Abstract
Cystic fibrosis (CF) is a disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR). Initially, Cl− conductance in the sweat duct was discovered to be impaired in CF1, a finding that has been extended to all CFTR-expressing cells2–4. Subsequent cloning of the gene5,6 showed that CFTR functions as a cyclic-AMP-regulated Cl− channel7; and some CF-causing mutations inhibit CFTR Cl− channel activity2–4,8. The identification of additional CF-causing mutants with normal Cl− channel activity indicates, however, that other CFTR-dependent processes contribute to the disease. Indeed, CFTR regulates other transporters3,4, including Cl−-coupled transport9,10. Alkaline fluids are secreted by normal tissues, whereas acidic fluids are secreted by mutant CFTR-expressing tissues11, indicating the importance of this activity. and pH affect mucin viscosity12,13 and bacterial binding14,15. We have examined Cl−-coupled transport by CFTR mutants that retain substantial or normal Cl− channel activity. Here we show that mutants reported to be associated with CF with pancreatic insufficiency do not support transport, and those associated with pancreatic sufficiency show reduced transport. Our findings demonstrate the importance of transport in the function of secretory epithelia and in CF.
We compared the activity of wild-type CFTR to that of 17 disease-causing mutants. Several criteria were used for selection of mutants and the methods to evaluate Cl− and transport. All mutants selected code for properly processed proteins16–21. The macroscopic Cl− current and single-channel activity of the mutants have been shown to be significant, normal or even elevated16–21. The mutants are from all cytoplasmic domains of CFTR and have been reported to be associated with CF with pancreatic sufficiency or pancreatic insufficiency. Table 1 in the Supplementary Information lists the mutants and the reported clinical status of the patients. Two of the mutations, I148T and R117H, are relatively common, and thus the clinical data are solid. Initially we analysed these mutations to establish the relationship between the CF phenotype and transport.
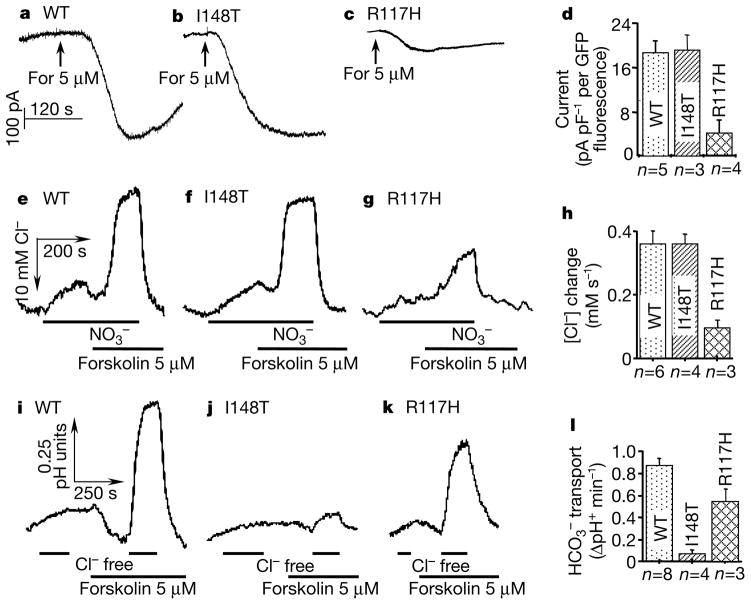
CFTR-dependent transport is dependent on Cl− transport and is not affected by the membrane potential9,10, suggesting that it is an electroneutral process that can only be followed by measuring intracellular concentration. Hence, changes in concentration were evaluated from changes in intracellular pH (pHi) (see refs 9, 10). To evaluate Cl− transport under the same conditions, we monitored intracellular chloride concentrations ([Cl−]i) with N-6(6-methoxyquinolyl) acetoethyl ester (MQAE). To validate the ability of this procedure to accurately report Cl− channel activity, we determined the correlation between expression efficiency (green fluorescent protein (GFP) fluorescence), Cl− current and changes in [Cl−]i in cells transfected with CFTR, CFTR(I148T) and CFTR(R117H). Figure 1a–h shows that similar results are reported by measurement of Cl− current and [Cl−]i for CFTR and the mutants. Therefore, the Cl− transport capacity of all other CFTR mutants was evaluated from changes in [Cl−]i.
Figure 1.
cAMP-stimulated Cl− and transport by wild-type (WT) CFTR and the CFTR mutants I148T and R117H. The whole-cell Cl− current of HEK293 cells transfected with the indicated constructs was determined (a–c). For, forskolin. The current was normalized with respect to membrane capacitance and GFP fluorescence before averaging (d). Transfected cells were also loaded with MQAE (e–h) or BCECF (i–l) for measurements of [Cl−]i and pHi, respectively. Cells loaded with MQAE were exposed to a solution in which Cl− was replaced with and then stimulated with 5 μM forskolin. For pH measurements, Cl− was replaced with gluconate. After calibration, initial rates of changes in [Cl−]i (h) and pHi (l) were averaged.
Figure 1e–l shows the experimental protocols used to measure the effect of the I148T and R117H mutations on Cl− and transport. The I148T mutant is normally processed and mediates macroscopic Cl− current (Fig. 1d; ref. 16), single-channel properties16 and Cl− fluxes (Fig. 1h) indistinguishable from those of wild-type CFTR, but is associated with CF with pancreatic insufficiency. Replacing external Cl− with in non-stimulated cells caused a slow Cl− efflux in cells expressing CFTR or the I148T mutant. Stimulation of CFTR-expressing cells with forskolin resulted in a change in [Cl−]i at a rate of 0.36±0.04 (n = 6) mM s−1. Similar rates were measured for the I148T, G178R, A1067T, G1244E, S1255P and G1349D mutants (see Fig. 3 for location of these mutations in CFTR), all of which are associated with CF with pancreatic insufficiency. The rates of changes in [Cl−]i measured here, and the macroscopic Cl− currents from previous studies are listed in Table 1 in the Supplementary Information. In all cases, the presence of forskolin-stimulated Cl− fluxes confirmed that the CFTR mutants matured and travelled properly to the plasma membrane.
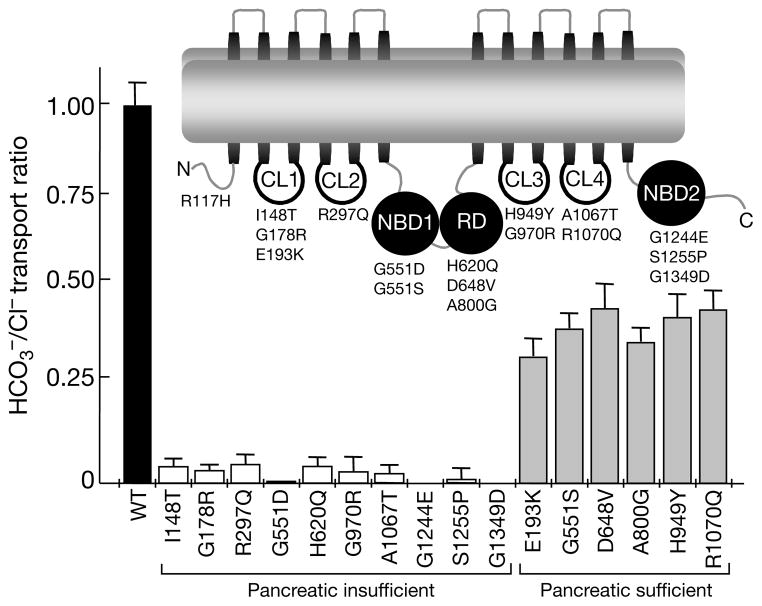
Figure 3.
The transport ratio of CFTR mutants associated with CF. The transport ratios were calculated from the averaged rates summarized in Table 1 of the Supplementary Information. The ratio measured in wild-type CFTR was set to 1. Inset illustrates the different cytoplasmic domains of CFTR. CL1, cytoplasmic loop 1; CL2, cytoplasmic loop 2; NBD1, nucleotide-binding domain 1; RD, regulatory domain; CL3, cytoplasmic loop 3; CL4, cytoplasmic loop 4; NBD2, nucleotide-binding domain 2.
The similarity of Cl− transport by CFTR and the mutants implies that aberrant Cl− transport cannot alone account for these severe forms of CF. Stimulation of CFTR markedly increases Cl−-coupled transport (Fig. 1i) by an unknown mechanism. Several studies have reported that CFTR functions as both a Cl− and a channel22–24, which might account for the fluxes reported here. In a continuing work, however, we confirmed that Cl− efflux is obligatory for CFTR-dependent influx, and that depolarizing the cells does not inhibit the transport by CFTR and the mutants. Therefore, we consider the CFTR-dependent transport as Cl−-coupled transport. Remarkably, all mutations associated with CF with pancreatic insufficiency tested here, without exception, eliminate the ability of CFTR to support transport. Results for the I148T mutant are shown in Fig. 1, and the averaged results for all mutants are listed in Table 1 in the Supplementary Information.
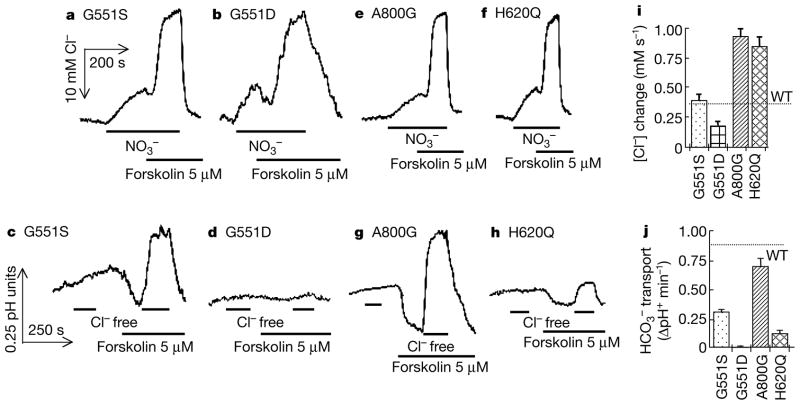
We next analysed Cl− and transport in CFTR mutants associated with pancreatic sufficiency. The results obtained with the R117H mutation are illustrated in Fig. 1. In sharp contrast with the results obtained with the mutants associated with CF with pancreatic insufficiency, and in agreement with previous reports25, the R117H mutation reduced Cl− current and the MQAE response by about 70%. By contrast, the R117H mutation reduced the ability of CFTR to support transport by only 37%. The E193K, D648V, H949Yand R1070Q mutants, all associated with CF with pancreatic sufficiency, had no effect on Cl− transport but reduced transport by 50–65%. Two sets of particularly interesting mutants are G551D and G551S and H620Q and A800G. G551S, which is associated with CF with pancreatic sufficiency, had no effect on Cl− transport but reduced transport by 59% (Fig. 2). As reported previously17,18,26, G551D reduced Cl− transport to 53% of the control value. By contrast, this mutation, which is associated with CF with pancreatic insufficiency, completely inhibited CFTR-dependent transport (Fig. 2b, d).
Figure 2.
cAMP-stimulated Cl− and transport by CFTR mutants associated with a severe or a mild form of CF. [Cl−]i (a, b, e, f) and (c, d, g, h) transport were measured as in Fig. 1. Summaries of averaged rates are given for Cl− (i) and for (j).
When expressed in oocytes, the H620Q and A800G mutants increased the macroscopic Cl− current about threefold and the channel open probability by 150–180% (ref. 19). We confirmed these findings, as Cl− transport by these mutants is about 2.4-fold higher than that by CFTR (Fig. 2e, f). Despite these markedly enhanced Cl− fluxes, the A800G mutation, which causes CF with pancreatic sufficiency, only stimulated transport to 75% of the wild-type CFTR value. The H620Q mutant, found in one CF patient with pancreatic insufficiency, only stimulated transport about 13% as effectively as did CFTR. The enhanced Cl− transport by the two mutants in heterologous systems seems to be caused by increased expression of the protein in the plasma membrane19. If this enhanced expression is not maintained in vivo, the mutations will further compromise transport to exacerbate the pancreatic phenotypes. In this respect, the R1070Q mutation, in the few documented cases, has been found to be associated with CF with either pancreatic sufficiency or pancreatic insufficiency. Our results of substantial transport by this mutant would predict a phenotype of CF with pancreatic sufficiency.
To account for variable Cl− transport and possible variable expression of the proteins in the plasma membrane, we normalized the capacity of the CFTR mutants to transport with respect to their capacity to transport Cl−. Figure 3 shows the correlation between the reported pancreatic status of the patients and the transport ratio. When the ratio measured with CFTR is taken as 1, all mutants associated with CF with pancreatic insufficiency show an transport ratio of less than 0.1. By comparison, all mutations associated with CF with pancreatic sufficiency show an transport ratio of between 0.31 and 0.46. Notably, although a few of these mutants exhibit altered channel gating or reduced processing, in all of the mutants CFTR-dependent transport is affected more than CFTR-mediated Cl− transport. In this regard, although the R117H mutation markedly reduces Cl− channel activity and Cl− transport (Fig. 1), it is associated with a mild form of CF, probably because it supports substantial transport (Fig. 1). Consequently, the CFTR-dependent transport ratio seems to correlate well with the reported pancreatic function of CF patients.
Cl− transport across the apical membrane of secretory epithelia is the rate-limiting step in fluid and electrolyte transport1–4,8, high-lighting the importance of the Cl− channel function of CFTR. The existence of CF-causing CFTR mutations that support normal or even elevated Cl− transport indicates, however, that factors other than abnormal Cl− transport can also lead to CF. An alternative view of the role of CFTR in epithelial transport was introduced with the finding that CFTR can affect the transport of other ions27–30, including (refs 9, 10, 22–24). The aberrant transport by the CF-causing mutations examined here indicates that transport by CFTR-expressing epithelia is critical for normal tissue physiology, and that impaired transport is sufficient to derange pancreatic function even in the presence of Cl− channel activity. Acidic fluid secretion by CFTR-expressing tissues in the disease state may lead to precipitation of mucins and plugging of ductal systems12,13, and facilitate bacterial infection through binding to the precipitated mucins14,15. In the special case of the pancreas, acidic pH would lead to premature activation of digestive enzymes, destruction of the pancreas and pancreatic insufficiency8. Thus, the aberrant transport model can account for diverse pathologies observed in the disease. Our findings suggest that simple correction of Cl− transport is not likely to ameliorate the symptoms of CF. Enhancing transport by epithelial cells, even in the absence of CFTR, or increasing the content on the apical surface of affected tissues, should be considered as additional means of reducing the debilitating effects of CF.
Methods
Site-directed mutagenesis
The pCMVNot 6.2 plasmid carrying the human wild-type CFTR gene was a gift from J. Rommens (Hospital for Sick Children, Toronto, Canada). Site-directed mutagenesis was performed using the QuickChange site-directed mutagenesis kit from Stratagene (La Jolla, CA). All mutations were verified by sequencing of four separate clones from each mutant. The activity of all four clones was measured to verify that all have similar activity.
Expression of CFTR and cell transfection
All wild-type and CFTR mutants were expressed in HEK293 cells by transient transfection using the lipofectamine reagent. To control for expression of CFTR and identify the CFTR-expressing cells, we co-transfected the cells with a GFP-expressing plasmid (Life Technologies). In previous work, we showed that there is good agreement between expression of GFP and CFTR9. GFP fluorescence was measured before current recording or loading the cells with 2′,7′-bis-(2-carboxyethyl)-5-(6)-carboxyfluorescein (BCECF) or after loading with MQAE. Hence, all experiments were performed with cells expressing comparable amount of GFP.
Cl− currents, [Cl−]i and pHi
and Cl− transport were measured with matched pairs of cells transfected in the same dish. In brief, cells grown on two cover slips in each dish were transfected with the same solution and maintained in culture together until use 48–72 h after transfection. On the day of experiment, cells on one of the cover slips were loaded with BCECF (15 min at room temperature) and used immediately to measure transport. At the same time, cells on the matching cover slip were loaded with MQAE by 1.5–2 h at 37 °C in culture medium containing 5mM MQAE and then used to measure [Cl−]i.
The procedures and solutions used to measure the CFTR-mediated, whole-cell Cl− current in the HEK 293 cells transfected with wild-type CFTR and the CFTR mutants were identical to those described for measurement of CFTR-mediated Cl− current in NIH 3T3 cells9. Measurement of pHi and with BCECF and MQAE techniques, respectively, in single HEK293 cells, calibration of fluorescent signals and composition of solutions have been described9,10.
Supplementary Material
Acknowledgments
We thank H. Cuppens from the European CF Consortium for informing us that the G970R mutation results in pancreatic insufficiency. This work was supported by a grant from the NIH.
Footnotes
Supplementary information is available on Nature’s World-Wide Web site (http://www.nature.com) or as paper copy from the London editorial office of Nature.
References
- 1.Quinton PM. Chloride impermeability in cystic fibrosis. Nature. 1983;301:421–422. doi: 10.1038/301421a0. [DOI] [PubMed] [Google Scholar]
- 2.Quinton PM. Physiological basis of cystic fibrosis: a historical perspective. Physiol Rev. 1999;79:S3–S22. doi: 10.1152/physrev.1999.79.1.S3. [DOI] [PubMed] [Google Scholar]
- 3.Pilewski JM, Frizzell RA. Role of CFTR in airway disease. Physiol Rev. 1999;79:S215–S255. doi: 10.1152/physrev.1999.79.1.S215. [DOI] [PubMed] [Google Scholar]
- 4.Grubb BR, Boucher RC. Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol Rev. 1999;79:S193–S213. doi: 10.1152/physrev.1999.79.1.S193. [DOI] [PubMed] [Google Scholar]
- 5.Rommens JM, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- 6.Riordan JR, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 7.Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol Rev. 1999;79:S23–S45. doi: 10.1152/physrev.1999.79.1.S23. [DOI] [PubMed] [Google Scholar]
- 8.Argent BE, Case RM. In: Physiology of the Gastrointestinal Tract. 3. Johnson LR, editor. Raven; New York: 1994. pp. 1478–1498. [Google Scholar]
- 9.Lee MG, et al. Regulation of exchange by cystic fibrosis transmembrane conductance regulator expressed in NIH 3T3 and HEK 293 cells. J Biol Chem. 1999;274:3414–3421. doi: 10.1074/jbc.274.6.3414. [DOI] [PubMed] [Google Scholar]
- 10.Lee MG, et al. Cystic fibrosis transmembrane conductance regulator regulates luminal exchange in mouse submandibular and pancreatic ducts. J Biol Chem. 1999;274:14670–14677. doi: 10.1074/jbc.274.21.14670. [DOI] [PubMed] [Google Scholar]
- 11.Kopelman H, et al. Impaired chloride secretion, as well as bicarbonate secretion, underlies the fluid secretory defect in the cystic fibrosis pancreas. Gastroenterology. 1988;95:349–355. doi: 10.1016/0016-5085(88)90490-8. [DOI] [PubMed] [Google Scholar]
- 12.Veerman EC, Valentijn-Benz M, Nieuw Amerongen AV. Viscosity of human salivary mucins: effect of pH and ionic strength and role of sialic acid. J Biol Buccale. 1989;17:297–306. [PubMed] [Google Scholar]
- 13.Bhaskar KR, et al. Profound increase in viscosity and aggregation of pig gastric mucin at low pH. Am J Physiol. 1991;261:G827–G832. doi: 10.1152/ajpgi.1991.261.5.G827. [DOI] [PubMed] [Google Scholar]
- 14.Wanke CA, Cronan S, Goss C, Chadee K, Guerrant RL. Characterization of binding of Escherichia coli strains which are enteropathogens to small-bowel mucin. Infect Immun. 1990;58:794–800. doi: 10.1128/iai.58.3.794-800.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veerman EC, et al. Sulfated glycans on oral mucin as receptors for Helicobacter pylori. Glycobiology. 1997;7:737–743. doi: 10.1093/glycob/7.6.737. [DOI] [PubMed] [Google Scholar]
- 16.Seibert FS, et al. Disease-associated mutations in cytoplasmic loops 1 and 2 of cystic fibrosis transmembrane conductance regulator impede processing or opening of the channel. Biochemistry. 1997;36:11966–11974. doi: 10.1021/bi9712652. [DOI] [PubMed] [Google Scholar]
- 17.Illek B, et al. Defective function of the cystic fibrosis-causing missense mutation G551D is recovered by genistein. Am J Physiol. 1999;277:C833–C839. doi: 10.1152/ajpcell.1999.277.4.C833. [DOI] [PubMed] [Google Scholar]
- 18.Anderson M, Welsh MJ. Regulation by ATP and ADP of CFTR chloride channels that contain mutant nucleotide-binding domains. Science. 1992;257:1701–1704. doi: 10.1126/science.1382316. [DOI] [PubMed] [Google Scholar]
- 19.Vankeerberghen A, et al. Characterization of 19 disease-associated missense mutations in the regulatory domain of the cystic fibrosis transmembrane conductance regulator. Hum Mol Genet. 1998;7:1761–1769. doi: 10.1093/hmg/7.11.1761. [DOI] [PubMed] [Google Scholar]
- 20.Seibert FS, et al. Cytoplasmic loop three of cystic fibrosis transmembrane conductance regulator contributes to regulation of chloride channel activity. J Biol Chem. 1996;271:27493–27499. doi: 10.1074/jbc.271.44.27493. [DOI] [PubMed] [Google Scholar]
- 21.Seibert FS, et al. Disease-associated mutations in the fourth cytoplasmic loop of cystic fibrosis transmembrane conductance regulator compromise biosynthetic processing and chloride channel activity. J Biol Chem. 1996;271:15139–15145. doi: 10.1074/jbc.271.25.15139. [DOI] [PubMed] [Google Scholar]
- 22.Gray MA, Plant S, Argent BE. cAMP-regulated whole cell chloride currents in pancreatic duct cells. Am J Physiol. 1993;264:C591–C602. doi: 10.1152/ajpcell.1993.264.3.C591. [DOI] [PubMed] [Google Scholar]
- 23.Poulsen JH, Fischer H, Illek B, Machen TE. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci USA. 1994;91:5340–5344. doi: 10.1073/pnas.91.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linsdell P, et al. Permeability of wild-type and mutant cystic fibrosis transmembrane conductance regulator chloride channels to polyatomic anions. J Gen Physiol. 1997;110:355–364. doi: 10.1085/jgp.110.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheppard DN, et al. Mutations in CFTR associated with mild-disease-form Cl− channels with altered pore properties. Nature. 1993;362:160–164. doi: 10.1038/362160a0. [DOI] [PubMed] [Google Scholar]
- 26.Fulmer SB, Schwiebert EM, Morales MM, Guggino WB, Cutting GR. Two cystic fibrosis transmembrane conductance regulator mutations have different effects on both pulmonary phenotype and regulation of outwardly rectified chloride currents. Proc Natl Acad Sci USA. 1995;92:6832–6836. doi: 10.1073/pnas.92.15.6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stutts MJ, et al. CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995;269:847–850. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- 28.Mall M, Bleich M, Greger R, Schreiber R, Kunzelmann K. The amiloride-inhibitable Na+ conductance is reduced by the cystic fibrosis transmembrane conductance regulator in normal but not in cystic fibrosis airways. J Clin Invest. 1998;102:15–21. doi: 10.1172/JCI2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddy MM, Light MJ, Quinton PM. Activation of the epithelial Na+ channel (ENaC) requires CFTR Cl− channel function. Nature. 1999;402:301–304. doi: 10.1038/46297. [DOI] [PubMed] [Google Scholar]
- 30.McNicholas CM, et al. Sensitivity of a renal K+ channel (ROMK2) to the inhibitory sulfonylurea compound glibenclamide is enhanced by coexpression with the ATP-binding cassette transporter cystic fibrosis transmembrane regulator. Proc Natl Acad Sci USA. 1996;93:8083–8088. doi: 10.1073/pnas.93.15.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.