Abstract
Steroid hormones are produced by the porcine uterus. We hypothesized that the uterus in pigs possesses active 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase (3β-HSD) responsible for progesterone and androstenedione production, that uterine steroids may supplement the amount of steroid hormones produced by embryos and corpus luteum and that these steroids are necessary for maintenance of pregnancy. In this study, we examined 1) endometrial and myometrial expression of 3β-HSD mRNA, 2) uterine 3β-HSD protein activity and 3) in vitro production of A4 and P4 by uterine slices harvested from pigs on days 10 to 11, 12 to 13 and 15 to 16 of pregnancy and the estrous cycle. The expression of 3β-HSD and the presence and activity of 3β-HSD protein were different in the endometrium and the myometrium during the examined periods of pregnancy and the estrous cycle. Production of A4 by the endometrium and myometrium was highest on days 12 to 13 of pregnancy and the estrous cycle. Endometrial secretion of P4 did not differ in the course of early pregnancy and on the respective days of the estrous cycle. The gravid myometrium was the highest source of P4 in pregnant pigs on days 12 to 13. The release of P4 by the cyclic myometrium rose during the examined days of the estrous cycle. The steroidogenic activity of the uterus, as described in this study, may support early pregnancy or the luteal phase of the estrous cycle in pigs.
Keywords: Androstenedione, Progesterone, Steroidogenesis, 3β-HSD, Uterus
Steroid hormones act as potent regulators of cyclic changes within the female reproductive system as well as in processes that lead to establishment and maintenance of pregnancy. The patterns of their episodic release are conserved across species with respect to the preovulatory rise in estrogens followed by progesterone (P4) secretion during the luteal phase [1, 2]. Most active steroids are derived from the gonads, adrenal glands and placenta [3]. It has been reported, however, that many other tissues, including nervous [4] and cardiac tissue [5], can synthesize active steroids that act locally in an autocrine and paracrine manner.
Our recent study conducted on a pig model showed that the uterus is a steroidogenic organ that produces androgens and estrogens de novo in both early pregnant and cyclic females [6,7,8]. We have concluded that uterine production of estrogens may supplement the amount of steroid hormones produced by porcine embryos and that uterine steroids may provide, as hypothesized, an alternative signal for pregnancy recognition and maintenance and initiation of implantation [6].
Synthesis of steroids in any tissue requires availability of substrates and activity of enzymes of two major classes–P450 heme-containing proteins and hydroxysteroid dehydrogenases, with 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase (3β-HSD) among the most important [9]. 3β-HSD catalyzes 3β-hydroxysteroid dehydrogenation and Δ5 to Δ4 isomerization of pregnenolone (PREG), 17α-hydroxypregnenolone, dehydroepiandrosterone (DHEA) and androstenediol into progesterone, 17α-hydroxyprogesterone, androstenedione (A4) and testosterone (T), respectively [10]. The presence of 3β-HSD has been reported in the human and murine placenta, skin, breast tissue, adrenal gland, liver, ovary and testis [2, 9, 10]. However, although the inherence of a different dehydrogenase, namely, 15-hydroxyprostaglandin dehydrogenase, in porcine uterine tissues was recently reported [11], there is still a lack of evidence related to the presence of active 3β-HSD in these tissues.
It was determined that A4 is a principal circulating androgen in gilts [12]. Past studies showed that the porcine pregnant endometrium produced higher amounts of A4in vitro than T in response to exogenous P4 [7]. In cyclic pigs around the time of luteolysis, the myometrium contributed mostly to the total secretion of A4 [7]. In response to P4, the porcine endometrium produced higher amounts of A4 compared with estrone (E1) or T on days 14 to 16 of pregnancy [7], and P4 is essential for maintenance of pregnancy in this species [13]. Thus, the presence of 3β-HSD, one of the most important enzymes of steroidogenesis responsible for P4 and A4 synthesis, may be crucial for the control of steroid hormones production in the uterus.
Our working hypothesis was that the presence and activity of 3β-HSD in the endometrium and myometrium creates the potential for synthesis of A4 and P4 in the uterus. We hypothesized that both uterine tissues produce A4 and P4 mainly during early pregnancy in pigs. To prove our hypothesis, we examined the activity of 3β-HSD and in vitro production of A4 and P4 using a model of uterine slices harvested from pregnant pigs on days 10 to 11, 12 to 13 and 15 to 16 of pregnancy. The activity of 3β-HSD and the level of A4 and P4 release in vitro determined in gravid pigs were compared with the levels observed in cyclic females at the respective days of the estrous cycle. The selected days of pregnancy in pigs, i.e., days 10 to 11, 12 to 13 and 15 to 16, are important for 1) migration of the embryos to and within the uterus, 2) maternal recognition of pregnancy and 3) corpus luteum (CL) protection against luteolysis and the onset of implantation, respectively. At approximately day 12 of pregnancy, pigs conceptuses initiate the process of attachment to the uterine luminal surface followed by a rapid morphological rearrangement of the trophoblast [14]. On days 15 to 16 of pregnancy, luteolysis is avoided, and the CL is protected to serve as an important source of P4. Thus, days 10 to 16 of pregnancy in pigs coincide with continued antiluteolysis and implantation, which are critical for the success of pregnancy [15].
In particular, the aim of the current study was to investigate if 1) the 3β-HSD gene and protein are present and active in the porcine endometrium and myometrium harvested during days 10 to 11, 12 to 13 and 15 to 16 of pregnancy and the estrous cycle; 2) endometrial and myometrial A4 and P4 secretion in vitro differs in the course of early pregnancy and the estrous cycle; and 3) A4 and P4 release from the uterus in vitro depends on the type of uterine tissue.
Materials and Methods
Animals and collection of endometrial and myometrial tissue for in vitro incubation
All experiments were approved by the Animal Ethics Committee, University of Warmia and Mazury in Olsztyn, Poland. Postpubertal pigs weighing 90–110 kg were used on days 10 to 11 (n=4), 12 to 13 (n=4) and 15 to 16 (n=4) of early pregnancy or days 10 to 11 (n=4), 12 to 13 (n=4) and 15 to 16 (n=4) of the estrous cycle. Gilts were observed for estrus behavior in the presence of an intact boar. The onset of the second estrus was designated as day 0 of the estrous cycle. Gilts assigned to the early pregnancy group were naturally bred on the second day of estrus. Pregnancy in mated gilts was confirmed by the presence of embryos after flushing each uterine horn with 20 ml of sterile saline. The stage of the estrous cycle was also confirmed by morphological changes of the ovaries and CL quality [16]. After slaughter, uteri were excised, placed in ice-cold sterile PBS supplemented with 100 IU/ml penicillin (Polfa, Tarchomin, Poland) and 100 μg/ml streptomycin (Polfa) and transported to the laboratory on ice within 30 min.
Determination of 3βHSD mRNA expression in porcine uterine tissues
Total RNA was extracted from slices of endometrium and myometrium (n=4 for each examined period) weighing 30 mg using an RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer's protocol. To obtain maximum purity of the RNA sample, DNAse (RNase-Free DNAse Kit, Qiagen) treatment was performed. RNA quality and quantity were determined with spectrophotometry (NanoDrop ND-1000, Thermo Scientific, Wilmington, DE, USA). Total RNA samples were transcribed to cDNA using an Omniscript RT Kit (Qiagen) and a mix of dNTPs and random hexamers as primers. Real-time PCR was performed in duplicate for each sample using a 7300 Real-Time PCR System and SYBR® Green PCR Master Mix (both Life Technologies, Grand Island, NY, USA). The conditions of thermal cycling were: initial denaturation for 10 min at 95 C, denaturation for 15 sec at 95 C, primer annealing for 1 min at 61 C and elongation for 1 min at 72 C followed by dissociation. Specific primers for hydroxy-delta-5-steroid dehydrogenase were designed with the Primer Express 3.0 software (Life Technologies) and their specificities were confirmed by comparison of their sequences with the sequence of HSD3B1 deposited in a database and calculation of the statistical significance of the match using the Basic Local Alignment Search Tool (BLAST). Levels of gene expression were calculated using the ΔΔCt method and normalized using the geometrical means of reference gene expression levels: glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and β-actin (ACTB). The primer sequences are shown in Table 1. Non-template controls were used to confirm amplification specificity for each set of primers (Table 1).
Table 1. Used primers sequences.
| Gene symbol | Primers sequences | Target sequence accession number | Reference |
| HSD3B1 | F: 5'- AGGTTCGCCCGCTCATC-3' | NM_001004049.1 | |
| R: 5'- CTGGGCACCGAGAAATACTTG-3' | |||
| GAPDH | F: 5'- CCTTCATTGACCTCCACTACATGGT-3' | NM_001206359.1 | Bogacka et al. 2006 [17] |
| R: 5'- CCACAACATACGTAGCACCACGAT -3' | |||
| ACTB | F: 5'- GGAGATCGTGCGGGACATCAAG-3' | AJ312193 | Staszkiewicz et al. 2007 [18] |
| R: 5'- GGCGTAGAGGTCCTTCCTGATG-3' |
Sequence analysis
The putative HSD3B1 amplicon was isolated from 2.0% agarose gel (GenElute™ Gel Extraction Kit, Sigma, St. Louis, MO, USA), and the specificity of the product was confirmed by automated sequencing on an Applied Biosystems 3730xl DNA Analyzer (Life Technologies).
3β-HSD histochemical study
Parts of uterine wall cross-sections containing both endometrium and myometrium were dissected, frozen in liquid nitrogen and stored in –80 C until determination of 3β-HSD expression. A day before histochemical staining, uterine tissues were brought to –20 C, incubated for 12 h and then cut at –20 C in a cryostat (Leica, Wetzlar, Germany) into 7-µm-thick slices. Slides were stored at –20 C. Before histochemical reaction, slides were brought to room temperature and then incubated for 3 h with pregnenolone (Sigma) or dehydroepiandrosterone (Sigma) which were use as substrates for 3β-HSD, nitrotetrazolium blue chloride (Sigma) and nicotinamide adenine dinucleotide (Sigma), which revealed the protein as blue granules under an optical microscope. After incubation, the slides were washed in PBS for 5 min and fixed in 4% formalin mix in 0.1 M PBS for 10 min. Subsequently, they were washed in PBS and redistilled water and then dehydrated. After dehydration, preparations were mounted with DPX (Sigma) and examined under a light microscope (Olympus, Tokyo, Japan). The activity of the enzyme expressed as the area of histochemical reaction was estimated by measuring the number of blue-colorized pixels in the field of vision and compared with the number of non-colorized pixels using the Cell^F software (Olympus). The colorized pixels were subtracted by applying a color mask with the specified intensity threshold (the same for each photograph) to the images. The specificity of histochemical staining was tested by incubation of cross-sections in the medium without substrates for 3β-HSD (negative controls).
In vitro incubation of endometrial and myometrial slices
Uterine horns were cut longitudinally, and the endometrium was separated by careful scraping using a scissors. The myometrium was obtained by tearing it off with tweezers. Tissues were cut into small pieces and washed twice with sterile PBS. Individual endometrial and myometrial slices (200–210 mg weight, 3 mm thick) were placed separately in culture vials containing 2 ml of Medium 199 (Sigma) supplemented with 0.1% BSA fraction V (Roth, Karlsruhe, Germany), 20 μg nystatin (Sigma) and 20 μg gentamicin (Krka, Novo Mesto, Slovenia) and then preincubated in vitro in an atmosphere of 95% O2 and 5% CO2 at 37 C for 18 h. After preincubation, the culture medium was replaced with fresh medium, and the slices were incubated in vitro for the next 12 h. After incubation, the culture media were collected and frozen at –20 C until the concentrations of A4 and P4 were determined with radioimmunoassay.
Androstenedione (A4) and progesterone (P4) determination
Concentrations of A4 and P4 were determined by radioimmunoassay according to the method described by Ciereszko [19]. Cross-reactivity of antisera against A4 and P4 has been reported by Szafranska et al. [20]. For the A4 assay, the extraction efficiency was 85.8 ± 0.7%, sensitivity of the assay was 1 pg/ml and the standard curve range was from 1 pg/ml to 500 pg/ml. The intra- and interassay coefficients of variation were 1.2% and 11.6%, respectively. For the P4 assay, the efficiency of extraction was 85.1 ± 0.3%, sensitivity of the assay was 2 pg/ml and the standard curve range was from 1 pg/ml to 1000 pg/ml. The intra- and interassay coefficients of variation were 0.8% and 3.7%, respectively.
Statistical analysis
All data were expressed as the mean ± SEM. The measured areas of histochemical reaction of 3β-HSD and HSD3B1 mRNA expression were compared between different days within one examined period with one-way ANOVA followed by the Fisher's LSD post hoc test. Comparisons between certain days of pregnancy and the estrous cycle were examined with the Student's t-test. Concentrations of A4 and P4 produced by endometrial and myometrial slices incubated in vitro were log-transformed and analyzed with the Student's t-test.
Results
HSD3B1 mRNA expression in porcine uterine tissues
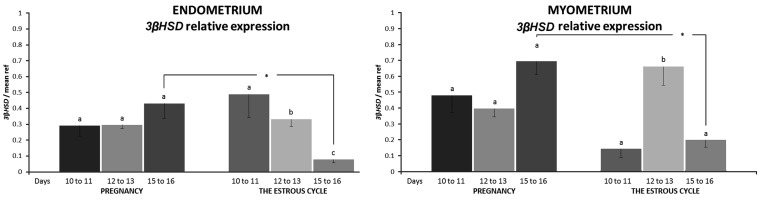
Both endometrial and myometrial expression of HSD3B1 mRNA did not change during the examined days of early pregnancy. The relative transcript abundance in the pregnant endometrium and myometrium harvested on days 15 to 16 was higher (P<0.05) than in tissues harvested from cyclic pigs. During the course of the estrous cycle, the expression level of HSD3B1 in the endometrium was the highest on days 10 to 11 and decreased on days 12 to 13 and days 15 to 16 (P<0.05). The myometrial expression of HSD3B1 mRNA was highest on days 12 to 13 of the estrous cycle (P<0.05) (Fig. 1).
Fig. 1.
The relative expression of the HSD3B1 gene in the endometrium and myometrium harvested from pigs during days 10 to 11, 12 to 13 and 15 to 16 of pregnancy (n=4 in each period) and the estrous cycle (n=4 in each period). The expression levels were normalized with the geometric mean expression of reference genes – GAPDH and ACTB. Data are expressed as the mean ± SEM. Different small letters (a, b, c) indicate statistical differences in HSD3B1 expression in tissues within one examined reproductive state (pregnancy or the estrous cycle; P<0.05). An asterisk (*) indicates statistical differences in HSD3B1 expression in the same tissues during corresponding days of pregnancy and the estrous cycle (* 0.01≤P≤0.05).
Activity and localization of 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase (3β-HSD) conversion of dehydroepiandrosterone into androstenedione
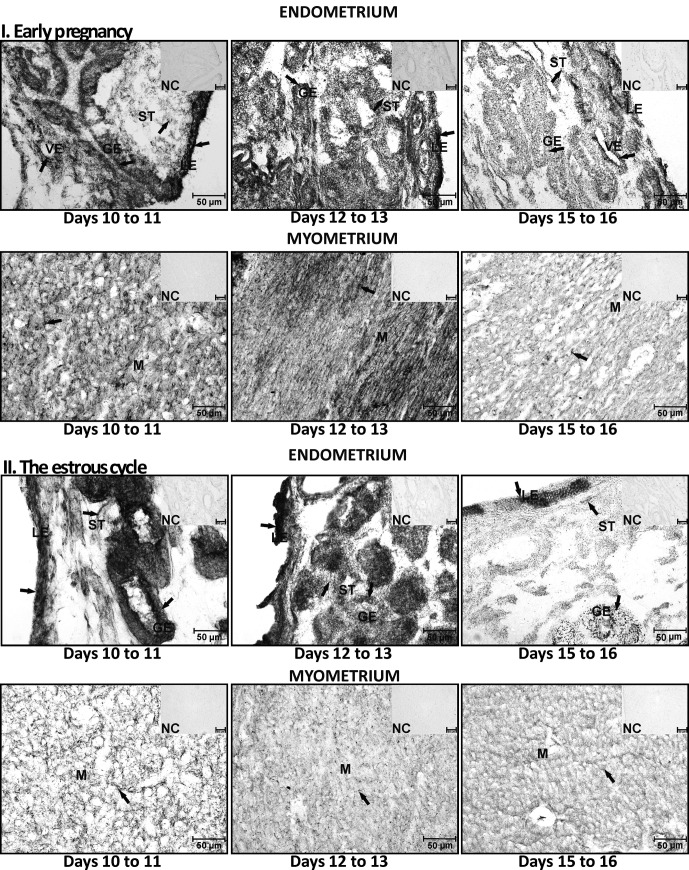
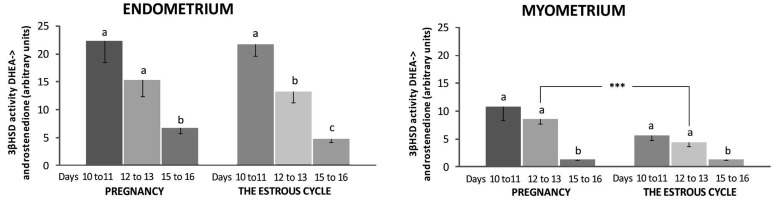
Conversion of DHEA into androstenedione by 3β-HSD was present and active in the porcine uterus harvested during days 10 to 11, 12 to 13 and 15 to 16 of pregnancy and the estrous cycle. The active enzyme was revealed in stromal, glandular and luminal epithelial cells of the endometrium and in myocytes (Fig. 2). The activity of 3β-HSD in conversion of DHEA into androstenedione did not differ in the endometrium harvested from pigs during days 10 to 11 and 12 to 13 of pregnancy (P>0.05) but was statistically higher than in tissues collected on days 15 to 16 (P<0.05) (Fig. 3). During the estrous cycle, the endometrial 3β-HSD activity was higher on days 10 to 11 and decreased on days 12 to 13 (P<0.05) and days 15 to 16 (P<0.05). The myometrial activity of 3β-HSD did not differ on days 10 to 11 and 12 to 13 (P>0.05) and decreased on days 15 to 16 during both pregnancy and the estrous cycle (P<0.05, respectively). The enzyme activity in tissues incubated without a substrate was undetectable (Fig. 2).
Fig. 2.
The localization of 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase (3β-HSD) conversion of DHEA into androstenedione in the porcine endometrium and myometrium collected during days 10 to 11, 12 to 13 and 15 to 16 of pregnancy and the estrous cycle. Exemplary areas of enzyme activity are marked with arrows. GE – glandular epithelium, VE – vascular epithelium, ST – stromal cells, LE – luminal epithelium, M – myometrium, NC – negative control.
Fig. 3.
The activity of 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase (3β-HSD) conversion of DHEA into androstenedione in the porcine endometrium and myometrium collected during days 10 to 11, 12 to 13 and 15 to 16 of pregnancy and the estrous cycle (n=4 in each period). Values are expressed as the mean ± SEM. Different small letters (a, b, c) indicate significant differences in 3β-HSD activity between different days within one examined period (P<0.05). Asterisks (***) indicate statistically significant differences in 3β-HSD activity between the same days of different periods (*** P≤0.001).
Activity and localization of 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase (3β-HSD) conversion of pregnenolone into progesterone
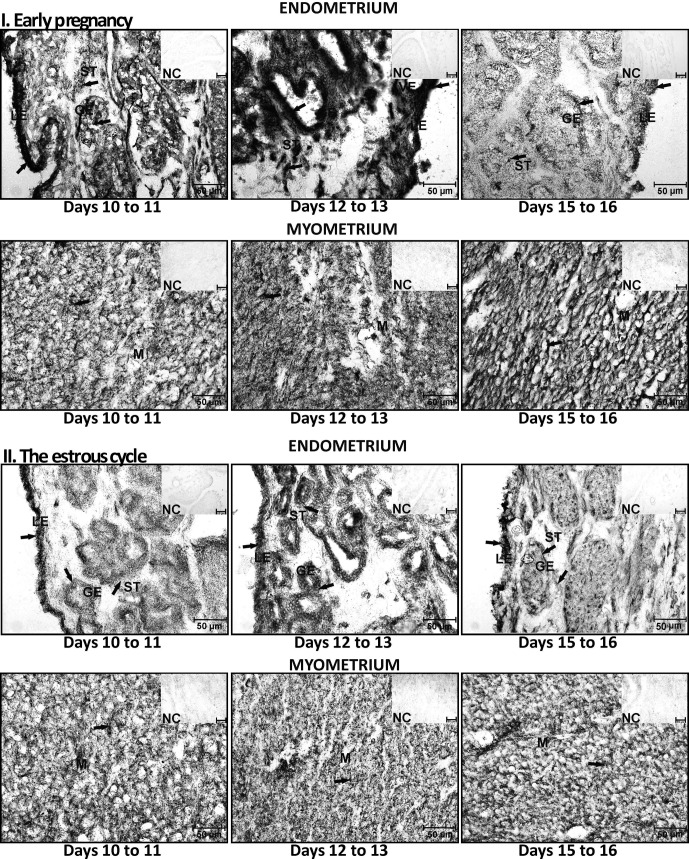
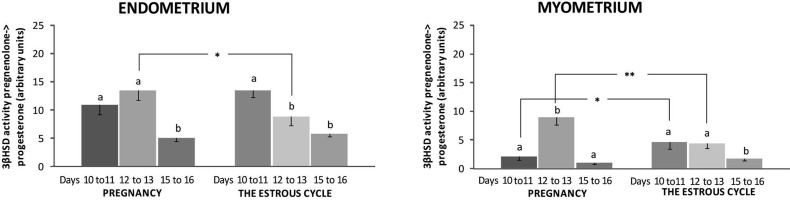
Conversion of PREG into P4 by 3β-HSD was present and active in the porcine uterus harvested during days 10 to 11, 12 to 13 and 15 to 16 of pregnancy and the estrous cycle. Enzyme activity was visualized in stromal, glandular and luminal epithelial cells as well as in myocytes (Fig. 4). Endometrial activity of 3β-HSD did not differ during days 10 to 11 and 12 to 13 and decreased on days 15 to 16 of pregnancy (Fig. 5). During the estrous cycle, the highest activity of 3β-HSD was observed in the endometrium obtained on days 10 to 11 (P<0.05), the activity was decreased on days 12 to 13 and 15 to 16. In tissues harvested on days 12 to 13 and 15 to 16 of the estrous cycle, the activity of the enzyme did not differ (P>0.05). The quantity of active 3β-HSD was higher in the pregnant endometrium than in the cyclic endometrium only on days 12 to 13 (P<0.05). The pregnant myometrium was the highest source of active 3β-HSD on days 12 to 13 when compared with the other examined days of pregnancy (P<0.05). During the estrous cycle, the enzyme activity was lower on days 15 to 16 than on days 10 to 11 and 12 to 13 (P<0.05). On days 10 to 11 of pregnancy, the myometrium expressed lower amounts of 3β-HSD than cyclic tissue harvested on days 10 to 11 of the estrous cycle (P<0.05). On days 12 to 13 of pregnancy, the myometrium expressed higher amounts of active 3β-HSD when compared with the myometrium harvested on days 12 to 13 of the estrous cycle (P<0.05). The enzyme activity in tissues incubated without a substrate was undetectable (Fig. 4).
Fig. 4.
The localization of 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase (3β-HSD) conversion of PREG into progesterone in the porcine endometrium and myometrium collected during days 10 to 11, 12 to 13 and 15 to 16 of pregnancy and the estrous cycle. Exemplary areas of enzyme activity are marked with arrows. GE – glandular epithelium, VE – vascular epithelium, ST – stromal cells, LE – luminal epithelium, M – myometrium, NC – negative control.
Fig. 5.
The activity of 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase (3β-HSD) conversion of PREG into progesterone in the porcine endometrium and myometrium collected during days 10 to 11, 12 to 13 and 15 to 16 of pregnancy and the estrous cycle (n=4 in each period). Values are expressed as the mean ± SEM. Different small letters (a, b) indicate significant differences in 3β-HSD activity between different days within the same examined period (P<0.05). Asterisks (*, **) indicate statistically significant differences in 3β-HSD activity between the same days of different periods (* 0.01≤P≤0.05, ** 0.001<P<0.01).
Endometrial and myometrial in vitro release of A4
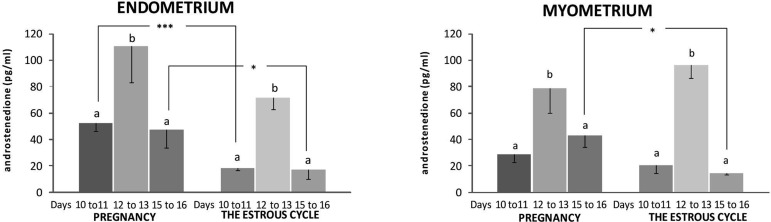
Release of A4 from the endometrium and the myometrium incubated in vitro was statistically higher during days 12 to 13 of both pregnancy and the estrous cycle than during other days of the examined periods (P<0.05) (Fig. 6). Endometrial and myometrial release of A4 on days 10 to 11 did not differ from the release on days 15 to 16 in both pregnant and cyclic tissues (P>0.05). The pregnant endometrium harvested during days 10 to 11 and 15 to 16 was a more than twofold higher source of A4 than the tissue collected on corresponding days of the estrous cycle (P<0.05). The pregnant myometrium obtained on days 15 to 16 released more A4 than that harvested during the estrous cycle (P<0.05).
Fig. 6.
Androstenedione release in vitro by endometrial and myometrial slices harvested from pigs during days 10 to 11, 12 to 13 and 15 to 16 of pregnancy (n=4 in each each period) and the estrous cycle (n=4 in each period) preincubated for 18 h and subsequently incubated for 12 h. Data are expressed as the mean ± SEM. Different small letters (a, b) indicate statistical differences in A4 secretion from tissues within one examined period (P<0.05). Asterisks (*) indicate statistical differences in A4 secretion from the same tissues during corresponding days of pregnancy and the estrous cycle (* 0.01≤P≤ 0.05, *** P≤0.001).
Endometrial and myometrial in vitro release of P4
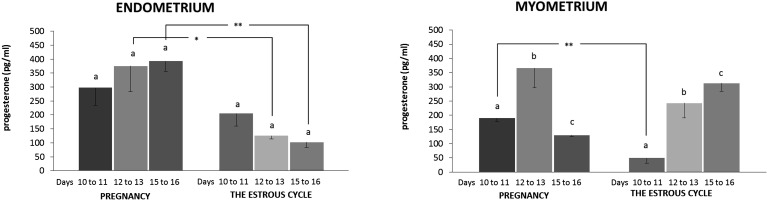
Endometrial release of P4 from tissues obtained from days 10 to 11, 12 to 13 and 15 to 16 did not differ between the studied days in the pregnant and cyclic pigs. During days 12 to 13 and 15 to 16, the gravid endometrium was a higher source of P4 than the endometrium harvested from cyclic pigs (P<0.05) (Fig. 7). The gravid myometrium released more P4 on days 12 to 13 than the myometrium harvested on days 10 to 11 (P<0.05) and 15 to 16 (P<0.05) of pregnancy. Production of P4 by the pregnant myometrium was lower on days 15 to 16 than on days 10 to 11 (P<0.05) and 12 to 13 (P<0.05). During the estrous cycle, myometrial release of P4 consequently increased during days 10 to 11, 12 to 13 (P<0.05) and 15 to 16 (P<0.05). The pregnant myometrium harvested on days 10 to 11 was a greater source of P4 than that harvested on days 10 to 11 of the estrous cycle (P<0.05).
Fig. 7.
Progesterone release in vitro by endometrial and myometrial slices harvested from pigs during days 10 to 11, 12 to 13 and 15 to 16 of pregnancy (n=4 in each period) and the estrous cycle (n=4 in each period). Slices were preincubated for 18 h and subsequently incubated for 12 h. Data are expressed as the mean ± SEM. Different small letters (a, b, c) indicate statistical differences in P4 secretion from tissues within one examined period (P<0.05). Asterisks (**) indicate statistical differences in P4 secretion from the same tissues during corresponding days of pregnancy and the estrous cycle (** 0.001<P<0.01).
Comparison of endometrial and myometrial A4 and P4 release
Endometrial A4 release did not differ from myometrial A4 release during any of the examined days of pregnancy and the estrous cycle (P>0.05). During days 10 to 11 and 15 to 16 of pregnancy, the endometrium released significantly higher amounts of P4 than the myometrium (P<0.05). In the course of the estrous cycle, the myometrial P4 production was greater than the endometrial P4 production on days 12 to 13 and 15 to 16 (P<0.05) (Table 2).
Table 2. The comparison of endometrial and myometrial P4 release.
| M | M | M | ||
| Days 10 to 11 | Days 12 to 13 | Days 15 to 16 | ||
| Pregnancy | E | E > M | - | - |
| Days 10 to 11 | ||||
| E | - | E = M | - | |
| Days 12 to 13 | ||||
| E | - | - | E > M | |
| Days 15 to 16 | ||||
| Estrous cycle | E | E = M | - | - |
| Days 10 to 11 | ||||
| E | - | E < M | - | |
| Days 12 to 13 | ||||
| E | - | - | E < M | |
| Days 15 to 16 | ||||
E – endometrial P4 release, M – myometrial P4 release.
Discussion
In the present study, we showed, for the first time to our knowledge, the expression of HSD3B1 mRNA and the localization and activity of 3β-HSD protein in the porcine uterus and in vitro production of A4 and P4 by uterine tissues harvested from pigs during days 10 to 11, 12 to 13 and 15 to 16 of pregnancy and the estrous cycle. We documented that the activity of 3β-HSDs, both converting DHEA into A4 and PREG into P4, differs in the endometrium and the myometrium during the examined periods of pregnancy and the estrous cycle. We found that endometrial and myometrial production of A4 was the highest on days 12 to 13 of pregnancy and the estrous cycle. Endometrial versus myometrial release of A4 did not differ. Endometrial release of P4 during the studied days of early pregnancy and the estrous cycle did not differ. The gravid myometrium was the highest source of P4 on days 12 to 13 of pregnancy, and the production of P4 by the myometrium of cyclic pigs increased over the course of the studied days of the estrous cycle. The endometrium released higher amounts of P4 on days 10 to 11 and 15 to 16 of pregnancy and lower amounts of P4 on days 12 to 13 and 15 to 16 of the estrous cycle than the myometrium.
Here we demonstrated the expression of a 3β-HSD-encoding gene in the porcine uterine tissues. The HSD3B1 transcript abundance in the endometrium and myometrium remained at the same level during the examined days of pregnancy. The changes in HSD3B1 gene expression levels during the estrous cycle were much more variable than during pregnancy. Interestingly, after luteolysis, both endometrial and myometrial HSD3B1expression wane. Thus, we documented for the first time that the temporal pattern of HSD3B1 gene expression depends on reproductive status of the gilt. Past studies have determined uterine 3β-HSD-encoding gene expression in the rodent [21] and human [22] endometrium.
Our findings showed that the uterus is a steroidogenic organ in which A4 can be synthesized from DHEA and P4 can be synthesized from PREG. We documented that A4 and P4 synthesis in the uterus is catalyzed by active 3β-HSDs enzymes. The presence and variable activity of 3β-HSDs was found in main populations of uterine cells type, i.e., stromal cells, glandular, luminal and endothelial cells and myocytes. We found that the endometrial and myometrial cell-specific localization of 3β-HSD is similar, but that the activity of 3β-HSD in the uterus changed during the course of pregnancy and the estrous cycle. Tissue- and developmentally specific expression of 3β-HSD was previously found in the human and mouse placenta, skin, breast tissue, adrenal gland, ovary and testis [for review, see 9]. The above notions again support our idea concerning the local de novo production of steroids in the porcine uterus, which is conditioned by specific steroidogenic enzymes [6,7,8].
The endometrium and myometrium harvested on days 12 to 13 of both pregnancy and the estrous cycle are high sources of A4. In pregnant pigs, the endometrial and myometrial activity of 3β-HSDs, conversion of DHEA into A4, was increased between days 10 to 13 of pregnancy. Moreover, the gravid endometrium harvested during days 10 to 11 and 15 to 16 was a more than twofold higher source of A4 than the tissue collected on corresponding days of the estrous cycle. The pregnant myometrium obtained on days 15 to 16 released more A4 than harvested during the estrous cycle. Endometrial A4 release during all examined days of pregnancy and the estrous cycle did not differ from the myometrial A4 release. These results suggest that both uterine tissues in pregnant pigs possess a huge potential for A4 production, especially during the critical time of maternal recognition of pregnancy, i.e., days 12 to 13.
Because A4 is converted to estrone (E1) by cytochrome P450 aromatase [23] and high levels of estrogens are present in uterine fluid around day 12 of pregnancy [14], we have proposed that uterine-derived A4 in pigs may serve as a substrate for estrogen production mainly around day 12 of pregnancy. At this time of pregnancy, estrogens play a pivotal role in sustaining CL function mainly by increasing PGE2 to PGF2α [24]. In a previous study, we showed that higher amounts of A4 than T were released from the endometrium in response to exogenous P4 [7]. On the other hand, the A4 secretion pattern in the uterine tissues of cyclic pigs may suggest an important role of A4 mainly on days 12 to 13, e.g., during the mid-luteal phase. Thus, the porcine uterus on days 12 to 13 of both pregnancy and the estrous cycle serve as an important source of A4. We suggest that uterine A4 produced during days 12 to 14 of the estrous cycle may act as a substrate for estrogen synthesis in the uterus. We propose that high production of E1 in the uterus, previously determined on days 14 to 16 in cyclic pigs [7], may be the result of A4 conversion in the uterus.
In pigs, A4 was found to be the principal circulating androgen [12]. Our results showed that the porcine pregnant uterus on days 15 to 16 is a higher source of A4 than the uterus of cyclic pigs. Thus, we confirmed our hypothesis that porcine uterine tissues produce A4 mainly after maternal recognition of pregnancy, e.g., on days 15 to 16. A previous study documented that the Δ5 pathways from pregnenolone to dehydroepiandrosterone and from androstenedione to estrogens are preferred in porcine embryos [25]. We suppose that a similar phenomenon may occur in the porcine endometrium and myometrium.
The results in vivo obtained by Stefanczyk-Krzymowska et al. [26] demonstrated a twofold decrease in the A4 concentration in the uterine artery in pigs after day 12 of pregnancy. Because in our study we also observed decreased release of uterine A4 after days 12 to 13 of pregnancy, we suggest that part of A4, estimated by Stefanczyk-Krzymowska et al. [26], could be of uterine origin. Moreover, we observed that the A4 concentration in culture medium obtained after 12 h of incubation of the pregnant endometrium was about twofold higher when compared with the concentration measured after 6 h of incubation (data not shown). This is evidence that supports our idea concerning active production of A4 in uterine tissues.
In the present study, the release of endometrial P4 did not differ during any examined periods of pregnancy and the estrous cycle. However, the endometrium was a higher source of P4 on days 12 to 13 and 15 to 16 of pregnancy in comparison with corresponding days of the estrous cycle. The gravid myometrium released more P4 on days 12 to 13 of pregnancy than during days 10 to 11 and 15 to 16. It was documented that days 12 to 13 are important for maternal recognition of pregnancy in gravid pigs and should be accompanied by a high level of P4 in uterine fluid [3]. In cyclic pigs, myometrial P4 production increased from days 10 to 11 through to days 15 to 16 of the estrous cycle. In our opinion, this is an intriguing observation that may indicate: 1) more dynamic changes in secretory activity of the myometrium when compared with the endometrium and 2) a more variable pattern of myometrial P4 release than endometrial P4 release in vitro.
We found, for the first time, that the pregnant endometrium releases higher amounts of P4 than the pregnant myometrium before and after maternal recognition of pregnancy, e.g., on days 10 to 11 and 15 to 16. In cyclic pigs, the myometrium was found to be a higher source of P4 than the endometrium on days 12 to 13 and 15 to 16 of the estrous cycle. We showed that the endometrium is a higher source of uterine P4 than the myometrium on days 10 to 11 and 15 to 16 of pregnancy. In cyclic pigs on days 12 to 13 and 15 to 16, the myometrium possesses a dominant role in uterine P4 production. Therefore, a different contribution of the endometrium and myometrium in P4 production was demonstrated.
According to Stefanczyk-Krzymowska et al. [26], no significant decrease was observed in vivo in the P4 concentration in uterine arteries close to the ovary as well as close to the cervix during the course of pregnancy from day 12 until day 35. Interestingly, these authors found that the concentrations of P4 in the uterine artery determined close to the ovary (36.7 ± 1.3 ng/ml) and close to the cervix (31.9 ± 1.0 ng/ml) were significantly higher than the concentration of P4 in peripheral blood measured in the jugular vein (28.4 ± 0.7 ng/ml) in pigs on day 12 of pregnancy. In our study, the higher in vitro endometrial production of P4 in pregnant pigs than in cyclic on days 12 to 13 suggests that the P4 determined in uterine arteries by Stefanczyk-Krzymowska et al. may be derived not only from the ovaries but also from the uterine tissues. Future study should determine if uterine production of P4 depends on the part of the uterine horn.
Based on the current study, we estimated that the total release of P4in vitro within 12 h from both uterine tissues was 2000 pg per gram of uterus at around day 12 of pregnancy. If the endometrium and myometrium together are approximately 600 g [27], about 1.2 µg of P4 will be produced by the uterus in vitro per 12 h, and about 2.4 µg will be produced daily. Thus, our results demonstrated that the porcine endometrium and myometrium are significant sources of P4 during early pregnancy in pigs. We propose that the local production of P4 is important for successful embryo-maternal cross-talk and maintenance of pregnancy.
Considering the role of uterine P4, it cannot be excluded that P4 of myometrial origin plays an important role acting on prostaglandin F2α (PGF2α) production by the uterus around the time of luteolysis. In cyclic pigs, luteolysis occurs around day 15 of the estrous cycle and is caused by PGF2α of endometrial [28] and myometrial [29] origin. It was found previously that the production and release of PGF2α into uterine blood vessels during the estrous cycle is triggered by about eleven days of stimulation of the endometrium by P4 [3]. Uterine P4 acting locally may support the action of ovarian P4 in the uterus by increasing its secretory activity and promoting angiogenesis [30, 31]. It was found that P4 blocks myometrial oxytocin-stimulated prostaglandin (PGs) secretion in pregnant pigs and serves as a factor of the mechanism of corpus luteum protection during pregnancy [32]. Moreover, during early pregnancy, P4 may inhibit the effect of oxytocin on intracellular Ca2+ release [24] and may decrease oxytocin receptor expression [33, 34] and cyclooxygenase-2 activity [35].
In summary, the current study revealed that 1) 3β-HSD mRNA and protein are present and active in the porcine endometrium and myometrium; 2) uterine tissues produce A4 and P4 during both early pregnancy and the estrous cycle and steroid secretion in vitro differs over the course of early pregnancy and the estrous cycle; 3) both porcine uterine tissues release high amounts of A4 during days 12 to 13 of pregnancy and the estrous cycle; 4) production of A4 did not differ between the endometrium and myometrium; 5) the endometrial P4 production rate is stable over the course of early pregnancy and the estrous cycle; 6) the endometrium from day 12 of pregnancy produces more P4 than that harvested during corresponding days of the estrous cycle; 7) myometrial production of P4 increases during days 10 to 16 of the estrous cycle; and 8) endometrial production of P4 in gravid pigs is higher than the myometrial production on days 10 to 11 and 15 to 16 of pregnancy, while that in cyclic pigs is lower between days 12 to 13 and 15 to 16. In conclusion, porcine uterine tissues express active 3β-HSD and therefore possess an ability to synthesize and release A4 and P4, which are important for regulation of early pregnancy and the estrous cycle.
Acknowledgment
The authors would like to thank Mrs J Bukowska and Dr A Zmijewska for their excellent assistance. This work was supported by the National Science Centre, Poland, through grant N N311 526940 (2011 to 2013) and grant N N311 068533 (2007–2010).
References
- 1.Conley AJ, Kaminski MA, Dubowsky SA, Jablonka-Shariff A, Redmer DA, Reynolds LP. Immunohistochemical localization of 3 beta-hydroxysteroid dehydrogenase and P450 17 alpha-hydroxylase during follicular and luteal development in pigs, sheep, and cows. Biol Reprod 1995; 52: 1081–1094 [DOI] [PubMed] [Google Scholar]
- 2.Simard J, Ricketts ML, Gingras S, Soucy P, Feltus FA, Melner MH. Molecular biology of the 3beta-hydroxysteroid dehydrogenase/delta5-delta4 isomerase gene family. Endocr Rev 2005; 26: 525–582 [DOI] [PubMed] [Google Scholar]
- 3.Spencer TE, Johnson GA, Burghardt RC, Bazer FW. Progesterone and placental hormone actions on the uterus: insights from domestic animals. Biol Reprod 2004; 71: 2–10 [DOI] [PubMed] [Google Scholar]
- 4.Baulieu EE. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology 1998; 23: 963–987 [DOI] [PubMed] [Google Scholar]
- 5.Kayes-Wandover KM, White PC. Steroidogenic enzyme gene expression in the human heart. J Clin Endocrinol Metab 2000; 85: 2519–2525 [DOI] [PubMed] [Google Scholar]
- 6.Franczak A, Kotwica G. Secretion of estradiol-17beta by porcine endometrium and myometrium during early pregnancy and luteolysis. Theriogenology 2008; 69: 283–289 [DOI] [PubMed] [Google Scholar]
- 7.Franczak A. Endometrial and myometrial secretion of androgens and estrone during early pregnancy and luteolysis in pigs. Reprod Biol 2008; 8: 213–228 [DOI] [PubMed] [Google Scholar]
- 8.Franczak A, Kotwica G. Androgens and estradiol-17β production by porcine uterine cells: In vitro study. Theriogenology 2010; 73: 232–241 [DOI] [PubMed] [Google Scholar]
- 9.Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev 2004; 25: 947–970 [DOI] [PubMed] [Google Scholar]
- 10.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev 2011; 32: 81–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franczak A, Bogacki M. Local and systemic effects of embryos on uterine tissues during early pregnancy in pigs. J Reprod Dev 2009; 55: 262–272 [DOI] [PubMed] [Google Scholar]
- 12.Simpson ER, Clyne C, Speed C, Rubin G, Bulun S. Tissue-specific estrogen biosynthesis and metabolism. Ann NY Acad Sci 2001; 949: 58–67 [DOI] [PubMed] [Google Scholar]
- 13.Spencer TE, Burghardt RC, Johnson GA, Bazer FW. Conceptus signals for establishment and maintenance of pregnancy. Anim Reprod Sci 2004; 82-83: 537–550 [DOI] [PubMed] [Google Scholar]
- 14.Geisert RD, Renegar RH, Thatcher WW, Roberts RM, Bazer FW. Establishment of pregnancy in the pig: I. Interrelationships between preimplantation development of the pig blastocyst and uterine endometrial secretions. Biol Reprod 1982; 27: 925–939 [DOI] [PubMed] [Google Scholar]
- 15.Bazer FW, Thatcher WW. Theory of maternal recognition of pregnancy in swine based on estrogen controlled endocrine versus exocrine secretion of prostaglandin F2alpha by the uterine endometrium. Prostaglandins 1977; 14: 397–400 [DOI] [PubMed] [Google Scholar]
- 16.Akins EL, Morrissette MC. Gross ovarian changes during estrous cycle of swine. Am J Vet Res 1968; 29: 1953–1957 [PubMed] [Google Scholar]
- 17.Bogacka I, Przała J, Siawrys G, Kaminski T, Smolinska N. The expression of short from of leptin receptor gene during early pregnancy in the pig examined by quantitative real time RT-PCR. J Physiol Pharmacol 2006; 57: 479–489 [PubMed] [Google Scholar]
- 18.Staszkiewicz J, Skowronski MT, Siawrys G, Kaminski T, Krazinski BE, Plonka KJ, Wylot B, Przala J, Okrasa S. Expression of proopiomelanocortin, proenkephalin and prodynorphin genes in porcine luteal cells. Acta Vet Hung 2007; 55: 435–449 [DOI] [PubMed] [Google Scholar]
- 19.Ciereszko R. Radioimmunoassay of steroid hormones in biological fluids. In: Przala J (ed.), Animal Physiology. Demonstrations and Methods. Olsztyn: UWM Press; 2009: 157-163 (In Polish).
- 20.Szafrańska B, Ziecik A, Okrasa S. Primary antisera against selected steroids or proteins and secondary antisera against gamma-globulins –– an available tool for studies of reproductive processes. Reprod Biol 2002; 2: 187–204 [PubMed] [Google Scholar]
- 21.Ben-Zimra M, Koler M, Melamed-Book N, Arensburg J, Payne AH, Orly J. Uterine and placental expression of steroidogenic genes during rodent pregnancy. Mol Cell Endocrinol 2002; 187: 223–231 [DOI] [PubMed] [Google Scholar]
- 22.Rhee HS, Oh SH, Ko BJ, Han DM, Jeon BH, Park H, Moon HB, Kim WS. Expression of 3beta-hydroxysteroid dehydrogenase and P450 side chain cleavage enzyme in the human uterine endometrium. Exp Mol Med 2003; 35: 160–166 [DOI] [PubMed] [Google Scholar]
- 23.Ryan KJ. Biochemistry of aromatase: significance to female reproductive physiology. Cancer Res 1982; 42: 3342s–3344s [PubMed] [Google Scholar]
- 24.Christenson LK, Farley DB, Anderson LH, Ford SP. Luteal maintenance during early pregnancy in the pig: role for prostaglandin E2. Prostaglandins 1994; 47: 61–75 [DOI] [PubMed] [Google Scholar]
- 25.Fischer HE, Bazer FW, Fields MJ. Steroid metabolism by endometrial and conceptus tissues during early pregnancy and pseudopregnancy in gilts. J Reprod Fertil 1985; 75: 69–78 [DOI] [PubMed] [Google Scholar]
- 26.Stefańczyk-Krzymowska S, Grzegorzewski W, Skipor J, Wasowska B, Krzymowski T. Local increase of steroid hormone concentrations in blood supplying the oviduct and uterus during early pregnancy of sow. Theriogenology 1998; 50: 1071–1080 [DOI] [PubMed] [Google Scholar]
- 27.Blackwell DM, Speth RC, Mirando MA. Morphometric analysis of the uterine endometrium of swine on days 12 and 16 postestrus. Anat Rec A Discov Mol Cell Evol Biol 2003; 270: 59–66 [DOI] [PubMed] [Google Scholar]
- 28.Moeljono MP, Thatcher WW, Bazer FW, Frank M, Owens LJ, Wilcox CJ. A study of prostaglandin F2alpha as the luteolysin in swine: II Characterization and comparison of prostaglandin F, estrogens and progestin concentrations in utero-ovarian vein plasma of nonpregnant and pregnant gilts. Prostaglandins 1977; 14: 543–555 [DOI] [PubMed] [Google Scholar]
- 29.Franczak A, Kurowicka B, Oponowicz A, Petroff BK, Kotwica G. The effect of progesterone on oxytocin-stimulated intracellular mobilization of Ca2+ and prostaglandin E2 and F2alpha secretion from porcine myometrial cells. Prostaglandins Other Lipid Mediat 2006; 81: 37–44 [DOI] [PubMed] [Google Scholar]
- 30.Knight JW, Bazer FW, Wallace HD. Effect of progesterone induced increase in uterine secretory activity of development of the porcine conceptus. J Anim Sci 1974; 39: 743–746 [DOI] [PubMed] [Google Scholar]
- 31.Jindal R, Cosgrove JR, Foxcroft GR. Progesterone mediates nutritionally induced effects on embryonic survival in gilts. J Anim Sci 1997; 75: 1063–1070 [DOI] [PubMed] [Google Scholar]
- 32.Franczak A, Kotwica G, Kurowicka B, Oponowicz A, Woclawek-Potocka I, Petroff BK. Expression of enzymes of cyclooxygenase pathway and secretion of prostaglandin E2 and F2α by porcine myometrium during luteolysis and early pregnancy. Theriogenology 2006; 66: 1049–1056 [DOI] [PubMed] [Google Scholar]
- 33.Grazzini E, Guillon G, Mouilac B, Zingg HH. Inhibition of oxytocin receptor function by direct binding of progesterone. Nature 1998; 392: 509–512 [DOI] [PubMed] [Google Scholar]
- 34.Wu WX, Ma XH, Yoshizato T, Shinozuka N, Nathalnielsz PW. Differential expression of myometrial oxytocin receptor and prostaglandin H synthase 2, but not estrogen receptor α and heat shock protein 90 messenger ribonucleic acid in the gravid horn and nongravid horn in sheep during betamethasone-induced labor. Endocrinology 1999; 140: 5712–5718 [DOI] [PubMed] [Google Scholar]
- 35.Doualla-Bell F, Guay JM, Bourgoin S, Fortier MA. Prostaglandin G/H synthase (PGHS)-2 expression in bovine myometrium: influence of steroid hormones and PGHS inhibitors. Biol Reprod 1998; 59: 1433–1438 [DOI] [PubMed] [Google Scholar]