Abstract
Background and Objective:
The surviving sepsis campaign treatment guideline (SSC) implementation is associated with improved outcome in adults with severe sepsis. The effect on outcome of pediatric sepsis is less clear.
Purpose:
To determine the clinical outcomes of SSC implementation and to investigate the prognostic value of initial plasma NT-proBNP and procalcitonin in children.
Materials and Methods:
Infants and children (aged 1month/0-15 years with severe sepsis or septic shock) were prospectively enrolled and treated according to the guidelines. Initial blood drawn was saved for NT-pro-BNP, procalcitonin measurements and clinical data were also recorded.
Results:
A total of 47 subjects were recruited. Since the application of the SSC, our mortality rate had significantly decreased from 42-19% (P = 0.003) as compared to the data in the previous 3 years. Clinical factors that significantly increased the mortality rate were: Initial central venous oxygen saturation < 7 0% after fluid resuscitation [odds ratio (OR) = 23.3; 95% confidence interval (CI) 3.7-143; P = 0.001], and initial albumin level (≤ 3 g/dl, OR = 6.7; 95% CI 1.2-37.5, P = 0.03). There was asignificant difference between the initial NT-proBNP levels between survivors and non survivors, (6280.3 ± 9597 ng/L, P < 0.001), but not for procalcitonin (12.7 ± 24.8, 29.3 ± 46 μg/L, P = 0.1), respectively. An initial NT-proBNP level of more than 11,200 pg/ml predicted Pediatric Intensive Care Unit (PICU) mortality with a sensitivity of 85.7% and a specificity of 90%.
Conclusions:
A modified SSC for severe sepsis and septic shock significantly reduced the mortality rate in our PICU. High initial NT-ProBNP level was associated with mortality.
Keywords: Biomarker, decrease mortality, mortality, pediatric sepsis, severe sepsis, surviving sepsis campaign, SSC guideline
Introduction
Sepsis is a common clinical condition with a significant impact on healthcare resources and expense. According to World Health Organization estimates, sepsis accounts for 60-80% of lost lives per year in childhood.[1] It accounts for approximately 20% of all admissions to the intensive care unit and is remain the leading cause of morbidity and mortality in the pediatric intensive care unit (PICU). Data from the surviving sepsis campaign showed a mortality of 34.8% and the number is higher in developing country.[2,3] Recent data from Italian PICU (n = 22) reported mortality as high as 50% in children with septic shock.[3] Their mortality was close to our previous report.[4] The growing number of patients with severe sepsis and septic shock with significant mortality requires changes in the current management protocols. In 2002, the American college of critical care medicine first published clinical practice parameters for hemodynamic support of pediatric and neonatal with septic shock.[5] Han et al., later reported that early recognition and aggressive resuscitation of pediatric-neonatal septic shock by community physicians can save lives.[6] It has been associated with better outcome.[7,8]
Previous reports have shown a reduction of incidence with using bundled care such as catheter-related infection or morbidity and mortality in mechanically ventilated patients by changing clinical practice.[9,10] The current guidelines recommend stepwise use of fluid resuscitation and inotropic support to attain optimal blood pressure and achieve adequate tissue perfusion.[2] We believe that if the bundle guidelines are well-organized, and performed reliably and timely, we can achieve the desired outcome of reducing sepsis-related death at our institution by at least 5-10%. Thus, the purpose of our study was to determine the clinical outcomes after using surviving sepsis campaign guideline also assess the prognostic value of initial NT-ProBNP and plasma procalcitonin in children with severe sepsis and septic shock.
Materials and Methods
We prospectively employed a before and after study in the PICU of University referral hospital, Bangkok, Thailand. The study compares time periods before (2007-2009) implementation of the interventions, and the period of intervention from April, 2010 to May, 2011 with wash-out period from January, 2010-March, 2010. The educational program was done through a protocol based on surviving sepsis campaign guideline including lectures by ER personnel, healthcare workers at pediatric ward, pediatric residents and fellows. The protocol addressed the importance of early diagnosis and therapeutic interventions, quality indicators and the data collection (See details of protocol in Appendix 1).
Patients aged between 1 month through 15 years with diagnosis of severe sepsis or septic shock who were admitted to the pediatric ward prior to PICU admission or directly to PICU were recruited to our study. This investigation was approved by our institutional review board. Informed consent was required for enrollment to the study. Clinical data collected included age, sex, admission date, the time of diagnosis before PICU admission, location, time of antibiotics given, length of stay and PRISM score. Blood was also drawn at the time of diagnosis and saved for further analysis. Once a patient met the inclusion criteria, the acute interventions including fluid resuscitation to achieve adequate tissue perfusion, administration of antibiotic, hydrocortisone if clinically indicated, early use of inotrope or vasopressor and post acute intervention bundle care to be completed within 24 h. Blood was collected for a basic laboratory work up including blood culture prior to antibiotic administration.
The Pediatric Critical Care Society sepsis definition was used for recruiting the subjects. (Appendix I).[11] The PICU physicians, fellows, and nurses were notified simultaneously of the cases enrolled.
A modified early goal directed in children with Severe Sepsis and Septic shock for 6 hrs and 24 hrs bundle.
Statistical analysis
Data are presented with mean ± standard deviation or median depending of the normality of their distribution. If data were normally distributed, the comparisons were performed using Student's t-test, otherwise, Wilcox on rank-sum (Mann-Whitney) test was used. Fischer's exact test was used to compare proportion variability. Multivariate logistic regression analysis was performed for different risk factors associate with mortality. Receiver-operating characteristic (ROC) curves were constructed to illustrate a cut off value of PCT and NT-proBNP levels. All tests of significance were two-tailed. A P values of < 0.05 was considered significant. All statistical analyses were performed using the software SPSS, version 16 (Chicago, IL, USA).
Results
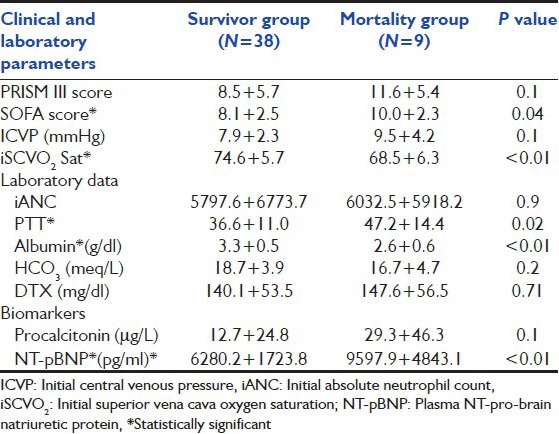
There were 47 patients enrolled in our 1-year prospective study. Their age was at 5.4 ± 4.9 years (range: 1 month-15 years). There were 22 males (46.8%) and 25 females (53.2%). Nine patients (9/47, 19%) expired. Thirty patients (63.8%) had underlying diseases in addition to sepsis, leukemia (25.5%) was the most common. Chronic liver disease was found in 6 (12.7%), neuromuscular disease in 4 (8.5%), and immunodeficiency in 3 (6.4%). There were 31 (66%) patients who developed respiratory failure and required mechanical ventilation. Their mean initial PRISM III score was 9.1 ± 5.8. Mean initial superior vena cava oxygen saturation (iScvO2) at admission to PICU was 73.4% ± 6.2% (range: 52-87%) as shown in Table 1. Initial oxygen index was 9.9 + 8.6 and PaO2 /FiO2 ratio was 210.1 + 95.1. Initial positive inspiratory pressure (PIP) for those mechanical ventilated was at 23.7 + 6.90 (cmH2 O). Maximum PIP was significantly lower in survivors compared with nonsurvivors (21.9 ± 7.2, 28.4 ± 2.7 cm H2 O, P = 0.02). Initial arterial pH was significantly lower in the non survival group compared to the survival group (7.3 ± 0.1 and 7.4 ± 0.1, P = 0.04) [Table 2].
Table 1.
Baseline demographic data of subjects enrolled in the study compared between preinterventional period (2007-2009) and interventional period (2010-2011). (Data represent as mean ± standard deviation)
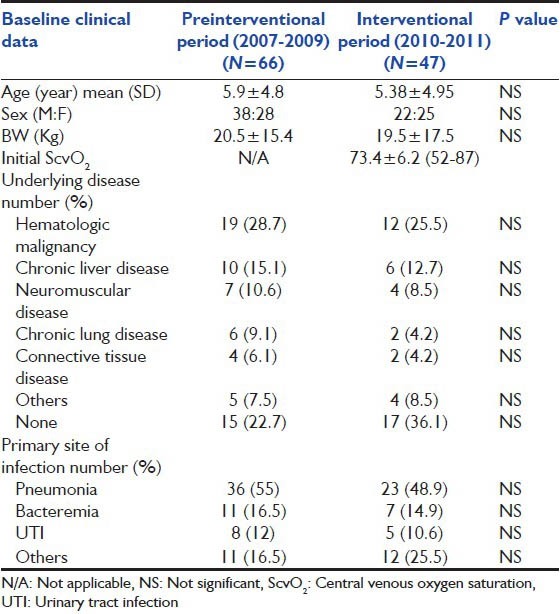
Table 2.
Baseline clinical characteristic and Laboratory parameters admitted to pediatric intensive care unit compared between survivor and nonsurvivor group. (*significant P<0.05) (Data represent as mean±standard deviation)
Sepsis bundled care compliance
The overall compliance of our sepsis bundle was at 70% by measuring from each item. To achieve optimum oxygen delivery by keeping Hb at 10 g/dl,[7] 17 patients (36%) were given blood transfusions. FFP and platelets were given in 21 patients (44.7%). A total of 42 patients (87.2%) were given antibiotics within an hour of severe sepsis or septic shock diagnosis. All patients were transferred out of ER to PICU within 1.1 ± 0.2 h.
Hemodynamic resuscitation compliance
A total of 0.9% NSS was selected as a first choice in 95% of initial fluid resuscitation after enrollment. 18.8 ± 3.4 CC/kg was given in the first 15 min, 43.1 ± 13.1 CC/kg in the 1st hour, and 68.3 ± 19.22 CC/kg in the first 6 h of fluid resuscitation. Inotropic or vasopressor agents were started via peripheral line after fluid resuscitation within 33.8 ± 5.5 min to achieve optimum cardiac output (by using update pediatric hemodynamic parameters guideline).[12] Dopamine was used as a first-line inotrope in 95.7% after fluid refractory shock was suspected. Central venous line (internal jugular 38.3%, subclavian 23.4%, and femoral 38.3%) were inserted in children with fluid refractory septic shock within 2 ± 0.3 h after initiation of fluid resuscitation to achieve a CVP goal of 8-12 mmHg. The initial CVP upon insertion was measured at 8.2 ± 2.8 mmHg.
ScvO2 monitoring
Central venous oxygen saturation (ScvO2) level of at least 70% was achieved in 89.4% of patients in the first 6 h. Levels were significantly different between survivors and non-survivors [Figure 1, Table 2, P < 0.01]. Children who did not achieve ScvO2 ≥ 70% at 6 h (3/9, 33%) after resuscitation expired. [Figure 1] If they had a follow-up ScvO2 < 70% at 6 h after resuscitation, they would have a significant risk of dying [odds ratio (OR) =23.3; 95% confidence interval (CI) =3.7 − 143; P = 0.001].
Figure 1.
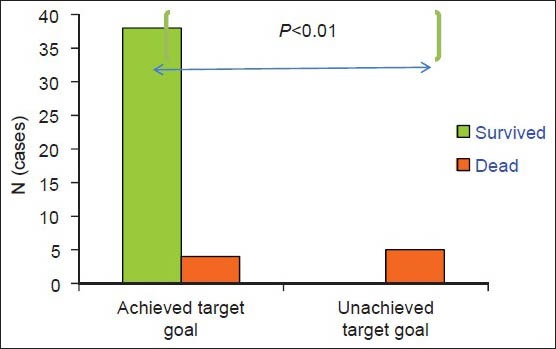
Demonstrates the difference of sepsis mortality compared between the group that achieved central venous oxygen saturation target goal >70% after initial fluids resuscitation and the group that had ScvO2 < 70% after fluids resuscitation (*P < 0.01)
Primary outcome
After full implementation of the SSC bundle, we found significant reduction of our septic shock 28-days PICU mortality from 42% (28/66) in the 3 years prior period down to 19.1% (9/47,*P = 0.003) in the 1-year intervention period and duration of PICU admission from 14.1 ± 14.5 (d) down to 8.6 ± 12.4 (d) (P = 0.04). Baseline clinical characteristics were comparable between two periods.[Tables 1 and 3] Among children with septic shock the amount of resuscitative fluids administered differed significantly between survivors and non-survivors at 1 and 6 h [P < 0.04, Figure 2].
Table 3.
Compare baseline clinical characteristic between septic shock children preintervention period (2007-2009) and post intervention period (2010-2011)
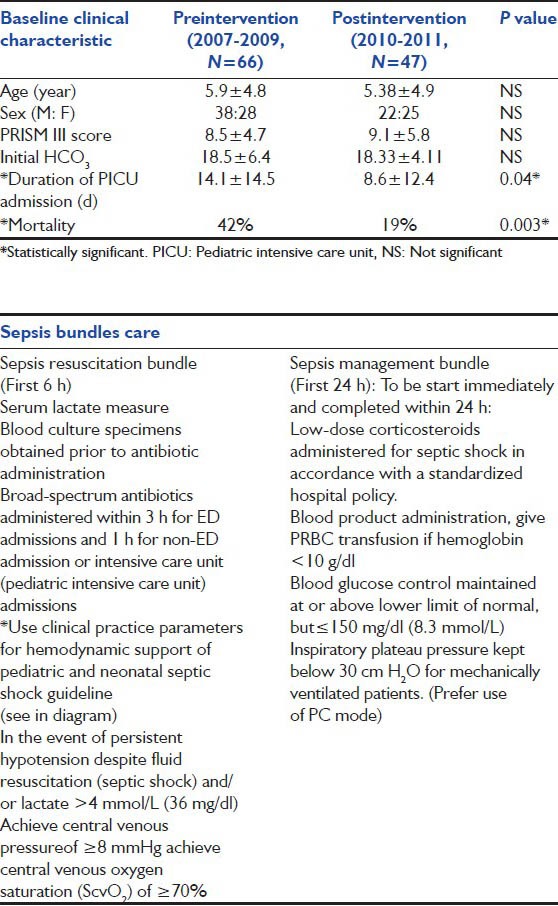
Figure 2.
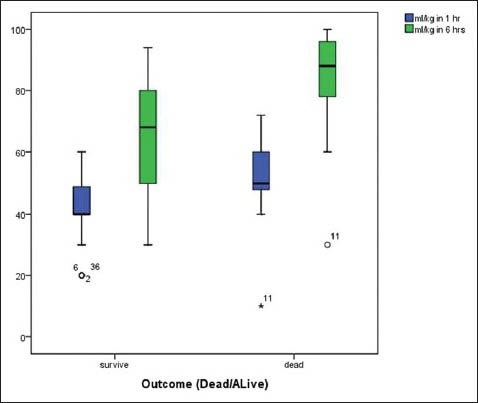
Demonstrates the total amount of volume resuscitation (cc/ kg) in children with septic shock compared between survivors and non survivors at 1 and 6 h after fluid resuscitation (*P < 0.04)
Sepsis biomarkers
Mean initial procalcitonin and NT-proBNP levels were at 15.7 ± 29.8 (μg/L) and 9780.5 ± 12531(ng/L), respectively. There was a significant increased difference in the initial NT-proBNP levels survivors versus non survivors, [Figure 3, P < 0.001], but not on initial procalcitonin levels. There was a significant correlation between initial procalcitonin level and initial plasma NT-proBNP level (R = 0.49, P = 0.001,) In the ROC curve analysis for NT-proBNP level and predictor of mortality, the area under the curve (AUC) for PICU mortality was 0.93 (95% CI, 0.79-1) compared to AUC = 0.68 (95% CI, 0.48-0.88) for procalcitonin [Figure 4].
Figure 3.
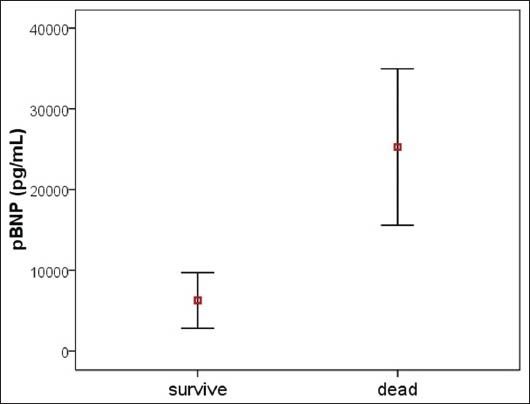
Demonstrates the levels of initial pBNP was significantly higher in non survivors compared with survivors (P < 0.01)
Figure 4.
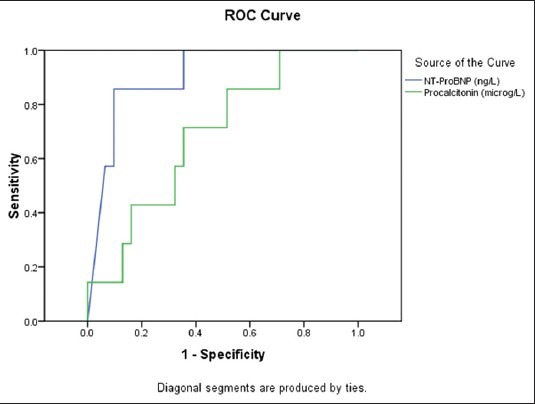
Demonstrates receiver-operator characteristic curves for predictive of mortality in children with septic shock compared between initial NT-proBNP (levels > 11,200 ng/L, sensitivity 85%, specificity 93%) and initial procalcitonin (levels > 1.42 ƒÝ/L, sensitivity 85%, specificity 49%)
Discussion
The mortality rate of severe sepsis and septic shock in our PICU was previously quite high similar to other developing countries.[1,3] Therefore, new sepsis management protocol needed to be implemented. Sepsis is associated with imbalance between oxygen delivery and demand. The treatment strategy to correct this imbalance is referred to as goal-directed therapy. Rivers et al.,[13] firstly introduced early goal-directed therapy management algorithm in adults sepsis since admission to emergency department. Early resuscitation in severe sepsis and septic shock can modulate inflammation and result in significant reduction morbidity and mortality as well as health care resource consumption.[14,15] Han et al.,[6] previously reported that early reversal of pediatric-neonatal septic shock by community physicians is associated with improved outcome. It showed the importance of early and aggressive hemodynamic resuscitation in children with septic shock. Additionally, previous analysis of utilized bundled care for adult septic shock demonstrated consistent and significant improvement in survival.[16,17] This study also shows that implementing a sepsis bundle in our hospital could significantly improve outcome, given the fact that our previous baseline clinical data for the preintervention period (3 years prior) is comparable with 14 months of interventional period [Tables 1 and 3].
Early recognition of sepsis, hemodynamic optimization with fluid resuscitation, and rapid vasopressor used may contribute to our success in reducing sepsis mortality. Intravenous antimicrobial therapy is a key element in treating septic patients. Every hour of delay in appropriate antibiotic administration was associated with a significant increase in mortality.[18,19,20] This study demonstrated that 87.2% (n = 41) of our subjects received antibiotics within 1 h of enrollment. The compliance of the sepsis bundle played a significant part of improving outcome. Prior to the implementation of a written sepsis protocol and educational program, our overall compliance was not good. Our compliance of the bundle was at 30% at the baseline and continues to increase over time. This study achieved a high compliance rate (70%) of the sepsis bundle at the end of the study which was much better compared to most of the previous published data that ranged between 30% and 55%.[21] Additionally, recent studies in adult sepsis from most of the Asian countries had reported even lower compliance rate (7.6-54%).[22,23]
We utilized a modified model of rapid response team (trained fellow, pediatric residents, and nurses) with rapid transfer of critically ill sepsis patients from their initial location (ER, medical, and surgical ward) to the PICU or wards for continued EGDT. Multiple clinical staff models may be required to achieve a consistent level of quality care for the treatment of sepsis.[24,25,26] This team model plays a vital part in achieving sepsis bundle compliance which resulted in early therapeutic interventions.[22] Logistic issues regarding early hemodynamic optimization limit its generalizability because of inadequate resources even in industrialized country. Recent multicenter study[27] reported overall in-hospital mortality rate reduction from 23.1% to 18.8% without including ScvO2 > 70% as a directed goal therapy. In contrast, another multicenter study showed that the only intervention from the sepsis bundle that impacts on mortality was the achievement of ScvO2 > 70%.[14,28,29] Nevertheless, we found in our study that targeted ScvO2 (>70%) at 6 has a better outcome (89.4% in first 6 h, OR: 0.8 (0.7-0.9), 95% CI, P = 0.02). Although a normal ScvO2 does not exclude tissue hypoxia, a low ScvO2 is an important sign of inadequate tissue perfusion.[30]
Several biomarkers have been investigated in severe sepsis and septic shock. Procalcitonin, a calcitonin precursor, seems to be among the most promising.[31] It has recently been linked to severity of bacterial infection.[32] In addition, children are more likely to have cardiac dysfunction than adults in severe sepsis or septic shock, with 58% having low cardiac output with high systemic vascular resistance and 22% having both low cardiac output and low systemic vascular resistance.[33,34] Thus, a biomarker that could be linked to cardiac dysfunction in children with septic shock would be helpful. Plasma NT-PBNP is a biomarker, synthesized and secreted by myocytes and fibroblasts in the atria and ventricle that has been associated with cardiac dysfunction.[35,36] Our study reveals significant correlation between initial procalcitonin level and plasma NT-proBNP levels drawn at initial resuscitation. This may indicate the severity of infection related with higher level of NT-proBNP. We observed that initial plasma NT-PBNP levels were significantly elevated in all septic patients and were significantly higher in non survivors compared with survivors. Moreover, initial plasma NT-proBNP levels were also found to be an independent prognostic marker of hospital mortality and were a better predict PICU mortality than initial procalcitonin. A recent article reported that BNP level at intensive care unit entry correlates with mortality and could have a role in guiding fluid therapy in septic patients.[37] They reported that the volume of positive fluid balance was independently associated with rise in BNP level. Although early goal-directed therapy requires volume resuscitation, negative fluid balance should be targeted after hemodynamic stabilization.[38] Further studies on the role of this marker in relation to cardiac dysfunction in sepsis and guiding fluid management are required.
Conclusion
Applying the sepsis management bundle in a single center tertiary care setting resulted in reduced mortality for children presenting with severe sepsis and septic shock. The sepsis bundle is a quality improvement program that should be implemented in all hospital settings and effort should be made to continue improving compliance. It also requires a coordinated effort among healthcare team such as emergency room physicians, ward physicians, nurses, and intensivists. However, the importance of each component in the bundle should be studied perhaps the bundle could be modified to suit individual institution capabilities. Last, continued education for all healthcare providers involved in sepsis care is mandatory for sustained improvement in patient outcomes.
Acknowledgment
Ratchadapiseksompotch fund, Faculty of Medicine, Chulalongkorn University.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Kissoon N, Argent A, Devictor D, Madden MA, Singhi S, van der Voort E, et al. World federation of pediatric intensive and critical care societies-its global agenda. Pediatr Crit Care Med. 2009;10:597–600. doi: 10.1097/PCC.0b013e3181a704c6. [DOI] [PubMed] [Google Scholar]
- 2.Oliveira C, Nogueira F, Oliveira D, Gottschald AF, Moura JD, Shibata AR, et al. Time-and fluid sensitive resuscitation for hemodynamic support of children in septic shock: Barriers to the implementation of the American College of Critical Care Medicine/Pediatric Advanced Life Support Guidelines in a pediatric intensive care unit in a developing world. Pediatr Emerg Care. 2008;24:810–5. doi: 10.1097/PEC.0b013e31818e9f3a. [DOI] [PubMed] [Google Scholar]
- 3.Wolfer A, Silvani P, Musicco M, Antonelli M, Salvo I Italian Pediatric Sepsis Study (SISPe) group. Incidence of and mortality due to sepsis, severe sepsis and septic shock in Italian Pediatric Intensive Care units: A prospective national survey. Intensive Care Med. 2008;34:1690–7. doi: 10.1007/s00134-008-1148-y. [DOI] [PubMed] [Google Scholar]
- 4.Samransamruajkit R, Hiranrat T, Prapphal N, Sritippayawan S, Deerojanawong J, Poovorawan Y. Levels of protein C activity and clinical factors in early phase of pediatric septic shock may be associated with the risk of death. Shock. 2007;28:518–23. doi: 10.1097/shk.0b013e318054de02. [DOI] [PubMed] [Google Scholar]
- 5.Carcillo JA, Fields A American College of Critical Care Medicine Task Force Committee Members. Clinical practice parameters for hemodynamic support of pediatric and neonatal patients in septic shock. Crit Care Med. 2002;30:1365–78. doi: 10.1097/00003246-200206000-00040. [DOI] [PubMed] [Google Scholar]
- 6.Han Y, Carcillo JA, Dragotta M, Bills DM, Watson RS, Westerman ME, et al. Early reversal of Pediatric-neonatal septic shock by community physicians is associated with improved outcome. Pediatrics. 2003;112:793–9. doi: 10.1542/peds.112.4.793. [DOI] [PubMed] [Google Scholar]
- 7.Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, et al. Surviving Sepsis Campaign Management Guidelines Committee. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858–73. doi: 10.1097/01.ccm.0000117317.18092.e4. [DOI] [PubMed] [Google Scholar]
- 8.Kissoon N, Carcillo JA, Espinosa V, Argent A, Devictor D, Madden M, et al. Global Sepsis Initiative Vanguard Center Contributors. World Federation of Pediatric Intensive Care and Critical Care Societies: Global Sepsis Initiative. Pediatr Crit Care Med. 2011;12:494–503. doi: 10.1097/PCC.0b013e318207096c. [DOI] [PubMed] [Google Scholar]
- 9.Pronovost P, Berenholtz S, Ngo K, McDowell M, Holzmueller C, Haraden C, et al. Developing and testing quality indicators in the intensive care unit. J Crit Care. 2003;18:145–55. doi: 10.1016/j.jcrc.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, et al. An intervention to decrease catheter-related blood stream infections in the ICU. N Engl J Med. 2006;355:2725–32. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein B, Giroir BP, Randolph A. International consensus conference on pediatric sepsis. International consensus on pediatric sepsis. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 12.Brierley J, Carcillo JA, Choong K, Cornell T, Decaen A, Deymann A, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: Update from American College of Critical Care Medicine. Crit Care Med. 2009;37:666–88. doi: 10.1097/CCM.0b013e31819323c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rivers EP, Nguyen HB, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early Goal-Directed Therapy Collaborative Group. Early goal directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;346:1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 14.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Shiramizo S, Marra A, Durao M, Paes ÂT, Edmond MB, Pavão dos Santos OF. Decreasing mortality in severe sepsis and septic shock patients by implementing a sepsis bundle in a hospital setting. PLoS One. 2011;6:e26790. doi: 10.1371/journal.pone.0026790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrer R, Artigas A, Suarez D, Palencia E, Levy MM, Arenzana A, et al. Edusepsis Study Group. Effectiveness of treatments for severe sepsis: A prospective, multicenter, observational study. Am J Respir Crit Care Med. 2009;180:861–6. doi: 10.1164/rccm.200812-1912OC. [DOI] [PubMed] [Google Scholar]
- 17.Baronchia A, CUI X, Vitberg D, Suffredini AF, O’Grady NP, Banks SM, et al. Bundled Care for septic shock: An Analysis of clinical trials. Crit Care Med. 2010;38:668–78. doi: 10.1097/CCM.0b013e3181cb0ddf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall JC, Al Naqbi A. Principles of source control in the management of sepsis. Crit Care Nurs Clin North Am. 2011;23:99–114. doi: 10.1016/j.ccell.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Bochud PY, Bonten M, Marchetti O, Calandra T. Antimicrobial therapy for patients with severe sepsis and septic shock: An evidence-based review. Crit Care Med. 2004;32(Suppl 11):S495–512. doi: 10.1097/01.ccm.0000143118.41100.14. [DOI] [PubMed] [Google Scholar]
- 20.Cohen J, Brun-Buisson C, Torres A, Jorgensen J. Diagnosis of infection in sepsis: An evidence-based review. Crit Care Med. 2004;32(Suppl 11):S466–94. doi: 10.1097/01.ccm.0000145917.89975.f5. [DOI] [PubMed] [Google Scholar]
- 21.Gao F, Melody T, Daniels DF, Giles S, Fox S. The impact of compliance with 6-hour and 24-hour sepsis bundles on hospital mortality in patients with severe sepsis: A prospective observational study. Crit Care. 2005;9:R764–70. doi: 10.1186/cc3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Na S, Kuan WS, Mahadevan M, Li CH, Shrikhande P, Ray S, et al. Atlas Investigators. Implementation of early goal-directed therapy and the surviving sepsis campaign resuscitation bundle in Asia. Int J Qual Health Care. 2012;24:452–62. doi: 10.1093/intqhc/mzs045. [DOI] [PubMed] [Google Scholar]
- 23.Phua J, Koh Y, Du B, Tang YQ, Divatia JV, Tan CC, et al. Mosaics Study Group. Management of severe sepsis in patients admitted to Asian intensive care units: Prospective cohort study. BMJ. 2011;342:d3245. doi: 10.1136/bmj.d3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giuliano KK. Physiological monitoring for critically ill patients: Testing a predictive model for the early detection of sepsis. Am J Crit Care. 2007;16:122–30. [PubMed] [Google Scholar]
- 25.Robson WP. From A and E to ICU: How nurses can support the Surviving Sepsis Campaign. Intensive Crit Care Nurs. 2004;20:113–5. doi: 10.1016/j.iccn.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Slade E, Tamber PS, Vincent JL. The Surviving Sepsis Campaign: Raising awareness to reduce mortality. Crit Care. 2003;7:1–2. doi: 10.1186/cc1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peake S, Bailey M, Bellomo R, Cameron PA, Cross A, Delaney A, et al. ARISE Investigators, for the Australian and New Zealand Intensive Care Society Clinical Trials Group. Australasian resuscitation of sepsis evaluation (ARISE): A multicenter, prospective, inception cohort study. Resuscitation. 2009;80:811–8. doi: 10.1016/j.resuscitation.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 28.van Beest PA, Hofstra JJ, Schultz MJ, Boerma EC, Spronk PE, Kuiper MA. The incidence of low venous oxygen saturation on admission to the intensive care unit: A multi-center observational study in The Netherlands. Crit Care. 2008;12:R33. doi: 10.1186/cc6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castellanos-Ortega A, Suberviola B, Garcia-Astudillo LA, Holanda MS, Ortiz F, Llorca J, et al. Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: Results of a three-year follow-up quasi-experimental study. Crit Care Med. 2010;38:1036–43. doi: 10.1097/CCM.0b013e3181d455b6. [DOI] [PubMed] [Google Scholar]
- 30.de Oliveira LT, De Oliveira DS, Gottschald A, Moura J, Costa G, Ventura A, et al. ACCM/PALS hemodynamic support guidelines for pediatric septic shock: An outcome comparison with and without monitoring central venous oxygen saturation. Intensive Care Med. 2008;34:1065–75. doi: 10.1007/s00134-008-1085-9. [DOI] [PubMed] [Google Scholar]
- 31.Kopterides P, Siampos I, Tsangaris I, Tsantes A, Armaganidis A. Procalcitonin-guided algorithms of antibiotic therapy in the Intensive care unit: A systematic review and Meta-analysis of randomized controlled trials. Crit Care Med. 2010;38:2229–41. doi: 10.1097/CCM.0b013e3181f17bf9. [DOI] [PubMed] [Google Scholar]
- 32.Reinhart K, Carlet JM. Procalcitonin-A new marker of severe infection and sepsis. Intensive Care Med. 2000;26(Suppl 2):S145. doi: 10.1007/BF02900726. [DOI] [PubMed] [Google Scholar]
- 33.Ceneviva G, Paschall J, Maffei F, Carcillo JA. Hemodynamic support in fluid-refractory pediatric septic shock. Pediatrics. 1998;102:e19. doi: 10.1542/peds.102.2.e19. [DOI] [PubMed] [Google Scholar]
- 34.Parker MM, Hazelzet JA, Carcillo JA. Septic shock: Pediatric considerations. Crit Care Med. 2004;32(Suppl 11):591–S4. doi: 10.1097/01.ccm.0000145904.97821.0d. [DOI] [PubMed] [Google Scholar]
- 35.Brueckmann M, Huhle G, Lang S, Haase KK, Bertsch T, Weiss C, et al. Prognostic value of plasma N-terminal pro-brain natriuretic peptide in patients with severe sepsis. Circulation. 2005;112:527–34. doi: 10.1161/CIRCULATIONAHA.104.472050. [DOI] [PubMed] [Google Scholar]
- 36.Roch A. What does high NT-ProBNP mean in septic shock patients? A part of the puzzle. Crit Care. 2007;11:122. doi: 10.1186/cc5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Z, Zhang Zhen, Xue Y, Xu X, Ni H. Prognostic value of B-type natriuretic peptide (BNP) and its potential role in guiding fluid therapy in critically ill septic patients. Scan J Trauma Resusc Emerg Med. 2012;20:86. doi: 10.1186/1757-7241-20-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: A positive fluid balance and elevated CVP are associated with increased mortality. Crit Care Med. 2011;39:259–65. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]


