Abstract
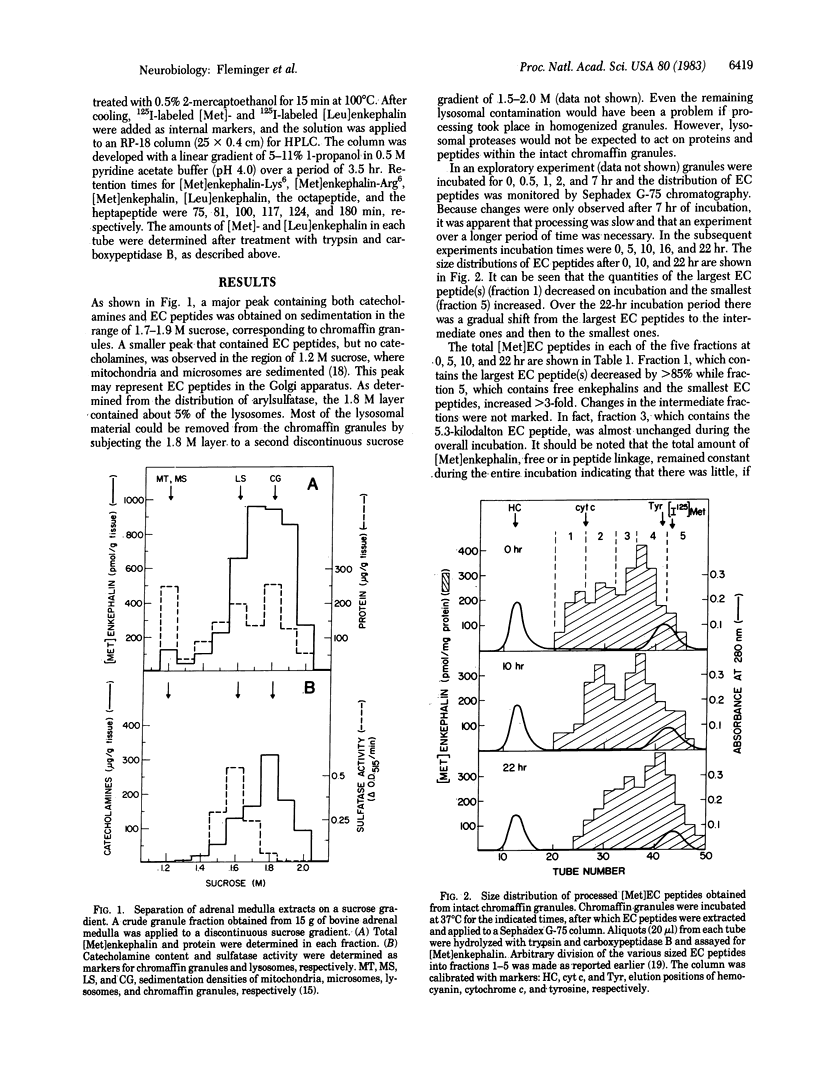
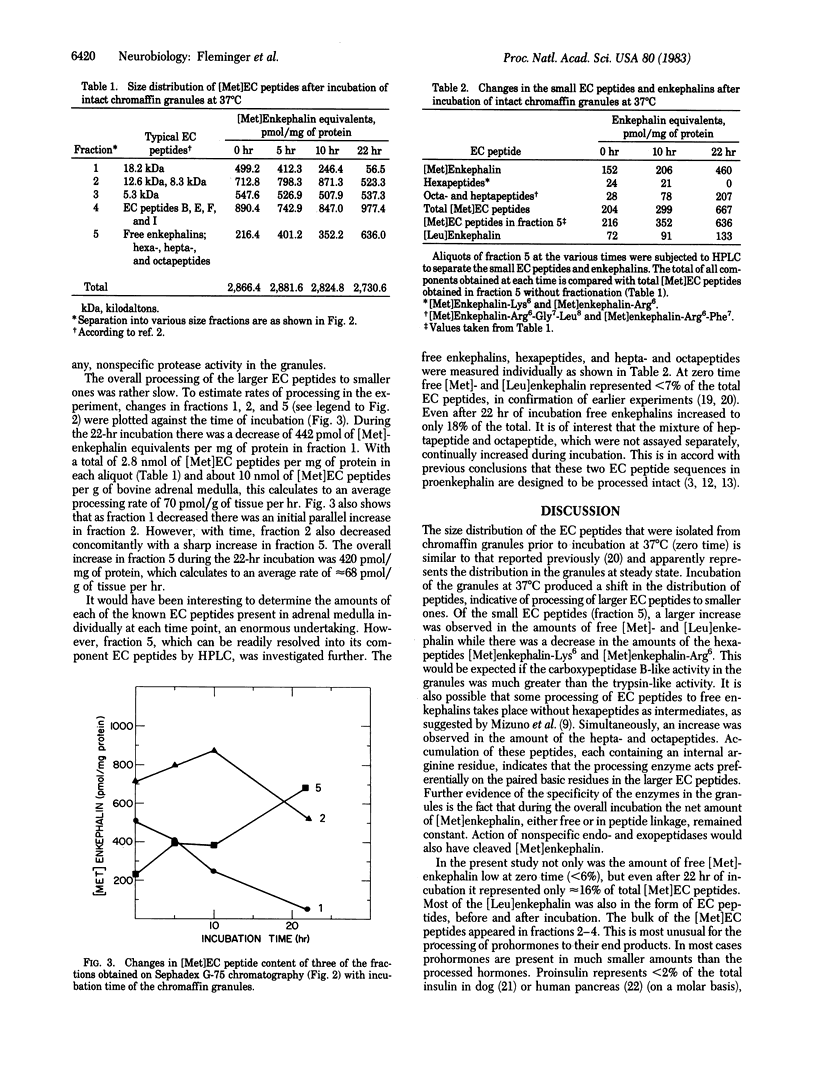
Intact chromaffin granules isolated from bovine adrenal medulla were incubated at 37 degrees C for up to 22 hr. Processing of enkephalin-containing (EC) peptides in the granules was followed by the change in their size distribution as shown by chromatography on Sephadex G-75 columns. A gradual shift toward lower molecular weight EC peptides was observed during the incubation, indicating processing of the higher molecular weight to lower molecular weight EC peptides. The total amount of [Met]-enkephalin, free and in peptide linkage, remained constant indicating that little or no nonspecific degradation occurred during the experiment. HPLC resolution of the fraction containing the low molecular weight EC peptides showed that free enkephalins as well as [Met]enkephalin-Arg6-Phe7 and [Met]enkephalin-Arg6-Gly7-Leu8 accumulated while [Met]enkephalin-Arg6 and [Met]enkephalin-Lys6 disappeared. All the above data indicate the presence of an atypical trypsin activity and the presence of a carboxypeptidase B-like activity within the granules. From the rates of accumulation of the low molecular weight EC peptides and the disappearance of the higher molecular weight EC peptides, a processing rate of 65-70 pmol/g tissue per hr was estimated, which calculates to a lifetime of 6-8 days for EC peptides in the granules. Under steady-state conditions this rate of processing appears to be too low to produce significant amounts of free enkephalins from larger EC peptides. This is well in accord with previous observations that relatively small amounts of free enkephalins are found in bovine adrenal medulla.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- Cohn D. V., Macgregor R. R., Chu L. L., Kimmel J. R., Hamilton J. W. Calcemic fraction-A: biosynthetic peptide precursor of parathyroid hormone. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1521–1525. doi: 10.1073/pnas.69.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista R., Ray P., Lewis R. V. A "trypsin-like" enzyme in adrenal chromaffin granules: a proenkephalin processing enzyme. Biochem Biophys Res Commun. 1982 Jun 15;106(3):895–902. doi: 10.1016/0006-291x(82)91795-8. [DOI] [PubMed] [Google Scholar]
- Fricker L. D., Snyder S. H. Enkephalin convertase: purification and characterization of a specific enkephalin-synthesizing carboxypeptidase localized to adrenal chromaffin granules. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3886–3890. doi: 10.1073/pnas.79.12.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainer H., Sarne Y., Brownstein M. J. Biosynthesis and axonal transport of rat neurohypophysial proteins and peptides. J Cell Biol. 1977 May;73(2):366–381. doi: 10.1083/jcb.73.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Seeburg P., Hoffman B. J., Gage L. P., Udenfriend S. Molecular cloning establishes proenkephalin as precursor of enkephalin-containing peptides. Nature. 1982 Jan 21;295(5846):206–208. doi: 10.1038/295206a0. [DOI] [PubMed] [Google Scholar]
- Hughes J., Smith T. W., Kosterlitz H. W., Fothergill L. A., Morgan B. A., Morris H. R. Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature. 1975 Dec 18;258(5536):577–580. doi: 10.1038/258577a0. [DOI] [PubMed] [Google Scholar]
- Kakidani H., Furutani Y., Takahashi H., Noda M., Morimoto Y., Hirose T., Asai M., Inayama S., Nakanishi S., Numa S. Cloning and sequence analysis of cDNA for porcine beta-neo-endorphin/dynorphin precursor. Nature. 1982 Jul 15;298(5871):245–249. doi: 10.1038/298245a0. [DOI] [PubMed] [Google Scholar]
- Kemper B., Habener J. F., Potts J. T., Jr, Rich A. Proparathyroid hormone: identification of a biosynthetic precursor to parathyroid hormone. Proc Natl Acad Sci U S A. 1972 Mar;69(3):643–647. doi: 10.1073/pnas.69.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D. L., Jones B. N., Kojima K., Udenfriend S. Identification of the octapeptide [Met]enkephalin -Arg6-Gly7-Leu8 in extracts of bovine adrenal medulla. Biochem Biophys Res Commun. 1981 Nov 30;103(2):698–705. doi: 10.1016/0006-291x(81)90506-4. [DOI] [PubMed] [Google Scholar]
- Kojima K., Kilpatrick D. L., Stern A. S., Jones B. N., Udenfriend S. Proenkephalin: a general pathway for enkephalin biosynthesis in animal tissues. Arch Biochem Biophys. 1982 May;215(2):638–643. doi: 10.1016/0003-9861(82)90125-4. [DOI] [PubMed] [Google Scholar]
- Lewis R. V., Stern A. S., Kimura S., Rossier J., Stein S., Udenfriend S. An about 50,000-dalton protein in adrenal medulla: a common precursor of [Met]- and [Leu]enkephalin. Science. 1980 Jun 27;208(4451):1459–1461. doi: 10.1126/science.7384787. [DOI] [PubMed] [Google Scholar]
- Lewis R. V., Stern A. S., Rossier J., Stein S., Udenfriend S. Putative enkephalin precursors in bovine adrenal medulla. Biochem Biophys Res Commun. 1979 Aug 13;89(3):822–829. doi: 10.1016/0006-291x(79)91852-7. [DOI] [PubMed] [Google Scholar]
- Lindberg I., Yang H. Y., Costa E. An enkephalin-generating enzyme in bovine adrenal medulla. Biochem Biophys Res Commun. 1982 May 14;106(1):186–193. doi: 10.1016/0006-291x(82)92076-9. [DOI] [PubMed] [Google Scholar]
- Lindberg I., Yang H. Y., Costa E. Enzymatic production of Met5- and Leu5-enkephalin in adrenal chromaffin granules. Adv Biochem Psychopharmacol. 1982;33:183–191. [PubMed] [Google Scholar]
- Mizuno K., Miyata A., Kangawa K., Matsuo H. A unique proenkephalin-converting enzyme purified from bovine adrenal chromaffin granules. Biochem Biophys Res Commun. 1982 Oct 15;108(3):1235–1242. doi: 10.1016/0006-291x(82)92132-5. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Inoue A., Kita T., Nakamura M., Chang A. C., Cohen S. N., Numa S. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature. 1979 Mar 29;278(5703):423–427. doi: 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Furutani Y., Takahashi H., Toyosato M., Hirose T., Inayama S., Nakanishi S., Numa S. Cloning and sequence analysis of cDNA for bovine adrenal preproenkephalin. Nature. 1982 Jan 21;295(5846):202–206. doi: 10.1038/295202a0. [DOI] [PubMed] [Google Scholar]
- Pierluissi J., Campbell J. Growth hormone and metasomatotrophic diabetes: effects on insulin and proinsulin of serum and pancreas in dogs. Diabetologia. 1981 Dec;21(6):558–562. doi: 10.1007/BF00281549. [DOI] [PubMed] [Google Scholar]
- Rigopoulou D., Valverde I., Marco J., Faloona G., Unger R. H. Large glucagon immunoreactivity in extracts of pancreas. J Biol Chem. 1970 Feb 10;245(3):496–501. [PubMed] [Google Scholar]
- Rubinstein M., Stein S., Gerber L. D., Udenfriend S. Isolation and characterization of the opioid peptides from rat pituitary: beta-lipotropin. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3052–3055. doi: 10.1073/pnas.74.7.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. D., Winkler H. The localization of lysosomal enzymes in chromaffin tissue. J Physiol. 1966 Mar;183(1):179–188. doi: 10.1113/jphysiol.1966.sp007859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner D. F., Cunningham D., Spigelman L., Aten B. Insulin biosynthesis: evidence for a precursor. Science. 1967 Aug 11;157(3789):697–700. doi: 10.1126/science.157.3789.697. [DOI] [PubMed] [Google Scholar]
- Stern A. S., Jones B. N., Shively J. E., Stein S., Undenfriend S. Two adrenal opioid polypeptides: proposed intermediates in the processing of proenkephalin. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1962–1966. doi: 10.1073/pnas.78.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaka Y., Takei M., Hirata Y. C-peptide, insulin and proinsulinlike components in diabetic and nondiabetic human pancreas. Endocrinol Jpn. 1979 Jun;26(3):313–318. doi: 10.1507/endocrj1954.26.313. [DOI] [PubMed] [Google Scholar]
- Troy C. M., Musacchio J. M. Processing of enkephalin precursors by chromaffin granule enzymes. Life Sci. 1982 Oct 18;31(16-17):1717–1720. doi: 10.1016/0024-3205(82)90193-x. [DOI] [PubMed] [Google Scholar]
- UDENFRIEND S., COOPER J. R., CLARK C. T., BAER J. E. Rate of turnover of epinephrine in the adrenal medulla. Science. 1953 Jun 12;117(3050):663–665. doi: 10.1126/science.117.3050.663. [DOI] [PubMed] [Google Scholar]
- Udenfriend S., Kilpatrick D. L. Biochemistry of the enkephalins and enkephalin-containing peptides. Arch Biochem Biophys. 1983 Mar;221(2):309–323. doi: 10.1016/0003-9861(83)90149-2. [DOI] [PubMed] [Google Scholar]



