Abstract
The aim of the present study was to describe the temperature of the different portions of the female genital tract and their relation to rectal temperature and to investigate the effect of steroid hormones profiles on these variables over the estrous cycle in cattle. Four nonpregnant Japanese Black cows were investigated daily over two successive estrous cycles using a digital thermometer with a long probe and rounded-end sensor to record the temperature of the rectum (RT), vagina (VT), cervix (CT), uterine body (UBT) and uterine horns (UHT). Blood samples were collected immediately before temperature recording to assay peripheral levels of progesterone (P4) and estradiol-17β (E2). Moreover, transrectal ultrasonography was carried out after temperature recording to monitor the ovulatory follicle and track ovulation. During the experiment, the ambient temperature and relative humidity were recorded for further calculation of the temperature humidity index (THI). The temperature within the genital tracts in these cows progressively increased towards the uterine horns from the vagina. The VT, CT, UBT and UHTs were significantly higher in association with peripheral P4 concentrations greater than 4 ng/ml (mid-luteal phase) when compared with lower peripheral P4 concentrations. The VT was more significantly (P<0.01) correlated to the CT, UBT and UHTs than RT. In conclusion, a temperature gradient was present among the vagina, cervix and uterus over the estrous cycle, and changes in peripheral P4 concentrations were associated with the thermal variations within these portions. The VT could be more beneficial than RT in monitoring temperature of deeper portions of the female genital tract in bovine.
Keywords: Digital thermometer, Estradiol-17β (E2), Estrous cycle, Female genital tract, Progesterone (P4), Temperature gradient
In the female reproductive organs, a temperature gradient has been shown between the isthmus and ampulla of the oviduct of the rabbit [1], and across the porcine ovarian tissues [2]. The establishment of these gradients has been shown to depend on estrous cycle phases; for example, the isthmus is cooler than the ampulla in the rabbit during the preovulatory period, and mature graafian follicles in swine had a lower temperature than the ovarian stroma at the follicular phase [1, 2]. However, studies showing the thermal gradient among the organs of the female genital tract from the vagina to the uterus have not been conducted so far.
Sexual cycle phases affect not only the establishment of temperature gradients within some genital organs, but also body temperature. In humans, basal body temperature (BBT) shows a biphasic pattern during the menstrual cycle [3]. This pattern was related to ovulation, and the temperature increment in the luteal phase was attributed to the thermogenic action of progesterone (P4) or its synergistic action with estrogen [4]. Subsequently, elevated peripheral P4 concentrations stimulate the release of norepinephrine from its storage sites in the sympathetic nervous system, affecting the temperature control center in the hypothalamus, with a consequent elevation in body temperature. This change is reflected by an increase in the oral, axillary, vaginal or rectal temperature [3,4,5,6]. Moreover, the dose-dependent thermogenic action of P4 has been described in laboratory animals [7]. In bovines, rectal (RT) and vaginal temperatures (VT) are associated with each other and could be used to monitor diurnal changes in body temperature with the minimal body temperature in the early morning [8, 9]. As with BBT in humans, once daily or continuous daily recording of VT in dairy cows revealed an elevation (≥0.1 C) of the body temperature during the mid-luteal phase, followed by a decrease 2–3 days before estrus [10,11,12]. This pattern was attributed to the thermogenic action of P4, and the pre-estrous reduction of VT reflected CL regression [13,14,15]. On the other hand, studies in bovine [9], equine [16] and swine [17] based only on monitoring RT did not show such a pattern during the estrous cycle.
It is not known if the temperature of deeper portions of the bovine genital tract is associated with that measured in the rectum and vagina [18, 19]. Moreover, the urgent need for a study investigating the temperature of these portions during the estrous cycle has been raised recently [19].
The current study aimed to describe the temperatures of the different portions of the female genital tract and their relationship to rectal temperature and to investigate the effects of steroid hormone profiles over the estrous cycle on these variables in cattle.
Materials and Methods
Animals and experimental location
Four nonpregnant, multiparous Japanese Black cows were enrolled in the current study. This investigation was carried out from March to May 2011 at the Experimental Farm of the University of Miyazaki, Japan (latitude 31°49'38" N, longitude 131°24'49" E). The animals were fed twice daily at 0800 and 1600 h, and water was available ad libitum. All experimental procedures were approved by the institutional review board for animal experiments of the University of Miyazaki (Approval No. 2009-001-3).
Experimental procedures
Transrectal ultrasonography equipped with a 10 MHz linear array transducer (LOGIQ Book XP, GE Healthcare, Tokyo, Japan) was used to identify the ovarian structures and determine estrous cycles. When transrectal ultrasonography showed that a cow had a mature corpus luteum (CL; ≥20 mm), luteolysis was induced by intramuscular injection of 500 µg prostaglandin F2α (Cloprostenol, Resipron®, Asuka, Tokyo, Japan). Then the animals were investigated daily (between 0630–0800 h; 15–20 min per cow) over two successive estrous cycles. The daily experimental procedures were carried out in the following sequence: blood sampling for the steroid assay and then measurement of the temperatures of the rectum and different portions of the genital tract. Finally, transrectal ultrasonography was used to monitor ovarian structures and track ovulation. The first day on which an ovulatory follicle was not observed was designated as Day 1 (ovulation). During all procedures, the on-site ambient temperature and relative humidity were recorded for further calculation of temperature humidity index (THI).
The temperatures of the rectum (RT), vagina (VT), cervix (CT), uterine body (UBT) and uterine horns (UHT; average of the right and left uterine horn temperatures) were recorded using a digital thermometer (TX10-02, Yokogawa, Tokyo, Japan) attached to a long probe with a rounded-end temperature sensor (length, 500 mm; diameter, 3.2 mm) with a response time of 1.4 sec. Two identical probes were used: one for measuring RT and the other for measuring the temperature of different portions of the genital tract. Briefly, after cleaning and disinfecting the perineum and external genitalia, the disinfected probe was introduced gently into the vagina, guided into the cervix by manipulation via the rectum and then introduced into the uterine body. Then, it was guided into the right and left uterine horns. RT was measured at an insertion depth of approximately 10 cm, while genital tract temperatures were recorded near the middle of the dorsal aspect of each portion to avoid interaction with genital secretions as much as possible.
Ambient air temperature (AT, C) and relative humidity (RH, %) within the experimental location were recorded every 5 min during the examination period using a thermometer/hygrometer (72948 A, Shinwa Rules, Sanjo, Japan), which was secured 1.5 m above the ground and in the shade. The recorded AT and RH were averaged for each cow. THI was calculated according to a previously reported equation [20]: THI = (0.8 × AT) + [(RH/100) × (AT − 14.4)] + 46.4.
Blood sampling
Blood samples were collected via coccygeal venipuncture into heparinized vacutainer tubes (Venoject II, Terumo, Tokyo, Japan) and were immediately centrifuged (3000 rpm × 15 min at 4 C). The plasma was harvested and stored at –30 C until the hormonal assay.
Hormonal assay
Plasma P4 concentrations were measured using an Enzyme-Linked Fluorescent Assay (ELFA) kit (VIDAS® Progesterone, SYSMEX bioMérieux, Tokyo, Japan), which has been validated for bovine plasma [21]. The current assay showed high correlation (r=0.98) to the reference method (radioimmunoassay; RIA). Moreover, the curve resulting from serial dilution of bovine plasma was parallel to the standard curve. The intra- and interassay coefficients of variation for the P4 assays were <4.0% and <8.1%, respectively, according to the validation of the kit [21]. The detection limit of the assay was 0.2 ng/ml (measurement range, 0.25–80 ng/ml; specificity, 100%). Estradiol-17β (E2) was extracted from plasma (800 µl) samples using diethyl ether (3 ml), and substances that interfere with the E2 assay were removed twice using the partition method of 0.5 ml acetonitrile and 2 ml n-hexane as described elsewhere [22, 23]. The E2 concentrations were determined using a 17beta-Estradiol ELISA kit (IBL International GmbH, Hamburg, Germany). The sensitivity and intra- and interassay coefficients of variation for E2 were 9.7 pg/ml, 6.8% and 9.4%, respectively, according to the manufacturer data sheet. E2:P4 ratios were then calculated.
Data classification and statistics
The normal distribution of the data was investigated by the D'Agostino and Pearson omnibus normality test using the GraphPad Prism software for Windows (version 5.0; GraphPad software, San Diego, CA, USA) [24]. Most of the data were normally distributed, with the exception of the endocrinological parameters. Both parametric and nonparametric tests were used for normally and non-normally distributed data, respectively.
Since, each portion of the female genital tract is subjected to the same conditions as the other portions in the same cow at every check, the overall differences between the RT, VT, CT, UBT and UHT were compared using one-way ANOVA followed by a pairwise comparison of means using Tukey's Honestly Significant Difference (HSD) [25]. Moreover, Pearson's correlation coefficient was used to determine the association between these variables. Significant correlation coefficients were interpreted as having marginal (<0.25), moderate (0.25–0.50), good (0.51–0.75) and high correlation (>0.75) [26, 27]. To compare the correlation of RT and VT to CT, UBT and UHT, Steiger's Z-test was carried out using the FZT Computator software, version 1.0 [28, 29]. To study the effect of endogenous P4 concentrations on the different recorded temperatures, the data were classified based on the corresponding plasma P4 levels into the following ranges: P4<1 ng/ml (nonsecretory CL, n=30), P4 between 1–4 ng/ml (evolving and regressing CL, n=40) and P4> 4 ng/ml (mid-cycle CL, n=78) [30, 31]. In this classification, nonparametric Kruskal-Wallis tests were used to compare the steroid hormone profiles in the different peripheral P4 concentrations ranges, while one-way ANOVA was used to compare the thermal changes within each temperature recording site in the different peripheral P4 concentrations ranges. To investigate whether the ovary-bearing CL affects the temperature of the ipsilateral or contralateral uterine horn, an independent t-test was carried out to compare the temperatures of the two horns during the luteal phase. Furthermore, partial and Spearman's correlation coefficients were applied to investigate the relation between steroid profiles and different recorded temperatures [32].
Except for the normality and correlation comparison tests, SPSS for Windows (version 17.0; SPSS, Chicago, IL, USA) was used for statistical analyses. Differences of P<0.05 were considered to be statistically significant. All results are expressed as the mean ± SEM, unless otherwise stated.
Results
Estrous cycles and clinical findings
One cow had developed a luteal cyst during the second estrous cycle; thus, the data for this cycle was excluded. In total, seven estrous cycles were investigated using the four Japanese Black cows. The mean cycle length was 21.1 ± 1.1 days (mean ± SD). Moreover, during the entire experimental period, no genital tract abnormalities were recorded.
THI
The average THI recorded during the experimental period was 56.39 ± 5.32 (mean ± SD). THI did not show any significant differences with regard to the different peripheral P4 concentrations ranges.
The temperature of the rectum and different portions of the genital tract
The genital tract temperature increased progressively toward the uterine horns over the estrous cycle. UHT was significantly higher than VT (P<0.001) and RT (P<0.05). Moreover, RT was significantly (P<0.001) higher than VT (Fig. 1). There was a strong correlation (r=0.79; P<0.001) between RT and VT. Both RT and VT were positively correlated with the different genital temperatures (Table 1); however, VT was significantly more correlated to CT (z=7.74, P<0.01), UBT (z=3.54, P<0.01) and UHT (z=2.67, P<0.01) in comparison with RT correlations.
Fig. 1.
Presence of a temperature gradient between different portions of the female genital tract and the difference between the RT and temperature of each portion of the female genital tract. Different letters indicate significant differences between the different portions of the genital tract (bc–c, P=0.05; a–b & b–c, P<0.01; a–c, P<0.001). Asterisks indicate significant differences between the RT and temperatures of other portions of the genital tract (*P<0.05; **P<0.001). The different recording sites for temperatures are shown as RT for rectal temperature, VT for vaginal temperature, CT for cervical temperature, UBT for uterine body temperature and UHT for uterine horn temperature. The data are represented as means ± SEM of the temperatures throughout two successive estrous cycles (n=148 for each variable).
Table 1. Pearson's correlation coefficients among the temperature humidity index and temperatures recorded in the rectum and different portions of the reproductive tract over the estrous cycle in beef cows (n=148 paired measurements).
| Parameters | THI | RT | VT | CT | UBT | UHT |
| THI | 1 | 0.38* | 0.43* | 0.42* | 0.43* | 0.42* |
| RT | 1 | 0.79* | 0.76* | 0.68* | 0.60* | |
| VT | 1 | 0.93* | 0.80* | 0.70* | ||
| CT | 1 | 0.91* | 0.82* | |||
| UBT | 1 | 0.93* | ||||
| UHT | 1 |
THI, temperature humidity index; RT, rectal temperature; VT, vaginal temperature; CT, cervical temperature; UBT, uterine body temperature; UHT, uterine horns temperature. * P<0.001.
Effect of steroid hormone profiles on the temperature of the rectum and different portions of the genital tract over the estrous cycle
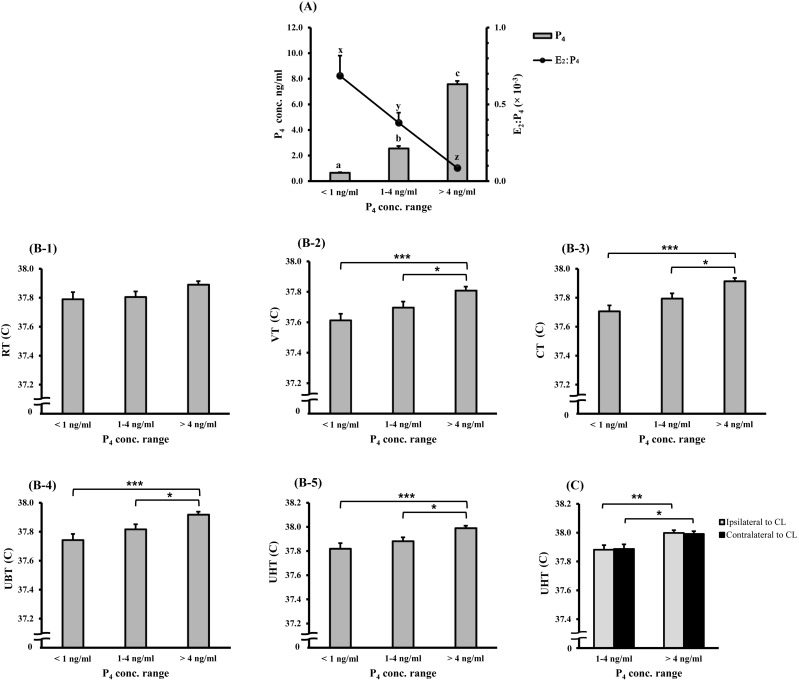
Regarding to the data classification based on the ranges of peripheral P4 concentrations, the corresponding E2:P4 ratios and P4 concentrations are shown in Fig. 2-A. Notably, each portion of the female genital tract exhibited a significant increase in its temperature when the peripheral P4 concentrations was more than 4 ng/ml (mid-cycle CL) in comparison with its temperatures at lower peripheral P4 concentrations (Fig. 2-B2 to B5). On the other hand, the RT did not show any significant difference among the different peripheral P4 concentrations ranges (Fig. 2-B1). There was no difference between the uterine horns ipsilateral and contralateral to the ovary-bearing CL, and the temperatures of both horns increased significantly when the peripheral P4 level was greater than 4 ng/ml in comparison with their temperatures in the range of 1–4 ng/ml (Fig. 2-C). Peripheral P4 concentrations showed a positive correlation with rectal and genital tract temperatures (Table 2). On the other hand, the E2:P4 ratio had exerted a negative correlation with these temperature variables. However, the correlation of these endocrinological parameters with RT was marginal.
Fig. 2.
Effect of different peripheral concentration ranges of P4 on the different recorded temperatures: A) The P4 and E2:P4 values at different P4 concentration ranges; different letters (a–c and x–z) indicate significant differences at P<0.001 for P4 concentrations and E2:P4 ratios, respectively. B) Changes in the RT, VT, CT, UBT and UHT with different concentration ranges of endogenous P4. C) Changes in the temperatures of the uterine horns ipsilateral and contralateral to an ovary-bearing CL. Asterisks indicate significant differences between variables (*P<0.05; **P<0.01; ***P<0.001). The different recording sites for temperatures are shown as RT for rectal temperature, VT for vaginal temperature, CT for cervical temperature, UBT for uterine body temperature and UHT for uterine horn temperature. The data are represented as means ± SEM.
Table 2. Correlation coefficients among steroidal parameters and temperatures recorded in the rectum and different organs of the reproductive tract over the estrous cycle in beef cows (n=148 paired measurements).
| Parameters | RT | VT | CT | UBT | UHT |
| P4 (a) | 0.19* | 0.27** | 0.31*** | 0.29*** | 0.27** |
| E2 (b) | –0.07 | –0.07 | –0.009 | 0.003 | 0.003 |
| E2:P4 (c) | –0.19* | –0.29*** | –0.32*** | –0.28*** | –0.25** |
P4, Progesterone; E2, Estradiol-17β ; RT, rectal temperature; VT, vaginal temperature; CT, cervical temperature; UBT, uterine body temperature; UHT, uterine horns temperature. (a) Partial correlation coefficient between peripheral P4 concentrations and the different recorded temperatures, controlling for the effect of E2. (b) Partial correlation coefficient between peripheral E2 concentrations and the different recorded temperatures, controlling for the effect of P4. (c) Spearman's correlation between peripheral E2:P4 ratios and the different recorded temperatures. * P<0.05; ** P<0.01; *** P<0.001.
Discussion
To the best of our knowledge, there is no report describing the temperature of deeper portions of the female genital tract in relation to the bovine estrous cycle. In humans, uterine temperature measurement is restricted to patients who are in labor, when the cervix is dilated and the uterine lumen is accessible [33]; however, under such conditions, the recorded uterine temperature is affected by fetal temperature, amniotic fluid and uterine contraction [33, 34]. In the current study, by using a digital thermometer with a long probe guided by recto-cervical manipulation in the bovine model, we were able to monitor the temperature of the deeper portions of the female genital tract. In dairy cows, a similar technique using a thermistor fixed to a probe has been used to record the uterine temperature in comparison with RT before and after insemination; this tool was used to study the effect of different THIs on conception rate [35]. It is notable that recto-cervical manipulation is used in a wide range of reproductive technologies in bovines, including artificial insemination, embryo collection and transfer and endometrial biopsy [36]. Thus, the temperature recording technique stated in the current article could be applicable for future research concerning the temperature of the deeper portions of the female genital tract in bovine.
The presence of a thermal gradient with increasing temperature from the vagina towards the uterine horns shown here could be explained on the anatomical basis that the deeper portions of the female genitalia are nearer to the warmer body core. This thermal gradient could affect gene expression in the spermatozoa after insemination and/or different genital tract tissues [18]. Moreover, these findings may change the concept of sperm thermotaxis being limited to the fallopian tube [37].
The RT measurement could be affected by the thermometer type and depth of penetration in the rectum [38]. VT recording is likely influenced by the measurement method, urination and air influx into the vagina [12]. In the current study, both RT and VT were measured using the same digital thermometer. Our results showed that RT was significantly higher than VT. In agreement with our findings, Lea et al. [39], Hillman et al. [40] and Burdick et al. [41] used identical or calibrated thermometers for recording both RT and VT in cattle and also reported that RT was higher than VT. In contrast to our results, a study on beef cows used a digital thermometer for RT recording and data loggers attached to intravaginal device for VT monitoring and found that RT was lower than VT in both winter and summer [42]. It is worth saying that the accuracy of digital thermometers is almost ± 0.1 C, while that of temperature loggers is ± 0.3 C [8]. Thus, it is recommended to use a single type of thermometer or temperature logger in studies concerning temperature recording from different sites in the animal body [12, 39]. Moreover, the current study reported that UHT was higher than RT. Similarly, Gwazdauskas et al. [35] reported that the average of uterine temperature in dairy cows was higher than the average RT by 0.2 C.
In the current study, the positive correlation between RT and VT in beef cows is nearly similar to that reported in pregnant (r=0.90) [40], healthy postpartum (r=0.75) [8] and cyclic dairy cows (r=0.95) [43] and beef heifers (r=0.92) [41]. The high association between VT and RT reflects the possibility of using VT to monitor diurnal changes in body temperature [8]. Moreover, our results show that both RT and VT were positively correlated with CT, UBT and UHT, but VT was significantly more associated with these variables. Thus, VT could be a more appropriate indicator of thermal changes in the deeper portions of the female genital tract than RT.
It is well known that both RT and VT could be affected by daily rhythms and environmental conditions [12]. The average THI during the experimental period was lower than the alert level (i.e., THI ≤ 74) for heat stress in beef cows [44]. Moreover, in the current study, THI was moderately correlated with VT when compared with another study carried out under temperate conditions, which reported a higher correlation between THI and VT [45]. This result may reflect the relatively moderate environmental conditions during our experimental period. It is worth noting that under heat stress, the uterine temperature increase as a result of reduction in uterine blood flow caused by catecholamine-mediated vasoconstriction of uterine arteries. This condition ultimately leads to a reduction in the conception rate and increased early embryonic loss [46, 47]. Further studies are required to elucidate the effect of seasonal variation of THI on the temperatures of the different portions of the female genital tract with special reference to the deeper portions.
In this study, the temperatures of different portions of the female genital tract showed significant increases when peripheral P4 concentrations were greater than 4 ng/ml (mid-cycle CL) in comparison with other lower concentrations of P4. This result is in agreement with previous studies that reported an increment in bovine VT during the mid-luteal phase followed by reduction 2–3 days before estrus [10, 11, 14, 15, 48]. This phenomenon was attributed to the thermogenic action of P4; however, none of these studies measured RT. Comparison of the recorded RTs in different peripheral P4 concentration ranges showed that there were no significant differences. Similarly, a report studying the estrous cycle based on RT only reported no such pattern in bovines [9]. Based on these findings, we postulated that this pattern is more likely to be a result of a localized effect of P4 on the genital tract than the consequence of generalized thermogenic actions. We attributed this localized effect to the vasoconstrictive effect of elevated P4 or the lowered E2:P4 ratio on genital blood vessels [49]. With the subsequent decrease in the blood flow and heat loss in the genital tract, there was a subsequent increase in the genital-tract temperature [49, 50]. P4 acts as a vasoconstrictor through two mechanisms: first, by increasing norepinephrine release from the sympathetic nervous system, and second, through increasing the responsiveness of vascular smooth muscle by increasing the number and/or the activity of α adrenergic receptors in adrenergic (vasoconstrictor) nerves [51]. In support of our hypothesis, parenteral administration of estradiol (i.e., increase of the E2:P4 ratio) was associated with a reduction in uterine temperature and an increase in the vaginal thermal conductance in cyclic dairy heifers. This event was attributed to the vasodilation of the uterine and vaginal arteries, respectively [52, 53]. Additionally, our results revealed positive and negative correlations of P4 and E2:P4, respectively, with the temperatures of the different portions of the female genital tract. Although these correlations were of moderate degree, this phenomenon could be explained by the fact that the vasoconstrictor effect of P4 is mediated by other substances [54]. In the current study, there was no significant difference between the temperature of the uterine horns ipsilateral and contralateral to the ovary-bearing CL. A point of inconsistency is that Pope et al. [55] and Weems et al. [56] had reported higher concentrations of P4 in the tissues of uterine horns and arteries ipsilateral to the ovary-bearing CL than those contralateral to the ovary-bearing CL. However, Ford [49] demonstrated that this pattern did not affect the uterine blood flow in a unilateral manner, as there was no significant difference in the blood flow in the uterine arteries ipsilateral and contralateral to the ovary-bearing a CL throughout the estrous cycle [57]. The knowledge concerning thermal changes within the female genitalia during the estrous cycle may be of practical significance for optimizing the in vitro regimes for gamete and embryo culture [58, 59]. At the biological level, these variations may result in modification of the gene expressions and/or epithelial tissues of the different tissues of the female genital tract [18].
So far, frequent monitoring of genital tract temperatures is not carried out. Further studies will be necessary to elucidate the hourly changes in relation to estrus. However, innovation of a device to be lodged in the female genital tract for continuous temperature monitoring of different portions at the same time may be difficult, due to the orientation and structure of the genitalia.
In conclusion, 1) the female genital tract shows a temperature gradient that increases progressively toward the uterine horns; 2) genital temperatures increase during the mid-luteal phase (P4>4ng/ml) in comparison with the other phases of the estrous cycle; and 3) VT could be more beneficial than RT in monitoring the temperatures of deeper portions of the female genital tract in bovine.
Acknowledgment
The authors would like to thank all of the members of the Laboratory of Theriogenology, University of Miyazaki, Japan, for their assistance in experimental procedures. This work was supported in part by a research grant (2011) from the Graduate School of Medicine and Veterinary Medicine, University of Miyazaki. EL-SHEIKH ALI, H. is supported by a PhD scholarship from the Egyptian government.
References
- 1.Bahat A, Eisenbach M, Tur-Kaspa I. Periovulatory increase in temperature difference within the rabbit oviduct. Hum Reprod 2005; 20: 2118–2121 [DOI] [PubMed] [Google Scholar]
- 2.Hunter RH, Bogh IB, Einer-Jensen N, Muller S, Greve T. Pre-ovulatory graafian follicles are cooler than neighbouring stroma in pig ovaries. Hum Reprod 2000; 15: 273–283 [DOI] [PubMed] [Google Scholar]
- 3.Martinez AR, van Hooff MH, Schoute E, van der Meer M, Broekmans FJ, Hompes PG. The reliability, acceptability and applications of basal body temperature (BBT) records in the diagnosis and treatment of infertility. Eur J Obstet Gynecol Reprod Biol 1992; 47: 121–127 [DOI] [PubMed] [Google Scholar]
- 4.Zuspan FP, Rao P. Thermogenic alterations in the woman. I. Interaction of amines, ovulation, and basal body temperature. Am J Obstet Gynecol 1974; 118: 671–678 [DOI] [PubMed] [Google Scholar]
- 5.Driver HS, Baker FC. Menstrual factors in sleep. Sleep Med Rev 1998; 2: 213–229 [DOI] [PubMed] [Google Scholar]
- 6.McCreesh Z, Evans NE, Scanlon WG. Vaginal temperature sensing using UHF radio telemetry. Med Eng Phys 1996; 18: 110–114 [DOI] [PubMed] [Google Scholar]
- 7.Freeman ME, Crissman JK, Jr, Louw GN, Butcher RL, Inskeep EK. Thermogenic action of progesterone in the rat. Endocrinology 1970; 86: 717–720 [DOI] [PubMed] [Google Scholar]
- 8.Vickers LA, Burfeind O, von Keyserlingk MA, Veira DM, Weary DM, Heuwieser W. Technical note: Comparison of rectal and vaginal temperatures in lactating dairy cows. J Dairy Sci 2010; 93: 5246–5251 [DOI] [PubMed] [Google Scholar]
- 9.Piccione G, Caola G, Refinetti R. Daily and estrous rhythmicity of body temperature in domestic cattle. BMC Physiol 2003; 3: 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis GS, Newman SK. Changes throughout estrous cycles of variables that might indicate estrus in dairy cows. J Dairy Sci 1984; 67: 146–152 [DOI] [PubMed] [Google Scholar]
- 11.Wrenn TR, Bitman J, Sykes JF. Body Temperature variations in dairy cattle during the estrous cycle and pregnancy. J Dairy Sci 1958; 41: 1071–1076 [Google Scholar]
- 12.Suthar VS, Burfeind O, Patel JS, Dhami AJ, Heuwieser W. Body temperature around induced estrus in dairy cows. J Dairy Sci 2011; 94: 2368–2373 [DOI] [PubMed] [Google Scholar]
- 13.Wrenn TR, Bitman J, Sykes JF. The thermogenic influence of progesterone in ovariectomized cows. Endocrinology 1959; 65: 317–321 [DOI] [PubMed] [Google Scholar]
- 14.Redden KD, Kennedy AD, Ingalls JR, Gilson TL. Detection of estrus by radiotelemetric monitoring of vaginal and ear skin temperature and pedometer measurements of activity. J Dairy Sci 1993; 76: 713–721 [DOI] [PubMed] [Google Scholar]
- 15.Kyle BL, Kennedy AD, Small JA. Measurement of vaginal temperature by radiotelemetry for the prediction of estrus in beef cows. Theriogenology 1998; 49: 1437–1449 [DOI] [PubMed] [Google Scholar]
- 16.Ammons SF, Threlfall WR, Kline RC. Equine body temperature and progesterone fluctuations during estrus and near parturition. Theriogenology 1989; 31: 1007–1019 [DOI] [PubMed] [Google Scholar]
- 17.Elmore RG, Garverick HA. The relationship of circulating plasma progesterone levels and rectal temperatures in gilts. Theriogenology 1979; 12: 319–325 [Google Scholar]
- 18.Hunter RHF. Temperature gradients in female reproductive tissues and their potential significance. Anim Reprod 2009; 6: 7–15 [Google Scholar]
- 19.Roelofs J, Lopez-Gatius F, Hunter RH, van Eerdenburg FJ, Hanzen C. When is a cow in estrus? Clinical and practical aspects. Theriogenology 2010; 74: 327–344 [DOI] [PubMed] [Google Scholar]
- 20.Amundson JL, Mader TL, Rasby RJ, Hu QS. Environmental effects on pregnancy rate in beef cattle. J Anim Sci 2006; 84: 3415–3420 [DOI] [PubMed] [Google Scholar]
- 21.Duchens M, Maciel M, Gustafsson H, Forsberg M, Rodríguez-Martínez H, Edqvist LE. Influence of perioestrous suprabasal progesterone levels on cycle length, oestrous behaviour and ovulation in heifers. Anim Reprod Sci 1995; 37: 95–108 [Google Scholar]
- 22.Nagata S, Kondou M, Kaneko H, Araki K, Nambo Y, Oikawa M, Watanabe G, Taya K. A simple defatting method using a partition method of acetonitrile and n-hexane for radioimmunoassay of low blood levels of estradiol-17β. J Reprod Dev 1996; 42: j43–j49 [Google Scholar]
- 23.Wijayagunawardane MP, Miyamoto A, Cerbito WA, Acosta TJ, Takagi M, Sato K. Local distributions of oviductal estradiol, progesterone, prostaglandins, oxytocin and endothelin-1 in the cyclic cow. Theriogenology 1998; 49: 607–618 [DOI] [PubMed] [Google Scholar]
- 24.D' Agostino R, Pearson ES. Tests for departure from normality. Empirical results for the distributions of b2 and √b1. Biometrika 1973; 60: 613–622 [Google Scholar]
- 25.Carmer SG, Walker WM. Pairwise multiple comparisons of treatment means in agronomic research. J Agron Edu 1985; 14: 19–26 [Google Scholar]
- 26.Herzog K, Koerte J, Flachowsky G, Bollwein H. Variability of uterine blood flow in lactating cows during the second half of gestation. Theriogenology 2011; 75: 1688–1694 [DOI] [PubMed] [Google Scholar]
- 27.Everitt BS. The Cambridge Dictionary of Statisitcs. New York: Cambridge University Press; 2006 [Google Scholar]
- 28.Steiger JH. Tests for comparing elements of a correlation matrix. Psychol Bull 1980; 87: 245–251 [Google Scholar]
- 29.Meng X-l, Rosenthal R, Rubin DB. Comparing correlated correlation coefficients. Psychol Bull 1992; 111: 172–175 [Google Scholar]
- 30.Battocchio M, Gabai G, Mollo A, Veronesi MC, Soldano F, Bono G, Cairoli F. Agreement between ultrasonographic classification of the CL and plasma progesterone concentration in dairy cows. Theriogenology 1999; 51: 1059–1069 [DOI] [PubMed] [Google Scholar]
- 31.Veronesi MC, Gabai G, Battocchio M, Mollo A, Soldano F, Bono G, Cairoli F. Ultrasonographic appearance of tissue is a better indicator of CL function than CL diameter measurement in dairy cows. Theriogenology 2002; 58: 61–68 [DOI] [PubMed] [Google Scholar]
- 32.Mondal M, Rajkhowa C, Prakash BS. Relationship of plasma estradiol-17β, total estrogen, and progesterone to estrus behavior in mithun (Bos frontalis) cows. Horm Behav 2006; 49: 626–633 [DOI] [PubMed] [Google Scholar]
- 33.Sciscione AC, Zainia T, Leet T, Winn JN, Winn HN. A new device for measuring intrauterine temperature. Am J Obstet Gynecol 2001; 184: 1431–1435, discussion :1434–1435 [DOI] [PubMed] [Google Scholar]
- 34.Macaulay JH, Randall NR, Bond K, Steer PJ. Continuous monitoring of fetal temperature by noninvasive probe and its relationship to maternal temperature, fetal heart rate, and cord arterial oxygen and pH. Obstet Gynecol 1992; 79: 469–474 [DOI] [PubMed] [Google Scholar]
- 35.Gwazdauskas FC, Thatcher WW, Wilcox CJ. Physiological, environmental, and hormonal factors at insemination which may affect conception. J Dairy Sci 1973; 56: 873–877 [DOI] [PubMed] [Google Scholar]
- 36.Chapwanya A, Meade KG, Narciandi F, Stanley P, Mee JF, Doherty ML, Callanan JJ, O' Farrelly C. Endometrial biopsy: a valuable clinical and research tool in bovine reproduction. Theriogenology 2010; 73: 988–994 [DOI] [PubMed] [Google Scholar]
- 37.Bahat A, Tur-Kaspa I, Gakamsky A, Giojalas LC, Breitbart H, Eisenbach M. Thermotaxis of mammalian sperm cells: a potential navigation mechanism in the female genital tract. Nat Med 2003; 9: 149–150 [DOI] [PubMed] [Google Scholar]
- 38.Burfeind O, von Keyserlingk MA, Weary DM, Veira DM, Heuwieser W. Short communication: repeatability of measures of rectal temperature in dairy cows. J Dairy Sci 2010; 93: 624–627 [DOI] [PubMed] [Google Scholar]
- 39.Lea JM, Niemeyer DDO, Reed MT, Fisher AD, Ferguson DM. Development and validation of a simple technique for logging body temperature in free-ranging cattle. Australian Journal of Experimental Agriculture 2008; 48: 741–745 [Google Scholar]
- 40.Hillman PE, Gebremedhin KG, Williard ST, Lee CN, Kennedy AD. Continuous measurements of vaginal temperature of female cattle using a data logger encased in a plastic anchor. Applied Engineering in Agriculture 2009; 25: 291–296 [Google Scholar]
- 41.Burdick NC, Carroll JA, Dailey JW, Randel RD, Falkenberg SM, Schmidt TB. Development of a self-contained, indwelling vaginal temperature probe for use in cattle research. Journal of Thermal Biology 2012; 37: 339–343 [Google Scholar]
- 42.Sakatani M, Balboula AZ, Yamanaka K, Takahashi M. Effect of summer heat environment on body temperature, estrous cycles and blood antioxidant levels in Japanese Black cow. Animal Science Journal 2012; 83: 394–402 [DOI] [PubMed] [Google Scholar]
- 43.Rajamahendran R, Robinson J, Desbottes S, Walton JS. Temporal relationships among estrus, body temperature, milk yield, progesterone and luteinizing hormone levels, and ovulation in dairy cows. Theriogenology 1989; 31: 1173–1182 [DOI] [PubMed] [Google Scholar]
- 44.Mader TL, Davis MS, Brown-Brandl T. Environmental factors influencing heat stress in feedlot cattle. J Anim Sci 2006; 84: 712–719 [DOI] [PubMed] [Google Scholar]
- 45.Kendall PE, Nielsen PP, Webster JR, Verkerk GA, Littlejohn RP, Matthews LR. The effects of providing shade to lactating dairy cows in a temperate climate. Livestock Science 2006; 103: 148–157 [Google Scholar]
- 46.Roman-Ponce H, Thatcher WW, Caton D, Barron DH, Wilcox CJ. Thermal stress effects on uterine blood flow in dairy cows. J Anim Sci 1978; 46: 175–180 [DOI] [PubMed] [Google Scholar]
- 47.Nabenishi H, Ohta H, Nishimoto T, Morita T, Ashizawa K, Tsuzuki Y. Effect of the temperature-humidity index on body temperature and conception rate of lactating dairy cows in southwestern Japan. J Reprod Dev 2011; 57: 450–456 [DOI] [PubMed] [Google Scholar]
- 48.Bobowiec R, Studzinski T, Babiarz A. Thermoregulatory effects and electrical conductivity in vagina of cow during oestrous cycle. Arch Exp Veterinarmed 1990; 44: 573–579 [PubMed] [Google Scholar]
- 49.Ford SP. Control of uterine and ovarian blood flow throughout the estrous cycle and pregnancy of ewes, sows and cows. J Anim Sci 1982; 55(Suppl 2): 32–42 [PubMed] [Google Scholar]
- 50.Abrams RM, Caton D, Clapp JF , 3rd, Barron DH. Thermal aspects of uterine blood flow in nonpregnant sheep. Am J Obstet Gynecol 1970; 108: 919–924 [DOI] [PubMed] [Google Scholar]
- 51.Ford SP, Weber LJ, Stormshak F. Role of estradiol-17 beta and progesterone in regulating constriction of ovine uterine arteries. Biol Reprod 1977; 17: 480–483 [DOI] [PubMed] [Google Scholar]
- 52.Abrams RM, Thatcher WW, Bazer FW, Wilcox CJ. Effect of estradiol-17beta on vaginal thermal conductance in cattle. J Dairy Sci 1973; 56: 1058–1062 [DOI] [PubMed] [Google Scholar]
- 53.Gwazdauskas FC, Abrams RM, Thatcher WW, Bazer FW, Caton D. Thermal changes of the bovine uterus following administration of estradiol-17beta. J Anim Sci 1974; 39: 87–92 [DOI] [PubMed] [Google Scholar]
- 54.Forman RG, Chapman MC, Steptoe PC. The effect of endogenous progesterone on basal body temperature in stimulated ovarian cycles. Hum Reprod 1987; 2: 631–634 [DOI] [PubMed] [Google Scholar]
- 55.Pope WF, Maurer RR, Stormshak F. Distribution of progesterone in the uterus, broad ligament, and uterine arteries of beef cows. Anat Rec 1982; 203: 245–249 [DOI] [PubMed] [Google Scholar]
- 56.Weems CW, Lee CN, Weems YS, Vincent DL. Distribution of progesterone to the uterus and associated vasculature of cattle. Endocrinol Jpn 1988; 35: 625–630 [DOI] [PubMed] [Google Scholar]
- 57.Ford SP, Chenault JR, Echternkamp SE. Uterine blood flow of cows during the oestrous cycle and early pregnancy: effect of the conceptus on the uterine blood supply. J Reprod Fertil 1979; 56: 53–62 [DOI] [PubMed] [Google Scholar]
- 58.Shi DS, Avery B, Greve T. Effects of temperature gradients on in vitro maturation of bovine oocytes. Theriogenology 1998; 50: 667–674 [DOI] [PubMed] [Google Scholar]
- 59.Rivera RM, Hansen PJ. Development of cultured bovine embryos after exposure to high temperatures in the physiological range. Reproduction 2001; 121: 107–115 [PubMed] [Google Scholar]