Abstract
Background:
Sickle cell disease (SCD) often leads to chronic hemolytic anemia of varying severity, and blood transfusion may be employed in the management of SCD complications.
Objective:
The aim of the study was to evaluate the effect of blood transfusion on the activities of some antioxidant enzymes as well as lipid peroxide and to relate transfusion status to these enzymes and malondialdehyde (MDA) in SCD patients.
Materials and Methods:
Glutathione peroxidase (GPX), superoxide dismutase, catalase, MDA, and lipoproteins were assayed in 87 SCD and 20 age- and sex-matched subjects with normal hemoglobin. Of the 87 SCD patients, 30 had multiple transfusions, 21 had been transfused once while 36 had not been transfused within the last 3 months.
Results:
Statistically significant decrease in the mean levels of GPX (P = 0.045) and Cu/Zn SOD (P = 0.001) and increased (P = 0.001) MDA were observed in the transfused compared to non-transfused patients. Similarly, significant decrease (P = 0.001) in Cu/Zn SOD and increase (P = 0.01) in MDA were observed in multi transfused compared to those who had been transfused once. Transfusion status correlated (P <0.047) inversely with Cu/Zn SOD and positively with MDA.
Conclusion:
Reduced activity levels of serum antioxidant enzymes and increased mean levels of MDA were observed in transfused compared to non-transfused SCD patients and these changes correlated with transfusion status.
Keywords: Antioxidant enzymes, malondialdehyde, sickle cell disease, transfusion
Introduction
Sickle cell disease (SCD) is a common genetic disorder of red blood cell, resulting in chronic hemolytic anemia of varying severity. It may lead to painful crisis caused by occlusion of small blood vessels by spontaneous intravascular sickling which also affects other organs. Circulating sickle cells are susceptible to peroxidative damage due to changes in membrane structure, exposure to high oxygen tension, and the presence of excess serum iron.[1,2] The associated oxidative damage may also be due to increased levels in resting oxygen consumption as well as increased levels of pro-oxidative free hemoglobin.[3] Sickle cell hemoglobin is unstable and constantly generates reactive oxygen species (ROS) that contribute to cellular enzymes and membrane lipid damage.[4] We reported in our previous study that there were increased levels of lipid peroxidation and various oxidative biomarkers in plasma of SCD patients compared to subjects with sickle cell trait and normal hemoglobin.[5]
Prevention of sickle cell-related complications is critical in the management of patients with this disease and blood transfusion is often used to dilute the circulating sickle cells in acute emergencies. It could also decrease hemolysis by suppressing the production of abnormal red cells and improving the nutritional status of the patients. However, the increased generation of ROS due to iron overload in transfused patients may be detrimental.[3] Studies on antioxidant enzymes and lipid peroxides have not been reported in transfused SCD patients from this center. This study was therefore designed to assess the effect of blood transfusion on the activities of some antioxidant enzymes as well as lipid peroxides and to relate transfusion status to these enzymes and malondialdehyde (MDA) in SCD patients.
Materials and Methods
The study was conducted at Aminu Kano Teaching Hospital, Kano, Nigeria, and was approved by the Ethical committee of the Hospital. Informed consent was obtained from all participants. The study population comprised 87 confirmed SCD patients who were consecutively recruited from the hematology clinic of the hospital between January 2006 and December 2007. The age range of the patients was 14 to 25 years with a mean of 21.2 ± 4 years. The patients were stratified into two groups; those who have never received blood transfusion and those who have been transfused within the last 4 months. Transfused subjects were further divided into two groups; those who had received multiple transfusions and those who had been transfused only once. Twenty age- and sex-matched apparently healthy subjects with normal hemoglobin who had never been transfused were recruited from among staff and students of the hospital. Demographic, transfusion history, and clinical examination findings were obtained using structured questionnaire. Those with edema, severe jaundice, abnormal chest and abdominal findings were excluded from the study. Four months time interval was taken between transfusion and sampling for both multi-transfused and those transfused once. None of the patients was on hydroxyurea because it was not available at our center. Also, none was on iron chelation because we did not have any patient with iron overload.
Five milliliters of blood was collected in a fasting state and emptied into plain specimen bottles which were allowed to clot at room temperature for 30 minutes. The specimens were centrifuged at 3,000 rpm for 10 minutes to obtain sera. The sera were stored at −20° C and analyses was done within two weeks of collection. MDA was assayed using thiobarbituric acid reacting substances by Northwest life Science specialties, Canada. Glutathione peroxidase (GPX) and superoxide dismutase (Cu/Zn SOD) were assayed using reagents supplied by Northwest Life Science specialties, Canada, while catalase was determined colorimetrically using kit supplied by Sigma, Missouri, USA. Lipoproteins were assayed using enzyme-catalyzed colorimetric method, using reagents supplied by Randox laboratories, UK. The LDL cholesterol was calculated using Friedewald equation.[6]
Statistical analysis
Results were expressed as mean± SEM and were analyzed by unpaired Students t-test. Values of P = 0.05 were considered significant and Pearson correlation coefficient was calculated to determine the association of antioxidant enzymes and MDA with transfusion status.
Results
The results are as shown in Tables 1 and 2. A total of 87 confirmed SCD patients; 39 males, mean age 22.1 ± 3.1 years and 48 females, mean age 21.8 ± 2.1 years. Of the 36 who have never been transfused, 18 were males and 18 were females while 51 of them who had received blood transfusion, 21 were males and 30 were females. Of the 51 subjects who had been transfused, 30 of them had received multiple transfusions and 21 had been transfused once. The decrease in mean levels of GPX (P = 0.045) and Cu/Zn SOD (P = 0.001) were statistically significant in the transfused SCD patients when compared to non-transfused patients. On the other hand, statistically significant increases in mean levels of MDA (P = 0.001), LDL cholesterol (P = 0.05), and total cholesterol (P<0.02) were observed in the transfused patients compared to non-transfused. Statistically significant decreases were also observed in total cholesterol (P = 0.05; NS), GPX (P = 0.001; P = 0.5), Cu/Zn SOD (P = 0.001), and CAT (P = 0.001; NS) in both transfused and non-transfused patients, respectively, when compared to control subjects, while significant increase (P = 0.001; P = 0.002) was observed for MDA in transfused and non-transfused patients compared to controls.
Table 1.
Antioxidant enzymes, Malondialdehyde, and lipoproteins in transfused and non-transfused sickle cell disease patients and controls (Mean±SEM)
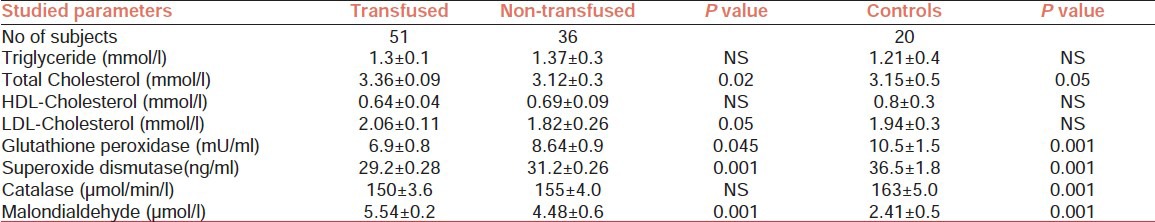
Table 2.
Antioxidant enzymes in multi transfused, transfused once, and non-transfused sickle cell disease patients
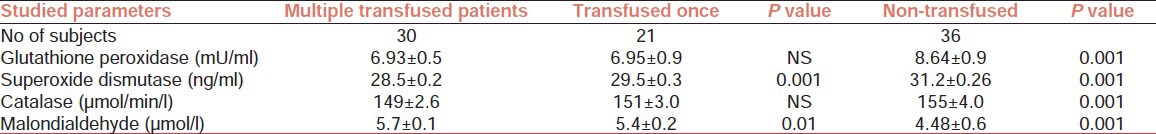
Table 2 indicates that significant decrease (P = 0.001) was observed for serum Cu/Zn SOD in multi transfused patients compared with those who had been transfused only once. Conversely, significant increase (P = 0.01) was observed for MDA in multi transfused subjects compared to those who had been transfused once. Those who had received multiple transfusions had decreased levels (P = 0.001) of GPX, Cu/Zn SOD, and CAT compared to those who have not been transfused. The mean MDA level in multi transfused subjects were significantly higher (P = 0.001) compared to those who had not been transfused. The serum levels of Cu/Zn SOD correlated negatively (r = 0.625; P = 0.05) while MDA correlated positively (r = 0.615; P < 0.047) with transfusion status in these subjects.
Discussion
The study shows that transfusion status affects the levels of MDA and activities of antioxidant enzymes in SCD patients. The levels of Cu/Zn SOD correlated negatively while MDA correlated positively with transfusion status in the study group. There was no significant change in the mean levels of CAT in transfused compared to non-transfused patients. The result is consistent with other studies.[7,8] Marwah et al.[7] reported that antioxidant capacity correlated with transfusion status in a group of SCD patients. They observed that antioxidant capacity was significantly lower in the regularly transfused compared to patients who had not received transfusion in last 3 months. In this study, we evaluated the effect of transfusion on antioxidant enzymes and lipid peroxidation in SCD patients as against vitamin E studied by the authors above.[7] Conversely, others observed that non-transfused SCD patients had reduced levels of zinc, selenium, glutathione, and vitamins as against transfused subjects.[9,10] They opined that chronic transfusions could decrease hemolysis by suppressing the production of abnormal red blood cells and can also improve the nutritional status of the patients. They however concluded that increased generation of free radicals due to iron overload in transfused patients may cancel the above benefits.[3,11]
The pathophysiological implications of our observations are that transfusion may promote increase lipid peroxidation as indicated by increased concentration of MDA and increased oxidative stress as shown by reduced activity levels of antioxidant enzymes. These may promote further intravascular hemolysis due to altered red blood cell membrane integrity. Oxidative stress biomarkers were reported to be increased in transfused SCD patients and correlated with non-transferrin bound levels.[3,8] Low levels of antioxidant capacity were also reported in chronically transfused compared to non-transfused thalassemia patients. These deficiencies correlated with increased levels of lipid peroxidation.[11]
The suggested mechanism whereby transfusion leads to reduced antioxidant capacity is that transfused iron-overload may lead to increased activity of reactive oxygen radicals on membrane components, contributing to hemolytic and vaso-occlusive complications.[7,12] Excess transfusional iron causes accumulation of labile plasma iron, the pathological form of non-transferrin-bound iron. This labile plasma iron is redox active and is rapidly taken up by cells, leading to a rise in the labile iron pool and catalyzing the generation of ROS. The resultant oxidative stress and associated decrease in antioxidants lead to oxidation of lipids, proteins, and DNA;causing cell death; and organ damage.[13] Transfused red blood cells apart from generation of free radicals may also cause infection and fibrosis consequent upon the breakdown of heme released from transfused erythrocytes. Heme is broken down by heme oxygenase (HO) to iron, carbon monoxide and bilirubin.[14] Under normal circumstances, the products of HO activity are beneficial to the organisms, but when HO activity is excessive as in SCD, the products are potentially damaging. Free iron if not sequestered with protein or urate will generate highly toxic free radicals via the Fenton and Heber-Weiss reactions and predispose the tissue to infection and fibrosis.[14,15,16] The lung is particularly sensitive to iron-induced HO activity where it may promote the production of proinflammatory cytokines and reduced levels of glutathione.[14] The imbalance between antioxidant capacity and iron overload may result in further depletion of oxidative capacity and membrane-associated iron in intact sickle cells and result in superoxide-dependent formation of hydroxyl radicals.[2] Consequently, reduction in antioxidant capacity and transfusional iron-overload may create suitable conditions for the formation of ROS that could cause damage to sickle red blood cell membrane.[17] In addition, resting energy expenditure which is elevated in patients with SCD is further increased after transfusion, despite decreased erythropoietic activity.[18]
Conclusion
Reduced activity levels of serum antioxidant enzymes and increased mean levels of MDA were observed in transfused compared to non-transfused SCD patients and these changes correlated with transfusion status.
Acknowledgements
We acknowledge the contributions of Residents and staff of Haematotology Clinic and Department of Chemical Pathology, especially Mr Usman Maigatari and Suleiman Dadinkowa.
Footnotes
Source of Support: Nil
Conflicting Interest: None declared.
References
- 1.Chiu D, Elliott V, Maggie Y, Klara K, Bertram L. Peroxidation, vitamin E and sickle cell anaemia. Am NY Acad Sci. 1982;393:323–33. doi: 10.1111/j.1749-6632.1982.tb31272.x. [DOI] [PubMed] [Google Scholar]
- 2.Sugihara T, Repka T, Hebbel P. Detection, characterization and bioavailability of membrane associated Iron in the intact sickle red cells. J Clin Invest. 1992:2327–32. doi: 10.1172/JCI116121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Claster S, Wood JC, Noetzli L, Carson SM, Hofstra TC, Khanna R, et al. Nutritional deficiencies in Iron overloaded patients with hemoglobinopathies. Am J Hematol. 2009;84:344–8. doi: 10.1002/ajh.21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Repka T, Hebbel RP. Hydroxyl radical formation by sickle erythrocyte membranes:Role of pathologic iron deposits and cytoplasmic reducing agents. Blood. 1991;78:2753–8. [PubMed] [Google Scholar]
- 5.Emokpae MA, Uadia PO, Kuliya-Gwarzo A. Antioxidant enzymes and acute phase proteins correlate with marker of lipid peroxide in adult Nigerian sickle cell disease patients. Iran J Basic Med Sci. 2010;13:177–82. [Google Scholar]
- 6.Friedewald WT, Levy RL, Fredickson DS. Estimation of concentration of low density lipoprotein cholesterol in plasma without use of preparative ultracentrifuge. Clin Chem. 1972;10:499–502. [PubMed] [Google Scholar]
- 7.Marwah SS, Blann AD, Phillips JD, Wright J, Bareford D. Reduced vitamin E antioxidant capacity in sickle cell disease is related to transfusion status but not to sickle crisis. Am J Hematol. 2002;69:144–6. doi: 10.1002/ajh.10033. [DOI] [PubMed] [Google Scholar]
- 8.Walter PB, Fung EB, Killilea DW, Jiang Q, Hudes M, Madden J, et al. Oxidative stress and inflammation in iron overloaded patients with beta-thalassaemia or sickle cell disease. Br J Haematol. 2006;135:254–63. doi: 10.1111/j.1365-2141.2006.06277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buison AM, Kawchak DA, Schall J, Ohene-Frempong K, Stallings VA, Zemel BS. Low vitamin D status in children with sickle cell disease. J Pediatr. 2004;145:622–7. doi: 10.1016/j.jpeds.2004.06.055. [DOI] [PubMed] [Google Scholar]
- 10.Tangney CC, Phillips G, Bell RA, Fernandes P, Hopkins R, Wu SM. Selected indices of micro nutritient status in adult patients with sickle cell anaemia. Am J Hematol. 1989;32:161–6. doi: 10.1002/ajh.2830320302. [DOI] [PubMed] [Google Scholar]
- 11.Chiou SS, Chang TT, Tsai SP, Jang RC, Lin SK, Lee SC, et al. Lipid peroxidation and anti-oxidative status in beta-thalassaemia major patients with or without hepatitis C virus infection. Clin Chem Lab Med. 2006;44:1226–33. doi: 10.1515/CCLM.2006.219. [DOI] [PubMed] [Google Scholar]
- 12.Marwah SS, Wheelwright D, Blann AD, Rea C, Beresford R, Phillips JD, et al. Decreased vitamin E correlates inversely with non-transferrin bound iron in sickle cell disease. Br J Haematol. 2001;114:917–9. doi: 10.1046/j.1365-2141.2001.03018.x. [DOI] [PubMed] [Google Scholar]
- 13.Ghoti H, Fibauch E, Merkel D, Perez-Avraham G, Grisariu S, Rachmilewitz EA. Changes in parameters of oxidative stress and iron biomarkers during treatment with deferasirox in iron-overloaded patients with myelodysplastic syndromes. Haematologica. 2010;95:1433–4. doi: 10.3324/haematol.2010.024992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collard KJ. Is there a causal relationship between the receipt of blood transfusions and the development of chronic lung disease of prematurity? Med Hyp. 2006;66:355–64. doi: 10.1016/j.mehy.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 15.Livrea MA, Tesoriere L, Pintaudi AM, Calabrese A, Maggio A, Freisleben HJ, et al. Oxidative stress and antioxidant status in beta-thalassemia major:Iron overload and depletion of lipid-soluble antioxidants. Blood. 1996;88:3608–14. [PubMed] [Google Scholar]
- 16.Dani C, Martelli E, Bertini G, Pezzati M, Rossetti M, Buonocore G, et al. Effect of blood transfusion on oxidative stress in preterm infants. Arch Dis Child Fetal Neonatal ED. 2004;89:F408–11. doi: 10.1136/adc.2003.037085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kehrer JP. The role of oxygen radicals in human disease with particular references to the vascular system. Haemostasis. 1993;23(Suppl 1):118–26. doi: 10.1159/000216921. [DOI] [PubMed] [Google Scholar]
- 18.Harmatz P, Heyman MB, Cunningham J, Lee PD, Styles L, Quirolo K, et al. Effects of red blood cell transfusion on resting energy expenditure in adolescents with sickle cell anaemia. J Pediatr Gastroenterol Nutr. 1999;29:27–31. doi: 10.1097/00005176-199908000-00006. [DOI] [PubMed] [Google Scholar]


