Abstract
Purpose
Chimeric antigen receptor (CAR) transduced T cells represent a promising immune therapy that has been shown to successfully treat cancers in mice and humans. However, CARs targeting antigens expressed in both tumors and normal tissues have led to significant toxicity. Preclinical studies have been limited by the use of xenograft models that do not adequately recapitulate the immune system of a clinically relevant host. EGFRvIII is a constitutively activated mutant of the naturally occurring epidermal growth factor receptor and is antigenically identical in both human and mouse glioma, but is also completely absent from any normal tissues.
Experimental Design
We developed a third-generation, EGFRvIII-specific murine CAR (mCAR), and performed tests to determine its efficacy in a fully immune-competent mouse model of malignant glioma.
Results
At elevated doses, infusion with EGFRvIII mCAR T cells led to cures in all mice with brain tumors. Additionally, antitumor efficacy was found to be dependent on lymphodepletive host conditioning. Selective blockade with EGFRvIII soluble peptide significantly abrogated the activity of EGFRvIII mCAR T cells in vitro and in vivo, and may offer a novel strategy to enhance the safety profile for CAR-based therapy. Lastly, mCAR-treated, cured mice were resistant to rechallenge with EGFRvIIINEG tumors, suggesting generation of host immunity against additional tumor antigens.
Conclusion
All together, these data support that third-generation, EGFRvIII specific mCARs are effective against gliomas in the brain and highlight the importance of syngeneic, immune-competent models in the preclinical evaluation of tumor immunotherapies.
Keywords: central nervous system neoplasms, epidermal growth factor receptor, immunotherapy, glioma, glioblastoma, GBM, chimeric antigen receptor, CAR, murine model
Introduction
Glioblastoma (GBM) is the most common brain tumor and also the most deadly. Despite aggressive treatment including surgical resection, dose-intensive radiation and multimodal chemotherapy, prognosis remains exceedingly grim with a median survival of less than 15 months from the time of diagnosis (1). Moreover, the effects of current therapies are often non-specific, causing significant collateral damage to healthy cells and adjacent brain.
In contrast, immunotherapy offers an extremely precise approach with the potential to eliminate cancer specifically while leaving normal tissues intact. Substantial evidence suggests that T cells in particular have the ability to eradicate large, well-established tumors in mice and humans (2–7). As such, a number of attempts have been made to establish large populations of tumor-reactive T cells in vivo, mainly via adoptive transfer of expanded tumor-infiltrating lymphocytes (TILs) or autologous T cells that have been transduced to express specific T-cell receptors (TCRs) (2, 3, 5). Although promising, these approaches have been limited by a number of technical and functional drawbacks. While TILs are difficult to isolate in most cancers, TCR-transduced T cells recognize only specific major histocompatibility complex (MHC) alleles, restricting them to a subset of patients and making them vulnerable to MHC down regulation by tumors (8).
To address these limitations, a versatile class of receptors known as chimeric antigen receptors (CARs) has been generated by combining the variable region of an antibody with a T-cell signaling molecule, usually CD3 (9). Because their capacity for antigen recognition is derived from antibody binding, CARs have the ability to mimic endogenous TCR-mediated activation without the drawbacks of classical MHC restriction. Moreover, whereas physiological TCRs are restricted by thymic selection, antibody-redirected CARs can accommodate virtually infinite antigenic diversity and operate at affinities even in the nanomolar range (10, 11).
An additional advantage of the CAR platform is the incorporation of costimulatory molecules such as CD28 and 4-1BB into the CD3 signaling domain to improve T-cell expansion, survival, cytokine secretion and tumor lysis (12–14). Clinical trials utilizing these second and third-generation CARs have now targeted a variety of antigens and malignancies and have demonstrated their remarkable potential (15–18). However, severe adverse events have occurred when these CARs have been directed against antigens shared by normal tissues, such as ERBB2/HER2 (19). As such, the lack of truly tumor-specific targets for CARs and a poor toxicity profile to date represent critical barriers to the safe and effective translation of this promising therapy.
EGFRvIII is a tumor-specific mutation of the epidermal growth factor receptor that is absent from normal tissues, but commonly expressed on the surface of GBMs and other neoplasms (20). Functionally, EGFRvIII is a constitutively active version of the wild-type receptor, conferring enhanced tumorgenicity (21, 22), invasiveness (23), and therapeutic resistance (24) to tumor cells. Because this mutation results in the translation of a unique extracellular epitope, it is readily recognized by a number of previously described monoclonal antibodies (20); EGFRvIII thus represents an ideal target for CAR-based therapeutic development.
With few exceptions, the great majority of preclinical studies for CARs have been performed in vitro or in vivo with xenogeneic models wherein human T cells are tested against human tumors implanted into immune-compromised mice (25–30). This strategy is often the only available option, due to the lack of immune-competent rodent models possessing surface molecules of equivalent binding affinities and function to those found in humans. Unfortunately, preclinical reports of gene-modified T cells in xenograft systems have not been predictive of dramatic toxicities that occurred upon translation in early clinical trials (13, 19). In addition to inadequately assessing autoimmune toxicity, these xenograft models also do not permit realistic analyses of parameters that may critically impact efficacy in humans, such as the influence of host-conditioning regimens, species-specific immunosuppressive factors, and the potential generation or priming of endogenous immunity (26).
In this study, we directly address the limitations of previous immune-compromised models by generating a murine-derived, third-generation, EGFRvIII-specific CAR (EGFRvIII mCAR) for evaluation in a fully immune-competent mouse model of malignant glioma (31). Additionally, we target a murine homologue of EGFRvIII that demonstrates identical antibody-binding characteristics to the human EGFRvIII (32). Our results demonstrate that murine T cells transduced with the EGFRvIII mCAR (mCAR T cells) express interferon-gamma (IFNγ) specifically in the presence of target cells expressing the EGFRvIII mutation. Despite conventional notions of immune-privilege, treatment with mCAR T cells led to complete eradication of 3–5-day established, syngeneic, EGFRvIII-expressing gliomas located subcutaneously and in the brain. Therapeutic effects were shown to be dose-dependent and required host lymphodepletion prior to adoptive transfer for efficacy. We also show the ability to block this mCAR T-cell function in vivo with systemic administration of EGFRvIII peptide. Lastly, successfully cured mice did not develop tumors upon rechallenge with EGFRvIIINEG matched tumor, suggesting that adoptive transfer in this setting may generate host immunity against novel tumor antigens, thereby circumventing tumor-antigen loss and preventing tumor recurrence (33).
Together, our findings support that third-generation CARs can be used to produce effective immune responses against tumors in the brain. Additionally, these data highlight the requirement of lymphodepletive host-conditioning for efficacy, and also suggest that epitope spreading may contribute to favorable outcomes in the setting of CAR-mediated therapies. Ultimately, these studies lend credence to the utilization of fully immune-competent models in the setting of adoptive transfer, with the potential to inform critical aspects of clinical trial design.
Materials and Methods
Cell lines and media
The murine glioma parental cell line SMA560 (EGFRvIIINEG), and its subline, SMA560vIII (EGFRvIIIPOS) have been previously described (31). Murine T cells were stimulated in complete RPMI-1640 media (R10) (HEPES, Pen/Strep, NEAA, L-Glut, 2ME, RPMI-1640 plus 10% FBS) supplemented with 50 IU/mL rh interleukin (IL) 2 or 1 ng/mL mIL-7 for two days, followed by expansion in 50 IU/mL IL-2.
Retrovirus design and T-cell transduction
The murine (m) 139 single-chain antibody variable fragment (scFv) mCAR retrovirus was generated similarly to the published human (h) 139 scFv hCAR (34). The 139 scFv was inserted in tandem with mCD8 trans-membrane, mCD28, m4-1BB, and mCD3ζ intracellular regions in the MSGV1 retroviral vector as previously described (13, 35). Retroviral supernatant was generated by transient co-transfection of HEK 293T cells by Lipofectamine 2000 Transfection Reagent (Invitrogen), along with pCL-Eco helper plasmid (Imgenex). The resulting retroviral supernatant was used to transduce murine splenocytes as previously described (36). Briefly, mouse splenocytes were collected from VM/Dk mice, disaggregated and passed through a 70 μm mesh filter to generate a single-cell suspension. Cells were cultured in complete R10 mouse T-cell media supplemented with 1 ng/mL rmIL-7 and activated on day 0 with 2.5 μg/mL Concanavalin A (ConA). T cells were transduced on RetroNectin-coated plates (Takara Inc., Japan) at a density of 1×106/mL on day 2 after stimulation. Cells were then maintained at 1–2×106/mL in mouse T-cell media with 50 IU/mL rhIL-2 for 4–7 days.
Flow cytometric analysis
Live cells were gated by FSC/SSC. Transduced T cells were stained for mCAR surface expression using goat-anti-human F(ab’)2-biotin primary and streptavidin-phycoerythrin (SA-PE) secondary antibodies as previously described (34), or using a PEPvIII-PE multimer generated in the lab. Transduction efficiency was confirmed by paired GFP-transduced controls. SMA560 and SMA560vIII tumors were stained for surface EGFRvIII expression using the L8A4 mAb with a goat-anti-mouse Ig-PE secondary antibody.
IFNγ enzyme-linked immunosorbent spot (ELISpot) assay
T-cell responses to EGFRvIIIPOS tumor target cells were measured ex vivo by direct IFNγ ELISpot assay. Single-cell suspensions of splenic lymphocytes from naïve or mice treated with GFP or EGFRvIII mCAR T cells in R10 medium were prepared by physical disprution. After red blood cell lysis and two washes with R10 medium, lymphocytes were isolated by density gradient centrifugation on Lympholyte M (Cedarlane), washed twice and resuspended in fresh R10 medium. Splenic lymphocytes (2.5×105/well) were added to duplicate wells of ELISpot assay plates coated with anti-mouse IFNγ monoclonal antibody (AN18) and allowed to settle for 1 hour. SMA560 or SMA560vIII tumor targets were subsequently added after one hour at an effector-to-tumor cell ratio of 1:1 and incubated overnight at 37°C. Plates were washed with PBS Tween-20, incubated with biotinylated, anti-mouse IFNγ mAb (R4-6A2 (2h), avidin-peroxidase complex (1h), and substrate (3–amino-9-ethylcarbazole) for 4 min),, separated by PBS washes at room temperature. Plates were dried and shipped to Zellnet Consulting (New York, NY) for spot enumeration by automated analysis with a Zeiss KS ELISpot system. This assay was MIATA compliant.
Syngeneic murine model for brain tumor immunotherapy
SMA560vIII cells were harvested with trypsin, washed once in serum-containing medium, and washed twice in Dulbecco's phosphate-buffered saline (DPBS). Cell pellets were resuspended in DPBS at the appropriate concentration of viable cells as determined by trypan blue dye exclusion, mixed with an equal volume of 10% methylcellulose in Zinc Option Modified Eagle’s medium (MEM) and loaded into a 250-μl syringe (Hamilton, Reno, NV) with an attached 25-gauge needle. In VM/Dk mice, the tip of the needle was positioned at bregma and 2 mm to the right of the cranial midline suture and 4 mm below the surface of the cranium using a stereotactic frame (David Kopf Instruments, Tujunga, CA). Each mouse received 5×104 tumor cells implanted into the brain in a volume of 5 μL. Three to five days following implantation, mice were treated with 1×105 to 1×107 mCAR or GFP control VM/Dk T cells delivered systemically via tail-vein injection. Absolute numbers of T cells were used for calulations, rather than number of mCAR positive T cells (mCAR surface expression ranged from 50%-75%). In experiments evaluating efficacy against subcutaneous tumors, 5×105 SMA560vIII tumor cells were injected into the right flank, in a volume of 100 μL PBS, followed three days later by 5×106 mCAR or GFP control T cells delivered systemically via tail-vein injection. Where indicated, mice were conditioned with 5Gy total body irradiation (TBI) immediately prior to T-cell transfer. For each in vivo experiment, groups consisted of eight mice, unless stated otherwise. All experiments were repeated a minimum of two times. According to Duke University and Institutional Animal Care and Use Committe (IACUC) protocols, animal survival was monitored, with mortality recorded or mice sacrificed upon reaching pre-determined morbidity endpoints. For subcutaneous experiments, tumors were measured for width and length at widest diameters to obtain size in mm2, animals were sacrificed upon any single diameter reaching 20 mm or upon ulceration.
Soluble PEPvIII blockade in vitro
The effect of peptide blocking on EGFRvIII mCAR T cells was measured utilizing the ELISpot assay described above. 24-well tissue-culture plate wells were coated with 50 μg/mL of soluble EGFRvIII peptide, PEPvIII (LEEKKGNYVVTDHC) or control. EGFRvIII mCAR or GFP control T cells were added to the wells in presence or absence of 10 or 100 μg/mL of PEPvIII or irrelevant isocitrate dehydrogenase (IDH) peptide (GWVKPIIIGHHAYGDQYR). Cells (5 × 104/well) were incubated for 18 hours and submitted for IFNγ ELISpot analysis.
Soluble PEPvIII blockade in vivo
PEPvIII peptide was prepared as a stock solution in DMSO, then diluted 10× in PBS prior to injecting 100 μg in 100 μL via tail vein into mice that had previously received mCAR or GFP control T cells. Injections were administered 2–4 hours after T-cell transfer, and repeated 10 days later.
In vivo passaging of SMA560p tumor
The SMA560 tumor line was cultured in Zinc Option MEM media for one week prior to implantation. At the time of implantation, cells were harvested with trypsin, washed once in serum-containing medium, washed twice in Dulbecco's phosphate-buffered saline (DPBS), and adjusted to a concentration of 5×106 cells/mL. 100 μL of cells were subcutaneously injected into the right flank of VM/Dk mice and were allowed to grow and establish for 14 days. Tumors were then harvested and made into a single-cell suspension by passing them through a 70 micrometer cell strainer and submitted to EGFRvIII staining as described above.
Analysis of CAR persistence in murine PBL
Whole blood obtained by retro-orbital bleeding was analyzed for EGFRvIII mCAR T cells as follows: 50 μL of blood was incubated with the antibody CD3-APC, and PEPvIII-PE multimer in 150 μL FACS buffer protected from light for 15 minutes at room temperature. Red blood cells (RBCs) were lysed and cells were fixed using 1 mL 1X BD FACS Lysing Solution (BD Bioscience, Cat# 349202) and incubated overnight at 4°C. Cells were then washed and resuspended in 2% paraformaldehyde and submitted to flow cytometry analysis. All samples were analyzed on a FACSCalibur flow cytometer (BD Biosciences). Absolute numbers per microliter of blood were calculated using Flowcount® beads from Beckman Coulter© according to manufacturer instructions.
Rechallenge with EGFRvIIINEG tumor
Either tumor naïve mice, tumor naïve mice receiving T cells, or mice that had been cured of previous subcutaneous SMA560vIII tumors following treatment with EGFRvIII mCAR T cells were rechallenged with 5×105 SMA560 (EGFRvIIINEG) tumors injected subcutaneously into the left (contralateral) flank.
Results
Generation of a, third-generation, EGFRvIII-specific murine CAR
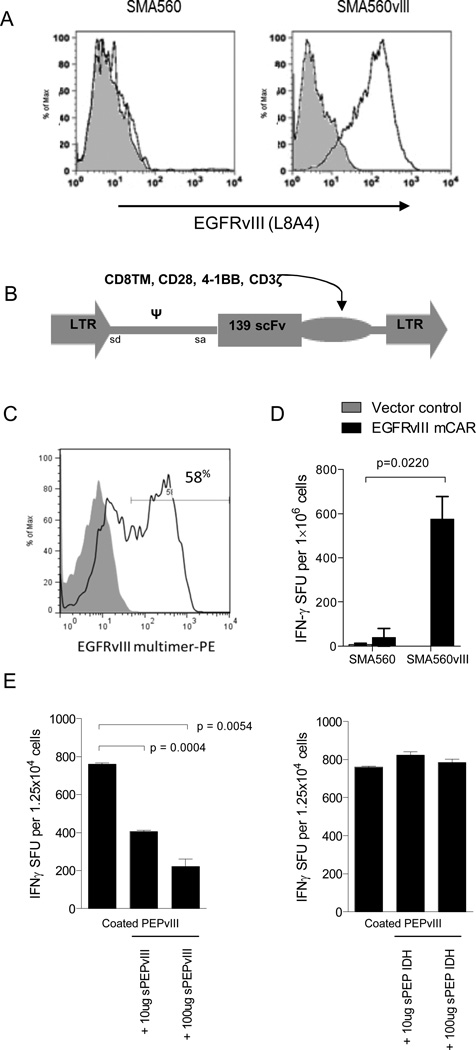
To evaluate CAR T-cell function in a syngeneic, immune-competent brain tumor model, we chose the murine glioma cell line, SMA560, derived from an astrocytoma that arose spontaneously in a VM/Dk mouse (37), in its parental form (SMA560) or stably transfected with EGFRvIII (SMA560vIII) (Figure 1A). This astrocytoma mirrors human gliomas phenotypically and morphologically, and secretes immunosuppressive cytokines including transforming growth factor (TGF)-betas secreted by human gliomas (38). To develop an appropriate mCAR, we combined murine signaling domains for CD8TM, CD28, 4-1BB, and CD3ζ (35) with a scFv isolated from a human antibody clone specific for EGFRvIII, mAb 139, inserted into the MSGV1 retroviral backbone (13) (Figure 1B). The selection of mAb 139 was based on observations that this clone, compared to six others, could be consistently expressed in retrovirally-transduced cells and recognize EGFRvIII antigen with high avidity and specificity (34). Also, because 139 possesses known specificity for the naturally occurring EGFRvIII epitope in both mouse and human tumors, its incorporation into preclinical models offers translational potential as well as biological principle.
Figure 1. EGFRvIII mCAR retroviral gene delivery to murine T cells confers antigen-specific activity.
(A) VM/Dk-derived glioma SMA560vIII shows EGFRvIII expression compared with SMA560 by surface antibody staining (L8A4) and flow cytometry; grey represents negative staining control. (B) The EGFRvIII mCAR MSGV1 retroviral vector contains the human anti-EGFRvIII scFv 139, in tandem with murine CD8TM, CD28, 4-1BB, and CD3ζ intracellular regions. EGFRvIII mCAR MSGV1 retrovirus containing supernatant was generated by transient transfection of HEK 293T cells with the corresponding retroviral vectors and the helper pCL-Eco plasmid. Supernatants harvested 48h after transfection were used to transduce previously ConA activated murine VM/Dk splenocytes. (C) Retroviral mCAR gene expression was evaluated by flow cyometric analysis 3 days after transduction; grey represents negative staining control. (D) Antigen specificity of EGFRvIII mCAR transduced and vector control (MSGV1-GFP) T cells was evaluated against EGFRvIII-expressing SMA560 cells (SMA560vIII) or the parental EGFRvIIINEG line (SMA560) by IFNγ ELISpot assay. Statistical analysis was performed by two-way ANOVA. Horizontal bar represents a statistical significance of P < 0.05 (E) Soluble EGFRvIII peptide (PEPvIII) at 50 μg/mL was used to coat tissue culture plates prior to addition of EGFRvIII mCAR or GFP control T cells. Cells were cultured 18h in the presence of increasing dosages of soluble PEPvIII or irrelevant peptide (IDH) and subjected to IFNγ ELISpot. Statistical analysis was performed by unpaired t test comparing groups defined by EGFRvIII target expression. Horizontal bar represents a statistical significance of P < 0.05. Experiments are representative of two independent repeats with similar results.
Next, we sought to determine the expression of the EGFRvIII mCAR on the surface of retrovirally-transduced splenocytes isolated from VM/Dk mice. The same retrovirus encoding GFP was used to transduce control T cells. Flow cytometric analysis following transduction revealed expression of GFP (data not shown), and surface expression of the EGFRvIII mCAR at 58% (MFI 328 versus negative control of <1%, MFI 8, Figure 1C). Importantly, when tested for reactivity against tumor cell lines, GFP control T cells did not display a detectable response to EGFRvIIIPOS SMA560vIII by IFNγ ELISpot, while T cells transduced with the EGFRvIII mCAR yielded a significant number of spot-forming units (P < 0.05, Figure 1D), which was found to be dependent on EGFRvIII expression on target tumor cells.
To test receptor specificity in vitro, we leveraged a previously-described EGFRvIII-derived soluble peptide, PEPvIII, that corresponds with the extracellular antigenic epitope of the EGFRvIII tumor mutation (33). We pre-coated tissue culture plates with PEPvIII or a negative-control peptide, and added EGFRvIII mCAR or vector control T cells. Only EGFRvIII mCAR T cells plated on PEPvIII showed reactivity by producing IFNγ, and this reactivity was blocked up to 75% in a concentration-specific manner by adding increasing amounts of soluble PEPvIII (P < 0.01), but not by the non-specific IDH control peptide (Figure 1E).
Murine CAR T cells show efficacy against intracerebral tumors that is dependent upon lymphodepletive pre-conditioning
Recent clinical trials of TCR- and CAR-engineered T-cell therapy have used systemic delivery to treat widely disseminated disease (2, 3, 5, 15, 17, 39, 40). Previous studies have demonstrated that adoptively-transferred T cells administered intravenously can even treat intracerebral tumor metastases (2, 15, 41); however, in contrast to tumors arising from CNS parenchyma, metastatic lesions tend to be associated with vascular border zones at the gray and white matter junction (42) and may not accurately recapitulate challenges associated with tumors arising from tissues originating behind the blood-brain barrier. In order to overcome the criticisms of previously used xenograft models (26), we selected an immune-intact murine model with a syngeneic, invasive glioma to evaluate the preclinical efficacy of EGFRvIII mCAR T cells in vivo.
Previous studies have shown that the survival and anti-tumor function of adoptively transferred T cells in vivo can be dependent upon lymphodepletive host-conditioning (43–45). However, the impact of this effect has been underappreciated for CAR T cells, given the few available immune-competent animal models for preclinical testing. Using our syngeneic murine system, we sought to determine if EGFRvIII mCAR T cells with or without lymphodepletive host-conditioning with TBI could demonstrate efficacy against tumors in the brain.
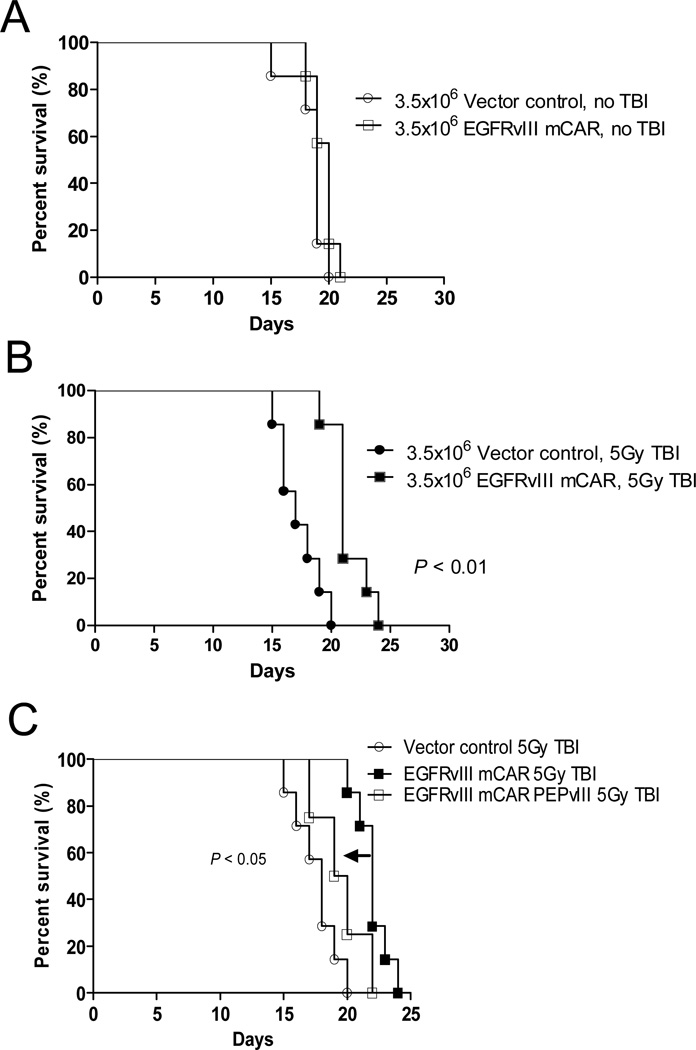
To investigate the ability of EGFRvIII mCAR T cells to localize to and treat gliomas in the CNS, VM/Dk mice were implanted intracerebrally with SMA560vIII gliomas. Three to five days later, mice were treated with or without 5Gy TBI to induce lymphodepletion, and were then injected intravenously with either control or EGFRvIII mCAR T cells and monitored for survival endpoints. In the absence of TBI host-conditioning, mice treated with EGFRvIII mCAR T cells did not display any survival benefit over mice receiving negative control GFP T cells (Figure 2A). However, significantly prolonged survival was observed using the same number of EGFRvIII mCAR T cells, compared with GFP T cells in the setting of 5Gy TBI (P < 0.01, Figure 2B). Thus, in our syngeneic, immune-competent mouse model, lymphodepletion is shown to be a critical factor in promoting the efficacy of CAR-modified T cells against tumors in the CNS.
Figure 2. EGFRvIII mCAR T-cell therapy against intracerebral tumors requires lymphodepletive host-conditioning for efficacy, and can be abrogated by soluble peptide administration.
To evaluate the impact of lymphopenia on the antitumor efficacy of EGFRvIII mCAR T cells, VM/Dk mice (8 per group) with 3-day established intracerebral SMA560vIII tumors were left untreated (A, no TBI) or irradiated (B, 5Gy TBI) prior to intravenous infusion with 3.5×106 vector control (MSGV1-GFP) or EGFRvIII mCAR T cells. (C) To evaluate whether a short peptide corresponding to the antigenic epitope expressed on target cells could be applied to competitively inhibit and reduce the functional activity of EGFRvIII mCAR T cells in vivo, mice with 3 day implanted SMA560vIII treated as in (B) above, were injected with 100 μg PEPvIII or saline 4h following mCAR T cell infusion intravenously, followed by a second injection 10 days later. Mice were monitored for morbidity end points approved by the Duke University IACUC and sacrificed when end points were met. Survival analysis was performed using the Log-Rank (Mantel Cox) test. Statistical significance was determined at P < 0.05.
EGFRvIII mCAR T-cell activity is abrogated by soluble peptide
Perhaps the greatest drawback of CAR T-cell therapy has been the lethal toxicity resulting from interaction between T cells and cognate antigen co-expressed in both tumors and normal tissues (19). Thus, a great need exists for novel strategies to reduce the toxicity profile of CARs targeting shared antigens on non-tumor cells. As proof-of-concept, we sought to determine whether a short peptide corresponding to the antigenic epitope expressed on target cells could be applied to competitively inhibit and reduce the functional activity of EGFRvIII mCAR T cells in vivo, similar to the effects we saw in vitro.
In order to perform antigen-specific blockade in vivo in our model system, VM/Dk mice bearing intracerebral SMA560vIII tumors were irradiated and treated with EGFRvIII mCAR T cells followed by PEPvIII via intravenous infusion. Using only two separate injections of 100 μg PEPvIII, given 10 days apart, we observed a significant reduction in EGFRvIII mCAR T-cell activity in vivo, demonstrated by a reduction in overall survival compared with animals receiving EGFRvIII-targeted T cells alone (P < 0.05, Figure 2C). These data support that soluble cognate peptide may be used to selectively inhibit mCAR T cells in vivo. In addition, this selective blockade also provides evidence supporting the antigen specificity of the 139 scFv EGFRvIII mCAR.
EGFRvIII mCAR T cells treat 3–5 day established, syngeneic subcutaneous and intracranial tumors in a dose-dependent manner
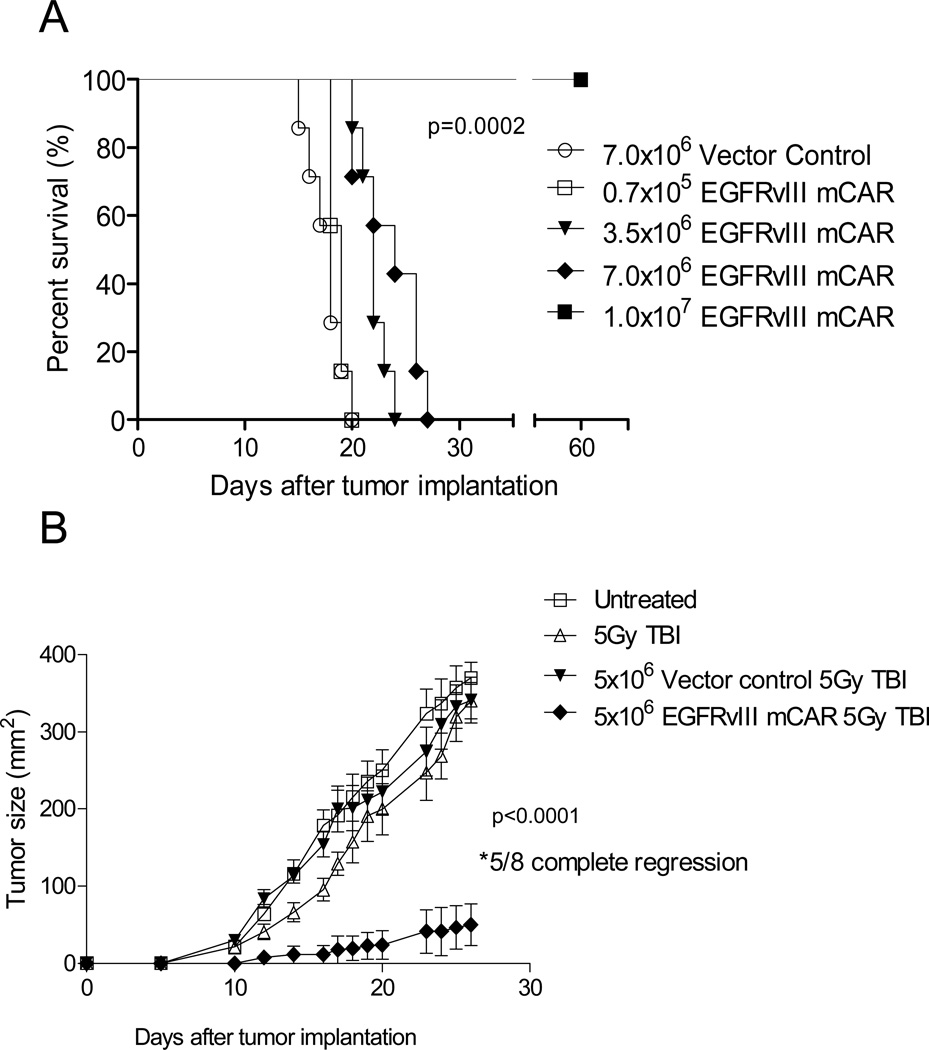
To evaluate if treatment effect was dose-dependant, we repeated the intracranial glioma treatment experiment, using increasing numbers of EGFRvIII mCAR T cells. T-cell treatment numbers were based upon total number of cells and were transduced 50–75%. At the lowest dose (0.7×105), there was no apparent impact on survival over control T-cell treated mice. However, there was a significant, dose-dependent improvement in survival in mice receiving 3.5×106-1.0×107 EGFRvIII mCAR T cells, and at the highest dose, all animals were cured of intracerebral tumors without apparent toxicity (P < 0.0002, Figure 3A). Taken together, these data demonstrate that systemic administration of EGFRvIII mCAR T cells can potently eliminate EGFRvIII-expressing tumors, even in the immunologically-privileged CNS.
Figure 3. Adoptive T-cell therapy with EGFRvIII mCAR T cells leads to regression and eradication of subcutaneous and intracerebral syngeneic tumors in a dose-dependent fashion.
VM/Dk mice (8 mice per group) with 3-day established (A) intracerebral, or (B) subcutaneous SMA560vIII tumors were irradiated (5Gy TBI) prior to intravenous infusion with various doses of vector control (MSGV1-GFP) or EGFRvIII mCAR T cells as indicated. Mice were monitored for morbidity end points approved by the Duke University IACUC and sacrificed when end points were met. Survival analysis was performed using the Log-Rank (Mantel Cox) test. Tumor growth was monitored in two dimensions. Statistical significance was determined at P < 0.05 using two-way ANOVA. Experiments are representative of three independent repeats with similar results.
To follow tumor growth characteristics in the context of CAR treatment, VM/Dk mice were implanted subcutaneously with SMA560vIII, and tumor growth was recorded to measure treatment effect. Tumors in mice lymphodepleted with 5Gy TBI prior to receiving GFP control T cells did not exhibit significantly altered tumor growth compared with untreated mice. However, lymphodepleted mice receiving EGFRvIII mCAR T cells exhibited compelling treatment effects with subcutaneous tumor growth completely eliminated in 5 of 8 mice (P < 0.0001, Figure 3B).
EGFRvIII mCAR T cells show long-term persistence in vivo
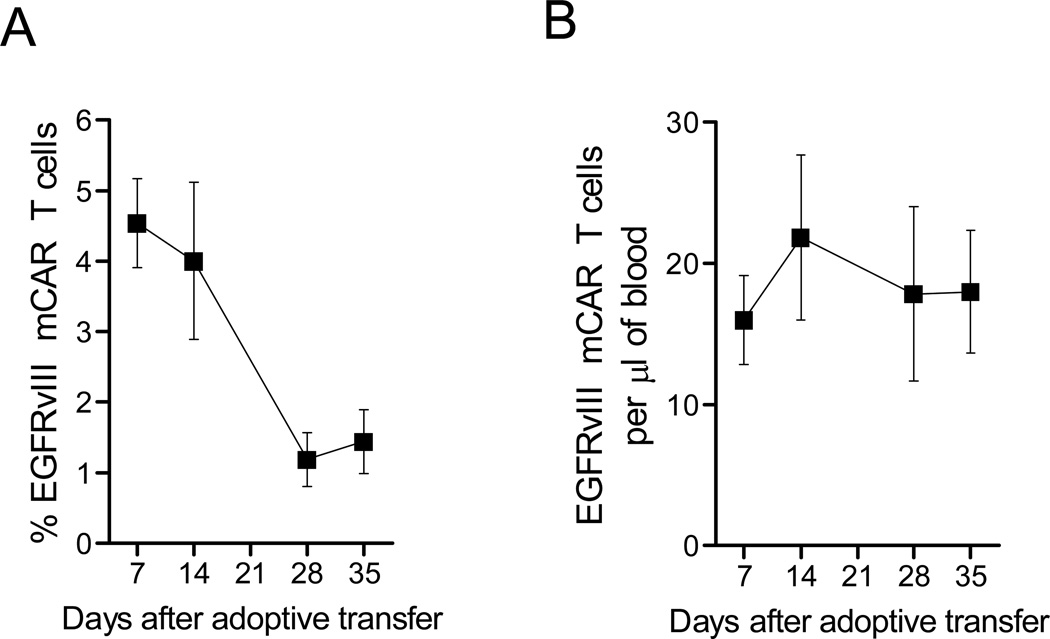
T-cell persistence was evaluated in peripheral blood lymphocytes (PBL) of 5Gy irradiated mCAR-treated mice over 35 days, and mCAR T cells were detected at all collection timepoints. Over time, the percentage of circulating PBL that were EGFRvIII mCAR-positive declined, from 4.5% after 7 days, to 1.5% on day 35, with the steepest decline occurring between days 14 and 28. (Figure 4A). As this is the same time period in which endogenous lymphocytes would be expected to begin recovering from TBI in irradiated mice, we also assessed the total absolute number of mCAR T cells in the periphery. The total numbers of circulating EGFRvIII mCAR T cells in mice did not change significantly between weeks one through five after infusion, maintaining approximately 15–25 per microliter of blood volume (Figure 4B). The same trend was observed in mice receiving GFP control T cells (data not shown). This suggested that the overall number of transferred EGFRvIII mCAR T cells were maintained in vivo over time, remaining relatively constant, while endogenous lymphocytes recovered and increased in numbers in the periphery, reducing the percentage of circulating EGFRvIII mCAR T cells, but not the total amount.
Figure 4. Constant numbers of EGFRvIII mCAR T cells persisted in peripheral blood lymphocytes over 5 weeks while endogenous lymphocytes recovered.
Constant numbers of EGFRvIII mCAR T cells persisted in peripheral blood lymphocytes (PBL) over 4 weeks. 5Gy irradiated VM/Dk mice (N = 7) had PBL sampled 1, 2, 4 and 5 weeks after intravenous EGFRvIII mCAR T-cell transfer. PBL were stained with mAb against CD3 and PEPvIII-PE multimer was used to identify EGFRvIII mCAR T cells. Absolute numbers per microliter of blood were evaluated by flow cytometry using Flowcount® beads from Beckman Coulter© according to manufacturer instructions. (A) Percentages, and (B) absolute numbers of EGFRvIII mCAR T cells in blood were calculated.
EGFRvIII mCAR treatment of mice with EGFRvIII-expressing tumors provides long-term protection against rechallenge and EGFRvIII tumor-antigen loss
Substantial evidence supports that immune-based therapies have the ability to eradicate tumors based on the expression of specific antigens (7). However, a limitation of current strategies is that they often result in tumor escape owing to heterogeneous antigen expression; indeed while EGFRvIII peptide vaccines have been shown to successfully eliminate EGFRvIII-expressing tumor cells in mice and humans, outgrowth of EGFRvIIINEG cells ultimately leads to tumor recurrence (33).
One suggested mechanism by which T-cell-based therapies in particular may be able to protect against tumor immune escape is through the triggering of epitope spreading, initiated by a localized endogenous immune response by the release of inflammatory cytokines at the tumor. The resulting recruitment of the endogenous immune system in the face of tumor destruction may confer the ability to generate new responses to tumor cells that do not express the original target antigen. To date, the ability of third-generation CAR T cells to effectively initiate this activity remains unknown, again owing to the relative dearth of available preclinical models with fully competent immune systems capable of mounting such a response.
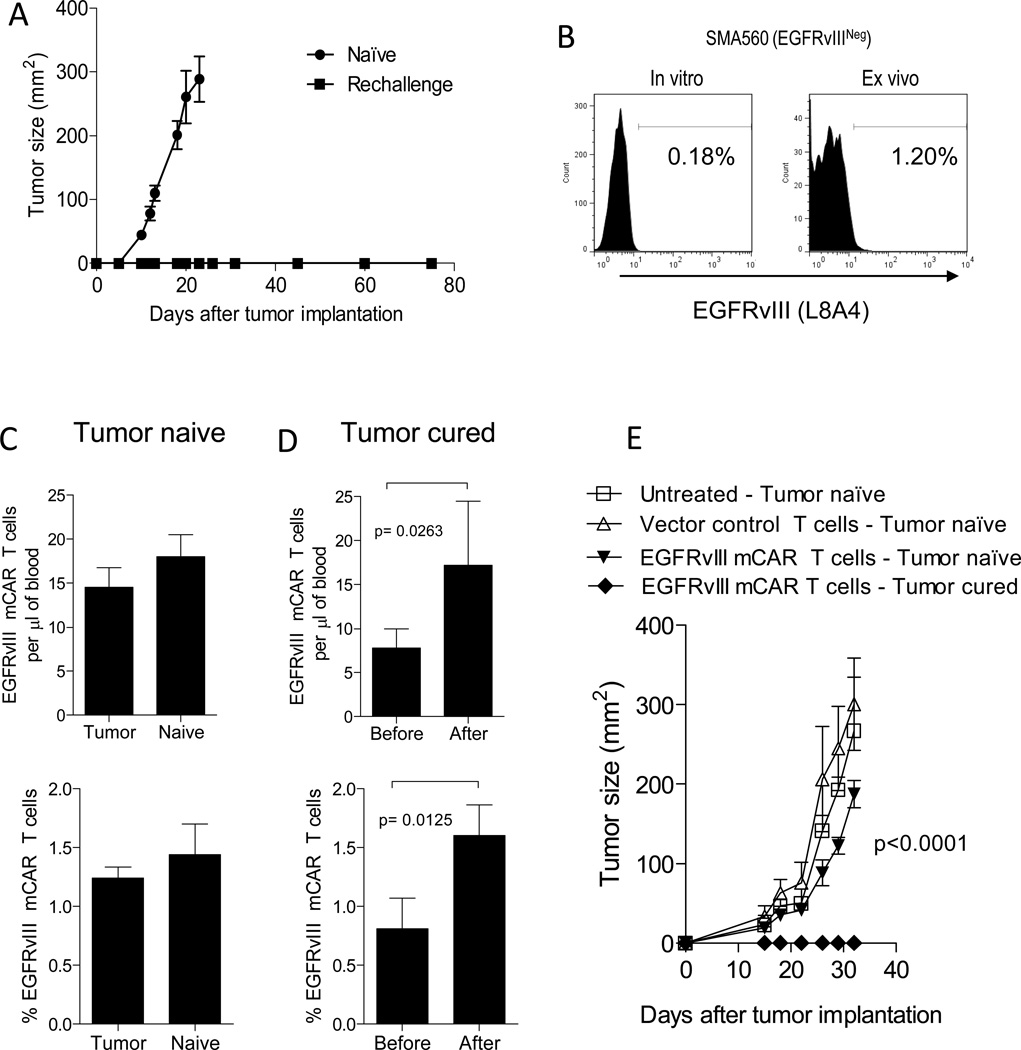
We sought to determine whether treatment with EGFRvIII mCAR T cells could elicit de novo priming against additional tumor antigens, which could then serve as protection against tumor-antigen loss. Specifically, we wanted to test whether mice that were previously treated with mCARs could acquire protective immunity against tumors that did not contain the original targeted antigen of interest, in this case, EGFRvIII. To do this, we challenged mice—either tumor naïve mice with no treatment, or those previously given EGFRvIII mCAR T cells and cured of SMA560vIII tumor. In the control group, tumors grew rapidly and all reached humane size endpoints within 40 days. In contrast, previously-cured mice were completely protected against rechallenge with EGFRvIIINEG tumor (Figure 5A).
Figure 5. Treatment of mice bearing EGFRvIIIPOS tumors with mCARs provides long-term protection against EGFRvIIINEG tumor.
(A) To evaluate whether treatment with EGFRvIII mCAR T cells could elicit de novo priming against antigen-loss variants, long-term survivors (greater than 60 days after treatment) of subcutaneous SMA560vIII tumors by EGFRvIII mCAR therapy were rechallenged with 5×105 EGFRvIIINEG SMA560 cells on the contralateral flank and their survival was monitored. (B) SMA560 (EGFRvIIINEG) gliomas were maintained in vitro or passaged in a mouse subcutaneously for 14 days, then harvested and both were stained for EGFRvIII expression using L8A4 mAb and analyzed by flow cytometry. Total numbers and percentages of mCAR T cells in blood was evaluated in (C) tumor-naïve mice, and (D) SMA 560vIII (EGFRvIIIPOS) tumor-cured mice before and 7 days after subcutaneous challenge with SMA560 (EGFRvIIINEG) tumors. Statistical significance was determined using a paired t test. Horizontal bar represents a statistical significance of P < 0.05 (C-E) Tumor naïve VM/Dk mice were either left untreated, received vector control, or EGFRvIII mCAR T cells 1 day after receiving a lymphodepletive conditioning regimen by 5Gy TBI. All mice were challenged subcutaneously with SMA560 (EGFRvIIINEG) tumors 28 days after adoptive-T-cell transfer. Additionally, VM/Dk mice that were previously inoculated with 5×105 SMA560vIII (EGFRvIIIPOS) tumor cells and were cured of tumor after receiving 5Gy TBI and EGFRvIII mCAR T cells (tumor cured) were rechallenged subcutaneously with 5×105 SMA560 (EGFRvIIINEG) tumor cells at the same time. (E) Tumor growth was monitored in two dimensions. Mice were examined for morbidity end points approved by the Duke University IACUC and sacrificed when end points were met. Experiment is representative of two independent repeats with similar results. Statistical significance of P < 0.0001was determined using two-way ANOVA.
The ability of EGFRvIIINEG glioma cell lines to express EGFRvIII upon transfer in vivo has been recently demonstrated (46). We sought to rule out the possibility that this was the reason for protection against EGFRvIIINEG tumor challenge. To evaluate whether SMA560 upregulates EGFRvIII in vivo, we implanted SMA560 tumors subcutaneously in mice. Fourteen days later, we excised the tumors, stained them for EGFRvIII expression and performed a flow cytometric analysis. Figure 5B shows that although the EGFRvIII expression did increase slightly, from 0.18% in vitro, to 1.20% post-passaging in vivo, the majority (> 98%) of tumor cells did not exhibit EGFRvIII expression, making it unlikely to be the cause of tumor rejection.
To further evaluate the possibility of EGFRvIII antigen expression in vivo on SMA560, we looked for mCAR T cell proliferation in vivo in response to SMA560 challenge. We used groups of mice that had received EGFRvIII mCAR T cells in the absence of tumor, and mice that had received the same T cells in the presence of SMA560vIII, and had successfully cleared the tumor. Both T cell-injected tumor naïve and cured mice were found to have circulating EGFRvIII mCAR T cells in their blood five weeks after injection, prior to SMA560 challenge (Figure 5C, D). Both groups then received the EGFRvIIINEG SMA560 tumor implanted subcutaneously, and were re-evaluated for circulating mCAR T cells seven days later. In the tumor naïve mice, there was no change in absolute numbers or percentages of EGFRvIII mCAR T cells before and after tumor challenge. However, unexpectedly, the cured mice showed a significant (P < 0.05) increase in both absolute numbers and percentages of circulating EGFRvIII CAR T cells after the antigen-negative tumor rechallenge. This was surprising, as we found no significant increase in EGFRvIII expression on the tumor in vivo, and because EGFRvIII CAR T cells alone were not sufficient to protect the mice against SMA560 rechallenge (Figure 5E and Table I). Importantly, this data suggests that the mechanism responsible for rejection of the re-challenge parental tumor in vivo may have included EGFRvIII CAR T cells, however additional elements were necessary for anti-tumor response. These results show that the mCAR T cells have some response to antigen-negative tumor rechallenge in vivo, whether by direct tumor interaction, or indirectly by another generalized immune stimulation in vivo. Together, these results suggest that third-generation, mCAR modified T cells may contribute to the development of host immunity against tumor-antigen escape.
Table I.
Eradication of SMA560vIII by EGFRvIII 139 CAR T cells is required for immunity against SMA560 (EGFRvIII-negative) parental tumors
| Group | Number of mice |
SMA560vIII challenge |
CAR T cells |
SMA560vIII cures |
SMA560 challenge |
SMA560 cures |
|---|---|---|---|---|---|---|
| Untreated, SMA560vIII naïve | 8 | − | − | N/A | + | 0/8 |
| Untreated, SMA560vIII exposed | 15 | + | − | 0/15 | N/A | N/A |
| mCAR T cells, SMA560vIII naïve | 4 | − | + | N/A | + | 0/4 |
| mCAR T cells, SMA560vIII exposed | 15 | + | + | 10/15 | + | 10/10 |
Day 0, mice received SMA560vIII tumors subcutaneously; day 5, mice received 5Gy TBI followed by T cells intravenously; day 28, mice were challenged with SMA560 parental tumors.
Discussion
Several groups have demonstrated that T cells can be successfully redirected by CARs to induce potent antitumor immune responses; however, unexpected toxicity has been observed due to shared target antigen expression on normal tissues (11, 19). Due to its exquisite tumor-specificity, EGFRvIII has been previously shown to be a promising target for gene-modified T-cell therapy immune responses both in vitro and in xenogeneic, immunodeficient mouse models (25, 34, 47). Our findings in the present study demonstrate that a third-generation, EGFRvIII-specific mCAR can be used to treat 3 to 5-day established intracerebral glioma in a fully immune-competent, syngeneic murine model.
With three notable exceptions, CD19 (48, 49), VEGFR-2 (48) and FAP (50), previous preclinical studies of CAR-redirected T cells have been performed in vitro or have been limited to in vivo studies against xenografts in immune-deficient mice (25, 28–30, 51). Xenograft models, while enabling important experiments using human tumors and reagents, suffer from numerous shortcomings that have been reviewed in detail elsewhere (26). Briefly, because these mice lack physiological immune systems, a number of signaling ligands, effector molecules, and other circulating factors are absent that could otherwise impact T-cell function, trafficking and persistence. Likewise, human tumor cells generally do not grow in mouse tissues as they do in patients, owing to several species-specific stromal interactions and autologous growth factors thought to promote highly-aggressive and invasive characteristics of human tumors in situ. In the current study, our utilization of an immune-competent mouse model with tumors derived from a syngeneic, spontaneously arising glioma, addresses many of these limitations.
The utilization of our immune-competent mouse model revealed a number of findings that had previously been impossible to evaluate in a xenograft setting. One finding was the need for lymphodepletive host-pre-conditioning to achieve anti-tumor efficacy using adoptively transferred mCAR T cells. We speculate this may be due to a number of factors, including making ‘space’ for the engineered lymphocytes to expand in the periphery, lack of cytokine sinks for lymphoid survival factors, removal of circulating regulatory cells, and possible increased Toll-like receptor signalling in immune cells, induced by damaging radiation (52, 53).
Total mCAR T cell numbers in PBL were maintained in mice over a 35 day period, although percentage of mCAR T cells diminished over time due to reconstitution of endogenous lymphocytes (Figure 4). It is not known what proportion of T cells may have migrated into tumor, tissue or lymphoid organs, however, so this may be an underestimate of total mCAR T cell retention in the VM/Dk model. It remains unknown what happens to the cells in vivo following ACT. A number of factors may, and likely do contribute, including body size, metabolic processes, extent of tumor infiltration, and potential suppressive, or immune activation factors in the periphery or at the tumor site. While total numbers of surviving mCAR T cells in our mouse model are unknown, it is possible that each CAR T cell could function to kill many tumor cells, effectively generating ‘serial killers’, as has been reported clinically for the CD19 CAR in patients with leukemia (17, 54), suggesting that clinically, the quality of the cell may be more important than the quantity.
One of the biggest clinical problems with GBM is its infiltrative micro-metastases. While GBM rarely metastasizes outside the brain, it does recur generally in the area around the initial tumor excision, indicating that tumor cells remain after gross total resection. One unexpected finding in our study is that mice treated with EGFRvIII mCARs developed long-lasting immunity against syngeneic EGFRvIIINEG tumors, suggesting that this approach may be superior to prior peptide vaccine approaches that have been limited by antigen escape (33). A major mechanism by which tumors have been shown to evade the immune system is through the process of immune editing, whereby tumor cells are favorably selected for the ability to either mutate or down-regulate a targeted antigen of interest (55). These phenomenae likely contributed to observations in our previous clinical studies of an EGFRvIII peptide vaccine, in which multiple patients achieved significant survival benefits but eventually had tumor recurrence owing to the outgrowth of EGFRvIII antigen-loss variant tumors (33). Unlike the EGFRvIII vaccine, which was found to elicit a predominantly humoral immune response, EGFRvIII CARs directly mediate a potent, highly-avid T-cell response. Importantly, T-cell immunity in particular is known to play central roles in the promotion of endogenous priming associated with epitope spreading (56), and is also capable of protecting against antigen loss through direct bystander effects (57).
We speculate that the expanded immune response we see may be occurring due to mCAR T-cell interaction with cognate target-bearing tumors, causing T-cell stimulation, and resulting in proliferation and production of multiple cytokines, including the inflammatory IL-1, IL-6 and IL-8, plus TNFα and IFNγ. These inflammatory cytokines are known to have direct effects on tumors, including upregulation of antigen expression on tumor cells, and additionally can recruit endogenous innate immune cells, including professional antigen presenting cells (APC) such as dendritic cells (DCs) and macrophages to the site of tumor destruction. The combination of mCAR T cells causing tumor cell lysis, recruitment of APC, and an inflammatory milieu of pro-Type 1 immune cytokines would create an optimal environment for the priming and activation of DCs in the presence of tumor antigen. Previously referred to as a cytokine storm (58, 59), this ‘perfect storm’ may be sufficient to cause activated APCs to engulf and present tumor-specific antigens to naïve T cells in situ, or at the draining lymph nodes, resulting in priming of endogenous T cells against novel tumor antigens. Although we cannot rule out a possible immune response against foreign antigens found on the tumors (i.e., fetal calf serum from in vitro culture), this would likely require the same immune priming process in vivo.
The development of endogenous antitumor (non-EGFRvIII) immunity in our mCAR treated mice was somewhat unexpected, as our mice were lymphodepleted, though not myeloablated with radiation (5Gy) at the time of their mCAR T-cell transfer. Even more surprising was the in vivo expansion of EGFRvIII mCAR T cells observed upon rechallenge with an EGFRvIIINEG tumor. It is unknown whether this increase in mCAR T cells was the result of proliferation induced by cognate antigen binding, or if another mechanism allowed for expansion of these cells in vivo, such as bystander proliferation as a result of IL-2 production by other activated T cells. We also postulate that the lymphodepleted periphery may have provided an ideal environment for adoptively-transferred mCAR T cells with endogenous tumor-reactive TCRs, or newly emergent naive lymphocytes from the bone marrow with endogenous TCR recogniton of tumor antigens, to selectively expand. Without competition for pro-growth cytokines or suppression by T regulatory and other suppressive cells, activation of endogenous tumor-specific TCRs would provide a selective advantage to those cells to proliferate and expand into the lymphopenic periphery. Given that recent clinical studies have associated favorable clinical responses with the ability to produce broad immunity to previously untargeted antigenic determinants (60), further study regarding the ability for CARs to elicit these secondary responses should be an emerging priority.
The remarkable cytotoxic potential of CAR-based T-cell therapy is perhaps most unfortunately illustrated by the dramatic toxicities observed in clinical trials of CARs targeting antigens co-expressed in both tumors and normal tissues. Thus, the selection of a potential target antigen must be carefully considered in order to reduce the risk of life-threatening autoimmunity. To our knowledge, the EGFRvIII-specific CAR represents the first truly tumor-specific application of the CAR platform, and promises to circumvent toxicities that have been previously observed for genetically-engineered T cells targeting shared antigens including MART-1 (5), gp100 (2), CEA (40), CAIX (11), ERBB2 (19), and CD19 (18, 39). However, in situations where these antigens continue to be targets for CAR therapy, our data presented here support that strategies utilizing soluble peptide blockade may be further developed to provide a novel antidote for reducing undesired CAR-mediated T-cell activation in vivo.
Although not directly addressed in the present study, our immune-competent murine model could prove useful in the evaluation of endogenous suppressive factors that have emerged as major impediments to successful antitumor immune-therapy. Many of these interactions are physiologically complex and as such, are not adequately modeled by in vitro systems or immune-deficient animal models of human tumor xenograft. Molecular interactions such as those observed between PD-1, CTLA-4 and their respective ligands (61), as well as the impact of suppressive regulatory T cells and TGF-β in glioma patients (62) have been cited as promising therapeutic targets for the reversal of tumor-associated immune suppression. Studies designed to address these and other questions in immune-competent animal models may provide useful alternatives and will certainly gain relevance as CAR-mediated therapies progress through clinical trials. Here, we begin to provide evidence that immune-replete, preclinical models can reveal critical details for therapy that may inform clinical trial design.
Together, these data support that third-generation EGFRvIII CAR T cells have the ability to treat established syngeneic gliomas in the brain and may additionally confer host immunity against tumor-antigen loss variants through epitope spreading. As shown here, preclinical models with competent immune systems represent an opportunity to greatly advance our understanding of adoptive transfer therapies. While it will be important to evaluate treatment at later timepoints following tumor implantation to better mirror the clinical situation, EGFRvIII-targeted CAR T cells may provide a highly-specific, promising therapeutic candidate for patients with tumors in the CNS and are now in phase I clinical trials at the NCI Surgery Branch (NCT01454596, www.clinicaltrials.gov).
Translational Relevance.
Our study describes a new model for testing chimeric antigen receptor (CAR) gene-engineered T lymphocytes against solid brain tumors in vivo in a syngeneic, immune intact mouse. Using a naturally-arising murine glioma expressing the tumor-specific mutation EGFRvIII, we were able to treat these mice by systemic adoptive transfer of syngeneic T cells transduced with a 3rd generation CAR encoding anti-EGFRvIII mAb scFv linked to murine CD28:4-1BB:CD3zeta intracellular domain. Intravenous delivery of these murine CAR T cells was able to treat tumors in a lymphodepletive and dose-dependent fashion. Long-term ‘cured’ animals were able to reject rechallenge with an EGFRvIII-negative tumor, suggesting endogenous immune system contribution to immunity. This work describes a relevant in vivo model for translational cancer research, namely, using immune-intact mice for adoptive T-cell-based cancer therapy, and has direct clinical implications that have previously been largely overlooked.
Acknowlegements
This work was supported by funding to LAJ from the National Cancer Institute and Office of the Director NIH Grant DP2CA174502; Voices Against Brain Cancer; and the American Brain Tumor Association. This work was also supported by an NIH NCI grant 1R01CA177476-01 to J.H. Sampson. We would also like to thank Dr. Darell Bigner for his kind gift of the VM/Dk mice.
Footnotes
Disclosure statement: JHS, BDC and LAJ are named in an intellectual property filing by Duke University based upon the use of EGFRvIII peptide to abrogate CAR T-cell function in vivo. There are no other potential conflicts of interest to declare.
References
- 1.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 2.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg SA. Cell transfer immunotherapy for metastatic solid cancer--what clinicians need to know. Nat Rev Clin Oncol. 2011;8:577–585. doi: 10.1038/nrclinonc.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nature reviews Immunology. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg SA, Yang JC, Robbins PF, Wunderlich JR, Hwu P, Sherry RM, et al. Cell transfer therapy for cancer: lessons from sequential treatments of a patient with metastatic melanoma. Journal of immunotherapy. 2003;26:385–393. doi: 10.1097/00002371-200309000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gross G, Gorochov G, Waks T, Eshhar Z. Generation of effector T cells expressing chimeric T cell receptor with antibody type-specificity. Transplant Proc. 1989;21:127–130. [PubMed] [Google Scholar]
- 10.Hollyman D, Stefanski J, Przybylowski M, Bartido S, Borquez-Ojeda O, Taylor C, et al. Manufacturing validation of biologically functional T cells targeted to CD19 antigen for autologous adoptive cell therapy. J Immunother. 2009;32:169–180. doi: 10.1097/CJI.0b013e318194a6e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamers CH, Sleijfer S, Vulto AG, Kruit WH, Kliffen M, Debets R, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24:e20–e22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 12.Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17:1453–1464. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Y, Wang QJ, Yang S, Kochenderfer JN, Zheng Z, Zhong X, et al. A herceptin-based chimeric antigen receptor with modified signaling domains leads to enhanced survival of transduced T lymphocytes and antitumor activity. J Immunol. 2009;183:5563–5574. doi: 10.4049/jimmunol.0900447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong XS, Matsushita M, Plotkin J, Riviere I, Sadelain M. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol Ther. 2010;18:413–420. doi: 10.1038/mt.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. The New England journal of medicine. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brentjens RJ, Riviere I, Park JH, Davila ML, Wang X, Stefanski J, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wikstrand CJ, Hale LP, Batra SK, Hill ML, Humphrey PA, Kurpad SN, et al. Monoclonal antibodies against EGFRvIII are tumor specific and react with breast and lung carcinomas and malignant gliomas. Cancer Res. 1995;55:3140–3148. [PubMed] [Google Scholar]
- 21.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 22.Inda MM, Bonavia R, Mukasa A, Narita Y, Sah DW, Vandenberg S, et al. Tumor heterogeneity is an active process maintained by a mutant EGFR-induced cytokine circuit in glioblastoma. Genes Dev. 2010;24:1731–1745. doi: 10.1101/gad.1890510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boockvar JA, Kapitonov D, Kapoor G, Schouten J, Counelis GJ, Bogler O, et al. Constitutive EGFR signaling confers a motile phenotype to neural stem cells. Mol Cell Neurosci. 2003;24:1116–1130. doi: 10.1016/j.mcn.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Lammering G, Hewit TH, Holmes M, Valerie K, Hawkins W, Lin PS, et al. Inhibition of the type III epidermal growth factor receptor variant mutant receptor by dominant-negative EGFR-CD533 enhances malignant glioma cell radiosensitivity. Clin Cancer Res. 2004;10:6732–6743. doi: 10.1158/1078-0432.CCR-04-0393. [DOI] [PubMed] [Google Scholar]
- 25.Ohno M, Natsume A, Ichiro Iwami K, Iwamizu H, Noritake K, Ito D, et al. Retrovirally engineered T-cell-based immunotherapy targeting type III variant epidermal growth factor receptor, a glioma-associated antigen. Cancer science. 2010;101:2518–2524. doi: 10.1111/j.1349-7006.2010.01734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan RA. Human tumor xenografts: the good, the bad, and the ugly. Mol Ther. 2012;20:882–884. doi: 10.1038/mt.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dotti G, Savoldo B, Brenner M. Fifteen years of gene therapy based on chimeric antigen receptors: "are we nearly there yet?". Hum Gene Ther. 2009;20:1229–1239. doi: 10.1089/hum.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed N, Salsman VS, Kew Y, Shaffer D, Powell S, Zhang YJ, et al. HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clin Cancer Res. 2010;16:474–485. doi: 10.1158/1078-0432.CCR-09-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balyasnikova IV, Ferguson SD, Sengupta S, Han Y, Lesniak MS. Mesenchymal stem cells modified with a single-chain antibody against EGFRvIII successfully inhibit the growth of human xenograft malignant glioma. PLoS One. 2010;5:e9750. doi: 10.1371/journal.pone.0009750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kahlon KS, Brown C, Cooper LJ, Raubitschek A, Forman SJ, Jensen MC. Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells. Cancer Res. 2004;64:9160–9166. doi: 10.1158/0008-5472.CAN-04-0454. [DOI] [PubMed] [Google Scholar]
- 31.Sampson JH, Ashley DM, Archer GE, Fuchs HE, Dranoff G, Hale LP, et al. Characterization of a spontaneous murine astrocytoma and abrogation of its tumorigenicity by cytokine secretion. Neurosurgery. 1997;41:1365–1372. doi: 10.1097/00006123-199712000-00024. discussion 72–3. [DOI] [PubMed] [Google Scholar]
- 32.Sampson JH, Crotty LE, Lee S, Archer GE, Ashley DM, Wikstrand CJ, et al. Unarmed, tumor-specific monoclonal antibody effectively treats brain tumors. Proc Natl Acad Sci U S A. 2000;97:7503–7508. doi: 10.1073/pnas.130166597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:4722–4729. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan RA, Johnson LA, Davis JL, Zheng Z, Woolard KD, Reap EA, et al. Recognition of Glioma Stem Cells by Genetically Modified T Cells Targeting EGFRvIII and Development of Adoptive Cell Therapy for Glioma. Hum Gene Ther. 2012;23:1043–1053. doi: 10.1089/hum.2012.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chinnasamy D, Yu Z, Theoret MR, Zhao Y, Shrimali RK, Morgan RA, et al. Gene therapy using genetically modified lymphocytes targeting VEGFR-2 inhibits the growth of vascularized syngenic tumors in mice. The Journal of clinical investigation. 2010;120:3953–3968. doi: 10.1172/JCI43490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kerkar SP, Sanchez-Perez L, Yang S, Borman ZA, Muranski P, Ji Y, et al. Genetic engineering of murine CD8+ and CD4+ T cells for preclinical adoptive immunotherapy studies. J Immunother. 2011;34:343–352. doi: 10.1097/CJI.0b013e3182187600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serano RD, Pegram CN, Bigner DD. Tumorigenic cell culture lines from a spontaneous VM/Dk murine astrocytoma (SMA) Acta neuropathologica. 1980;51:53–64. doi: 10.1007/BF00688850. [DOI] [PubMed] [Google Scholar]
- 38.Heimberger AB, Crotty LE, Archer GE, McLendon RE, Friedman A, Dranoff G, et al. Bone marrow-derived dendritic cells pulsed with tumor homogenate induce immunity against syngeneic intracerebral glioma. Journal of neuroimmunology. 2000;103:16–25. doi: 10.1016/s0165-5728(99)00172-1. [DOI] [PubMed] [Google Scholar]
- 39.Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Molecular therapy : the journal of the American Society of Gene Therapy. 2011;19:620–626. doi: 10.1038/mt.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong JJ, Rosenberg SA, Dudley ME, Yang JC, White DE, Butman JA, et al. Successful treatment of melanoma brain metastases with adoptive cell therapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:4892–4898. doi: 10.1158/1078-0432.CCR-10-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hwang TL, Close TP, Grego JM, Brannon WL, Gonzales F. Predilection of brain metastasis in gray and white matter junction and vascular border zones. Cancer. 1996;77:1551–1555. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1551::AID-CNCR19>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 43.Dummer W, Niethammer AG, Baccala R, Lawson BR, Wagner N, Reisfeld RA, et al. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J Clin Invest. 2002;110:185–192. doi: 10.1172/JCI15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wrzesinski C, Paulos CM, Kaiser A, Muranski P, Palmer DC, Gattinoni L, et al. Increased intensity lymphodepletion enhances tumor treatment efficacy of adoptively transferred tumor-specific T cells. J Immunother. 2010;33:1–7. doi: 10.1097/CJI.0b013e3181b88ffc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Del Vecchio CA, Giacomini CP, Vogel H, Jensen KC, Florio T, Merlo A, et al. EGFRvIII gene rearrangement is an early event in glioblastoma tumorigenesis and expression defines a hierarchy modulated by epigenetic mechanisms. Oncogene. 2013;32:2670–2681. doi: 10.1038/onc.2012.280. [DOI] [PubMed] [Google Scholar]
- 47.Bullain SS, Sahin A, Szentirmai O, Sanchez C, Lin N, Baratta E, et al. Genetically engineered T cells to target EGFRvIII expressing glioblastoma. J Neurooncol. 2009;94:373–382. doi: 10.1007/s11060-009-9889-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santos EB, Yeh R, Lee J, Nikhamin Y, Punzalan B, Punzalan B, et al. Sensitive in vivo imaging of T cells using a membrane-bound Gaussia princeps luciferase. Nat Med. 2009;15:338–344. doi: 10.1038/nm.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kochenderfer JN, Yu Z, Frasheri D, Restifo NP, Rosenberg SA. Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood. 2010;116:3875–3886. doi: 10.1182/blood-2010-01-265041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tran E, Chinnasamy D, Yu Z, Morgan RA, Lee CC, Restifo NP, et al. Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia. The Journal of experimental medicine. 2013;210:1125–1135. doi: 10.1084/jem.20130110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009;106:3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nature reviews Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, Cassard L, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. The Journal of clinical investigation. 2007;117:2197–2204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. The New England journal of medicine. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 56.Pilon SA, Kelly C, Wei WZ. Broadening of epitope recognition during immune rejection of ErbB-2-positive tumor prevents growth of ErbB-2-negative tumor. J Immunol. 2003;170:1202–1208. doi: 10.4049/jimmunol.170.3.1202. [DOI] [PubMed] [Google Scholar]
- 57.Zhang B, Karrison T, Rowley DA, Schreiber H. IFN-gamma- and TNF-dependent bystander eradication of antigen-loss variants in established mouse cancers. J Clin Invest. 2008;118:1398–1404. doi: 10.1172/JCI33522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hombach AA, Holzinger A, Abken H. The weal and woe of costimulation in the adoptive therapy of cancer with chimeric antigen receptor (CAR)-redirected T cells. Current molecular medicine. 2013;13:1079–1088. doi: 10.2174/1566524011313070003. [DOI] [PubMed] [Google Scholar]
- 59.D'Elia RV, Harrison K, Oyston PC, Lukaszewski RA, Clark GC. Targeting the "cytokine storm" for therapeutic benefit. Clinical and vaccine immunology : CVI. 2013;20:319–327. doi: 10.1128/CVI.00636-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Corbiere V, Chapiro J, Stroobant V, Ma W, Lurquin C, Lethe B, et al. Antigen spreading contributes to MAGE vaccination-induced regression of melanoma metastases. Cancer Res. 2011;71:1253–1262. doi: 10.1158/0008-5472.CAN-10-2693. [DOI] [PubMed] [Google Scholar]
- 61.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer research. 2006;66:3294–3302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]