Abstract
Curcumin is a dietary diphenol with antioxidant, antinflammatory and antitumor activity. We describe facile procedures for the synthesis of [14C2]curcumin (4 mCi/mmol), [d6]curcumin, [d3]curcumin, [13C5]curcumin, and [d6]bicyclopentadione, the major oxidative metabolite of curcumin. We also describe synthesis of the labeled building blocks [14C]vanillin, [d3]vanillin, and [13C5]acetylacetone. The overall molar yields of the labeled products were 52% ([14C]) and 47% ([d3]) for vanillin and 25% ([14C2]) and 27% ([d6]) for curcumin. The compounds can be used as radiotracers in biotransformation studies and as isotopic standards for mass spectrometry-based quantification in pharmacokinetic analyses.
Keywords: bicyclopentadione, methyliodide, acetylacetone, polyphenol, oxidative metabolism, biotransformation, dietary
Introduction
The dietary diphenol curcumin is widely studied for use as an anti-inflammatory and anticancer agent.[1] A plethora of cellular targets of curcumin have been identified,[2] and its “polypharmacology” of affecting diverse cellular processes make curcumin a potentially powerful therapeutic agent.[3] Application of curcumin in clinical studies has been hampered by its low bioavailability and rapid metabolism.[4, 5] A large number of medicinal chemistry approaches have focused on improving biological activity or bioavailability of curcumin.[6–10]
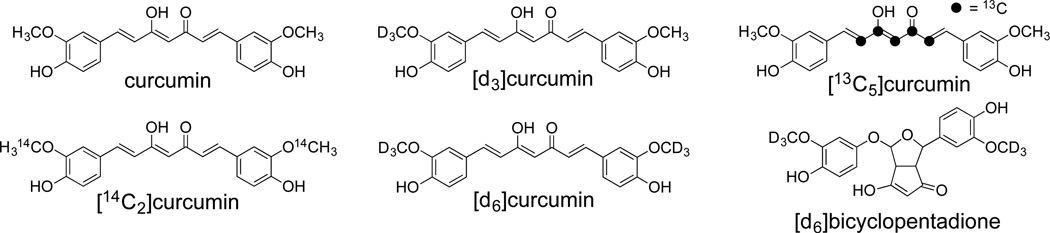
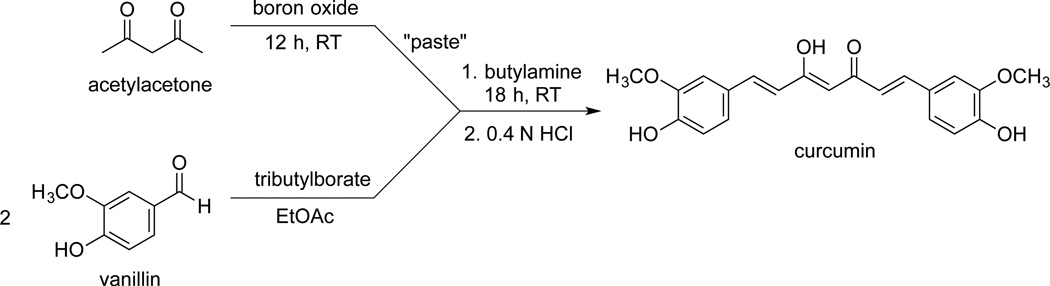
The labeled curcumin derivatives synthesized are shown in Fig. 1. A straightforward synthesis of curcumin was originally developd by Pabon.[11] The procedure is a two-step reaction with the formation of a boron complex of acetylacetone (“paste”) and its butylamine-catalyzed reaction with two molar equivalents of vanillin (Fig. 2). Slight modifications of this approach have been reported.[12–16] A readily accessible position for introduction of label into curcumin is the methoxy group of vanillin that can accomodate a [14C]radiolabel and three deuterium atoms, respectively. The apparent stability of the methoxy group during chemical and metabolic transformation of curcumin is a further advantage.[5, 17]
Fig. 1.
Curcumin and its [14C2]-, [d6]-, [d3]-, and [13C5]-labeled derivatives.
Fig. 2.
Synthesis of curcumin from vanillin and acetylacetone.[11]
We are interested in defining the mechanism, products, and biological consequences of oxidative transformation of curcumin in vitro and in vivo. Oxidative transformation of curcumin is a spontaneous reaction in buffer of physiological pH.[18] Reactive intermediates from the early stages of oxidative transformation of curcumin – rather than curcumin itself – are responsible for poisoning of human topoisomerase.[19, 20] The major end product of oxidative transformation is a dioxygenated bicyclopentadione.[21] In addition, formation of a number of less abundant products has been observed.[18, 22] In order to identify all possible transformation products and to analyze their formation in vivo we decided to prepare [14C]labeled, [13C]labeled, and deuterated curcumin to use in mechanistic studies and as isotopic standards for quantification using LC-MS.
Results and Discussion
[14C]- and [d3]Vanillin
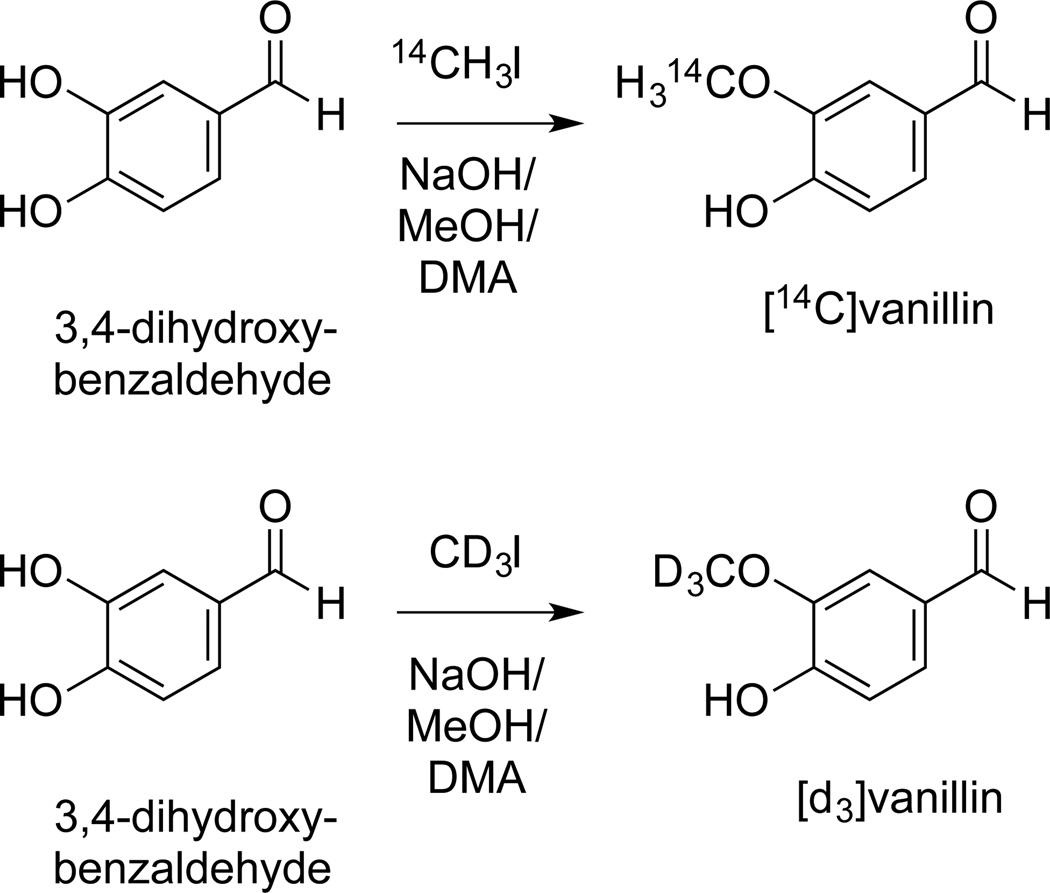
Synthesis of [14C]- as well as [d3]vanillin was accomplished by following the method by Schneider and Rolando with some modifications (Fig. 3). The authors employed a strong base in ethanol to direct methylation in the meta position of the aromatic ring of 3,4-dihydroxybenzaldehyde in moderate yield (38%).[23] Methylation of the meta position of 3,4-dihydroxybenzaldehyde allows introduction of label ([d3] or [14C]) into vanillin in a single step and, therefore, was preferred over a six-step alternative for [14C]labeling of the carbonyl.[14]
Fig. 3.
Introduction of [14C]- or [d3]label into the methoxy group of vanillin.[23]
We found that 3,4-dihydroxybenzaldehyde had only limited solubility in ethanol which was overcome by using methanol instead. Addition of 4 volumes of dimethylacetamide as a co-solvent increased yield and reduced the reaction time.[24] Methyl iodide ([14C] and [d3], respectively) was added as a dilution in 100 µl toluene without any apparent influence of toluene on yield or positional specificity. For synthesis of [14C]vanillin a twofold molar excess of 3,4-dihydroxybenzaldehyde relative to [14C]methyl iodide was used to enhance consumption of the expensive reagent. The isolated yields for [14C]- and [d3]vanillin were 52% and 47%, respectively. The isotopic incorporation of [d3] into vanillin exceeded 99.9%.
[14C2]- and [d6]Curcumin
We found that the yield of curcumin largely depended on the reaction time used to form the boron oxide-acetylacetone paste. When the paste was allowed to form overnight (16 h), the yield of curcumin detected by HPLC increased to >80% in pilot reactions of unlabled reagents (100 mg vanillin).
A technical inconvenience in scaling down curcumin synthesis to a low mg-scale was the preparation and handling of a small amount of the paste. Therefore, an excess of the paste was prepared, dissolved in ethyl acetate, and an aliqout added into the reaction with labeled vanillin. Both [14C2]- as well as [d6]curcumin were isolated in good overall yield (25% and 27%, respectively) using RP-HPLC. The specific activity of [14C2]curcumin was 4 mCi/mmol. The incorporation of deuterium into [d6]curcumin was >99.9%.
We have used [14C2]curcumin as a tracer for HPLC analyses of autoxidation products of curcumin. This approach has led to the identification of at least 10 different products formed by oxidative transformation of curcumin, including unstable reaction intermediates and novel end products.
[d3]Curcumin
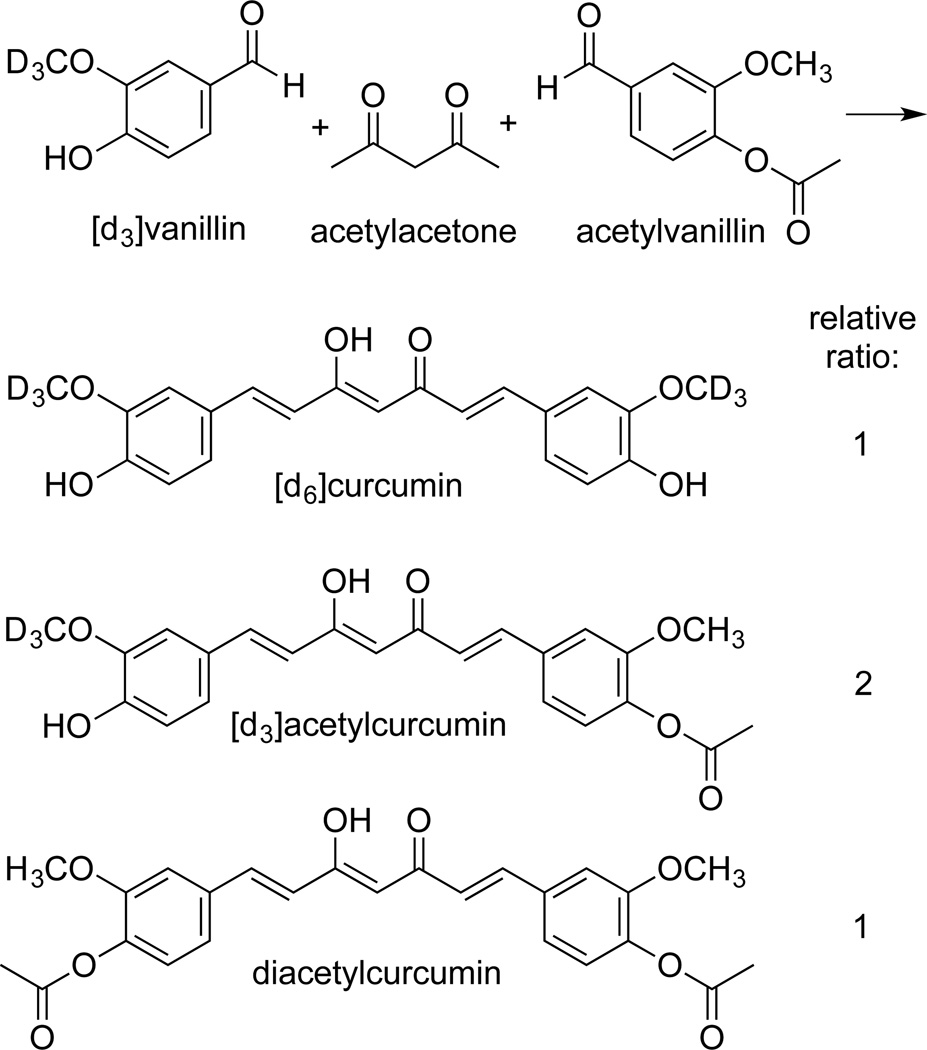
The approach of fusing two molar equivalents of vanillin with acetylacetone can be modified for the preparation of curcumin derivatives, for example, [d3]curcumin. For asymmetric derivatives one of the two vanillin equivalents is substituted with a desired analog. Thus, an equimolar mixture of vanillin and 3,4-dimethoxybenzaldehyde gives curcumin, 4’-methylcurcumin, and 4’,4”-dimethylcurcumin in a 1:2:1 ratio.[18] For synthesis of asymmetric [d3]curcumin this approach will give curcumin, [d3]curcumin, and [d6]curcumin, when starting with a mixture of vanillin and [d3]vanillin. Chromatographic resolution of the [d0], [d3], and [d6]isotopologues by RP-HPLC is expected to be tedious if not impossible. Therefore, we devised an alternative strategy in order to enhance the difference in polarity of [d3]curcumin relative to the unwanted [d0] and [d6]isomers. Using a 1:1 mixture of [d3]vanillin and acetylvanillin, the products were a mixture of [d6]curcumin, [d3]acetylcurcumin (the desired product), and diacetylcurcumin in 1:2:1 ratio (Fig. 4). [d3]Acetylcurcumin was readily resolved from the other products using RP-HPLC, and [d3]curcumin was obtained by hydrolysis with K2CO3 in ethyl acetate.
Fig. 4.
Synthesis of [d3]acetylcurcumin.
[d6]Bicyclopentadione
An aliquot of synthesized [d6]curcumin was subjected to autoxidation for preparation of [d6]bicyclopentadione. Autoxidation of curcumin is a spontaneous and rapid reaction in slightly alkaline buffer (pH 7.5–8) that can be monitored by following the disappearance of the curcumin chromophore at 430 nm in a UV/Vis spectrophotometer.[18] Products are recovered from the reaction by using a C18 solid phase cartridge and purified using RP-HPLC.
[d6]Curcumin and [d6]bicyclopentadione will be used as isotopic standards for quantitative LC-MS analysis of curcumin metabolism in cultured cells and in vivo. [d3]Curcumin will be added to samples after collection in order to quantify artifactual autoxidative transformation to [d3]bicyclopentadione during sample work-up. [d6]Curcumin is also available commercially.
[13C5]Curcumin
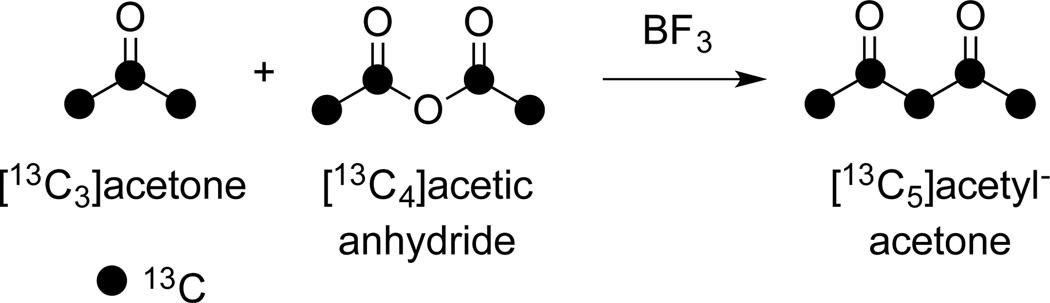
The label of [13C5]curcumin is located in the five central carbons of the heptadienone chain of curcumin. In this case, the label was introduced from [13C5]acetylacetone which was synthesized by fusion of [13C3]acetone and [13C4]acetic anhydride in the presence of BF3 (Fig. 5). Separating [13C5]acetylacetone from the solvent used for extraction (dichloromethane) was challenging because distillation was not feasible due to small volumes. Instead, we decided to concentrate the dichloromethane extract (≈1 ml volume) under a gentle stream of nitrogen to about 120 µl, and to use this solution directly in the next step. The small amount of remaining dichloromethane (≈100 µl) did not interfere with formation of the acetylacetone-B2O3 paste although in pilot experiments a larger amount of solvent (>500 µl) was inhibitory.
Fig. 5.
Synthesis of [13C5]acetylacetone.
[13C5]Curcumin was prepared anticipating two potential applications. It can serve as a standard or tracer in MS experiments where loss of the methoxy group during metabolic transformation is expected or a concern. Our main incentive was to enrich the [13C]content of the heptadienone chain in order to enhance signal intensities for 13C NMR spectroscopic identification of oxidative transformation products of curcumin.
Experimental
Materials
[14C]Methyl iodide (2 mCi/mmol) was from American Radiolabeled Chemicals, Inc., MO. [13C3]Acetone (99%) and [13C4]acetic anhydride (99%) were from Cambridge Isotope Laboratories, Inc., MA. [d3]Methyl iodide (99.5%), acetylvanillin, 3,4-dihydroxybenzaldehyde, and other reagents used for synthesis were from Sigma, St. Louis, MO.
[14C]Vanillin
3,4-Dihydroxybenzaldehyde (15 mg, 0.1 mmol) was dissolved in 4 M methanolic NaOH (50 µl) and diluted with dimethylacetamide (200 µl). [14C]Methyl iodide (7 mg, 0.05 mmol, 2 mCi/mmol) in toluene (100 µl) was added dropwise to the solution. The reaction was stirred for 2 h, acidified with 1 M HCl, and extracted 3 times with dichloromethane (500 µl). The solvent was evaporated and the product was isolated using RP-HPLC (Method A) to yield 3.5 mg [14C]vanillin (52 µCi, 52% radiochemical yield; purity 98% by HPLC). The identity of [14C]vanillin was confirmed by comparison of its RP-HPLC retention time and UV spectra with an authentic standard. LC-MS analysis was not performed in order to avoid contamination of the instrument with radioactive material.
[d3]Vanillin
[d3]Vanillin was prepared as described for [14C]vanillin starting from 3,4-dihydroxybenzaldehyde (50 mg, 0.35 mmol) and [d3]methyl iodide (50 mg, 0.35 mmol) in toluene (100 µl). The product was isolated using RP-HPLC (Method B) to yield [d3]vanillin (26 mg; 47% yield; purity 91% by HPLC). LC-MS: 154.1 ([M-H]−). 1H NMR (CD3OD, 600 MHz): δ = 5.28 (s, 1H), 6.77 (d, 1H, J = 8.1 Hz), 6.86 (dd, 1H, J = 8.1; 1.8 Hz), 6.98 (d, 1H, J = 1.8 Hz), 9.75 (s, 1H) ppm.
[14C2]Curcumin
Boron oxide (20 mg, 0.3 mmol) and acetylacetone (40 µl, 0.4 mmol) were stirred for 16 h to form a white paste. An aliquot (20 µl) of the paste dissolved in ethyl acetate (360 µl) was added to 3.5 mg 14C-vanillin (0.03 mmol, 52 µCi) dissolved in tributyl-borate (21 µl). The mixture was stirred for 5 min after which a 10% dilution of butylamine in ethyl acetate (2 µl) was added every 10 min for 40 min. The reaction was stirred for 16 h overnight. The next day, 0.4 M HCl (100 µl; heated to 60°C) was added to the reaction and stirred for 1 h. [14C2]Curcumin was extracted into ethyl acetate (4 × 200 µl) and purified using RP-HPLC (Method A) to yield 1.2 mg purified [14C2]curcumin (13 µCi; 4 mCi/mmol; 25% radiochemical yield; purity 97% by HPLC). 1H NMR (CD3OD, 600 MHz): δ = 3.91 (s, 6H), 5.97 (s, 1H), 6.64 (d, 2H, J = 15.0 Hz), 6.82 (d, 2H, J = 7.9 Hz), 7.1 (d, 2H, J = 7.5 Hz), 7.22 (s, 2H), 7.57 (d, 2H, J = 15.0 Hz) ppm.
[d6]Curcumin
[d6]Curcumin was prepared as described for [14C2]curcumin starting from boron oxide (30 mg, 0.45 mmol), acetyl acetone (60 µl, 0.6 mmol) and 100 mg [d3]vanillin. [d6]Curcumin was purified using semi-preparative RP-HPLC (Method C) to yield 30 mg (27%) [d6]curcumin (purity 98% by HPLC). LC-MS: 373.2 ([M-H]−). 1H NMR (CD3OD, 600 MHz): δ = 5.97 (s, 1H), 6.64 (d, 2H, J = 15.7 Hz), 6.82 (d, 2H, J = 8.1 Hz), 7.10 (dd, 2H, J = 7.5; 1.1 Hz), 7.22 (d, 2H, J = 1.1 Hz), 7.58 (d, 2H, J = 15.7 Hz) ppm.
[d3]Curcumin
[d3]Curcumin was prepared as described for [14C2]curcumin starting from boron oxide (320 mg, 4.60 mmol) and acetylacetone (660 mg, 6.60 mmol). An aliquot (5%) of the boron complex (paste) was reacted with [d3]vanillin (60 mg, 0.40 mmol), vanillin acetate (77 mg, 0.40 mmol), tributyl borate (213 µl, 7.90 mmol), and butylamine (2 µl) in ethyl acetate (1 ml). The products were separated by semi-preparative RP-HPLC (Method D) to give 11 mg of purified [d3]acetylcurcumin (yield 18%). The acetate group was hydrolyzed using a saturated solution of K2CO3 (25 mg) in ethyl acetate (1 ml) at room temperature overnight (16 h) to release [d3]curcumin (purity 95% by HPLC). 1H NMR (CD3OD, 600 MHz): δ = 3.91 (s, 3H), 5.97 (s, 1H), 6.62 (d, 2H, J = 15.8 Hz), 6.82 (d, 2H, J = 8.3 Hz), 7.10 (d, 2, J = 8.3 Hz), 7.21 (s, 2H), 7.58 (d, 2H, J = 15.9 Hz) ppm.
[13C5]Curcumin
[13C3]Acetone (120 µl, 2 mmol) and [13C4]acetic anhydride (500 µl, 5 mmol) were placed in a 1 ml reaction vial and cooled in an ice-salt bath. Boron trifluoride diethyl etherate (400 µl, 3 mmol) was added slowly over the course of 3 min. The reaction was stirred for 4 h and then poured into 3 ml of 10 M sodium acetate heated to 80°C. The reaction was extracted twice using 500 µl dichloromethane to yield 12 mg (0.1 mmol; 5% yield) [13C5]acetylacetone.
[13C5]Curcumin was prepared as described for [14C2]curcumin starting from boron oxide (47 mg, 0.7 mmol) and [13C5]acetylacetone (12 mg) dissolved in dichloromethane (100 µl). Vanillin (250 mg), tributylborate (800 µl) in 800 µl ethyl acetate, and butylamine (5 µl) were added. The products were loaded onto a 2 g Supelco DSC-18 cartridge in 30% acetonitrile and eluted using 100% acetonitrile. [13C5]curcumin was purified using RP-HPLC (Method A; 51% yield; purity 92% by HPLC). LC-MS: 372.2 ([M-H]−). 1H NMR (CD3OD, 600 MHz): δ = 3.91 (s, 6H), 5.97 (s, 1H), 6.64 (d, 2H, J =15.7 Hz), 6.82 (d, 2H, J = 8.1 Hz), 7.1 (dd, 2H, J = 8.3; 1.8 Hz), 7.21 (d, 2H, J = 1.7 Hz), 7.58 (d, 2H, J = 15.7 Hz) ppm.
[d6]Bicyclopentadione
[d6]Curcumin (5 mg) was dissolved in ethanol (2.5 ml) to prepare a 5 mM solution. The entire solution was added to 50 mM sodium phosphate buffer pH 7.5 (250 ml). The reaction was allowed to proceed at room temperature for 8 h. Products were extracted using a preconditioned 2 g Supelco DSC-18 cartridge and eluted with 2 aliquots of 4 ml acetonitrile. The solvent was concentrated under a stream of nitrogen and [d6]bicyclopentadione was purified using RP-HPLC (Method A; purity 98% by HPLC). LC-MS: 405.1 ([M-H]−). 1H NMR (CD3OD, 600 MHz): δ = 3.35 (dd, 1H, J = 6.2; 1.7 Hz), 3.66 (d, 1H, J = 6.1 Hz), 4.93 (s, 1H, J = 1.8 Hz), 5.40 (d, 1H, J = 8.3 Hz), 5.87 (s, 1H), 6.67 (dd, 1H, J = 8.6, 2.8 Hz), 6.70 (d, 1H, J = 8.1 Hz), 6.72 (dd, 1H, J = 8.3; 1.8 Hz), 6.80 (d, 1H, J = 1.8 Hz), 6.84 (d, 1H, J = 1.1 Hz), 6.85 (d, 1H, J = 7.1 Hz) ppm.
Analytical procedures
Products were analyzed and purified by RP-HPLC using an Agilent 1200 series diode array system equipped with a Waters Symmetry C18 5 µm column (4.6 × 250 mm). The column was eluted with a linear gradient of MeCN/H2O/HOAc 20/80/0.01 to 80/20/0.01 (by vol.) over 20 min and a flow rate of 1 ml/min (Method A). Semi-preparative RP-HPLC using a Waters Symmetry C18 column (300 mm × 19 mm) was eluted with a solvent of MeOH/H2O/HOAc (40/60/0.01, by vol.; Method B) or MeCN/H2O/HOAc (55/45/0.01, by vol; Method C) or MeOH/H2O/HOAc (75/25/0.01, by vol.; Method D) at a flow rate of 10 ml/min. LC-MS was performed using a Thermo LTQ ion trap instrument equipped with an electrospray ionization interface. The instrument was operated in the negative ion mode, and mass spectra were acquired at a rate of 2 sec/scan. The settings for the heated capillary (300°C), spray voltage (4.0 kV), spray current (0.22 µA), auxiliary (37 mTorr) and sheath gas (16 mTorr) were optimized using direct infusion of a solution of curcumin (20 ng/µl) in MeCN/H2O 95/5, by vol., containing 10 mM NH4OAc. Samples were introduced using a Waters Symmetry Shield C18 3.5 µm column (2.1 × 100 mm) eluted with a gradient of MeCN/H2O (5/95, by vol., containing 10 mM NH4OAc) to MeCN/H2O (95/5, by vol., containing 10 mM NH4OAc) over 10 min followed by 3 min of isocratic elution and re-equilibration in the starting solvent (Method E).
NMR spectra were recorded using a Bruker AV-II 600 MHz spectrometer equipped with a TCI cryoprobe. Chemical shifts are reported in ppm relative to the non-deuterated solvent peak of methanol-d4 (δ = 3.34 ppm).
Acknowledgements
This work was supported awards CA159382 and AT006896 by NCI and NCCAM, respectively, of the National Institutes of Health. ONG acknowledges support by training grants 2T32GM07628 and a pre-doctoral fellowship award (F31AT007287) from NCCAM of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Cell. Mol. Life Sci. 2008;65:1631. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esatbeyoglu T, Huebbe P, Ernst IM, Chin D, Wagner AE, Rimbach G. Angew. Chem. Int. Ed. Engl. 2012;51:5308. doi: 10.1002/anie.201107724. [DOI] [PubMed] [Google Scholar]
- 3.Gupta SC, Prasad S, Kim JH, Patchva S, Webb LJ, Priyadarsini IK, Aggarwal BB. Nat. Prod. Rep. 2011;28:1937. doi: 10.1039/c1np00051a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Mol. Pharm. 2007;4:807. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 5.Pan MH, Huang TM, Lin JK. Drug. Metab. Dispos. 1999;27:486. [PubMed] [Google Scholar]
- 6.Fuchs JR, Pandit B, Bhasin D, Etter JP, Regan N, Abdelhamid D, Li C, Lin J, Li PK. Bioorg. Med. Chem. Lett. 2009;19:2065. doi: 10.1016/j.bmcl.2009.01.104. [DOI] [PubMed] [Google Scholar]
- 7.Wei X, Du ZY, Zheng X, Cui XX, Conney AH, Zhang K. Eur. J. Med. Chem. 2012;53:235. doi: 10.1016/j.ejmech.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Shahani K, Swaminathan SK, Freeman D, Blum A, Ma L, Panyam J. Cancer Res. 2010;70:4443. doi: 10.1158/0008-5472.CAN-09-4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sasaki H, Sunagawa Y, Takahashi K, Imaizumi A, Fukuda H, Hashimoto T, Wada H, Katanasaka Y, Kakeya H, Fujita M, Hasegawa K, Morimoto T. Biol. Pharm. Bull. 2011;34:660. doi: 10.1248/bpb.34.660. [DOI] [PubMed] [Google Scholar]
- 10.Kakkar V, Singh S, Singla D, Kaur IP. Mol. Nutr. Food Res. 2011;55:495. doi: 10.1002/mnfr.201000310. [DOI] [PubMed] [Google Scholar]
- 11.Pabon HJJ. Recl. Trav. Chim. Pays Bas. 1964;83:379. [Google Scholar]
- 12.Roughley PJ, Whiting DA. Journal of the Chemical Society-Perkin Transactions. 1973;1:2379. [Google Scholar]
- 13.Pedersen U, Rasmussen PB, Lawesson SO. Liebigs Annalen Der Chemie. 1985:1557. [Google Scholar]
- 14.Parveen I, Threadgill MD. J. Labelled Cpd. Radiopharm. 2000;43:883. [Google Scholar]
- 15.Ishida J, Ohtsu H, Tachibana Y, Nakanishi Y, Bastow KF, Nagai M, Wang HK, Itokawa H, Lee KH. Bioorganic & Medicinal Chemistry. 2002;10:3481. doi: 10.1016/s0968-0896(02)00249-3. [DOI] [PubMed] [Google Scholar]
- 16.Rao EV, Sudheer P. Indian Journal of Pharmaceutical Sciences. 2011;73:262. doi: 10.4103/0250-474X.93508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metzler M, Pfeiffer E, Schulz SI, Dempe JS. Biofactors. 2013;39:14. doi: 10.1002/biof.1042. [DOI] [PubMed] [Google Scholar]
- 18.Griesser M, Pistis V, Suzuki T, Tejera N, Pratt DA, Schneider C. J. Biol. Chem. 2011;286:1114. doi: 10.1074/jbc.M110.178806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez-Lazaro M, Willmore E, Jobson A, Gilroy KL, Curtis H, Padget K, Austin CA. J. Nat. Prod. 2007;70:1884. doi: 10.1021/np070332i. [DOI] [PubMed] [Google Scholar]
- 20.Ketron AC, Gordon ON, Schneider C, Osheroff N. Biochemistry. 2013;52:221. doi: 10.1021/bi3014455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider C, Amberg A, Feurle J, Ross A, Roth M, Tóth G, Schreier P. J. Mol. Catalysis B: Enzymatic. 1998;4:219. [Google Scholar]
- 22.Gordon ON, Schneider C. Trends Mol. Med. 2012;18:361. doi: 10.1016/j.molmed.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider S, Rolando C. J. Label Compd. Radiopharm. 1992;31:489. [Google Scholar]
- 24.Ciucanu I, Kerek F. J. Chromatogr. A. 1984;284:179. [Google Scholar]