Abstract
Background and Aims:
Intensive care unit acquired weakness (ICUAW) is a common occurrence in patients who are critically ill. It is most often due to critical illness polyneuropathy (CIP) or to critical illness myopathy (CIM). ICUAW is increasingly being recognized partly as a consequence of improved survival in patients with severe sepsis and multi-organ failure, partly related to commonly used agents such as steroids and muscle relaxants. There have been occasional reports of CIP and CIM in children, but little is known about their prevalence or clinical impact in the pediatric population. This review summarizes the current understanding of pathophysiology, clinical presentation, diagnosis and treatment of CIP and CIM in general with special reference to published literature in the pediatric age group.
Subjects and Methods:
Studies were identified through MedLine and Embase using relevant MeSH and Key words. Both adult and pediatric studies were included.
Results:
ICUAW in children is a poorly described entity with unknown incidence, etiology and unclear long-term prognosis.
Conclusions:
Critical illness polyneuropathy and myopathy is relatively rare, but clinically significant sequelae of multifactorial origin affecting morbidity, length of intensive care unit (ICU) stay and possibly mortality in critically ill children admitted to pediatric ICU.
Keywords: Children, critical illness myopathy, critical illness polyneuropathy, intensive care unit acquired weakness, intensive care unit, pediatric
Introduction
Intensive care unit acquired weakness (ICUAW) is being increasingly recognized as a significant clinical problem in critically ill patients who have spent longer periods of intensive care unit (ICU) stay and mechanical ventilation (usually greater than 7 days). Osler's 1892 description of the “rapid loss of flesh” in patients with prolonged sepsis was possibly first observation of this condition.[1] In 1984, Bolton et al. first reported the clinical, electrophysiological and histopathological features of ICU patients with newly acquired weakness and subsequently introduced the term critical illness polyneuropathy (CIP).[2] Lately, critical illness myopathy (CIM) has been increasingly recognized in both adult and pediatric patients. It is uncertain whether CIP and CIM are two different disorders. Recent data suggest that CIP and CIM co-exist; a condition that has been termed critical illness polyneuropathy and myopathy (CIPNM).[3,4] Data regarding ICUAW in children is restricted to small case series and reports[5,6,7,8,9,10,11,12,13,14,15,16] and only one prospective study has been reported so far in the pediatric age group.[17]
Data Collection and Synthesis
To identify relevant studies, we searched two databases from January 1980 to March 2013. For MedLine and Embase, we used the following text words and key words: Critical care, intensive care, “CIM” or “CIP” or “CIPNM” or “neuromuscular diseases”. Articles available in English language were considered. Both adult and pediatric studies were considered. We contacted the primary authors to obtain necessary permissions to use figure and table for this review.
Results
A total of 76 articles were found including both adults and children. Articles on Guillian Barre syndrome, stroke and meningitis were excluded. There were about 30 pediatric cases described in retrospective reviews and case studies. In one prospective pediatric study, 14 cases of ICUAW were described out of 830 admissions to the pediatric ICU. In this review, we describe the incidence, clinical features, diagnosis and management of ICUAW based on current available published adult and pediatric literature to date with special reference to pediatric age group as much as feasible.
Discussion
Incidence
Incidence of CIP and CIM varies depending on patient subpopulation, diagnostic method used and timing of examination. In an adult series De Jonghe et al. found ICUAW in 25% of patients who were mechanically ventilated for ≥ 7 days and were awake and able to cooperate with physical examination.[18] Substantial number of the patients could not be evaluated in this study most commonly because of death before regaining consciousness. Several studies that included unresponsive patients have reported higher incidence of acquired neuromuscular disease in similar cohorts. Most of those studies relied on electrophysiological testing (without a confirmed muscle biopsy), which might have resulted in overestimation of weakness.[18] Incidences of neuromuscular weakness have been reported from 50% to as high as 100% in patients with sepsis and multi-organ failure.[19,20] There is no published data on exact incidence of CIP/CIM in children. The only prospective study to date estimated an incidence of 1.7%, which is much lower than in adults,[17] perhaps due to failure to recognize this clinical syndrome in children as a distinct clinical entity.[21] This issue has not been systematically studied in pediatric ICUs with the exception of above mentioned prospective study where ICUAW was found in 14 children mostly pediatric transplant patients (post bone marrow or solid organ transplant) with sepsis (9 children) and multi-organ dysfunction (11 children) out of 830 patients admitted to pediatric ICU. Most of those patients had received corticosteroids (CS), neuromuscular blocking agents (NMBA) or aminoglycosides facing multiple attempts and failed extubations due to profound muscle weakness.[17]
Risk factors
A large number of studies have been carried out to identify critically ill patients at risk for CIM/CIP.[22,23,24,25,26] These studies have identified that sepsis, systemic inflammatory response syndrome and multi-organ failure are the crucial risk factors. CS administration is another common risk factor for ICUAW. ICU acquired myopathy has been reported in patients with severe asthma who received high doses of steroids in association with other risk factors as sepsis and use of muscle relaxants (neuromuscular blocking drugs).[26,27] Third major risk factor is the administration of neuromuscular blocking drugs. However ICUAW has been described in absence of sepsis,[28] use of CSs and neuromuscular blockade.[29,30] This emphasizes our incomplete understanding of this disease and complexity of the pathogenesis involved. Various other independent risk factors have been identified in association with CIM/CIP. These are female gender,[18] severity of illness,[22,29] duration of organ dysfunction,[18] renal failure and renal replacement therapy,[31] low serum albumin,[19] parenteral nutrition,[31] hyperosmolarity,[31] duration of ICU stay,[32] vasopressor and catecholamine use,[32] septic encephalopathy[31] and hyperglycemia.[32] Immobility has profound effects on skeletal muscles and is considered as a risk factor for muscle weakness during the critical illness.[33] Muscle weakness in critically ill patients due to immobility probably reflects the loss of mechanical loading as the cause.[34] Of children with CIP/CIM reported to date majority had sepsis and systemic inflammatory response syndrome. Few children were admitted with severe asthma and after bone marrow transplantation. Most of them received CS and neuromuscular blockers before the onset of weakness, but from the data available it is not possible to drive any cause and effect association between these factors due to lack of randomized prospective data for comparison.[21] It is generally recommended that these agents should be avoided once a diagnosis of ICUAW is made, until further evidence is available on this particular issue.
Clinical features
CIP and/or CIM cannot be clearly differentiated from each other on the basis of clinical signs and symptoms alone.[35] Patients generally present with diffuse skeletal muscle weakness. Muscle involvement is so profound that sometimes it mimics flaccid areflexic quadriparesis.[36] This clinical problem is often recognized during the time when sedation is discontinued and weaning from mechanical ventilation is initiated. Weaning problems are due to the involvement of the phrenic nerves, diaphragm, intercostal and other accessory respiratory muscles.[19,37] In Patients with CIP evidence of distal loss of pain, temperature, and vibration may be observed even in fully alert patients. Tendon reflexes may be present and this should not be held against the diagnosis of CIM/CIP.[38] Cranial nerve innervated muscle groups are less commonly involved than spinally innervated muscle groups and their involvement should point to other neurological disorders such as Guillian-Barre syndrome. Facial muscles are relatively spared, however, those may be involved and in rare cases ophthalmoplegia may occur.[2,39] In children with CIP/CIM, the cases described so far have been identified because of muscle weakness and failure to wean from the ventilator. The timing of onset of weakness was variable. In some cases, it was very early in the onset of critical illness.[5,6] In the only prospective study done so far, weakness was clinically noticed after a wide range of duration of illness (4-26 days) and 86% of these children were mechanically ventilated for > 5 days.[17]
Pathogenesis
Exact pathogenesis of CIM and CIP is not known. The frequent association between myopathy and neuropathy holds the theory that CIM and CIP are not isolated events, but rather an integral part of the complications resulting from multi-organ dysfunction and failure in critically ill. Therefore shared microcirculatory, cellular and metabolic pathophysiological mechanisms may be most likely in existence. Various pathophysiological mechanisms that have been proposed are shown in Figure 1. Sepsis-related disturbance of the microcirculation in peripheral nerves and muscles is a crucial event in the pathogenesis.[19,40] In CIP, this might be mediated by the enhanced expression of E-selectin, a marker of endothelial cell activation, in the vascular endothelium of the peripheral nerves.[40] This increased expression of E-selectin could activate leucocytes within the endoneurial space, with local cytokine production, increased microvascular permeability, and formation of endoneurial edema. These microvascular alterations lead to hypoperfusion of small capillaries of the nerves and loss of blood-nerve barrier.[41] Hyperglycemia and hypoalbuminemia can further enhance endoneurial edema.[42] Hyperglycemia and ensuing increased passive uptake of glucose might also contribute to bioenergetic failure which might explain the improvement in CIP that occurs with intensive insulin therapy.[32,43] Consequently, primary axonal degeneration might occur as a result of severe deficit in energy supply. Increased permeability and leakage of capillaries may also enhance the passage of neurotoxic factors in the endoneurium. In addition, endothelial cell-leucocyte adhesion and extravasation of activated leucocytes within the endoneurial space are promoted by septic state, where they can induce tissue injury with local cytokine production.[40]
Figure 1.
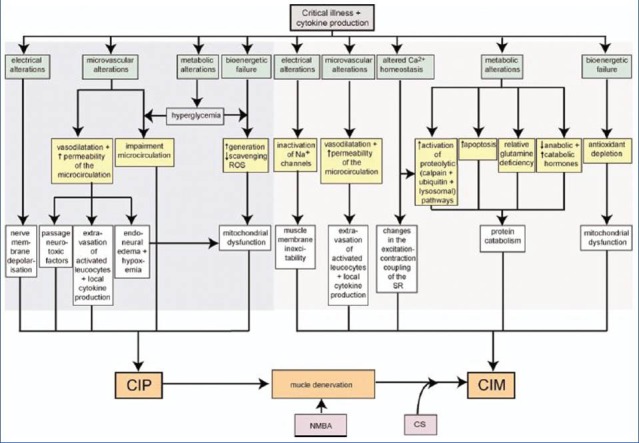
Pathophysiology of critical illness polyneuropathy/critical illness myopathy. Proposed pathophysiological mechanisms and their interactions involved in the development of critical illness polyneuropathy/critical illness myopathy. Adapted with permission from Hermans et al.[35] (CS: Corticosteroids; NMBA: Neuromuscular blocking agent; ROS: Reactive oxygen species; SR: Sarcoplasmatic reticulum)
The pathophysiology of CIM is complex and involves activation of protein degradation, inhibition of muscle protein synthesis, mitochondrial loss, mitochondrial dysfunction and abnormalities of myocyte Ca++ sequestration. Proteolytic pathways involve ubiquitin-proteasome (UPS)[44,45] and calcium dependent calpain.[46] Ubiquitination is the process in which ubiquitin moieties are selectively passed onto target proteins to “tag” them for degradation by proteasome or lysosomes among other cellular processes.[47,48] Studies have reported increased proteolysis, with activation of UPS in the skeletal muscle of human beings with atrophy associated with a variety of conditions, including CIM.[44,49] Calpains are calcium dependent, nonlysosomal proteases. They are found to be increased in ICUAW.[49] Their upregulation suggests alteration of calcium mediated homeostasis due to endotoxemia and inflammation possibly contributing to the pathogenesis of CIM. Another potential mechanism is the “Sodium channelopathy” which induces the loss of muscle membrane excitability, impairing the generation of action potentials and contraction.[50,51] In addition, impaired expression of nitric oxide synthetase could contribute to reduced muscle membrane excitability as nitric oxide takes part in maintaining resting membrane potential of fiber myocyte.[52] Disruption of skeletal muscle excitation-contraction coupling as well as altered regulation of calcium release by sarcoplasmic reticulum, are also thought to be involved in the pathogenesis of CIM.[53] Other mechanisms suggested to contribute to the pathogenesis of CIM are mitochondrial dysfunction, which is manifested as diminished mitochondrial density, oxygen consumption, oxidative phosphorylation and increased production of free radicals. Loss of mitochondrial function results in lower energy status, which causes muscle fatigability and weakness.[41]
Diagnosis
The diagnosis of ICUAW is suspected in patients who have the clinical features mentioned above, particularly flaccid muscle weakness and failure to wean from the ventilator in the setting of critical illness. CIM can be distinguished from CIP if preservation of sensory function (indicative of the former) can be demonstrated. However, sensory evaluation can be difficult in critically ill patients, particularly when obtunded, comatosed, or intubated and it might be difficult to distinguish CIM from CIP on the basis of clinical features and neurologic examination findings alone. Furthermore, some patients have features of combined CIM and CIP. Alert, cooperative patients with muscle weakness arising as a complication of critical illness should be assessed clinically with the medical research council (MRC) score. This score includes formal testing of three muscle groups in each limb on a scale of 1-5 rendering a maximum score of 60. CIP/CIM is arbitrarily diagnosed if the MRC sum score is less than 48.[18] This score has demonstrated excellent reliability, including evaluations of patients with Guillain-Barre syndrome on mechanical ventilation and can be utilized to document the extent of disease and to track changes over time.[54] Laboratory assessment of patients with ICUAW is usually unrevealing. The creatine phosphokinase is generally normal. However, if elevated it should raise the possibility of a toxic or inflammatory etiology of myopathy. Diagnosis of CIP, CIM, or combined CIP and CIM therefore relies on the clinical, electrophysiological and muscle biopsy investigations. No particular specific clinical diagnostic criteria exist in children except a clinical presentation in an ICU setting with similar features of profound muscle weakness in case reports described in the published literature.
Electrophysiological and histological features
Electrophysiological studies provide a bedside method to confirm the diagnosis and to exclude other neuromuscular causes of weakness. Unfortunately, routine electrophysiological examination has insufficient specificity to discriminate between CIP and CIM in critically ill, sedated, uncooperative, or extremely weak patients. The first electrophysiological sign that can occur very early, after the onset of critical illness, is a reduction in amplitude of the nerve conduction potentials compound muscle action potential (CMAP) or sensory nerve action potential (SNAP) or both with preserved conduction velocity.
This reduction in amplitude often precedes the clinical findings. As CIP is a primary axonal neuropathy, patients show a reduction in CMAPs and SNAPs. By contrast in CIM, CMAP duration is not prolonged. Varying degrees of fibrillation potentials and positive sharp waves will be recorded in both CIP and CIM. Direct muscle stimulation followed by calculation of the ratio between the nerve and muscle evoked action potential has been proposed as a useful test to differentiate between CIP and CIM.[55] A neCMAP: dmCMAP ratio ≥ 1 suggests either myopathy or a normal clinical picture. Differentiation between CIM and normal muscle function can be accomplished on the basis of the absolute value of the dmCMAP amplitude; myopathy causes a loss of electrically excitable muscle, leading to a reduction in the dmCMAP amplitude. A neCMAP: dmCMAP ratio < 1 is observed in neuropathy. In patients with CIPNM, ratios > 0.5 are typically observed. In such cases, dmCMAPs are depressed, and the reduction of neCMAPs depends on the extent to which axons are involved.[55] As routine electrophysiological examination in critically ill patients often cannot differentiate between neuropathy and myopathy and direct muscle stimulation is technically challenging, biopsy can be performed and is considered a gold standard for the diagnosis of involvement of muscles in the disease process. Three main types of ICU acquired myopathies have been identified based on histopathological finding collectively known as “acute quadriplegic myopathy” constituted by (a) CIM, (b) myopathy with selective loss of thick (myosin) filaments and (c) acute necrotizing myopathy.[38] CIM is characterized by generalized muscle cell atrophy, fatty degeneration, and fibrosis. It usually affects both type I and type II muscle fibers. In thick-filament myopathy, a selective loss of myosin filaments is the predominant finding. Acute necrotizing myopathy of critical illness is characterized by prominent myonecrosis, with vacuolization and phagocytosis of myocytes. In patients with CIP, histopathological studies show signs of axonal degeneration in both motor and sensory fibers.[43]
Differential diagnosis
Patient history and physical examination in an appropriate ICU setting associated with muscle weakness and difficult ventilator weaning should point toward a probable diagnosis of CIP/CIM. Limitations in this approach may include encephalopathy, tracheal intubation with limited patient communication, but this should not dissuade one from further clinical evaluation for a neuromuscular disorder.
Several disease processes involving the brain, spinal cord, peripheral nerves, neuromuscular transmission or muscles can cause muscle weakness or paralysis in a patient who is critically ill. Electrolyte abnormalities such as hypokalemia and hypophosphatemia can cause acute myopathic processes, hypermagnesemia can impair neuromuscular transmission. Several drugs can affect neuromuscular transmission, including NMBA, cancer chemotherapy, statins, and antiretroviral agents. Simple mnemonic muscles is helpful in remembering some of the most common causes of generalized weakness in the ICU [Table 1].[56]
Table 1.
Mnemonic used for differential diagnosis of generalized weakness in the intensive care unit
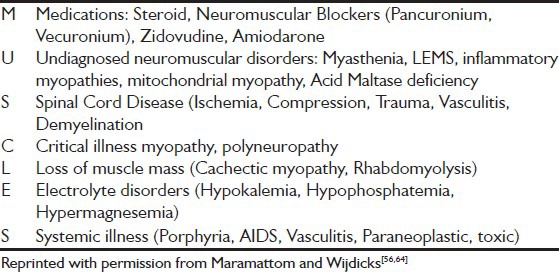
Since specific clinical diagnostic criteria don’t exist, a high index of suspicion for CIP/CIM should be kept in children and diagnosis is mainly made by excluding other known causes of muscle weakness.
Management
There are no proven therapies that prevent or reverse CIP or CIM. Several therapeutic strategies have been suggested to prevent CIP/CIM. These include nutritional interventions, supplement therapies, antioxidant therapy (glutamine, arginine, omega-3 fatty acids), testosterone derivatives, growth hormone, and immunoglobulins.[31,35,57] None of these therapies, however, have actually been shown to have beneficial effects on muscle function in ICU patients. Therefore, only preventive and supportive measures can be recommended. The best evidence for prevention comes from two prospective studies that studied the effect of intensive versus conventional insulin therapy in both surgical and medical ICU patients.[58,59] In the sub analysis, intensive insulin therapy significantly reduced the incidence of CIP/CIM and need for prolonged mechanical ventilation. This management seems appropriate with careful implementation and safeguards against hypoglycemia. The underlying systemic illness should be treated properly. Medications that increase the risk of weakness should undergo careful reviews. Strategies to mobilize patients either with passive stretching or active exercise with physical and occupational therapy reduces muscle atrophy.[60] A protocol of coordinated daily interruption of sedatives with spontaneous awakening and interruption of mechanical ventilation with spontaneous breathing trials is recommended to reduce the duration of ventilator dependence in many adult studies. Recently, one prospective randomized controlled pediatric study demonstrated good results with daily interruption of sedation in mechanically ventilated children with reduction in ventilator days and length of ICU stay.[61] Although this trial did not particularly look at incidence ICUAW in group of children who received continuous sedation versus group with daily interruption of sedation, the decreased length of stay and ventilator days in the intermittent sedation group was a significant clinical finding, which might suggest lesser incidence of adverse effects of prolonged sedation such as tolerance, withdrawal and delayed recovery.[62] Finally, it is important to maintain internal milieu of patients with attention to electrolyte disorder including phosphate and magnesium[64]
Prognosis
ICUAW (CIP/CIM) has been associated with increased length of stay in the ICU, increased number ventilated days and higher mortality rates, although data in pediatric age group is limited. Spontaneous recovery occurs within weeks in mild cases, and in months in moderate to severe cases, or does not occur at all in some of the cases.[42] Like in most axonopathies, distal muscle weakness and sensory deficits are the most common residual deficits[42] Although patients with CIM tend to have a poorer overall health status, CIM does appear to have a better prognosis as compared to CIP[63]
Conclusion
ICUAW due to CIP/CIM alone or in combination causes delay in ventilator weaning, compromises rehabilitation and is associated with significant morbidity and mortality. Diagnosis can be made by clinical examination, electrophysiological tests and muscle biopsies. Unfortunately, our understanding of this entity in pediatrics is limited. Prospective studies are required to better characterize the prevalence, natural history and clinical significance of ICUAW (CIP/CIM) in critically ill children.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Bolton CF. The discovery of critical illness polyneuropathy. Eur J Anaesthesiol Suppl. 2008;42:66–7. doi: 10.1017/S0265021508003530. [DOI] [PubMed] [Google Scholar]
- 2.Bolton CF, Gilbert JJ, Hahn AF, Sibbald WJ. Polyneuropathy in critically ill patients. J Neurol Neurosurg Psychiatry. 1984;47:1223–31. doi: 10.1136/jnnp.47.11.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bednarik J, Lukas Z, Vondracek P. Critical illness polyneuromyopathy: The electrophysiological components of a complex entity. Intensive Care Med. 2003;29:1505–14. doi: 10.1007/s00134-003-1858-0. [DOI] [PubMed] [Google Scholar]
- 4.De Letter MA, van Doorn PA, Savelkoul HF, Laman JD, Schmitz PI, Op de Coul AA, et al. Critical illness polyneuropathy and myopathy (CIPNM): Evidence for local immune activation by cytokine-expression in the muscle tissue. J Neuroimmunol. 2000;106:206–13. doi: 10.1016/s0165-5728(99)00252-0. [DOI] [PubMed] [Google Scholar]
- 5.Chetaille P, Paut O, Fraisse A, Kreitmann B, Camboulives J, Pellissier JF. Acute myopathy of intensive care in a child after heart transplantation. Can J Anaesth. 2000;47:342–6. doi: 10.1007/BF03020950. [DOI] [PubMed] [Google Scholar]
- 6.Sheth RD, Pryse-Phillips WE, Riggs JE, Bodensteiner JB. Critical illness neuromuscular disease in children manifested as ventilatory dependence. J Pediatr. 1995;126:259–61. doi: 10.1016/s0022-3476(95)70555-4. [DOI] [PubMed] [Google Scholar]
- 7.Tabarki B, Coffiniéres A, Van Den Bergh P, Huault G, Landrieu P, Sébire G. Critical illness neuromuscular disease: Clinical, electrophysiological, and prognostic aspects. Arch Dis Child. 2002;86:103–7. doi: 10.1136/adc.86.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan PW, Rocha W, Sanders DB, D’Souza B, Spock A. Acute steroid-induced tetraplegia following status asthmaticus. Pediatrics. 1986;78:121–3. [PubMed] [Google Scholar]
- 9.Dimachkie MM, Austin SG, Slopis JM, Vriesendorp FJ. Critical illness polyneuropathy in adolescence. J Child Neurol. 1995;10:409–11. doi: 10.1177/088307389501000515. [DOI] [PubMed] [Google Scholar]
- 10.Vondracek P, Bednarik J. Clinical and electrophysiological findings and long-term outcomes in paediatric patients with critical illness polyneuromyopathy. Eur J Paediatr Neurol. 2006;10:176–81. doi: 10.1016/j.ejpn.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Ohto T, Iwasaki N, Ohkoshi N, Aoki T, Ichinohe M, Tanaka R, et al. A pediatric case of critical illness polyneuropathy: Clinical and pathological findings. Brain Dev. 2005;27:535–8. doi: 10.1016/j.braindev.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Zifko UA, Zipko HT, Bolton CF. Clinical and electrophysiological findings in critical illness polyneuropathy. J Neurol Sci. 1998;159:186–93. doi: 10.1016/s0022-510x(98)00164-6. [DOI] [PubMed] [Google Scholar]
- 13.Tsao CY, Lo WD, Mendell JR, Batley RJ. Critical illness polyneuropathy in a 2-year-old girl with hemorrhagic shock encephalopathy syndrome. J Child Neurol. 1995;10:486–8. doi: 10.1177/088307389501000614. [DOI] [PubMed] [Google Scholar]
- 14.Geller TJ, Kaiboriboon K, Fenton GA, Hayat GR. Vecuronium-associated axonal motor neuropathy: A variant of critical illness polyneuropathy? Neuromuscul Disord. 2001;11:579–82. doi: 10.1016/s0960-8966(01)00200-0. [DOI] [PubMed] [Google Scholar]
- 15.Adamovic T, Willems A, Vanasse M, D’Anjou G, Robitaille Y, Litalien C, et al. Critical illness polyneuromyopathy in a child with severe demyelinating myelitis. J Child Neurol. 2009;24:758–62. doi: 10.1177/0883073808330166. [DOI] [PubMed] [Google Scholar]
- 16.Petersen B, Schneider C, Strassburg HM, Schrod L. Critical illness neuropathy in pediatric intensive care patients. Pediatr Neurol. 1999;21:749–53. doi: 10.1016/s0887-8994(99)00076-4. [DOI] [PubMed] [Google Scholar]
- 17.Banwell BL, Mildner RJ, Hassall AC, Becker LE, Vajsar J, Shemie SD. Muscle weakness in critically ill children. Neurology. 2003;61:1779–82. doi: 10.1212/01.wnl.0000098886.90030.67. [DOI] [PubMed] [Google Scholar]
- 18.De Jonghe B, Sharshar T, Lefaucheur JP, Authier FJ, Durand-Zaleski I, Boussarsar M, et al. Paresis acquired in the intensive care unit: A prospective multicenter study. JAMA. 2002;288:2859–67. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- 19.Witt NJ, Zochodne DW, Bolton CF, Grand’Maison F, Wells G, Young GB, et al. Peripheral nerve function in sepsis and multiple organ failure. Chest. 1991;99:176–84. doi: 10.1378/chest.99.1.176. [DOI] [PubMed] [Google Scholar]
- 20.Tepper M, Rakic S, Haas JA, Woittiez AJ. Incidence and onset of critical illness polyneuropathy in patients with septic shock. Neth J Med. 2000;56:211–4. doi: 10.1016/s0300-2977(00)00019-x. [DOI] [PubMed] [Google Scholar]
- 21.Williams S, Horrocks IA, Ouvrier RA, Gillis J, Ryan MM. Critical illness polyneuropathy and myopathy in pediatric intensive care: A review. Pediatr Crit Care Med. 2007;8:18–22. doi: 10.1097/01.pcc.0000256623.01254.40. [DOI] [PubMed] [Google Scholar]
- 22.Bednarík J, Vondracek P, Dusek L, Moravcova E, Cundrle I. Risk factors for critical illness polyneuromyopathy. J Neurol. 2005;252:343–51. doi: 10.1007/s00415-005-0654-x. [DOI] [PubMed] [Google Scholar]
- 23.Visser LH. Critical illness polyneuropathy and myopathy: Clinical features, risk factors and prognosis. Eur J Neurol. 2006;13:1203–12. doi: 10.1111/j.1468-1331.2006.01498.x. [DOI] [PubMed] [Google Scholar]
- 24.Hough CL, Needham DM. The role of future longitudinal studies in ICU survivors: Understanding determinants and pathophysiology of weakness and neuromuscular dysfunction. Curr Opin Crit Care. 2007;13:489–96. doi: 10.1097/MCC.0b013e3282efea3a. [DOI] [PubMed] [Google Scholar]
- 25.Young GB. Critical illness myopathy: Deeper insights. Crit Care Med. 2008;36:1977. doi: 10.1097/CCM.0b013e318176aa87. [DOI] [PubMed] [Google Scholar]
- 26.Danon MJ, Carpenter S. Myopathy with thick filament (myosin) loss following prolonged paralysis with vecuronium during steroid treatment. Muscle Nerve. 1991;14:1131–9. doi: 10.1002/mus.880141115. [DOI] [PubMed] [Google Scholar]
- 27.Amaya-Villar R, Garnacho-Montero J, García-Garmendía JL, Madrazo-Osuna J, Garnacho-Montero MC, Luque R, et al. Steroid-induced myopathy in patients intubated due to exacerbation of chronic obstructive pulmonary disease. Intensive Care Med. 2005;31:157–61. doi: 10.1007/s00134-004-2509-9. [DOI] [PubMed] [Google Scholar]
- 28.Coakley JH, Nagendran K, Yarwood GD, Honavar M, Hinds CJ. Patterns of neurophysiological abnormality in prolonged critical illness. Intensive Care Med. 1998;24:801–7. doi: 10.1007/s001340050669. [DOI] [PubMed] [Google Scholar]
- 29.de Letter MA, Schmitz PI, Visser LH, Verheul FA, Schellens RL, Op de Coul DA, et al. Risk factors for the development of polyneuropathy and myopathy in critically ill patients. Crit Care Med. 2001;29:2281–6. doi: 10.1097/00003246-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Deconinck N, Van Parijs V, Beckers-Bleukx G, Van den Bergh P. Critical illness myopathy unrelated to corticosteroids or neuromuscular blocking agents. Neuromuscul Disord. 1998;8:186–92. doi: 10.1016/s0960-8966(98)00003-0. [DOI] [PubMed] [Google Scholar]
- 31.Garnacho-Montero J, Madrazo-Osuna J, García-Garmendia JL, Ortiz-Leyba C, Jiménez-Jiménez FJ, Barrero-Almodóvar A, et al. Critical illness polyneuropathy: Risk factors and clinical consequences. A cohort study in septic patients. Intensive Care Med. 2001;27:1288–96. doi: 10.1007/s001340101009. [DOI] [PubMed] [Google Scholar]
- 32.Van den Berghe G, Schoonheydt K, Becx P, Bruyninckx F, Wouters PJ. Insulin therapy protects the central and peripheral nervous system of intensive care patients. Neurology. 2005;64:1348–53. doi: 10.1212/01.WNL.0000158442.08857.FC. [DOI] [PubMed] [Google Scholar]
- 33.Latronico N, Rasulo FA. Presentation and management of ICU myopathy and neuropathy. Curr Opin Crit Care. 2010;16:123–7. doi: 10.1097/MCC.0b013e328336a229. [DOI] [PubMed] [Google Scholar]
- 34.Chambers MA, Moylan JS, Reid MB. Physical inactivity and muscle weakness in the critically ill. Crit Care Med. 2009;37:S337–46. doi: 10.1097/CCM.0b013e3181b6e974. [DOI] [PubMed] [Google Scholar]
- 35.Hermans G, De Jonghe B, Bruyninckx F, Van den Berghe G. Clinical review: Critical illness polyneuropathy and myopathy. Crit Care. 2008;12:238. doi: 10.1186/cc7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zochodne DW, Bolton CF, Wells GA, Gilbert JJ, Hahn AF, Brown JD, et al. Critical illness polyneuropathy. A complication of sepsis and multiple organ failure. Brain. 1987;110:819–41. doi: 10.1093/brain/110.4.819. [DOI] [PubMed] [Google Scholar]
- 37.Maher J, Rutledge F, Remtulla H, Parkes A, Bernardi L, Bolton CF. Neuromuscular disorders associated with failure to wean from the ventilator. Intensive Care Med. 1995;21:737–43. doi: 10.1007/BF01704741. [DOI] [PubMed] [Google Scholar]
- 38.Eriksson LI. Acquired neuromuscular disorders in the critically ill patient. Semin Anesth Perioper Med Pain. 2002;21:135–9. [Google Scholar]
- 39.Bolton CF, Laverty DA, Brown JD, Witt NJ, Hahn AF, Sibbald WJ. Critically ill polyneuropathy: Electrophysiological studies and differentiation from Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry. 1986;49:563–73. doi: 10.1136/jnnp.49.5.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fenzi F, Latronico N, Refatti N, Rizzuto N. Enhanced expression of E-selectin on the vascular endothelium of peripheral nerve in critically ill patients with neuromuscular disorders. Acta Neuropathol. 2003;106:75–82. doi: 10.1007/s00401-003-0704-3. [DOI] [PubMed] [Google Scholar]
- 41.Dos Santos CC, Batt J. ICU-acquired weakness: Mechanisms of disability. Curr Opin Crit Care. 2012;18:509–17. doi: 10.1097/MCC.0b013e328357cb5e. [DOI] [PubMed] [Google Scholar]
- 42.Latronico N, Peli E, Botteri M. Critical illness myopathy and neuropathy. Curr Opin Crit Care. 2005;11:126–32. doi: 10.1097/01.ccx.0000155357.24360.89. [DOI] [PubMed] [Google Scholar]
- 43.Bolton CF. Neuromuscular manifestations of critical illness. Muscle Nerve. 2005;32:140–63. doi: 10.1002/mus.20304. [DOI] [PubMed] [Google Scholar]
- 44.Klaude M, Fredriksson K, Tjäder I, Hammarqvist F, Ahlman B, Rooyackers O, et al. Proteasome proteolytic activity in skeletal muscle is increased in patients with sepsis. Clin Sci (Lond) 2007;112:499–506. doi: 10.1042/CS20060265. [DOI] [PubMed] [Google Scholar]
- 45.Tiao G, Hobler S, Wang JJ, Meyer TA, Luchette FA, Fischer JE, et al. Sepsis is associated with increased mRNAs of the ubiquitin-proteasome proteolytic pathway in human skeletal muscle. J Clin Invest. 1997;99:163–8. doi: 10.1172/JCI119143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Showalter CJ, Engel AG. Acute quadriplegic myopathy: Analysis of myosin isoforms and evidence for calpain-mediated proteolysis. Muscle Nerve. 1997;20:316–22. doi: 10.1002/(SICI)1097-4598(199703)20:3<316::AID-MUS8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 47.Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315:201–5. doi: 10.1126/science.1127085. [DOI] [PubMed] [Google Scholar]
- 48.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: Destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 49.Constantin D, McCullough J, Mahajan RP, Greenhaff PL. Novel events in the molecular regulation of muscle mass in critically ill patients. J Physiol. 2011;589:3883–95. doi: 10.1113/jphysiol.2011.206193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rich MM, Pinter MJ. Crucial role of sodium channel fast inactivation in muscle fibre inexcitability in a rat model of critical illness myopathy. J Physiol. 2003;547:555–66. doi: 10.1113/jphysiol.2002.035188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rossignol B, Gueret G, Pennec JP, Morel J, Giroux-Metges MA, Talarmin H, et al. Effects of chronic sepsis on the voltage-gated sodium channel in isolated rat muscle fibers. Crit Care Med. 2007;35:351–7. doi: 10.1097/01.CCM.0000254335.88023.0E. [DOI] [PubMed] [Google Scholar]
- 52.Capasso M, Di Muzio A, Pandolfi A, Pace M, Di Tomo P, Ragno M, et al. Possible role for nitric oxide dysregulation in critical illness myopathy. Muscle Nerve. 2008;37:196–202. doi: 10.1002/mus.20907. [DOI] [PubMed] [Google Scholar]
- 53.Zink W, Kaess M, Hofer S, Plachky J, Zausig YA, Sinner B, et al. Alterations in intracellular Ca2+-homeostasis of skeletal muscle fibers during sepsis. Crit Care Med. 2008;36:1559–63. doi: 10.1097/CCM.0b013e318170aa97. [DOI] [PubMed] [Google Scholar]
- 54.Kleyweg RP, van der Meché FG, Schmitz PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barré syndrome. Muscle Nerve. 1991;14:1103–9. doi: 10.1002/mus.880141111. [DOI] [PubMed] [Google Scholar]
- 55.Seghelini E. Direct stimulation: A useful technique. Eur J Anaesthesiol Suppl. 2008;42:181–5. doi: 10.1017/S0265021507003365. [DOI] [PubMed] [Google Scholar]
- 56.Maramattom BV, Wijdicks EF. Acute neuromuscular weakness in the intensive care unit. Crit Care Med. 2006;34:2835–41. doi: 10.1097/01.CCM.0000239436.63452.81. [DOI] [PubMed] [Google Scholar]
- 57.Zink W, Kollmar R, Schwab S. Critical illness polyneuropathy and myopathy in the intensive care unit. Nat Rev Neurol. 2009;5:372–9. doi: 10.1038/nrneurol.2009.75. [DOI] [PubMed] [Google Scholar]
- 58.Hermans G, Schrooten M, Van Damme P, Berends N, Bouckaert B, De Vooght W, et al. Benefits of intensive insulin therapy on neuromuscular complications in routine daily critical care practice: A retrospective study. Crit Care. 2009;13:R5. doi: 10.1186/cc7694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hermans G, Wilmer A, Meersseman W, Milants I, Wouters PJ, Bobbaers H, et al. Impact of intensive insulin therapy on neuromuscular complications and ventilator dependency in the medical intensive care unit. Am J Respir Crit Care Med. 2007;175:480–9. doi: 10.1164/rccm.200605-665OC. [DOI] [PubMed] [Google Scholar]
- 60.Griffiths RD, Palmer TE, Helliwell T, MacLennan P, MacMillan RR. Effect of passive stretching on the wasting of muscle in the critically ill. Nutrition. 1995;11:428–32. [PubMed] [Google Scholar]
- 61.Gupta K, Gupta VK, Jayashree M, Singhi S. Randomized controlled trial of interrupted versus continuous sedative infusions in ventilated children. Pediatr Crit Care Med. 2012;13:131–5. doi: 10.1097/PCC.0b013e31820aba48. [DOI] [PubMed] [Google Scholar]
- 62.Vet NJ, Verlaat CW, de Wildt SN, Tibboel D, de Hoog M. Daily interruption of sedation in critically ill children. Pediatr Crit Care Med. 2012;13:122. doi: 10.1097/PCC.0b013e31822f1109. [DOI] [PubMed] [Google Scholar]
- 63.Guarneri B, Bertolini G, Latronico N. Long-term outcome in patients with critical illness myopathy or neuropathy: The Italian multicentre CRIMYNE study. J Neurol Neurosurg Psychiatry. 2008;79:838–41. doi: 10.1136/jnnp.2007.142430. [DOI] [PubMed] [Google Scholar]
- 64.64 Kukreti V, Shamim M, Khilnani P. Intensive care unit acquired weakness in children:Critical illness polyneuropathy and myopathy. Indian J Crit care med. 2014;18:36–42. doi: 10.4103/0972-5229.126079. [DOI] [PMC free article] [PubMed] [Google Scholar]


