Abstract
In this study, a dose-response assessment was performed to understand the relation between supplementation of media with L-ascorbic acid or vitamin C and porcine oocyte maturation and the in vitro development of parthenotes (PA) and handmade cloned (HMC) embryos. Various concentrations (0, 25, 50 and 100 µg/ml) of vitamin C supplemented in in vitro maturation (IVM) and culture (IVC) media were tested. None of these vitamin C additions affected nuclear maturation of oocytes, yet supplementation at 50 µg/ml led to significantly increased intracellular glutathione (GSH) levels and reduced reactive oxygen species (ROS). When cultured in IVM- and/or IVC-supplemented media, the group supplemented with 50 µg/ml of vitamin C showed improved cleavage rates, blastocyst rates and total cell numbers per blastocyst (P<0.05) compared with other groups (control, 25 µg/ml and 100 µg/ml). In contrast, supplementation with 50 µg/ml vitamin C decreased (P<0.05) the apoptosis index as compared with the groups supplemented with 100 µg/ml. In addition, even with a lower blastocyst rate to start with (37.6 vs. 50.3%, P<0.05), supplementation of HMC embryos with vitamin C ameliorated their blastocyst quality to the extent of PA embryos as indicated by their total cell numbers (61.2 vs. 59.1). Taken together, an optimized concentration of vitamin C supplementation in the medium not only improves blastocyst rates and total cell numbers but also reduces apoptotic indices, whereas overdosages compromise various aspects of the development of parthenotes and cloned porcine embryos.
Keywords: Culture system, Embryo development, Handmade cloning, Parthenote, Vitamin C
In an attempt to improve the efficiency of in vitro production (IVP) of embryos, we have come to a better understanding of the environmental factors that impact embryo development. In reality, in vitro culture superimposes onto embryos a number of stress factors, which nonetheless can be avoided in vivo [1]. Direct consequences of these in vitro stresses are physiological compromises of embryonic gene expression and development [2]. Today, the task of maintaining some mammalian embryos in culture is challenging, particularly in species such as the canine and porcine [3,4,5,6]. Recent studies have been reported with great improvement of in vitro culture systems by manipulating defined media via various combinatory supplements such as vitamins ([7,8,9,10,11,12], growth factors [13,14,15,16,17], cytokines [18, 19], hormones [20] and other selective intracellular and extracellular modulators for biochemical processes [9] in several species. Despite efforts to make improvements, the yield and quality of IVP porcine embryos are still low when compared with their in vivo counterparts [13, 21, 22]. In a long-standing practice, the hydrosoluble antioxidant vitamin C has often been used as a supplement in in vitro culture systems for oocytes and embryos [7, 9, 10, 12]. As a result of reduced O2 tension, IVP embryos acquired improved development evident by a decreased reactive oxygen species (ROS) content and DNA fragmentation [9, 23,24,25]. Glutathione (GSH), a major intracellular free thiol group, is involved in cellular proliferation, amino acid transport, DNA and protein syntheses and free radical scavenging in the oxidative microenvironment. It has been reported that GSH synthesis during oocyte maturation is beneficial to pronuclear formation and subsequent development [23, 26,27,28]. However, none of the past studies elucidated the functions of supplemented vitamin C in porcine oocyte maturation and further development in vitro or in the development of porcine parthenotes and handmade cloned (HMC) embryos. The objective of this study was to investigate the effects of vitamin C (L-ascorbic acid) when supplemented in media for 44 h at various doses on in vitro maturation (IVM) and during the overall culture of porcine embryos. Improved oocyte maturation rates and subsequent embryonic development after parthenogenetic activation and nuclear transplantation were demonstrated in this study.
Materials and Methods
All chemicals used in this study were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise indicated.
Oocyte recovery and IVM
Oocyte recovery and IVM were performed as described in previous studies [15, 29, 30]. Briefly, ovaries obtained from a local slaughterhouse were transported to the laboratory within 2 h in physiological saline containing penicillin (600 IU/ml). Oocytes were aspirated from follicles (3 to 7 mm in diameter), and cumulus-oocyte complexes (COCs) with at least two layers of cumulus cells and homogeneous ooplasm were selected for maturation in North Carolina State University 23 (NCSU-23) medium. Twenty to 30 oocytes were randomly allocated to each 100 µl droplet of IVM medium containing different concentrations of vitamin C (0, 25, 50 and 100 µg/ml), covered by mineral oil and cultured at 39 C in an incubator containing 5% CO2. For the first 22 h, the COCs were cultured in NCSU-23 medium supplemented with 10% porcine follicular fluid, cysteine (0.1 mg/ml), equine chorionic gonadotrophin (10 IU/ml) and human chorionic gonadotrophin (10 IU/ml), and then the COCs from all treatment groups were switched to the medium without hormones for another 22 h.
Measurement of ROS and intracellular GSH levels
The matured oocytes were sampled to determine intracellular ROS and GSH levels by methods described previously [24, 28]. Briefly, H2DCFDA (2',7'-dichlorodihydrofluorescein diacetate; Invitrogen, Eugene, OR, USA) and CellTracker Blue CMF2HC (4-chloromethyl-6.8-difluoro-7-hydroxycoumarin; Invitrogen) were used to detect intracellular ROS by green fluorescence and GSH level by blue fluorescence, respectively. Ten oocytes from each treatment group were incubated (in the dark) for 30 min in NCSU-23 supplemented with 10 μM H2DCFDA and 10 μM CellTracker. After incubation, oocytes were washed with D-PBS (Invitrogen) containing 0.1% (w/v) PVA and placed into 10 µl droplets, and the fluorescence was observed under an epifluorescence microscope (TE300; Nikon, Tokyo, Japan) with UV filters (460 nm for ROS and 370 nm for GSH). Fluorescent images were saved as graphic files, and their fluorescence intensities were analyzed with ImageJ software (Version 1.44p; National Institutes of Health, Bethesda, MD, USA). All images data were normalized by the untreated control oocytes for statistical analysis.
In vitro production of HMC embryos
The procedures for cloning HMC embryos were mainly based on previous studies [30, 31] with some modifications [16]. Briefly, in vitro matured oocytes with a first polar body were incubated in HEPES-buffered TCM-199 containing pronase (3.3 mg/ml) and FBS (33%) for 10 sec and then washed 2 times in HEPES-buffered TCM-199 supplemented with 10% FBS (T10). After washing, oocytes were lined up in 40 µl T10 containing 2.5 mg/ml cytochalasin B (16 oocytes in each droplet). Oocytes were rotated with a fire-polished glass pipette to identify the membrane protrusion or first polar body for oriented bisection with a microblade (ESE020, Bioniche Animal Health, Pullman, WA, USA) under a stereomicroscope. The demi-ooplasts were transferred to a T10 drop after bisection.
Cell fusion was performed with a two-step protocol containing two consecutive fusions. First, the enucleated cytoplast was transferred to the HEPES-TCM-199 droplet containing 1 mg/ml phytohaemagglutinin (PHA) for 5 sec and then put into the T10 droplet holding the fibroblasts, in which each cytoplast was allowed to pair with one fibroblast cell. The cytoplast-fibroblast pairs were incubated in fusion medium (0.3 M mannitol and 0.01% PVA) for 20 sec and transferred to a fusion chamber (with two electrodes separated by 1 mm). Under 0.6 kV/cm AC, the cell pairs were aligned between the wires with the fibroblasts farthest from the wires. Fusion was performed with one DC pulse at 2.0 kV/cm for 9 µsec, and the pairs were then transferred to the T10 drop and incubated for 1 h before the second fusion.
For the second fusion, the remaining cytoplasts and the fused cytoplast-fibroblast pairs were transferred to activation medium (0.3 M mannitol, 0.1 mM MgSO4, 0.1 mM CaCl2 and 0.01% PVA) for equilibration. Then they were aligned with the fused pairs farthest from the wires and then subjected to DC pulse (0.80 kV/cm, 80 µsec) fusion and initial activation simultaneously. After fusion and activation, the cytoplast-fibroblast triplets were incubated in T10 for 20 min to allow complete fusion prior to activation with 6-dimethylaminopurine (6-DMAP).
Oocyte activation and embryo culture
After 44 h of IVM, cumulus cells were removed from matured oocytes by gentle pipetting in DPBS containing 0.1% hyaluronidase. The oocytes with an extruded polar body were chosen under stereomicroscope and then washed twice in activation medium (0.28 M mannitol; 0.01% polyvinyl alcohol; 0.05mM HEPES; 0.1 mM CaCl2·2H2O and 0.1 mM MgCl2) before parthenogenetic activation. For activation, an electrical pulse (2.04 kV/cm, 30 µsec) generated by a BTX Electro-Cell Manipulator 2001 (BTX, San Diego, CA, USA) was applied to oocytes. After being washed twice with 2.5 mM 6-DMAP, activated oocytes were transferred to porcine zygote medium (PZM-3) containing 2.5 mM 6-DMAP for 4 h and then washed six times with PZM-3 medium. The activated oocytes were cultured continuously in PZM-3 medium following the same pattern of treatment as described above for 7 days to evaluate their developmental competence [4, 15, 30].
Apoptosis assays and total cell counts
Apoptosis was detected by terminal deoxynucleotidyl transferase-mediated d-UTP nick end-labeling (TUNEL) assay as described by Hao et al. [32] and Nguyen et al. [16] with minor modifications. Briefly, 7-day-old embryos were washed three times in DPBS/PVP (DPBS supplemented with 0.1% polyvinylpyrrolidone) and fixed in 4% (v/v) paraformaldehyde solution for 1 h at room temperature. Membranes were made permeable in 0.1% Triton X-100 in 0.1% citrate solution for 1 h at room temperature. Fixed embryos were incubated in TUNEL reaction medium (In Situ Cell Death Detection Kit, Fluorescein; Roche, Mannheim, Germany) for 1 h at 38.5 C in the dark. So, the broken DNA ends of the embryonic cells were labeled with TdT and fluorescein-dUTP. After the reaction stopped, the embryos were washed in DPBS/BSA (DPBS supplemented with 0.1% bovine serum albumin) and mounted on glass slides with DAKO Fluorescent Mounting Medium (S3023, Dako North America, Carpinteria, CA, USA) containing Hoechst 33342 for total cell counts. Whole-mount embryos were examined under an epifluorescence microscope (Nikon) by TUNEL assay and Hoechst staining. The numbers of apoptotic nuclei (by TUNEL assay) and total numbers of nuclei were determined from optical images. The apoptotic index was calculated as follows: apoptotic index = (number of TUNEL-positive nuclei / total number of nuclei) × 100.
Statistical analysis
Data for the percentage of apoptotic blastomeres were normalized by arcsine transformation prior to analysis. Statistical differences in embryo development among treatment effects on cleavage rates, total cell numbers, percentages of blastocysts and apoptotic indices were tested by the general linear model procedure (SAS Institute, Cary, NC, USA). Differences between treatment means were determined by using Tukey's test and were considered significant at P<0.05. All data are expressed as the mean ± SEM.
Results
Effects of vitamin C on oocyte maturation and intracellular levels of GSH and ROS
To optimize the effective concentration of vitamin C in IVM medium, oocytes were cultured in medium supplemented with 0, 25, 50 and 100 µg/ml of vitamin C. The results showed that there was no significant difference between treatment groups in various aspect of nuclear maturation such as proportion of oocytes at the germinal vesicle (GV), germinal vesicle break down (GVBD), metaphase I (MI) and metaphase II (MII) stages of meiosis as presented in Table 1. However, 50 µg/ml vitamin C in the maturation medium increased (P<0.05) the intracellular GSH level and decreased (P<0.05) ROS accumulation in MII oocytes (Table 2 and Fig. 1).
Table 1. Maturation of porcine oocytes cultured in IVM medium supplemented with various concentrations of vitamin C.
| Vitamin C (µg/ml) | Total No. oocytes | GV (%) | GVBD (%) | MI (%) | MII (%) |
| 0 | 68 | 5.3 ± 3.0 | 9.2 ± 3.3 | 13.7 ± 4.4 | 71.8 ± 7.6 |
| 25 | 73 | 8.3 ± 8.3 | 4.6 ± 2.3 | 4.4 ± 2.2 | 82.6 ± 7.6 |
| 50 | 82 | 5.2 ± 3.5 | 4.9 ± 1.3 | 5.9 ± 3.1 | 79.8 ± 7.8 |
| 100 | 69 | 6.5 ± 2.6 | 6.1 ± 1.7 | 7.2 ± 1.0 | 80.3 ± 3.3 |
No significant differences were detected among treatment groups: values of three replicates are shown as the mean ± SEM. IVM, in vitro maturation; GV, germinal vesicle; GVBD, germinal vesicle break down; MI, metaphase; MII, metaphase II.
Table 2. Relative levels of GSH and ROS in porcine oocytes cultured in IVM medium supplemented with vitamin C.
| Vitamin C (µg/ml) | Total No. oocytes | Relative level (pixels/oocyte) of | |
| GSH | ROS | ||
| 0 | 36 | 1.0 ± 0.1a | 1.0 ± 0.2a |
| 25 | 44 | 2.2 ± 0.6ab | 0.8 ± 0.1a |
| 50 | 49 | 3.4 ± 0.7b | 0.4 ± 0.1b |
| 100 | 44 | 2.5 ± 0.6ab | 0.7 ± 0.1a |
a, b Within the same column, numbers without the same superscripts differ (P<0.05). Values of four replicates are shown as the mean ± SEM. GSH, glutathione; ROS, reactive oxygen species; IVM, in vitro maturation.
Fig.1.
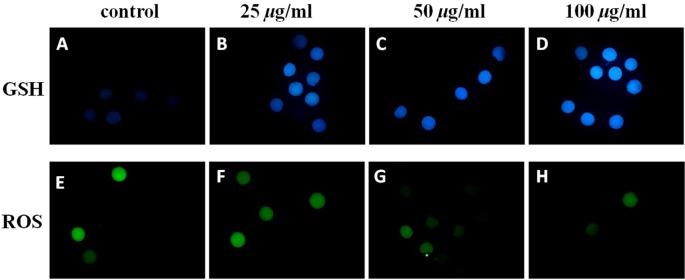
Epifluorescent microphotographic images of in vitro matured porcine oocytes. Oocytes were stained with CellTracker Blue (A–D) and H2DCFDA (E–H) to detect intracellular levels of glutathione (GSH) and reactive oxygen species (ROS), respectively. Metaphase II oocytes derived from the culture in medium supplemented with (B, C, D, F, G and H) or without (A and E) vitamin C. Matured oocytes from the group treated with 50 µg/ml of vitamin C treated group showed an increased cytoplasmic level of GSH (C) but a reduced ROS level.
Effects of vitamin C in the maturation medium on the development of parthenotes
For further developmental assessment (Table 3), our results showed that there was no significant difference in the cleavage rate regardless of the concentration of vitamin C used. The percentage of parthenogenetic embryos reaching the blastocyst stage was higher in the 50 µg/ml group (53.8%) than in the control group (39.8%). As for total cell numbers per blastocyst, embryos cultured at 50 µg/ml (55.2%) exhibited an improved total cell number as compared with those cultured at 100 µg/ml (39.9%). None of the treatments showed a significant difference in apoptotic indices (P>0.05).
Table 3. Development of porcine parthenotes derived from oocytes matured in IVM medium supplemented with vitamin C.
| Vitamin C (µg/ml) | Total No. oocytes | Cleavage rate (n) (%) | BL rate (n) (%) | TCN/BL (n) (%) | Apoptotic index (%) |
| 0 | 155 | 87.79 ± 3.11 (126) | 39.82 ± 1.70b (62) | 43.82 ± 3.94ab (41) | 7.02 ± 1.12 |
| 25 | 149 | 90.53 ± 2.59 (135) | 43.16 ± 4.25ab (64) | 47.37 ± 3.63ab (47) | 5.36 ± 0.57 |
| 50 | 144 | 92.53 ± 1.35 (133) | 53.87 ± 4.10a (76) | 55.23 ± 2.58a (54) | 4.19 ± 0.32 |
| 100 | 145 | 84.91 ± 4.84 (122) | 44.13 ± 3.09ab (63) | 39.91 ± 2.34b (45) | 4.17 ± 1.18 |
a, b Within columns, means without the same superscripts differ (P<0.05). Values from six replicates are shown as the mean ± SEM. IVM, in vitro maturation; TCN, total cell number; BL, blastocyst.
Effects of vitamin C in the culture medium on the development of parthenotes
This study aimed to identify the best working concentration of vitamin C for embryo development in the IVC medium. The results (Table 4) showed that different treatments had similar cleavage rates (P>0.05). The blastocyst rate was higher with 50 µg/ml supplementation (46.1%) than with the other treatments (28.5% for the control, 30.5% for 25 µg/ml and 24.5% for 100 µg/ml). Total cells numbers per blastocyst at 50 µg/ml (50) were also significantly higher than those of the control (41) and the 100 µg/ml group (41). As for the apoptotic index, supplementation at 50 µg/ml resulted in a significantly lower value (3.9%) than supplementation at 100 µg/ml (7.3%).
Table 4. Development of porcine parthenotes cultured in IVC medium supplemented with vitamin C.
| Vitamin C (µg/ml) | Total No. oocytes | Cleavage rate (n) | BL rate (n) | TCN/BL (n) (%) | Apoptosis index (%) |
| 0 | 132 | 97.03 ± 0.94 (128) | 28.51 ± 7.59a (38) | 41.17 ± 0.84a (34) | 6.91 ± 0.87ab |
| 25 | 123 | 91.31 ± 4.12 (112) | 30.48 ± 5.09a (37) | 44.55 ± 2.31ab (31) | 6.19 ± 0.95ab |
| 50 | 123 | 93.59 ± 2.05 (115) | 46.11 ± 6.07b (56) | 49.83 ± 2.21b (45) | 3.88 ± 0.69a |
| 100 | 126 | 90.40 ± 3.80 (114) | 24.48 ± 3.41a (31) | 40.41 ± 2.31a (25) | 7.29 ± 0.75b |
a, b Within the same column, means without the same superscripts differ (P<0.05). Values from six replicates are shown as the mean ± SEM. IVC, in vitro culture; BL, blastocyst; TCN, total cell number.
Effects of vitamin C addition in both IVM and IVC media on the development of porcine parthenotes
The developmental competence of the porcine parthenotes under the influence of vitamin C is shown as Table 5. Parthenotes supplemented with vitamin C at 50 μg/ml had better cleavage rates than those with 100 μg/ml (95.4 vs. 84.9%, P<0.05). Regarding the blastocyst rates, the rate was significantly higher (59.1%) for parthenotes supplemented with 50 μg/ml of vitamin C than for parthenotes in the control group and in 100 μg/ml group (40.8 and 36.0%, P<0.05). Embryos grown in 50 μg/ml vitamin C had the highest total cell number per blastocyst (55.9) among the groups (46, 45 and 44 for the control and 25 and 100 μg/ml groups respectively). Concomitantly, embryos supplemented with vitamin C at 50 μg/ml had a reduced apoptotic index compared with those supplemented with 100 μg/ml (4.8 vs. 7.6%, P<0.05).
Table 5. Development of porcine parthenotes cultured consecutively in IVM and IVC media supplemented with vitamin C.
| Vitamin C (µg/ml) | Total No. oocytes | Cleavage rate (n) (%) | BL rate (n) (%) | TCN/BL (n) (%) | Apoptosis index (%) |
| 0 | 151 | 90.15 ± 3.01(136)ab | 40.86 ± 4.2a (62)a | 45.78 ± 1.81a (57) | 6.31 ± 0.64ab |
| 25 | 154 | 90.08 ± 2.36 (139)a | 44.39 ± 4.54ab (64) | 45.12 ± 2.43a (56) | 5.93 ± 0.98ab |
| 50 | 141 | 95.36 ± 2.25 (135)b | 59.14 ± 8.46b (76) | 55.86 ± 1.61b (61) | 4.87 ± 0.37a |
| 100 | 149 | 84.94 ± 5.53 (126)a | 36.04 ± 5.83a (63) | 43.99 ± 0.98a (61) | 7.60 ± 0.75b |
a, b Within the same column, means without the same superscript differ (P<0.05). Values from six replicates are shown as the mean ± SEM. IVM, in vitro maturation; TCN, total cell number; BL, blastocyst.
Effects of vitamin C on the development of parthenotes and HMC embryos
As shown in Table 6, cleavage rates and total cell numbers did not differ between PA and HMC embryos regardless to the treatment group, despite the PA embryos showing a higher (P<0.05) blastocyst rate (50%) than HMC embryos (38%) in IVC supplemented with vitamin C (50 μg/ml), which in turn showed a higher blastocyst rate than the HMC embryos grown in medium without vitamin C. In terms of cell number per blastocyst, PA and HMC embryos for 50 μg/ml supplementation of vitamin C had higher cell numbers (61 and 59, respectively) compared with HMC embryos (44) cultured in medium without vitamin C.
Table 6. Developmental competence of porcine parthenotes and handmade cloned (HMC) embryos cultured in media supplemented with (50 µg/ml) or without vitamin C.
| Total No. embryos | Cleavage rate (n) (%) | Blastocyst rate (n) (%) | Total cell number (n) | |
| PA (+) | 99 | 89.6 ± 1.2 (88) | 50.3 ± 1.9a (49) | 61.2 ± 1.7a (44) |
| HMC (–) | 111 | 92.6 ± 1.4 (104) | 27.0 ± 4.8b (34) | 43.8 ± 4.6b (34) |
| HMC (+) | 114 | 95.1 ± 1.6 (109) | 37.6 ± 4.1c (45) | 59.1 ± 4.6a (40) |
a, b, c Numbers with different superscripts in the same column differ (P<0.05). Values from four replicates are shown as the mean ± SEM. PA, parthenotes; HMC, handmade cloned embryos; +, with (50 g/ml) in in vitro culture medium; –, without vitamin C in in vitro culture medium.
Discussion
Ameliorating the culture system is of great importance for gamete and embryo manipulation and production in vitro. One of the major problems encountered in the culture system has been oxidative stress and its adverse effects on oocytes and embryos [1, 23, 24, 33]. To alleviate this situation, special attention has been accorded to the addition of antioxidants to the culture system [7, 9, 10, 33,34,35], and vitamin C is one of the antioxidant being tested [7, 9, 10, 33, 34, 36]. Evaluating it based on its chemical structure, vitamin C is an electron donor and therefore a reducing agent. It thus has two different biochemical roles: as an antioxidant and an enzymatic cofactor [12]. In the present study, vitamin C did not affect the nuclear maturation status regardless of the concentrations used. Similarly, Tatemoto et al. [7] and Córdova and coworkers [37] found that the addition of vitamin C to the oocyte maturation medium exerted no effect on the maturation rates of bovine oocytes. Our results are also in agreement with those found in murine and bovine [11] oocytes. Nevertheless, Tao et al. [9, 10] showed that L-ascorbic acid promoted polar body extrusion in denuded porcine oocytes. Some antioxidants such as vitamin C, vitamin E and Trolox had no effect on oocyte maturation, but others such as propyl gallate and 2,4,5-trihydroxybutrophenone inhibited the spontaneous resumption of meiosis [38]. In the same study, denuded oocytes were shown to be less sensitive to an antioxidant in terms of resumption of meiosis. It is suggested that the effect of vitamin C supplemented in the IVM medium lies mainly in its capability to promote ooplasmic maturation.
High levels of ROS during early embryonic development caused lipid peroxidation of cell membranes [39, 40] and DNA fragmentation, and its presence also perturbed RNA transcription and protein synthesis [23, 41]. It has been reported that the inability of embryos to develop normally in vitro is caused by a cytoplasmic deficiency of protective enzyme activity, such as those of superoxide dismutase, catalase or the glutathione peroxidase/reductase couple [39]. Glutathione is one of the naturally synthesized antioxidants that protect cells from ROS toxicity and regulate the intracellular redox balance [28]. The intracellular level of GSH increases during oocyte maturation [23, 27, 42]. Its concentration in a matured oocyte has been used as a parameter for assessing ooplasmic maturation [28], and it has been in turn associated with the capability to form the male pronucleus after fertilization [7, 27] and the developmental competence of developing embryos [43]. Recent reports also showed that addition of anthocyanin, β-mercaptoethanol or cysteine addition to IVM media improved the cytoplasmic maturation of oocytes and embryo development [28, 43,44,45]. In the present study, 50 µg/ml of vitamin C supplementation during IVM exerted a strong antioxidant effect by decreasing the ROS content and increasing the GSH level in matured oocytes, which presumably allowed better ooplasmic maturation and subsequent embryo development. Concomitantly, we should notice that 100 µg/ml of vitamin C supplementation during IVM did not produce noticeable amelioration of the GSH or ROS status, allowing us to draw the conclusion that there is an existing dose-response window. These results differ in part from those of Tatemoto et al. [7], who found no effect on intracellular GSH content when ascorbic acid was added but obtained an increase in the intracellular GSH content. In addition, vitamin C did have some antioxidant effects, but it also impose some side effects by decreasing the maturation rate at 750 µM/ml [7] and increasing the ratio of abnormal oocytes for COCs at 250 µM/ml and denuded oocytes at 750 µM/ml [9].
In vitro produced embryos with a large number of cells are more likely to successfully implant and give rise to live births [46]. It has been shown that the quality of PA blastocysts is not better than that of IVF blastocysts in terms of their total cell numbers and apoptotic nuclei per blastocyst [9]. In this study, we demonstrated that embryos treated with vitamin C (50 µg/ml) had improved cleavage rates, blastocyst rates and total cell numbers per blastocyst and a reduced apoptotic index. Clearly, a suboptimal concentration of free-radical scavengers in the culture system would also contribute to poor embryo viability and, in turn, to progressive embryo losses during IVP [16, 47]. Nonetheless, supplementation of 100 µg/ml of vitamin C to the culture medium increased the apoptotic index, suggesting the existence of a narrow window for the concentration dependency [10, 48] of vitamin C treatment.
In the present study, parthenotes only showed improvement in their blastocyst rate (Table 6) after vitamin C addition. Supplementation of the medium with 50 µg/ml of vitamin C improved the blastocyst formation rate compared with that of the untreated group and ameliorated the embryo quality compared with that of the PA considering the total cell numbers per blastocyst. Our results are in agreements with those found by Kragh and coworkers [49], but the cell numbers were still low compared with those of embryos (cell numbers = 77) derived from the piglet previously obtained by Du and colleagues [6]. The quality of the developing HMC blastocysts was not impaired compared with those of the parthenogenetic embryos in medium supplemented with 50 µg/ml of vitamin C. The beneficial effect of vitamin C supplementation may be ascribed to an improved culture condition and/or a better cytoplasmic maturation of the oocytes due to a reduced intracellular oxidative status for embryo development [50]. Moreover, the in vitro environment can alter the delicate interdependency of the epigenetic state, metabolisms and development of the embryo [12, 51]. Huang and colleagues [36] related the enhanced acH4K5 signal to more efficient genome activation during reprogramming, as supported by the elevation of Oct4 and Sox2 expression levels observed in vitamin C-treated blastocysts.
In conclusion, the addition of a relatively low concentration of vitamin C during in vitro maturation of porcine oocytes enhanced their cytoplasmic maturation and subsequent development, whereas high concentrations of vitamin C induced apoptosis. Our study clearly also demonstrated that supplementation of 50 µg/ml of vitamin C in IVC medium improved, at least partially, the suboptimal culture conditions for porcine parthenotes and cloned embryos. Further studies are required to elucidate more in-depth mechanisms underlying oocyte maturation in the presence of vitamin C as well as potential additives in the culture medium.
Acknowledgment
This research was supported in part by grants from the National Science Council (NSC 98-2628-B005-019-MY3), Executive Yuan, and the Ministry of Education, Taiwan, Republic of China, under the ATU plan. Special thanks go to our colleagues in the reproductive biotechnology laboratory for technical assistance. We are also grateful to Taichung Abattoir for their kind provision of porcine ovaries.
References
- 1.Gardner DK. Dissection of culture media for embryos: the most important and less important components and characteristics. Reprod Fertil Dev 2008; 20: 9–18 [DOI] [PubMed] [Google Scholar]
- 2.Gardner DK, Lane M. Ex vivo early embryo development and effects on gene expression and imprinting. Reprod Fertil Dev 2005; 17: 361–370 [DOI] [PubMed] [Google Scholar]
- 3.Farstad W. Current state in biotechnology in canine and feline reproduction. Anim Reprod Sci 2000; 60-61: 375–387 [DOI] [PubMed] [Google Scholar]
- 4.Yoshioka K, Suzuki C, Tanaka A, Anas IM, Iwamura S. Birth of piglets derived from porcine zygotes cultured in a chemically defined medium. Biol Reprod 2002; 66: 112–119 [DOI] [PubMed] [Google Scholar]
- 5.Fayrer-Hosken R. Embryo transfer in the dog and cat. Theriogenology 2007; 68: 382–385 [DOI] [PubMed] [Google Scholar]
- 6.Du Y, Zhang Y, Li J, Kragh PM, Kuwayama M, Ieda S, Zhang X, Schmidt M, Bøgh IB, Purup S, Pedersen AM, Villemoes K, Yang H, Bolund L, Vajta G. Simplified cryopreservation of porcine cloned blastocysts. Cryobiology 2007; 54: 181–187 [DOI] [PubMed] [Google Scholar]
- 7.Tatemoto H, Ootaki K, Shigeta K, Muto N. Enhancement of developmental competence after in vitro fertilization of porcine oocytes by treatment with ascorbic acid 2-O-alpha-glucoside during in vitro maturation. Biol Reprod 2001; 65: 1800–1806 [DOI] [PubMed] [Google Scholar]
- 8.Bormann CL, Ongeri EM, Krisher RL. The effect of vitamins during maturation of caprine oocytes on subsequent developmental potential in vitro. Theriogenology 2003; 59: 1373–1380 [DOI] [PubMed] [Google Scholar]
- 9.Tao Y, Zhou B, Xia G, Wang F, Wu Z, Fu M. Exposure to L-ascorbic acid or alpha-tocopherol facilitates the development of porcine denuded oocytes from metaphase I to metaphase II and prevents cumulus cells from fragmentation. Reprod Domest Anim 2004; 39: 52–57 [DOI] [PubMed] [Google Scholar]
- 10.Tao Y, Chen H, Tian NN, Huo DT, Li G, Zhang YH, Liu Y, Fang FG, Ding JP, Zhang XR. Effects of L-ascorbic acid, alpha-tocopherol and co-culture on in vitro developmental potential of porcine cumulus cells free oocytes. Reprod Domest Anim 2010; 45: 19–25 [DOI] [PubMed] [Google Scholar]
- 11.Dalvit G, Lanes SP, Descalzo A, Insani M, Beconi M, Cetica P. Effect of alpha-tocopherol and ascorbic acid on bovine oocyte in vitro maturation. Reprod Domest Anim 2005; 40: 93–97 [DOI] [PubMed] [Google Scholar]
- 12.Belin S, Kaya F, Burtey S, Fontes M. Ascorbic acid and gene expression: another example of regulation of gene expression by small molecules? Curr Genomics 2010; 11: 52–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim S, Lee SH, Kim JH, Jeong YW, Hashem MA, Koo OJ, Park SM, Lee EG, Hossein MS, Kang SK, Lee BC, Hwang WS. Anti-apoptotic effect of insulin-like growth factor (IGF)-I and its receptor in porcine preimplantation embryos derived from in vitro fertilization and somatic cell nuclear transfer. Mol Reprod Dev 2006; 73: 1523–1530 [DOI] [PubMed] [Google Scholar]
- 14.Lange Consiglio A, Dell' Aquila ME, Fiandanese N, Ambruosi B, Cho YS, Bosi G, Arrighi S, Lacalandra GM, Cremonesi F. Effects of leptin on in vitro maturation, fertilization and embryonic cleavage after ICSI and early developmental expression of leptin (Ob) and leptin receptor (ObR) proteins in the horse. Reprod Biol Endocrinol 2009; 7: 113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen NT, Lin DP, Yen SY, Tseng JK, Chuang JF, Chen BY, Lin TA, Chang HH, Ju JC. Sonic hedgehog promotes porcine oocyte maturation and early embryo development. Reprod Fertil Dev 2009; 21: 805–815 [DOI] [PubMed] [Google Scholar]
- 16.Nguyen NT, Lin DP, Siriboon C, Lo NW, Ju JC. Sonic Hedgehog improves in vitro development of porcine parthenotes and handmade cloned embryos. Theriogenology 2010; 74: 1149–1160 [DOI] [PubMed] [Google Scholar]
- 17.Nguyen NT, Lo NW, Chuang SP, Jian YL, Ju JC. Sonic hedgehog supplementation of oocyte and embryo culture media enhances development of IVF porcine embryos. Reproduction 2011; 142: 87–97 [DOI] [PubMed] [Google Scholar]
- 18.de Moraes AA, Hansen PJ. Granulocyte-macrophage colony-stimulating factor promotes development of in vitro produced bovine embryos. Biol Reprod 1997; 57: 1060–1065 [DOI] [PubMed] [Google Scholar]
- 19.Nilsson EE, Schindler R, Savenkova MI, Skinner MK. Inhibitory actions of Anti-Müllerian Hormone (AMH) on ovarian primordial follicle assembly. PLoS One 2011; 6: e20087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nam DH, Lee SH, Kim HS, Lee GS, Jeong YW, Kim S, Kim JH, Kang SK, Lee BC, Hwang WS. The role of gonadotropin-releasing hormone (GnRH) and its receptor in development of porcine preimplantation embryos derived from in vitro fertilization. Theriogenology 2005; 63: 190–201 [DOI] [PubMed] [Google Scholar]
- 21.Abeydeera LR, Wang H, Cantley T, Rieke A, Prather RS, Day BN. Presence of epidermal growth factor during in vitro maturation of pig oocytes and embryo culture can modulate blastocyst development after in vitro fertilization. Mol Reprod Dev 1998; 51: 395–401 [DOI] [PubMed] [Google Scholar]
- 22.Ock SA, Lee SL, Kim JG, Kumar BM, Balasubramanian S, Choe SY, Rho GJ. Development and quality of porcine embryos in different culture system and embryo-producing methods. Zygote 2007; 15: 1–8 [DOI] [PubMed] [Google Scholar]
- 23.Tatemoto H, Sakurai N, Muto N. Protection of porcine oocytes against apoptotic cell death caused by oxidative stress during in vitro maturation: role of cumulus cells. Biol Reprod 2000; 63: 805–810 [DOI] [PubMed] [Google Scholar]
- 24.Kitagawa Y, Suzuki K, Yoneda A, Watanabe T. Effects of oxygen concentration and antioxidants on the in vitro developmental ability, production of reactive oxygen species (ROS), and DNA fragmentation in porcine embryos. Theriogenology 2004; 62: 1186–1197 [DOI] [PubMed] [Google Scholar]
- 25.Iwamoto M, Onishi A, Fuchimoto D, Somfai T, Suzuki S, Yazaki S, Hashimoto M, Takeda K, Tagami T, Hanada H, Noguchi J, Kaneko H, Nagai T, Kikuchi K. Effects of caffeine treatment on aged porcine oocytes: parthenogenetic activation ability, chromosome condensation and development to the blastocyst stage after somatic cell nuclear transfer. Zygote 2005; 13: 335–345 [DOI] [PubMed] [Google Scholar]
- 26.Meister A, Anderson ME. Glutathione. Annu Rev Biochem 1983; 52: 711–760 [DOI] [PubMed] [Google Scholar]
- 27.Yoshida M, Ishigaki K, Nagai T, Chikyu M, Pursel VG. Glutathione concentration during maturation and after fertilization in pig oocytes: relevance to the ability of oocytes to form male pronucleus. Biol Reprod 1993; 49: 89–94 [DOI] [PubMed] [Google Scholar]
- 28.You J, Kim J, Lim J, Lee E. Anthocyanin stimulates in vitro development of cloned pig embryos by increasing the intracellular glutathione level and inhibiting reactive oxygen species. Theriogenology 2010; 74: 777–785 [DOI] [PubMed] [Google Scholar]
- 29.Ju JC, Tsay C, Ruan CW. Alterations and reversibility in the chromatin, cytoskeleton and development of pig oocytes treated with roscovitine. Mol Reprod Dev 2003; 64: 482–491 [DOI] [PubMed] [Google Scholar]
- 30.Lin TA, Tsay C, Chen CH, Tang PC, Ju JC. Nuclear and cytoskeletal dynamics during oocyte maturation and development of somatic cell cloned pig embryos injected with membrane disintegrated donor cells. Anim Reprod Sci 2008; 103: 107–119 [DOI] [PubMed] [Google Scholar]
- 31.Li J, Du Y, Zhang YH, Kragh PM, Purup S, Bolund L, Yang H, Xue QZ, Vajta G. Chemically assisted handmade enucleation of porcine oocytes. Cloning Stem Cells 2006; 8: 241–250 [DOI] [PubMed] [Google Scholar]
- 32.Hao Y, Lai L, Mao J, Im GS, Bonk A, Prather RS. Apoptosis in parthenogenetic preimplantation porcine embryos. Biol Reprod 2004; 70: 1644–1649 [DOI] [PubMed] [Google Scholar]
- 33.Jeong YW, Park SW, Hossein MS, Kim S, Kim JH, Lee SH, Kang SK, Lee BC, Hwang WS. Antiapoptotic and embryotrophic effects of alpha-tocopherol and L-ascorbic acid on porcine embryos derived from in vitro fertilization and somatic cell nuclear transfer. Theriogenology 2006; 66: 2104–2112 [DOI] [PubMed] [Google Scholar]
- 34.Hossein MS, Hashem MA, Jeong YW, Lee MS, Kim S, Kim JH, Koo OJ, Park SM, Lee EG, Park SW, Kang SK, Lee BC, Hwang WS. Temporal effects of alpha-tocopherol and L-ascorbic acid on in vitro fertilized porcine embryo development. Anim Reprod Sci 2007; 100: 107–117 [DOI] [PubMed] [Google Scholar]
- 35.Wu GQ, Jia BY, Li JJ, Fu XW, Zhou GB, Hou YP, Zhu SE. L-carnitine enhances oocyte maturation and development of parthenogenetic embryos in pigs. Theriogenology 2011; 76: 785–793 [DOI] [PubMed] [Google Scholar]
- 36.Huang Y, Tang X, Xie W, Zhou Y, Li D, Zhou Y, Zhu J, Yuan T, Lai L, Pang D, Ouyang H. Vitamin C enhances in vitro and in vivo development of porcine somatic cell nuclear transfer embryos. Biochem Biophys Res Commun 2011; 411: 397–401 [DOI] [PubMed] [Google Scholar]
- 37.Córdova B, Morató R, Izquierdo D, Paramio T, Mogas T. Effect of the addition ofinsulin-transferrin-selenium and/or L-ascorbic acid to the in vitro maturation of prepubertal bovine oocytes on cytoplasmic maturation and embryo development. Theriogenology 2010; 74: 1341–1348 [DOI] [PubMed] [Google Scholar]
- 38.Takami M, Preston SL, Toyloy VA, Behrman HR. Antioxidants reversibly inhibit the spontaneous resumption of meiosis. Am J Physiol 1999; 276: E684–688 [DOI] [PubMed] [Google Scholar]
- 39.Nasr-Esfahani MH, Aitken JR, Johnson MH. Hydrogen peroxide levels in mouse oocytes and early cleavage stage embryos developed in vitro or in vivo. Development 1990; 109: 501–507 [DOI] [PubMed] [Google Scholar]
- 40.Noda Y, Matsumoto H, Umaoka Y, Tatsumi K, Kishi J, Mori T. Involvement of superoxide radicals in the mouse two-cell block. Mol Reprod Dev 1991; 28: 356–360 [DOI] [PubMed] [Google Scholar]
- 41.Takahashi M, Keicho K, Takahashi H, Ogawa H, Schultz RM, Okano A. Effect of oxidative stress on development and DNA damage in in-vitro cultured bovine embryos by comet assay. Theriogenology 2000; 54: 137–145 [DOI] [PubMed] [Google Scholar]
- 42.de Matos DG, Furnus CC, Moses DF. Glutathione synthesis during in vitro maturation of bovine oocytes: role of cumulus cells. Biol Reprod 1997; 57: 1420–1425 [DOI] [PubMed] [Google Scholar]
- 43.Furnus CC, De Matos DG, Moses DF. Cumulus expansion during in vitro maturation of bovine oocytes: relationship with intracellular glutathione level and its role on subsequent embryo development. Mol Reprod Dev 1998; 51: 76–83 [DOI] [PubMed] [Google Scholar]
- 44.Choe C, Shin YW, Kim EJ, Cho SR, Kim HJ, Choi SH, Han MH, Han J, Son DS, Kang D. Synergistic effects of glutathione and β-mercaptoethanol treatment during in vitro maturation of porcine oocytes on early embryonic development in a culture system supplemented with L-cysteine. J Reprod Dev 2010; 56: 575–582 [DOI] [PubMed] [Google Scholar]
- 45.Nakamura BN, Fielder TJ, Hoang YD, Lim J, McConnachie LA, Kavanagh TJ, Luderer U. Lack of maternal glutamate cysteine ligase modifier subunit (Gclm) decreases oocyte glutathione concentrations and disrupts preimplantation development in mice. Endocrinology 2011; 152: 2806–2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Soom A, Ysebaert MT, De Kruif A. Relationship between timing of development, morula morphology, and cell allocation to inner cell mass and trophectoderm in in vitro-produced bovine embryos. Mol Reprod Dev 1997; 47: 47–56 [DOI] [PubMed] [Google Scholar]
- 47.Nánássy L, Lee K, Jávor A, Macháty Z. Effects of activation methods and cultureconditions on development of parthenogenetic porcine embryos. Anim Reprod Sci 2008; 104: 264–274 [DOI] [PubMed] [Google Scholar]
- 48.Naruse K, Kim HR, Shin YM, Chang SM, Lee HR, Park CS, Jin DI. Low concentrations of MEM vitamins during in vitro maturation of porcine oocytes improves subsequent parthenogenetic development. Theriogenology 2007; 67: 407–412 [DOI] [PubMed] [Google Scholar]
- 49.Kragh PM, Du Y, Corydon TJ, Purup S, Bolund L, Vajta G. Efficient in vitro production of porcine blastocysts by handmade cloning with a combined electrical and chemical activation. Theriogenology 2005; 64: 1536–1545 [DOI] [PubMed] [Google Scholar]
- 50.Suzuki C, Yoshioka K, Sakatani M, Takahashi M. Glutamine and hypotaurine improves intracellular oxidative status and in vitro development of porcine preimplantation embryos. Zygote 2007; 15: 317–324 [DOI] [PubMed] [Google Scholar]
- 51.Chason RJ, Csokmay J, Segars JH, Decherney AH, Armant DR. Environmental and epigenetic effects upon preimplantation embryo metabolism and development. Trends Endocrinol Metab 2011; 22: 412–420 [DOI] [PMC free article] [PubMed] [Google Scholar]


