (GRN) |
| CLN12 disease, juvenile | (9) | CLN12 (ATP13A2) |
| CLN13 disease, adult (Kufs type B) | (8) | CLN13 (CTSF) |
| CLN14 disease, infantile | (11) | CLN14 (KCTD7) |
The genetic spectrum of NCL diseases
To date, 14 different NCL forms have been To date, 14 different NCL forms have been described (table1) (5-10). More NCL genes remain to be identified as in some patients mutations cannot be demonstrated in any of the known NCL genes although they present with typical NCL symptoms and characteristic cellular storage material. Intracellular localization and- if known- function of the defective NCL proteins are different: Four NCL types are caused by defects in lysosomal enzymes (CLN1, CLN2, CLN10, CLN13), others by defects in transmembrane proteins (CLN3, CLN6, CLN7, CLN8) (6). Also, mutations of an ATPase (CLN12) (9) or a potassium channel (CLN14) (11) seem to cause NCL disease. The CLN4 gene (also called DNAJC5) codes for a protein with putative function in synapses (10). The exact mechanism, how these defects lead to neurodegeneration, is still not understood. Despite their genetic heterogeneity, the different NCL diseases have clinically much in common. This is important both for diagnosis and palliative treatment. Curative therapies do not yet exist for any NCL disease.
The clinical spectrum of NCL diseases
In almost all NCL forms the patients initially present healthy and have a normal development. Main symptoms are a combination of dementia, visual loss, epilepsy, and motor deterioration that progress slowly but inexorably. The age at disease onset can range from birth till young adulthood. The order in which symptoms occur is variable. In a young child, first symptoms are standstill and regression of psychomotor development, or intractable epilepsy. In a school child, first symptoms are mostly visual loss, followed by dementia. The different disease courses are described below (2).For diagnostic tests refer to table 2.
Table 2.
Diagnostic Algorithm for NCL Diseases
| Clinical presentation | Necessary diagnostic tests | Possibly affected genes |
|---|---|---|
|
Newborn
With epilepsy and microcephaly |
Enzyme testing for cathepsin D (CtsD) (leucocytes fibroblasts) | CtsD deficient: CLN10 |
|
Young child
(>6months) with developmental or regression and/or newly severe epilepsy of unknown cause |
Enzyme testing for PPT1 and TPP1 (dry blood spots; confirmation in leucocytes or fibroblasts) |
|
| PPT1 | PPT1 deficient: CLN1 | |
| TPP1 | TPP1 deficient: CLN2 | |
| If PPT1 and TPP1 enzyme normal: Electron microscopic examination (skin biopsy or lymphocytes): If storage material is present: genetic testing. | CLN5, CLN6, CLN7, CLN8, CLN14 (KCTD7) | |
|
School child
with visual loss and / or dementia and epilepsy |
Search for lymphocyte vacuoles (light microscopy of blood smear). If lymphocyte vacuoles are present: genetic testing of the CLN3 gene | CLN3 |
| If no lymphocyte vacuoles, enzyme testing for PPT1, TPP1 and CtsD (see above) | PPT1 deficient: CLN1, TPP1 deficient: CLN2, CtsD deficient: CLN10 |
|
| If PPT1 and TPP1 enzyme normal: Electron microscopic examination (skin biopsy or lymphocytes). If storage material is present: genetic testing. |
CLN5, CLN6, CLN7, CLN8, CLN12 (ATP13A2) | |
|
Young adult
with non-specific mental, motor or behavioral abnormalities. |
Enzyme testing for PPT1, TPP1 and CtsD (see above) | PPT1 deficient: CLN1, TPP1 deficient: CLN2, CtsD deficient: CLN10 |
| If PPT1 and TPP1 enzyme activity is normal: Electron microscopic examination (skin biopsy or lymphocytes). If storage material is present: genetic testing (eventually in special cases even without detection of storage material), consider possible mode of inheritance. | If autosomal dominant:CLN4 (DNAJC5) If autosomal recessive: CLN6, CLN11 (GRN, CLN13 (CTSF) |
Congenital disease onset
This is the only NCL form where patients are severely affected already at birth. Intrauterine or immediate postnatal onset of epileptic seizures as well congenital microcephaly should lead to the suspected diagnosis.
Confirmation is based on demonstration of cathepsin D deficiency and a mutation in the CLN10 gene. The disease leads to death in early infancy.
Infantile disease onset
First symptoms are standstill and subsequent regression of psychomotor development occurring at the age of 10-18 months. These are followed by muscular hypotonia which may change to spasticity later on, as well as myoclonus and epilepsy. Fulminant brain atrophy leads to progressive microcephaly. The electroencephalogram (EEG) becomes flat. Visual contact is lost due to retinopathy. Diagnosis depends on demonstration of a lysosomal enzyme defect (PPT1) and a CLN1 mutation (see table 2). Death occurs in early childhood.
Late infantile disease onset
In this age group, several different gene defects and enzyme deficiencies may be responsible (table 2). First symptoms such as muscular hypotonia, ataxia and developmental regression occur at the age of 2-3 years. At the same age, therapy-resistant epileptic seizures frequently appear. The EEG may show posterior spikes under slow photic stimulation in several NCL forms (CLN2, CLN5, CLN6) (Fig. 1). Cerebral and cerebellar atrophy are seen by cerebral magnetic resonance imaging (Fig. 2). Visual loss is often diagnosed with delay due to the severe neurologic deficits of the patients. Additional problems are increasing spasticity and non-epileptic myoclonus. Most patients live until the age of 10-15 years.
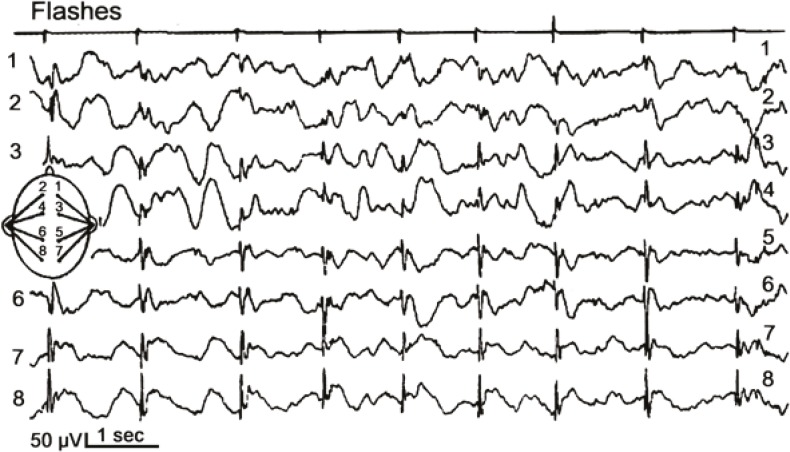
Fig 1.
Electroencephalogramm of a 5-year-old patient with CLN2 disease, late infantile, recorded under slow (1/sec) photic stimulation. Spikes over the occipital areas correspond to light flashes and illustrate increased neuronal excitability during this stage of the disease
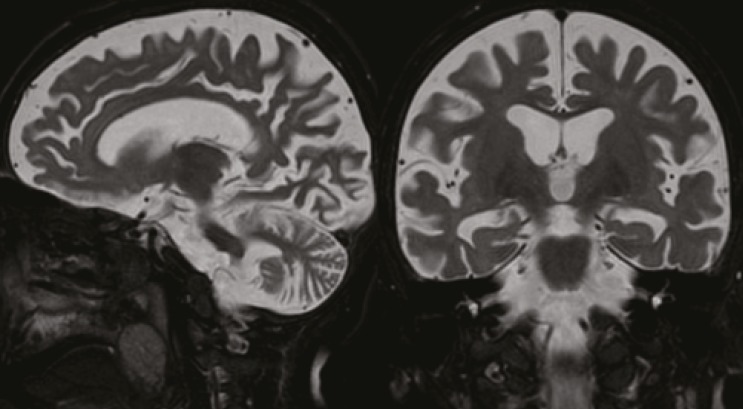
Fig 2.
Magnetic resonance tomograms, T2-weighted, of a 5½-year-old child with CLN2 disease, late infantile. Cerebral and cerebellar atrophy is evident
Juvenile disease onset
The juvenile disease typically starts with isolated visual failure due to retinal degeneration at early school age (5-7 years). An early ophthalmologic finding is the extinguished electroretinogram, later also a pigmentary retinopathy with thinning of vessels (frequently mistaken for “retinitis pigmentosa”, Fig. 3). Most patients in this group have an underlying CLN3 mutation and vacuolated lymphocytes in their peripheral blood (Fig. 4). Unexpected difficulties with abstract reasoning at school herald progressive dementia, grand mal seizures and motor difficulties appear, the latter mostly of a parkinsonian type with small-stepped gait and rigor. Spasticity and myoclonus are rare but may develop late during the disease course. During puberty a psychoorganic syndrome may occur with attacks of fear, panic, and hallucinations or with depressive or aggressive mood changes. These disturbances are a particular challenge to psychiatric management. Older patients may suffer from cool, livid extremities due to sinus bradycardia or other circulatory disturbances. Improved palliative management (e.g., gastric feeding tube for swallowing difficulties) has increased life expectancy up to the age of 30 years and more.
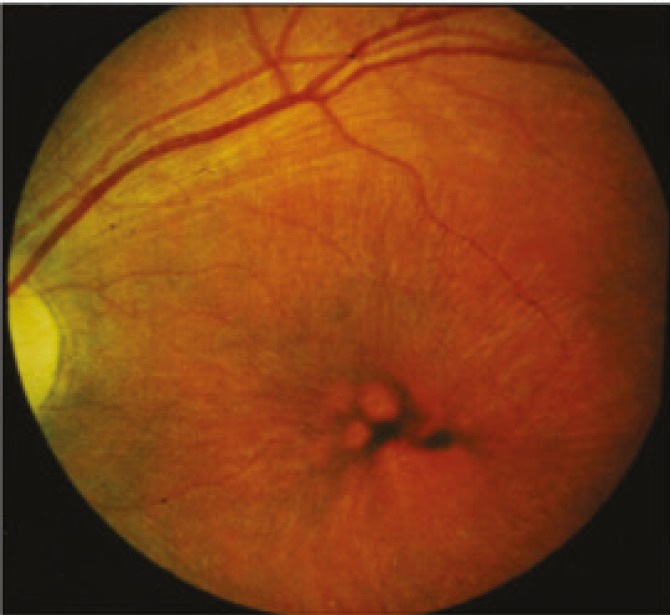
Fig 3.
Fundoscopic appearance of the retina of a patient with CLN3 disease, juvenile. Irregular pigment distribution and thin blood vessels are visible
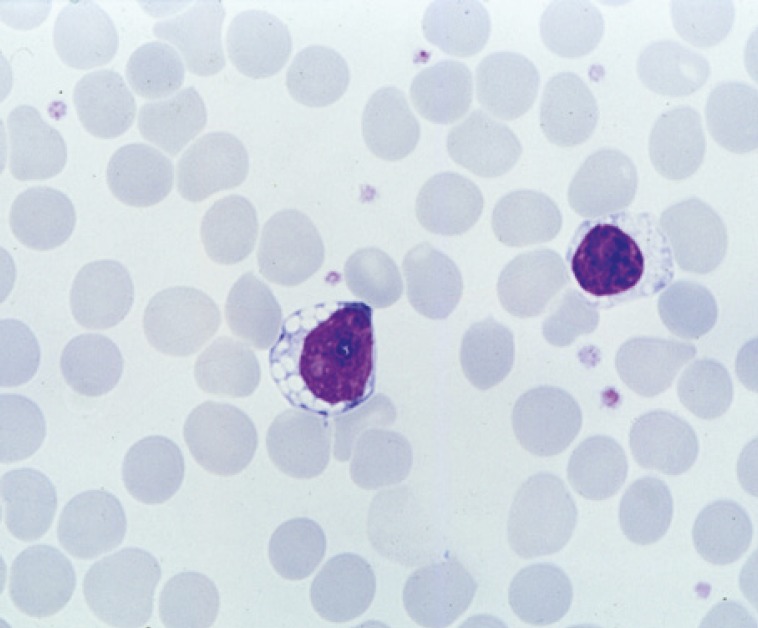
Fig 4.
Vacuoles in the cytoplasm of a peripheral blood lymphocyte from a patient with CLN3 disease, juvenile, routine blood smear
Adult disease onset
The age at disease onset in adult NCL forms has a wide range with average around 39 years (12, 13). First symptoms are variable. Clinically, a type A (initial progressive myoclonus epilepsy, dementia and ataxia) and type B (initial behavioral abnormalities and motor problems) have been differentiated, both without visual problems (12). The identification of affected genes as well as the number of diagnosed cases is currently increasing.
Special aspects of palliative therapy in NCL
Palliative management of a patient with an NCL disease represents a significant challenge due to a number of clinical problems some of which are disease-specific. Moreover, recognition of some complications is difficult as most patients are not able anymore to communicate verbally at later stages of their disease. Episodes of apparent pain require thorough clinical examination. Abdominal pain may be caused by constipation due to disease-related intestinal hypomotility or inadequate nutrition, but many other causes must be considered, such as unnoticed bone fractures or renal calculi, which both are a frequent consequence of immobility. Attacks of exacerbated spasticity (“spastic crisis”) may caused by gastro-esophageal reflux or an acute event such as testicular torsion. Collaboration with other physicians experienced in clinical NCL problems can help improving palliative medication. Table 3, which is based on the authors experience, gives an overview on palliative medication for treating the most common symptoms in NCL.
Table 3.
Overview on Medication for Palliative Therapies in NCL
| Symptom | Substance | Comment |
|---|---|---|
| Epilepsy* | Valproate | Advantage: mood stabilizing effect, useful in juvenile NCL patients with psychotic symptoms |
| Lamotrigine | ||
| Topiramate | Increase dosage SLOWLY to minimize side effects such as speech disturbance (starting dose 0.5 mg/kg/d). Agitation may be a side effect. In this case discontinue the drug. | |
| Levetiracetame | Severe agitation is a possible side effect in juvenile NCL. | |
| Diazepam, lorazepam | Acute therapy of prolonged grand mal seizures | |
| Myoclonus | Levetiracetame | Also effective as anti-epileptic medication (especially in late infantile NCL) |
| Zonisamide | ||
| Piracetame | High dosage required (300-350 mg/kg/d) | |
| Spasticity | Baclofen (1st choice) | Frequently high dosagerequired |
| Tizanidine (2nd choice) | Good effect also against dyskinesia | |
| Tetrahydrocannabinol | “Add-on” medication, increase dosage SLOWLY up to 0.07 mg/kg/d, | |
| Botulinumtoxine | Local application by injection to muscles; always accompanied by physical therapy |
* Avoid overtreatment, as most seizures in NCL are treatment-resistant
Epilepsy
Seizures in NCL are mostly treatment-resistant. Some commonly used antiepileptic drugs may have unusual effects on a patient whose brain is undergoing a degenerative process. We suggest the following guidelines for treating seizures in NCL:
(1) Complete absence of seizures or normalization of electroencephalogram are not realistic aims in NCL patients.
(2) The EEG in NCL serves mainly for monitoring. Therapy should be adjusted to clinical symptoms.
(3) Therapy with more than two antiepileptic drugs may lead to irritating side effects rather than to reduction of seizures.
(4) Some antiepileptic drugs are recommended in NCL patients (valproate and lamotrigine, table 3), others may have negative effects on the disease course and should be avoided (carbamazepin, phenytoine, vigabatrin).
(5) “Use only as many drugs as are necessary and as few as possible.”
Myoclonus
Myoclonus is a symptom which is difficult to treat. Patients, however, frequently seem to be less disturbed by the myoclonic jerks than caregivers. Levetiracetame, zonisamide and piracetame are effective treatments (table 3). Benzodiazepins should be avoided. Some antiepileptic drugs can aggravate myoclonus. Therefore, reduction of the number of different medications may improve myoclonus.
Spasticity
Painful spasticity is another symptom which should be treated: Substances such as baclofen and are effective. Tetrahydrocannabinol may be used as add on medication (table 3). Benzodiazepin as well as tetrazepam seem to be less effective (less potency, negative effect on disease course, increased mucous secretion). Physical therapies and the search for any underlying trigger of spastic crises should always be considered.
Other problems
Diagnosis and treatment of tormenting psychopathological symptoms such as sleep disturbances, fear, aggressive behaviour, depression, and hallucinations represent special challenges. Psychopharmacologic medication should be given in collaboration with child neurologists and child psychiatrists, parents, and caregivers. Only this way, side effects of drugs as well as undetected causes for fear and restlessness can be identified in time.
References
- 1.Haltia M. The neuronal ceroid-lipofuscinoses: from past to present. Biochim Biophys Acta. 2006;1762:850–6. doi: 10.1016/j.bbadis.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Mole SE, Williams R, Goebel HH, eds . The Neuronal Ceroid Lipofuscinoses (Batten Disease) 2 ed. Oxford: Oxford University Press; 2011. p. 480 pages. Contemporary Neurology Series. [Google Scholar]
- 3.Lebrun AH, Moll-Khosrawi P, Pohl S, Makrypidi G, Storch S, Kilian D, et al. Analysis of potential biomarkers and modifier genes affecting the clinical course of CLN3 disease. Mol Med. 2011;17:1253–61. doi: 10.2119/molmed.2010.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lebrun AH, Storch S, Ruschendorf F, Schmiedt ML, Kyttala A, Mole SE, et al. Retention of lysosomal protein CLN5 in the endoplasmic reticulum causes neuronal ceroid lipofuscinosis in Asian sibship. Hum Mutat. 2009;30:E651–61. doi: 10.1002/humu.21010. [DOI] [PubMed] [Google Scholar]
- 5.Kousi M, Lehesjoki AE, Mole SE. Update of the mutation spectrum and clinical correlations of over 360 mutations in eight genes that underlie the neuronal ceroid lipofuscinoses. Hum Mutat. 2011;33:42–63. doi: 10.1002/humu.21624. [DOI] [PubMed] [Google Scholar]
- 6.Jalanko A, Braulke T. Neuronal ceroid lipofuscinoses. Biochim Biophys Acta. 2009;1793:697–709. doi: 10.1016/j.bbamcr.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Arsov T, Smith KR, Damiano J, Franceschetti S, Canafoglia L, Bromhead CJ, et al. Kufs disease, the major adult form of neuronal ceroid lipofuscinosis, caused by mutations in CLN6. Am J Hum Genet. 2011;88:566–73. doi: 10.1016/j.ajhg.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith KR, Damiano J, Franceschetti S, Carpenter S, Canafoglia L, Morbin M, et al. Strikingly different clinicopathological phenotypes determined by progranulin-mutation dosage. Am J Hum Genet. 2012;90:1102–7. doi: 10.1016/j.ajhg.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bras J, Verloes A, Schneider SA, Mole SE, Guerreiro RJ. Mutation of the Parkinsonism Gene ATP13A2 Causes Neuronal Ceroid-Lipofuscinosis. doi: 10.1093/hmg/dds089. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noskova L, Stranecky V, Hartmannova H, Pristoupilova A, Baresova V, Ivanek R, et al. Mutations in DNAJC5, encoding cysteine-string protein alpha, cause autosomaldominant adult-onset neuronal ceroid lipofuscinosis. Am J Hum Genet. 2011;89:241–52. doi: 10.1016/j.ajhg.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staropoli JF, Karaa A, Lim ET, Kirby A, Elbalalesy N, Romansky SG, et al. A homozygous mutation in KCTD7 links neuronal ceroid lipofuscinosis to the ubiquitinproteasome system. Am J Hum Genet. 2012;91:202–8. doi: 10.1016/j.ajhg.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berkovic SF, Carpenter S, Andermann F, Andermann E, Wolfe LS. Kufs‘ disease: a critical reappraisal. Brain. 1988;111(Pt 1):27–62. doi: 10.1093/brain/111.1.27. [DOI] [PubMed] [Google Scholar]
- 13.Babacan-Yildiz G, Hanagasi H, Gurvit H, Sirin G, Solakoglu S, Kucuk OS. A rare dementing disease: adult neuronal ceroid lipofuscinoses. J Neuropsychiatry Clin Neurosci. 2012;24:493–8. doi: 10.1176/appi.neuropsych.11080182. [DOI] [PubMed] [Google Scholar]