Abstract
Neurons in the arcuate nucleus (ARC) that concomitantly express kisspeptin, neurokinin B (NKB) and dynorphin A are termed KNDy neurons and are likely candidates for the intrinsic gonadotropin-releasing hormone (GnRH) pulse generator. Our hypothesis is that KNDy neurons are functionally and anatomically interconnected to generate discrete neural signals that govern pulsatile GnRH secretion. Our goal was to address this hypothesis using electrophysiological and anatomical experiments in goats. Bilateral electrodes targeting KNDy neurons were implanted into ovariectomized goats, and GnRH pulse generator activity, represented by characteristic increases in multiple-unit activity (MUA volleys), was measured. Spontaneous and pheromone- or senktide (an NKB receptor agonist)-induced MUA volleys were simultaneously recorded from both sides of the ARC. An anterograde tracer, biotinylated dextran amine (BDA), was also injected unilaterally into the ARC of castrated male goats, and the distribution of fibers containing both BDA and NKB was examined using dual-labeling histochemistry. The results showed that MUA volleys, regardless of origin (spontaneous or experimentally induced), occur simultaneously between the right and left sides of the ARC. Tract tracing indicated that axons projecting from NKB neurons in the ARC were directly apposed to other NKB neuronal cells located bilaterally in the ARC. These results demonstrate that GnRH pulse generator activity occurs synchronously between both sides of the ARC in goats and that KNDy neurons are bilaterally interconnected in the ARC via NKB-containing fibers. Taken together, the results suggest that KNDy neurons form a neuronal circuit to synchronize burst activity among KNDy neurons and thereby generate discrete neural signals that govern pulsatile GnRH secretion.
Keywords: GnRH pulse generator, Goat, Kisspeptin, Neurokinin B
A key determinant of reproductive function in mammals is the hypothalamic gonadotropin-releasing hormone (GnRH) pulse generator. This pulse generator governs intermittent GnRH discharges into the portal vessels and thereby regulates pulsatile luteinizing hormone (LH) release into the peripheral circulation [1,2,3]. GnRH pulse generator activity can be monitored by recording multiple-unit activity (MUA) in the mediobasal hypothalamus (MBH) and has been represented as periodic bursts of MUA (MUA volleys) in monkeys [4, 5], rats [6, 7] and goats [8, 9]. These studies clearly demonstrated that neural substrates firing a high-frequency volley of action potentials govern the pulsatile discharge of GnRH into the portal vessels. However, no study has identified the specific neuronal population responsible for this activity within the MBH.
Kisspeptin plays a crucial role in the central control of GnRH release [10,11,12]. Neurons expressing kisspeptin are distributed exclusively in the medial preoptic area (the anteroventral periventricular nucleus in rodents) and the arcuate nucleus (ARC) in the brain [13,14,15]. In a variety of species such as mice [16], rats [17], goats [18], sheep [19] and monkeys [20], kisspeptin neurons in the ARC have been shown to concomitantly express other neuropeptides implicated in the control of reproduction, including neurokinin B (NKB) and dynorphin A (Dyn) [21,22,23]. ARC kisspeptin neurons that concomitantly express these other neuropeptides are termed KNDy (Kisspeptin/NKB/Dyn) neurons [24].
A recent study in goats demonstrated that GnRH pulse generator activity, as represented by an MUA volley, can be recorded at close proximity to KNDy neurons [11, 18], suggesting that the population of KNDy neurons is a likely candidate for the intrinsic GnRH pulse generator [25]. Several lines of evidence support this proposition. First, KNDy neurons exist in the ARC, where the GnRH pulse generator has been postulated to be located by a deafferentation study [26]. Second, it has been shown in human genetic studies that inactivating mutations of several genes, including Kiss1R, which encodes the kisspeptin receptor (GPR54), Tac3 and Tacr3, which encode NKB and its receptor (NK3R), respectively, result in reproductive dysfunction, including gonadotropin deficiency and pubertal failure [21, 27, 28]. Third, antagonists of GPR54 [29] and NK3R [20, 30] suppress pulsatile LH release. Forth, it is thought that the GnRH pulse generator mediates the negative feedback action of gonadal steroids, as virtually all KNDy neurons express both estrogen receptor alpha [25, 31] and the progesterone receptor [32, 33] in the female or androgen receptors in the male [34]. In support of this idea, we showed that the frequency of MUA volleys is significantly reduced by estradiol (E2) administration and further reduced by progesterone administration, perhaps reflecting the negative feedback action of gonadal steroids [18]. Moreover, pharmacological ablation of KNDy neurons in rats results in complete abolishment of the negative feedback action of E2 on LH secretion [35].
To generate neural signals that govern distinct GnRH pulses, a group of KNDy neurons should produce coordinated bursts of activity. Several studies have proposed the existence of an interconnected neural circuit that, through axon collaterals and/or dendrites, serves to synchronize neural activity among KNDy neurons [16, 22, 24, 25]. However, direct evidence for such a neural circuit is limited to only few studies at present. Cardenas et al. [4] reported that coordinated GnRH pulse generator activity occurred between the right and left sides of the MBH in monkeys. However, due to the relatively broad time scale (30-sec time bins) used for data analysis, the details of electrophysiological synchronization were unclear and potential cellular sources of the MUA volley could not be identified. Indirect anatomical evidence suggests the presence of the KNDy neuronal circuit. For example, NKB/Dyn neurons are found in close apposition with fibers containing NKB/Dyn in the rat ARC [31] and kisspeptin/NKB neurons are surrounded by a dense network of fibers containing kisspeptin/NKB [18]. Recently, in a tract-tracing study using the anterograde tracer biotinylated dextran amine (BDA), Krajewski et al. [36] revealed that NKB neurons in the ARC (a likely candidate for KNDy neurons) send both ipsilateral and contralateral projections to other NKB neurons in rats. Although these results demonstrate that NKB neurons are interconnected via axon collaterals, this relationship should be confirmed in other species. In addition, because the precise location of the tracer injection site in relation to the distribution of NKB neurons was unclear and the tracer was taken up only by a few NKB neurons, it is possible that the results of that study do not fully represent the extent of neural connectivity among NKB neurons.
The purpose of the present study was to further explore the relationships suggested by previous studies [4, 36] in goats using electrophysiological and anatomical methods. The electrophysiological investigation employed ovariectomized (OVX) goats implanted with bilateral electrodes aimed at KNDy neurons. In this investigation, the onsets of MUA volleys were simultaneously recorded from both the right and left sides of the ARC and analyzed to evaluate whether GnRH pulse generator activity occurs in a synchronized manner between bilateral KNDy neurons using spontaneous, pheromone-induced [37] and senktide-induced [38] MUA volleys. The anatomical investigation was applied in castrated male goats with BDA injection aimed at the caudal portion of the ARC, where the cluster of KNDy neurons is located and from which previous studies have successfully recorded the MUA volley in this species [11, 18]. The goal of the anatomical investigation was to determine the distribution of fibers containing BDA/NKB or BDA/kisspeptin by using dual-labeling histochemistry.
Materials and Methods
Animals
Four female and 8 male adult (4–8 years) Shiba goats weighing 20.0–28.0 kg were used. Goats underwent gonadectomy at least 6 months prior to beginning any experiment. Animals were maintained with a standard pelleted diet, dry hay, and had free access to water and supplemental minerals. All experimental procedures were approved by the Committee on the Care and Use of Experimental Animals at the National Institute of Agrobiological Sciences.
Surgery and MUA recording
Ovariectomized female goats were stereotaxically implanted with an array of bilateral recording electrodes aimed at the caudal region of the ARC where KNDy neurons are concentrated using the method described in previous studies [11, 18], in which the tip of the electrode was confirmed to be located in close vicinity to or among the cluster of KNDy neurons by histological observation. MUA was measured as previously described [11], with the exception of the right- and left-side electrodes being connected to 2 independent input boxes to facilitate separate analysis of bilateral MUA signals. After a recovery period of approximately 4 weeks, the goats were loosely held in individual stanchions in a condition-controlled room (12 L:12 D, 23 C and 50% relative humidity). The MUA signals were recorded simultaneously from the bilateral electrodes and stored as counts per sec on a personal computer. Previous studies using this method have demonstrated an invariable association between each MUA volley and an LH pulse in the plasma [11, 18, 38]. Therefore, characteristic increases in MUA (MUA volleys) were considered electrophysiological manifestations of the GnRH pulse generator.
Analysis of spontaneous MUA volley onset
MUA was recorded for 6 h in 4 OVX goats. The MUA signals were initially analyzed as counts/20 sec in order to assess the gross appearance of MUA during the observation period. Subsequently, each MUA volley was analyzed as counts/sec, and the onset of each MUA volley was determined at both the left and right sides of the ARC.
Effects of pheromone exposure and senktide administration on MUA volley onset
Prior to the start of the experiment, MUA was recorded for at least 2 h, and the mean inter-volley interval during this period (T) was calculated on each experimental day in each goat. A hair sample collected from a mature male goat was used as the pheromone source. Ovariectomized goats (n=2) were exposed to the pheromone source, according to previously described methods [37]. Briefly, goats were exposed for 1 sec at a timing of 3/4 T after a spontaneously occurring MUA volley. In addition, senktide, a selective NK3R agonist (Sigma-Aldrich, St. Louis, MO, USA), was dissolved in saline at a concentration of 15 nmol/ml. The senktide solution (2 ml) was intravenously (iv) administered to the OVX goats (n=2) through a jugular catheter at a timing of 1/2 T after a spontaneously occurring MUA volley, as previously described [38]. It has been confirmed in previous studies that each MUA volley induced by either exposure to a pheromone or peripheral administration of senktide (30 nmol) was invariably accompanied by pulsatile LH secretion [37, 38].
Determination of MUA volley onset
For the analysis of spontaneously occurring MUA volleys, the mean value and standard deviation (SD) of MUA counts/sec during a 60-sec period at the mid-point of two successive MUA volleys were calculated for both the left and right sides. The onset of the next volley was designated as the first time point at which the counts exceeded twice the SD.
For pheromone- and senktide-induced MUA volleys, the mean value and SD were calculated during a 60-sec period beginning immediately prior to pheromone exposure or senktide injection. Volley onset was determined in the same manner as for spontaneously occurring volleys.
Anterograde tracer injection to the ARC
Castrated male goats (n=6) were used for this experiment. As previously mentioned, the cluster of KNDy neurons is located in the caudal portion of the ARC. Tracer injection was aimed towards this population of neurons by referring to radio-ventriculographs. A 23-gauge stainless-steel guide cannula was placed unilaterally 4 mm dorsal to the caudal ARC. A 30-gauge injector, 4 mm longer than the guide cannula, was then inserted, and 10% BDA (MW=10,000, Life Technologies, Carlsbad, CA, USA) in 10 mM phosphate buffer (PB) was injected at a rate of 10 nl/min for 10 min using a microinjection pump (model ESP-32, Eicom, Kyoto, Japan). The injector remained in place for an additional 15 min following completion of the injection. The amount of BDA used for injection was determined based on previous studies performed in sheep [39, 40].
A survival period of 7 days after injection was chosen based on a previous study performed in rats [36]. After the survival period of 7 days, the goats were sacrificed using an overdose of sodium pentobarbital, and their heads were perfused bilaterally with 4 l of 10 mM PB (pH 7.4) containing 0.9% sodium chloride, 3000 U heparin/l and 0.7% sodium nitrate, followed by perfusion of 0.1 M PB containing 4% paraformaldehyde. A brain block containing the ARC was immersed in the same fixative overnight at 4 C, followed by immersion in 20% sucrose in 0.1 M PB until the block sank.
Histochemical detection of BDA
The brain blocks containing the ARC were sectioned, and every sixth section was used for BDA histochemistry in order to examine the injection site and the distribution of BDA-positive fibers in the ARC and adjacent areas. Free-floating sections were washed with 50 mM PB containing 0.9% sodium chloride and 0.3% Triton X-100 (PBST) and treated with 3% H2O2 in methanol for 30 min. After extensive washing with PBST, sections were incubated with the avidin-biotin complex (15 µl each/ml PBST; Elite ABC Kit, Vector Laboratories, Burlingame, CA, USA) for 1 h. Sections were then washed with PBST and immersed in 50 mM Tris-HCl (pH 7.6), followed by reaction with the chromogen solution consisting of 0.2% ammonium nickel (II) sulfate, hexahydrate, 0.04% 3,3′-diaminobenzidine (DAB) and 0.0026% H2O2 in Tris buffer for 5 min.
Dual-labeling histochemistry for BDA and NKB or kisspeptin
To detect BDA fibers containing either NKB or kisspeptin, fluorescence immunohistochemistry for NKB or kisspeptin was followed by fluorescence histochemistry for BDA with amplification using a tyramide amplification biotin kit (TSA Biotin, PerkinElmer, Waltham, MA, USA). After treatment with 3% H2O2 in methanol and washing with PBST, sections were sequentially incubated with the following: 10% normal goat serum (NGS) in PBST containing 1% BSA and 0.05% sodium azide (PBST-BSA) for 1 h, the primary antibody (either anti-NKB polyclonal antibody, 1:4,000, Peninsula Laboratories/Bachem, San Carlos, CA, USA, or anti-kisspeptin monoclonal antibody, 1:4,000, Takeda no. 245) in PBST-BSA containing 2% NGS for 48 h at 4 C and the secondary antibody (either NKB, Alexa 555 conjugated anti-rabbit IgG, 1:200, Life Technologies, or kisspeptin, Alexa 555 conjugated anti-mouse IgG, 1:200, Life Technologies) for 3 h. Sections were then sequentially incubated with the avidin-biotin complex (4 µl each/ml PBST) for 3 h, the TSA blocking solution for 30 min, TSA-biotin (1:200) for 5 min and streptavidin-Alexa 488 (1:200, Life Technologies) in PBST overnight at 4 C. The incubation steps for the primary and secondary antibodies, the avidin-biotin complex, TSA and streptavidin-Alexa 488 were followed by three 15-min washes with PBST. Incubations were performed at room temperature, unless otherwise specified. Specificities of the anti-NKB and anti-kisspeptin antibodies in goat tissues have been confirmed elsewhere [11, 18].
Sections were observed under a fluorescent microscope (Eclipse E800M, Nikon, Tokyo, Japan) equipped with a CCD camera (AxioCam HRc, Carl Zeiss, Jena, Germany), and the 2 fluorescent images were merged with the aid of computer software (AxioVision, Carl Zeiss). Cells and fibers were considered dual labeled for either BDA/NKB or BDA/kisspeptin when 2 positive signals overlapped in the same focal plane. Some sections were further analyzed using a confocal microscope (LSM780, ×20 and ×40 apochromatic lens, Carl Zeiss) with sequential imaging of the two channels. Photomicrographs were taken at the same focal plane (2 µm thickness).
Some sections containing the caudal portion of the ARC were prepared in castrated male goats (n=2) without BDA injection, and were subjected to NKB immunohistochemistry using the NKB antibody (1: 8,000) and the ABC kit. Positive signals were visualized with DAB as described [11].
Results
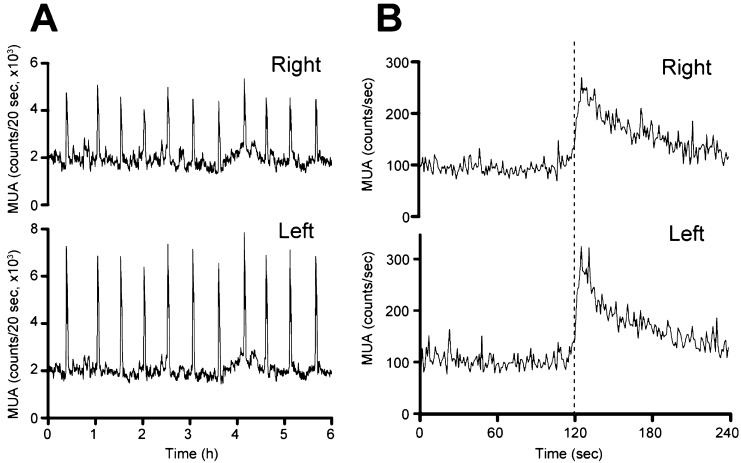
Figure 1 shows the MUA signals recorded simultaneously from bilateral electrodes. Spontaneous MUA volleys (counts/20 sec) occurred with an almost constant 30-min interval and with similar timing on the right (Fig. 1A, upper panel) and left (Fig. 1A, lower panel) sides of the ARC. More detailed MUA analysis (counts/sec) revealed that the onset of each MUA volley occurred simultaneously on the right (Fig. 1B, upper panel) and left (Fig. 1B, lower panel) sides of the ARC. All spontaneously occurring MUA volleys during the 6-h observation period in the 4 OVX goats followed this pattern. Of 35 volleys analyzed according to the criteria, the onsets of bilateral MUA volleys were exactly coincident in 23 and different by 1 sec in 5, by 2 sec in 5 and by 4 sec in 2.
Fig. 1.
Representative profiles of MUA in the OVX goat. A: MUA signals (counts/20 sec) simultaneously recorded (for 6 h) from the right (upper panel) and left (lower panel) sides of the ARC. B: Detailed MUA signals (counts/sec; upper panel, right side; lower panel, left side) measured around one spontaneously occurring MUA volley. The dotted line indicates the onset of the MUA volley.
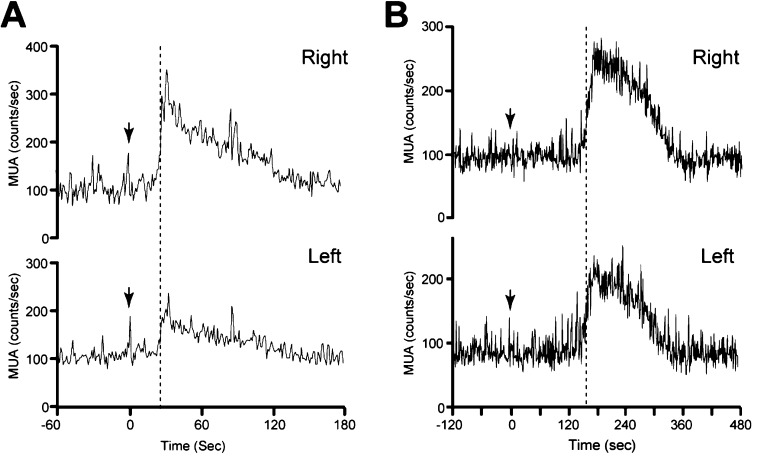
Exposure of OVX goats to the male goat hair (pheromone) induced an MUA volley with a brief time lag between pheromone exposure and volley onset, as previously described [37]. In the representative goat, the MUA volley onset occurred 29 sec after pheromone exposure in both the right (Fig. 2A, upper panel) and left (Fig. 2A, lower panel) sides of the ARC. In the other goat, MUA volley onset occurred 8 sec after pheromone exposure and was again bilaterally synchronized (data not shown). Similarly, intravenous senktide injection resulted in the occurrence of an MUA volley between 2 spontaneous volleys, as previously demonstrated [38]. In the representative goat, the MUA volley onset occurred 154 sec after senktide administration in both the right (Fig. 2B, upper panel) and left (Fig. 2B, lower panel) sides of the ARC. In the other goat, simultaneous bilateral MUA volley onset occurred 131 sec after senktide administration (data not shown).
Fig. 2.
Effect of pheromone exposure and senktide injection on the onset of bilateral MUA volleys. A: An OVX goat was exposed to male goat hair (pheromone) at the time indicated by the arrow. The MUA signals (counts/sec) were simultaneously recorded from the right (upper panel) and left (lower panel) sides of the ARC. B: An OVX goat received a bolus senktide injection (30 nmol/2 ml) at the time indicated by the arrow. MUA signals (counts/sec) simultaneously recorded from the right (upper panel) and left (lower panel) sides of the ARC are presented. The dotted lines indicate the onset of the MUA volleys.
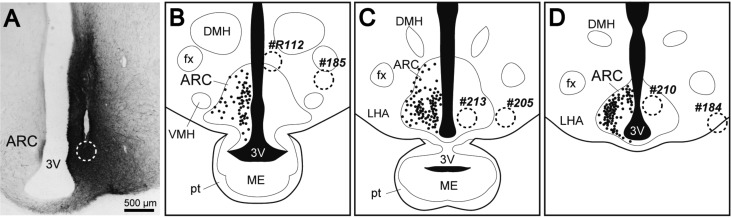
An anterograde tract-tracing study was performed in 6 goats to assess the neuronal circuitry among KNDy neurons. Figure 3A shows a photomicrograph of BDA histochemistry in one representative goat (#210). The injection site can be identified by the location of densely packed BDA-positive products (Fig. 3A, a dotted circle). The injection sites of all goats are schematically indicated in Fig. 3B–D. It was confirmed that the injection site was located among the cluster of KNDy neurons in 2 goats (#210 and #213), at the dorsal edge of the population of KNDy neurons in #R112 and laterally outside but adjacent to the population of KNDy neurons in #205. In the other 2 goats (#184, #185), the injection site was observed in the lateral hypothalamic area and was located at positions more than 500 µm lateral to the edge of the population of KNDy neurons. There were numerous BDA-containing cells around the injection site. BDA-positive neurons were observed approximately 1.2–1.5 mm distal to the injection site. BDA-positive fibers projected dorsally to the thalamic areas, laterally to the zona incerta through the lateral hypothalamus and ventrally to the median eminence (ME). In addition, BDA-positive fibers extended to the hypothalamic areas contralateral to the injection site through the internal layer of the ME and an area under the third ventricle (Fig. 3A). This area is considered to be a ventral part of the caudal ARC (between the caudal edge of the ME and the rostral edge of the mammillary body) in goats (Fig. 3C and D) [41]. While the projection pattern of BDA-positive fibers was similar among all 6 goats, the number of these fibers projecting to the ME and contralateral hypothalamus was correlated with the distance between the ARC and the injection site; they were abundant in #210 and #213, in which the injection site was located at the center of the ARC, moderate in number in #R112 and #205, in which the injection site was located at the edge of or adjacent to the ARC, and extremely few in number in #184 and #185, in which the injection site was located far from the ARC.
Fig. 3.
BDA injection into the unilateral ARC of castrated male goats. A: Photomicrograph showing BDA histochemistry in a section containing the injection site of a representative goat (#210). The injection site (a dotted circle) can be identified as densely packed BDA-positive products with a track of the injector. B–D: Schematic coronal view illustrations of the caudal portion of the goat ARC. Black dots represent the schematic distribution and relative density of KNDy neurons, but the number of dots does not necessarily correspond to the actual number of KNDy neurons. The BDA injection site of each goat is shown by the dotted line with the goat identification number. Panels B and C are separated by approximately 600 µm, while panels C and D are separated by approximately 300 µm. ARC, arcuate nucleus; DMH, dorsomedial nucleus; LHA, lateral hypothalamic area; VMH, ventromedial nucleus; fx, fornix; ME, median eminence; pt, pars tuberalis; 3V, third ventricle.
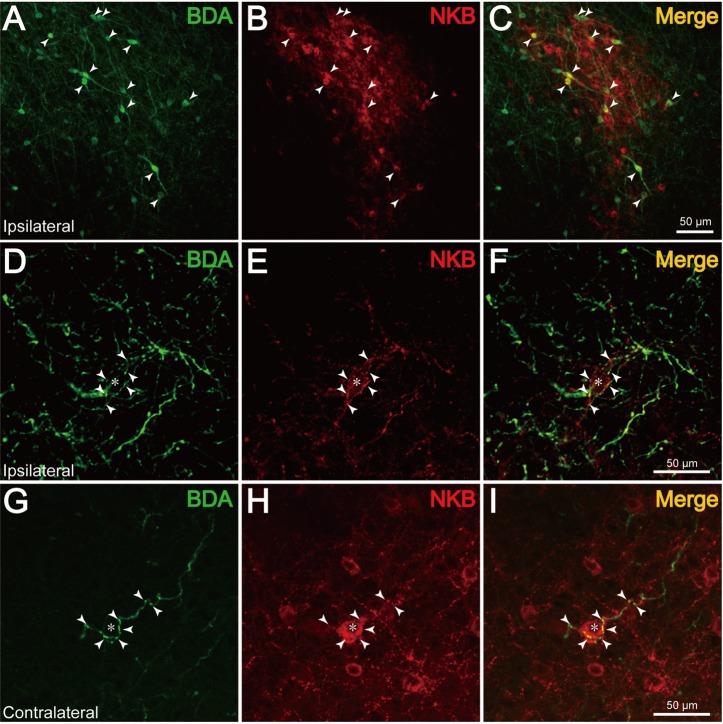
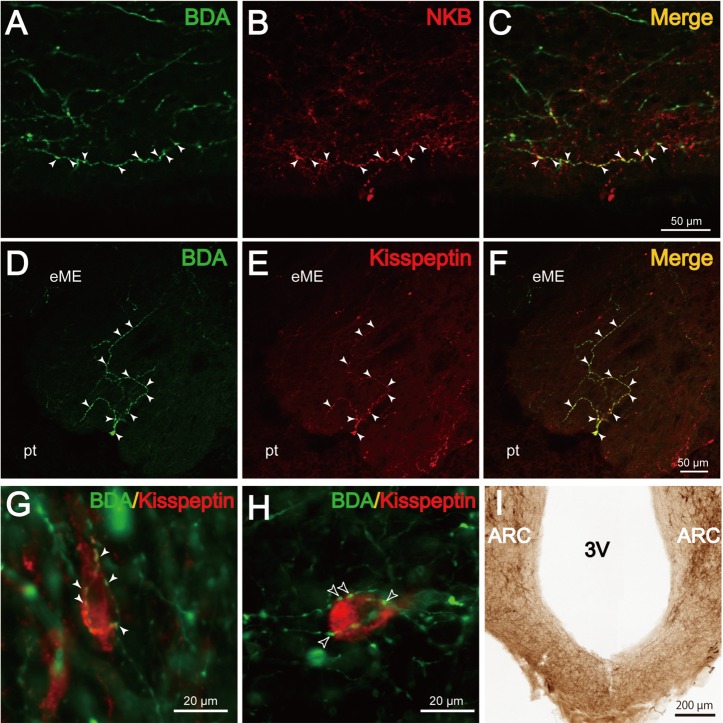
The distribution of NKB-immunoreactive (ir) neurons in the ARC was remarkably abundant in the caudal portion of the ARC, as illustrated in Fig. 3B–D. Dual-labeling histochemistry for BDA and NKB revealed that several NKB-ir neurons also contained BDA-positive products (Fig. 4A–C, arrowheads). The maximum number of BDA/NKB-positive neurons identified in single sections around the injection site was 3 (#R112 and #205) or 15 (#210 and #213). Although numbers of BDA- or NKB-positive neurons were not quantitatively analyzed, it appeared that the ratio of dual-labeled neurons to the entire population of BDA-positive cells was markedly small. Curiously, BDA/NKB-positive fibers extended from the dual-labeled neurons in only a few instances. In 2 goats (#184 and #185), no NKB-ir neurons were BDA positive. In 4 goats (#R112, #205, #210 and #213), a few BDA/NKB-positive fibers were observed in bilateral hypothalamic areas. BDA/NKB-positive fibers had varicosities and were relatively thin compared with fibers containing only BDA-positive products. In the rostral and middle portions of the ipsilateral ARC, some BDA/NKB-positive fibers were found to pass in close apposition to NKB-ir cell bodies. Observation under confocal microscopy revealed that the BDA/NKB-positive fibers in close apposition to NKB-ir cell bodies were located within the same optic plane (Fig. 4D–F, arrowheads). Apposition of BDA/NKB-positive fibers to a few NKB-ir neurons (0–5 neurons/section) in the middle and caudal portions of the contralateral ARC was also identified (Fig. 4G–I). In the caudal portion of the ipsilateral ARC, numerous BDA-positive products can be seen in Fig. 4A, and the densely packed NKB-ir neurons made it difficult to identify BDA/NKB-positive fibers.
Fig. 4.
Co-localization of BDA and NKB in the ARC. A–C (#210): Fluorescence photomicrographs showing dual-label histochemistry for BDA (A) and NKB (B) in a section containing KNDy neurons in the ARC. C: Merged image of A and B. The arrowheads indicate cell bodies containing both BDA- and NKB-positive products. D–I: Confocal microscopic images showing apposition of BDA/NKB fibers (arrowheads) to an NKB-ir neuron (asterisk) in the ARC, ipsilateral (D–F, #205) or contralateral (G–I, #210) to the injection site. F: Merged images of D and E. I: Merged images of G and H.
Some BDA-positive fibers passing in the ventral part of the caudal ARC under the third ventricle (Fig. 5A–C) and internal layer of the ME (data not shown) were found to contain NKB-ir materials. Dual-labeling histochemistry for BDA and kisspeptin showed that BDA/kisspeptin-positive fibers projected to the external layer of the ME (Fig. 5D–F). Under fluorescent microscopy, apposition of BDA/kisspeptin-positive fibers to a few kisspeptin-ir neurons in the bilateral ARC was also identified (Fig. 5G). In the ARC ipsilateral to the injection site, several kisspeptin- (Fig. 5H) or NKB-ir neurons were surrounded by fibers containing exclusively BDA-positive products (Fig. 5H, open arrowheads). By NKB immunohistochemistry, it was shown that abundant NKB-positive fibers are present at the ventral part of the caudal ARC under the third ventricle (Fig. 5I). Occasionally, a few NKB-positive neurons were also observed in this area (see, Fig. 3D).
Fig. 5.
Co-localization of BDA and either NKB or kisspeptin in the ME and ARC. A–C (#210): Confocal microscopic images showing a fiber located in the ventral part of the ARC containing both BDA- (A) and NKB (B)-positive products. C: Merged image of A and B. D–F (#205): Confocal microscopic images showing fibers concomitantly containing BDA (D)- and kisspeptin (E)-positive products in the external layer of the ME (eME). F: Merged image of D and E. Pt indicates the pars tuberalis. G and H (#210): Merged fluorescence photomicrographic images of sections dual labeled for BDA (green) and kisspeptin (red). G: Apposition of BDA/kisspeptin fibers (arrowheads) to a kisspeptin-ir neuron in the ARC contralateral to the injection site. H: Apposition of BDA-positive (kisspeptin negative, open arrowheads) fibers to a kisspeptin-ir neuron in the ARC ipsilateral to the injection site. I: A photomicrograph showing NKB-ir fibers passing through the ventral part of the ARC under the third ventricle (3V) of a castrated male goat.
Discussion
GnRH pulse generator activity, as represented by the MUA volley, has been extensively studied under a variety of experimental conditions in monkeys [4, 5], rats [6, 7], and goats [8, 9]. However, whether simultaneous GnRH pulse generator activity occurs at different locations within the MBH has yet to be fully examined. In the present study, MUA was simultaneously measured from the right and left sides of the ARC, and the MUA signals were analyzed in detail using 1-sec time bins. The results of this study show that the MUA volleys, regardless of origin, i.e., spontaneous (Fig. 1) or experimentally induced (Fig. 2), occur with an exactly identical timing between the right and left sides of the ARC. Previously, Cardenas et al. [4] examined MUA volleys recorded simultaneously from both sides of the MBH in monkeys. Using cluster analysis, they extracted single units from multiple signals and reported that volleys from individual units within the same recording occurred synchronously between implanted ipsilateral or contralateral electrodes in the MBH. Although several methodological differences exist between these 2 studies including use of different animal species (goat vs. monkey), unit analysis (multiple vs. single), time scale of analysis (1-sec vs. 30-sec time bins), and electrode location (the ARC vs. the MBH), the results obtained are consistent between the 2 studies and clearly indicate that GnRH pulse generator activity is synchronized among neurons contributing to generation of the activity.
The results from the current study cannot directly prove that GnRH pulse generator activity (MUA volleys) recorded in the ARC reflect the activity of KNDy neurons. However, the recording electrode was targeted at the caudal portion of the ARC where KNDy neurons are concentrated by the same method as used in previous studies [11, 18], in which the tip of the MUA recording electrode was confirmed to be located in close vicinity to or among the cluster of KNDy neurons by histological observation. Together with the lines of evidence previously discussed (see Introduction), it is suggested that the population of KNDy neurons is the source of the MUA volley, and that bursting activity that governs pulsatile GnRH secretion occurs synchronously among KNDy neurons located in the bilateral ARC.
The anterograde tract-tracing study using BDA was conducted to clarify the neuronal connections among KNDy neurons that would provide a neural framework for electrophysiological synchronization. BDA, which is taken up by cells at the site of injection and transported along their axons, has been used to reveal projections of primary afferents [36, 39, 40, 42, 43]. The pattern of projections of BDA fibers from the unilateral ARC observed in the present study is essentially similar to that reported in sheep [40] and rats [36]. Dual-labeling histochemistry for BDA and NKB revealed that axons projecting from NKB neurons in the ARC were directly apposed to cell bodies of other NKB neurons located in the ipsilateral (Fig. 4D–F) and contralateral (Fig. 4G–I) ARC. These results are similar to those reported in rats [36]. Moreover, we showed in this study that quite abundant NKB fibers pass through the ventral part of the caudal ARC (Fig, 5I), and some of them were dual labeled for BDA/NKB (Fig. 5A–C). It has been shown that NKB cell bodies are distributed almost exclusively within the ARC in the MBH of rats [31] and sheep [44] and that almost all kisspeptin neurons co-express NKB in goats [18]. Therefore, the interactions between NKB fibers and cell bodies demonstrated in this study may represent some, if not all, interconnections among KNDy neurons. It is also suggested that the bilateral communication of KNDy neurons is achieved via the ventral part of the caudal ARC under the third ventricle (present study) and internal layer of the ME [36]. Tract-tracing studies in rats [36] and goats (present study) provide compelling anatomical evidence for the formation of a neural circuit, which has been proposed by several groups to explain synchronization of neural activities among KNDy neurons [16, 22, 24, 25].
Several lines of evidence suggest that NKB directly stimulates neural activity in KNDy neurons. First, a majority of KNDy neurons contain NK3R in mice [16], rats [17, 31], and sheep [45]. Second, intracerebroventricular (icv) administration of senktide induces cFos in KNDy neurons in rats [17] and sheep [46]. Third, NKB elicits trains of action potentials in Kiss1 neurons in the ARC via an NK3R-mediated mechanism in mice [47]. Fourth, bolus icv administration of NKB immediately induces the MUA volley in goats [18]. Taken together, this evidence suggests that NKB may play a role in the synchronization of bursting activity among KNDy neurons at the time of GnRH pulse generation.
Krajewski et al. [36] reported a small number (~4 neurons/rat) of NKB neurons with BDA uptake when BDA was iontophoretically injected into the unilateral ARC of rats. The aim of the present study was to increase the number of BDA/NKB-positive neurons through direct injection of BDA into the caudal portion of the ARC, the location of the cluster of NKB (KNDy) neurons, and through injection of a relatively large amount of the tracer (100 nl of 10% BDA solution). In the cases in which injection was performed at the targeted site (#210 and #213), as many as 15 BDA/NKB neurons were identified in a single section. Nevertheless, fiber-cell apposition representing interconnections between bilateral NKB neurons was observed only in a few instances (0–5/section). There are several possible explanations for this result. Anterograde projections revealed using this method may depend on several factors, including the properties of the neurons of interest, distance from the injection site and the survival period [43]. It is possible that the survival period of 7 days was too short for the tract-tracing study of KNDy neurons in goats. Indeed, BDA/NKB-positive fibers extending from dual-labeled neurons were rarely observed. Alternatively, only a small population of KNDy neurons may send projections to the contralateral side and play a role in bilateral electrophysiological synchronization of KNDy neurons.
Dual-labeling histochemistry for BDA and kisspeptin demonstrated that kisspeptin neurons in the ARC, which may represent KNDy neurons, send axons containing kisspeptin to the external layer of the ME (Fig. 5D–F). Projections from kisspeptin fibers in the ME have been demonstrated in a variety of animals, including rats [48], goats [49], sheep [50] and monkeys [51]. Considering the fact that a majority of kisspeptin fibers in the ME also contain NKB [18, 49, 50], it has been proposed that the kisspeptin fibers in the ME (at least a portion of them) originate from KNDy neurons [24, 25]. Results from the present tract-tracing study strongly support this proposition. Kisspeptin fibers have extensive associations with GnRH axons in the ME [48,49,50,51]. Further, kisspeptin is episodically secreted into the ME, and the kisspeptin pulse is temporally associated with pulsatile GnRH secretion in monkeys [52]. Moreover, direct administration of a kisspeptin antagonist into the ME suppresses pulsatile GnRH release [29]. Therefore, it is likely that kisspeptin plays a role in the transmission of GnRH pulse generator activity, which originates in KNDy neurons, to GnRH axon terminals in the ME.
The current study showed that, similar to NKB (Fig. 4D–I), axons projecting from kisspeptin neurons are in apposition to cell bodies of other bilateral kisspeptin neurons in the ARC (Fig. 5G). Although it is unclear whether kisspeptin and NKB are concomitantly contained in the appositional fibers found on kisspeptin or NKB cell bodies, these results raise the possibility that kisspeptin may play a role in the KNDy neuronal circuit. However, peripheral injection of kisspeptin-10 had no effect on either the amplitude or frequency of the MUA volley in rats [53] and goats [11]. Moreover, expression of the kisspeptin receptor was not detected in KNDy neurons in mice [54] and sheep [50]. Therefore, the involvement of kisspeptin in the KNDy neuronal circuit has not yet been definitively proven. In the ARC ipsilateral to the injection site, several kisspeptin (Fig. 5H) or NKB neurons were surrounded by BDA fibers containing neither kisspeptin nor NKB. This suggests that KNDy neurons receive inputs from other neurons in the ARC, such as neuropeptide Y and proopiomelanocortin neurons [55].
In conclusion, the results of this study using an analysis of MUA volleys in close vicinity to KNDy neurons demonstrate that GnRH pulse generator activity occurs synchronously between both sides of the ARC in goats. Furthermore, the current tract-tracing study combined with NKB immunohistochemistry demonstrates that KNDy neurons are both ipsilaterally and contralaterally interconnected in the ARC via NKB-containing fibers. Taken together, these results suggest that KNDy neurons form a neuronal circuit to synchronize bursting activities among KNDy neurons and thereby generate discrete neural signals that govern pulsatile GnRH secretion.
Acknowledgment
This study was supported partly by a Grant-in-Aid for Young Scientists (B) (23780285) from the Japan Society for the Promotion of Science and the Research Program on Innovative Technologies for Animal Breeding, Reproduction, and Vaccine Development (REP2001) of the Ministry of Agriculture, Forestry and Fisheries of Japan. We thank for Dr K Moriya-Ito for taking photographs with a confocal microscope. We also thank Ms Y Sakairi for her technical assistance.
References
- 1.Knobil E. The neuroendocrine control of the menstrual cycle. Recent Prog Horm Res 1980; 36: 53–88 [DOI] [PubMed] [Google Scholar]
- 2.Karsch FJ. The hypothalamus and anterior pituitary gland. In: Austin CR, Short RV (eds.), Reproduction in Mammals: 3 Hormonal Control of Reproduction. 2 ed. Cambridge: Cambridge University Press; 1984: 1–20.
- 3.Lincoln DW, Fraser HM, Lincoln GA, Martin GB, McNeilly AS. Hypothalamic pulse generators. Recent Prog Horm Res 1985; 41: 369–419 [DOI] [PubMed] [Google Scholar]
- 4.Cardenas H, Ordog T, O' Byrne KT, Knobil E. Single unit components of the hypothalamic multiunit electrical activity associated with the central signal generator that directs the pulsatile secretion of gonadotropic hormones. Proc Natl Acad Sci USA 1993; 90: 9630–9634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knobil E. Patterns of hypophysiotropic signals and gonadotropin secretion in the rhesus monkey. Biol Reprod 1981; 24: 44–49 [DOI] [PubMed] [Google Scholar]
- 6.Kinsey-Jones JS, Grachev P, Li XF, Lin YS, Milligan SR, Lightman SL, O' Byrne KT. The inhibitory effects of neurokinin B on GnRH pulse generator frequency in the female rat. Endocrinology 2012; 153: 307–315 [DOI] [PubMed] [Google Scholar]
- 7.Nishihara M, Hiruma H, Kimura F. Interactions between the noradrenergic and opioid peptidergic systems in controlling the electrical activity of luteinizing hormone-releasing hormone pulse generator in ovariectomized rats. Neuroendocrinology 1991; 54: 321–326 [DOI] [PubMed] [Google Scholar]
- 8.Mori Y, Nishihara M, Tanaka T, Shimizu T, Yamaguchi M, Takeuchi Y, Hoshino K. Chronic recording of electrophysiological manifestation of the hypothalamic gonadotropin-releasing hormone pulse generator activity in the goat. Neuroendocrinology 1991; 53: 392–395 [DOI] [PubMed] [Google Scholar]
- 9.Tanaka T, Mori Y, Hoshino K. Long-term recording of hypothalamic GnRH pulse generator activity during programmed administration of progesterone and estradiol in the ovaryectomized goat. J Reprod Dev 1994; 40: 183–188 [Google Scholar]
- 10.Maeda K, Adachi S, Inoue K, Ohkura S, Tsukamura H. Metastin/kisspeptin and control of estrous cycle in rats. Rev Endocr Metab Disord 2007; 8: 21–29 [DOI] [PubMed] [Google Scholar]
- 11.Ohkura S, Takase K, Matsuyama S, Mogi K, Ichimaru T, Wakabayashi Y, Uenoyama Y, Mori Y, Steiner RA, Tsukamura H, Maeda K-I, Okamura H. Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J Neuroendocrinol 2009; 21: 813–821 [DOI] [PubMed] [Google Scholar]
- 12.Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev2009; 30: 713–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev 2007; 53: 367–378 [DOI] [PubMed] [Google Scholar]
- 14.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 2006; 147: 5817–5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci Lett 2006; 401: 225–230 [DOI] [PubMed] [Google Scholar]
- 16.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 2009; 29: 11859–11866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, Clifton DK, Tena-Sempere M, Steiner RA. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab 2011; 300: E202–E210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K-I, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci 2010; 30: 3124–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology 2007; 148: 5752–5760 [DOI] [PubMed] [Google Scholar]
- 20.Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology 2010; 151: 4494–4503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O' Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet 2009; 41: 354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rance NE, Krajewski SJ, Smith MA, Cholanian M, Dacks PA. Neurokinin B and the hypothalamic regulation of reproduction. Brain Res 2010; 1364: 116–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology 2004; 145: 2959–2967 [DOI] [PubMed] [Google Scholar]
- 24.Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 2010; 151: 3479–3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maeda K, Ohkura S, Uenoyama Y, Wakabayashi Y, Oka Y, Tsukamura H, Okamura H. Neurobiological mechanisms underlying GnRH pulse generation by the hypothalamus. Brain Res 2010; 1364: 103–115 [DOI] [PubMed] [Google Scholar]
- 26.Ohkura S, Tsukamura H, Maeda K. Effects of various types of hypothalamic deafferentation on luteinizing hormone pulses in ovaryectomized rats. J Neuroendocrinol 1991; 3: 503–508 [DOI] [PubMed] [Google Scholar]
- 27.Seminara SB. Mechanisms of Disease: the first kiss-a crucial role for kisspeptin-1 and iths receptor, G-protein-coupled receptor 54, in puberty and reproduction. Nat Clin Pract Endocrinol Metab 2006; 2: 328–334 [DOI] [PubMed] [Google Scholar]
- 28.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 2003; 100: 10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa E, Clarke IJ, Steiner RA, Millar RP. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci 2009; 29: 3920–3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noritake K, Matsuoka T, Ohsawa T, Shimomura K, Sanbuissho A, Uenoyama Y, Maeda K, Tsukamura H. Involvement of neurokinin receptors in the control of pulsatile luteinizing hormone secretion in rats. J Reprod Dev 2011; 57: 409–415 [DOI] [PubMed] [Google Scholar]
- 31.Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: Morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol 2006; 498: 712–726 [DOI] [PubMed] [Google Scholar]
- 32.Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN. Colocalization of progesterone receptors in parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinology 2002; 143: 4366–4374 [DOI] [PubMed] [Google Scholar]
- 33.Smith JT, Clay CM, Caraty A, Clarke IJ. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology 2007; 148: 1150–1157 [DOI] [PubMed] [Google Scholar]
- 34.Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of Kiss-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 2005; 146: 2976–2984 [DOI] [PubMed] [Google Scholar]
- 35.Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, Lai J, Ciofi P, McMullen NT, Rance NE. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology 2012; 153: 2800–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience 2010; 166: 680–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murata K, Wakabayashi Y, Sakamoto K, Tanaka T, Takeuchi Y, Mori Y, Okamura H. Effects of brief exposure of male pheromone on multiple-unit activity at close proximity to kisspeptin neurons in the goat arcuate nucleus. J Reprod Dev 2011; 57: 197–202 [DOI] [PubMed] [Google Scholar]
- 38.Wakabayashi Y, Ohkura S, Homma T, Sakamoto K, Murata K, Mori Y, Maeda K-I, Okamura H. Effects of senktide, a neurokinin B receptor agonist, on neural activity of arcuate kisspeptin neurons and luteinizing hormone secretion in the goat. In: Program of the 40th Society for Neuroscience Annual Meeting; 2010; San Diego, USA. Abstract 594.4.
- 39.Pompolo S, Ischenko O, Pereira A, Iqbal J, Clarke IJ. Evidence that projections from the bed nucleus of the stria terminalis and from the lateral and medial regions of the preoptic area provide input to gonadotropin releasing hormone (GNRH) neurons in the female sheep brain. Neuroscience 2005; 132: 421–436 [DOI] [PubMed] [Google Scholar]
- 40.Pompolo S, Rawson JA, Clarke IJ. Projections from the arcuate/ventromedial region of the hypothalamus to the preoptic area and bed nucleus of stria terminalis in the brain of the ewe; lack of direct input to gonadotropin-releasing hormone neurons. Brain Res 2001; 904: 1–12 [DOI] [PubMed] [Google Scholar]
- 41.Zuccolilli GO, Hayashi S, Mori Y. Hypothalamic structures of the goat on stereotaxic coordinates. J Vet Med Sci 1995; 57: 459–467 [DOI] [PubMed] [Google Scholar]
- 42.Reiner A, Veenman CL, Medina L, Jiao Y, Del Mar N, Honig MG. Pathway tracing using biotinylated dextran amines. J Neurosci Methods 2000; 103: 23–37 [DOI] [PubMed] [Google Scholar]
- 43.Novikov LN. Labeling of central projections of primary afferents in adult rats: a comparison between biotinylated dextran amine, neurobiotin and Phaseolus vulgaris-leucoagglutinin. J Neurosci Methods 2001; 112: 145–154 [DOI] [PubMed] [Google Scholar]
- 44.Goubillon ML, Forsdike RA, Robinson JE, Ciofi P, Caraty A, Herbison AE. Identification of neurokinin B-expressing neurons as an highly estrogen-receptive, sexually dimorphic cell group in the ovine arcuate nucleus. Endocrinology 2000; 141: 4218–4225 [DOI] [PubMed] [Google Scholar]
- 45.Amstalden M, Coolen LM, Hemmerle AM, Billings HJ, Connors JM, Goodman RL, Lehman MN. Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: colocalisation in neurokinin B cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurons. J Neuroendocrinol 2010; 22: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakamoto K, Murata K, Wakabayashi Y, Yayou K-I, Ohkura S, Takeuchi Y, Mori Y, Okamura H. Central administration of Neurokinin B activates kisspeptin/NKB neurons in the arcuate nucleus and stimulates luteinizing hormone secretion in ewes during the non-breeding season. J Reprod Dev 2012; 58:700–706 [DOI] [PubMed] [Google Scholar]
- 47.Navarro VM, Gottsch ML, Wu M, Garcia-Galiano D, Hobbs SJ, Bosch MA, Pinilla L, Clifton DK, Dearth A, Ronnekleiv OK, Braun RE, Palmiter RD, Tena-Sempere M, Alreja M, Steiner RA. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology 2011; 152: 4265–4275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uenoyama Y, Inoue N, Pheng V, Homma T, Takase K, Yamada S, Ajiki K, Ichikawa M, Okamura H, Maeda KI, Tsukamura H. Ultrastructural evidence of kisspeptin-gonadotrophin-releasing hormone (GnRH) interaction in the median eminence of female rats: implication of axo-axonal regulation of GnRH release. J Neuroendocrinol 2011; 23: 863–870 [DOI] [PubMed] [Google Scholar]
- 49.Matsuyama S, Ohkura S, Mogi K, Wakabayashi Y, Mori Y, Tsukamura H, Maeda K-I, Ichikawa M, Okamura H. Morphological evidence for direct interaction between kisspeptin and GnRH neurons at the median eminence of the male goat: an immunoelectron microscopic study. Neuroendocrinol 2011; 94: 323–332 [DOI] [PubMed] [Google Scholar]
- 50.Smith JT, Li Q, Yap KS, Shahab M, Roseweir AK, Millar RP, Clarke IJ. Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology 2011; 152: 1001–1012 [DOI] [PubMed] [Google Scholar]
- 51.Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology 2008; 149: 4387–4395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology 2008; 149: 4151–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kinsey-Jones JS, Li XF, Luckman SM, O' Byrne KT. Effects of kisspeptin-10 on the electrophysiological manifestation of gonadotropin-releasing hormone pulse generator activity in the female rat. Endocrinology 2008; 149: 1004–1008 [DOI] [PubMed] [Google Scholar]
- 54.Herbison AE, d' Anglemont de Tassigny X, Doran J, Colledge WH. Distribution and postnatal development of gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology 2010; 151: 312–321 [DOI] [PubMed] [Google Scholar]
- 55.Backholer K, Smith JT, Rao A, Pereira A, Iqbal J, Ogawa S, Li Q, Clarke IJ. Kisspeptin cells in the ewe brain respond to leptin and communicate with neuropeptide Y and proopiomelanocortin cells. Endocrinology 2010; 151: 2233–2243 [DOI] [PubMed] [Google Scholar]