Abstract
Background
The nonapeptide oxytocin (OT) has been repeatedly implicated in processes of parent-infant bonding in animal models; yet, its role in the development of human parenting has received less attention and no research has addressed the involvement of OT in the transition to fatherhood.
Methods
Using a prospective longitudinal design, 160 cohabitating mothers and fathers and their firstborn infant were visited at home during the first postpartum weeks and again at 6 months postpartum. Mothers’ and fathers’ plasma OT was analyzed at each time point with enzyme-linked immunosorbent assay methodology. Interactions between each parent and the infant were observed in the postpartum and microcoded for parenting behavior.
Results
Overall, parental OT increased across the study period and there were no differences between maternal and paternal OT at each time point. Oxytocin showed high intraindividual stability across the first 6 months of parenting and the OT levels of husband and wife were interrelated at both assessments. Maternal OT was related to the amount of affectionate parenting behaviors, including “motherese” vocalizations, the expression of positive affect, and affectionate touch, whereas paternal OT correlated with the degree of stimulatory parenting behaviors, including proprioceptive contact, tactile stimulation, and object presentation.
Conclusions
Results are the first to describe plasma OT levels in new fathers and mothers across the transition to parenthood in relation to maternal and paternal typical parenting behaviors. These data may provide a normative basis for the study of parenting under conditions of high risk.
Keywords: Infant development, neurobiology of parenting, oxytocin, parent-infant interaction, parenting, transition to parenthood
Sensitive parenting is considered the cornerstone of the individual’s well-being and adaptation throughout life (1,2). Uncovering the neurobiological basis of the parent-infant bonding process in normative populations may therefore be important for understanding disruptions to the bonding process that may lead to pathological development. Research has repeatedly implicated the neuropeptide oxytocin (OT) as one of the key hormones involved in parent-infant bonding in mammals, as well as in a range of social and affiliative behaviors (3–7). Oxytocin plays an important role in birth and lactation, and central OT injection in animals can quickly induce the full-blown repertoire of maternal behavior (8–12).
Studies on the associations between OT and parenting in mammals have pointed to several mechanisms by which central OT may affect the development of parenting behavior. For instance, maternal licking and grooming of rat pups during the postpartum period has been associated with greater OT receptor binding or increased activity in brain areas critical for the development of parenting in mammals, including the medial preoptic area, lateral septum, the paraventricular nucleus of the hypothalamus, and the nucleus accumbens (13–16). Cross-fostering studies indicated that such patterns of maternal behavior are transmitted from mother to daughter on the basis of early experience. In sheep, OT brain activity has been shown to regulate aspects of maternal interest and involvement, such as the inhibition of aggression and sexual motivation after birth (11), and in free-ranging Rhesus Macaques, plasma OT is related to increased nursing and grooming (17).
The role of OT in the development of human affiliation and affiliation-related behaviors has recently been demonstrated through the use of intranasal administration of OT. Oxytocin inhaling was found to increase trust (18), reduce negative behavior during couple conflict (19,20), decrease amygdala activation to fearful faces (21–23), and increase theory of mind skills (24), suggesting a potential direct OT involvement in processes of social bonding and interpersonal closeness, as well as in other social and emotional behaviors (25). Still, very little is known on the effects of OT on human parenting. Specifically, the normative distributions of peripheral measurements of maternal and paternal OT across the first months of parenting has not, to our knowledge, been systematically examined. Such information in healthy mothers and fathers may be important for research on high-risk parenting, including conditions such as postpartum depression, premature birth, child maltreatment, or contextual risk.
Several studies in human mothers have shown that peripheral OT release during birth, breastfeeding, and skin-to-skin contact between mothers and newborns is related to higher maternal responsiveness, lower maternal stress, and more optimal motherinfant bond (3,26). A study that followed maternal OT levels from early pregnancy to the postpartum showed that a significant increase from the first to the third trimester was associated with the degree of the mother’s emotional bonding to her fetus (27). In addition, higher levels of plasma OT in the first trimester predicted greater amounts of maternal behavior in the postpartum, including gaze toward the infant’s face, positive affect, and affectionate touch (28).
As such, the overall goal of the present study was to document the levels of plasma OT in first-time mothers and fathers during the first weeks of parenting and again when the infant was 6 months old. Three specific goals guided the study: 1) to compare plasma OT concentrations in mothers and fathers across the first 6 months of parenting and to assess interrelatedness in OT levels between cohabitating partners; 2) to assess whether OT levels in mothers and fathers are individually stable across the first postpartum months; and 3) to examine whether maternal and paternal OT are related to a specific set of maternal and paternal behaviors that are characteristic of human mothers and fathers.
Methods and Materials
Participants
Eighty cohabitating couples and their firstborn infant (n = 240 participants) participated in the study. Of these, 128 parents (66 mothers and 62 fathers) were seen again when the infant was approximately 6 months old (M = 24.8 weeks, SD = 4.38). Fathers’ age averaged 29.45 years (SD = 3.87) and education averaged 15.7 years (SD = 2.85), and mothers’ age averaged 27.24 years (SD = 3.67) and education averaged 16.08 years (SD = 2.22). Parents were all residing in central Israel, were of middle class background, had completed at least high school education, and were above 20 years old, and the infant was the first child to both mother and father. Infants (37 boys) were all healthy firstborns of a singleton birth. Sixty-five infants (81.2%) were born by vaginal birth and 15 infants were born by cesarean section. Most mothers (86.2%) breastfed at the first visit and 73.8% were still breastfeeding at the second visit. Participants were recruited through advertisements that were posted in the university and surrounding area and in parenting message boards online. The research was approved by the Institutional Review Board and conducted according to ethical standards, and all participants signed an informed consent.
Oxytocin
Parents were visited at home during the evening hours (4:00–8:00 pm). Parents first signed an informed consent and then completed self-report measures assessing a range of demographic and health variables (e.g., weight, height, smoking, menstrual cycle, contraceptives, and feeding patterns). Next, blood was drawn from the antecubital vein of the parents into 9 mL chilled vacutainer tubes containing lithium heparin that were supplemented with 400 KIU of Trasylol (Bayer, Leverkusen, Germany) per 1 mL blood. Oxytocin samples were kept ice-chilled for up to 2 hours before being centrifuged at 4°C at 1000g for 15 minutes. Supernatants were collected and stored at −70°C until assayed. Parents were asked to refrain from food intake for 30 minutes before blood draw. Maternal blood was drawn at least 30 minutes after nursing and 30 minutes before nursing. In the first home visit, blood was not drawn from two mothers, and in the second time point of the study, blood could not be drawn from eight mothers and three fathers for technical reasons.
Determination of OT was performed using a commercial OT enzyme-linked immunosorbent assay kit (Assay Design, Ann Arbor, Michigan) as described in earlier studies (27–30). Measurements were performed in duplicate and the concentrations of samples were calculated by using MATLAB-7 (The MathWorks, Inc., Natick, Massachusetts) according to relevant standard curves. The intra-assay and interassay coefficients were less than 12.4% and 14.5%, respectively.
Parenting Behavior
In the first visit, each parent was videotaped interacting with the infant for 10 minutes. Instructions were to play with your infant as you normally do and no specific position or toys were required. If the infant was asleep throughout the home visit or was crying and could not be soothed, interactions were not videotaped. Interactions were available for 58 fathers and 61 mothers. Infants’ mean age at the first time point was 7.1 weeks (SD = 2.11).
Interactions were microcoded by trained graduate students on The Observer computerized system (Noldus, Wageningen, Netherlands) in .01 second frames, consistent with previous research on parent-infant interaction in the neonatal period that utilized the same computerized system (31–33). Four nonverbal categories of parenting behavior were coded and each category included a set of mutually exclusive codes (an “uncodable” code was added to each category to address moments when codes could not be determined).
Categories and codes were as follows: parent gaze—to infant’s face, to infant’s body, to object or environment, gaze aversion; parent affect—positive, neutral, negative; parent vocalizations— motherese (high-pitched, sing-song vocalization), adult speech to infant, adult speech to other adult, none; and parent touch—affectionate touch (e.g., hugging, kissing, stroking), touch of infant extremities, functional touch, proprioceptive touch (i.e., changing infant position in space), object presentation, stimulatory touch, none.
Interrater reliability was conducted for 10% of the interactions and averaged 98% (kappa = .84). For each behavior, we computed the proportions of time out of the entire interaction this behavior had occurred. Two composite scores for parental behavior were created in light of previous microanalytic studies of parenting in the postpartum (28,32–35): affectionate parenting behavior was the sum of the proportions of positive affect, motherese vocalization, and affectionate touch, and stimulatory parenting behavior was the sum of the proportions of proprioceptive touch, object presentation, and stimulatory touch.
Results
Oxytocin
Maternal and paternal plasma OT levels in the second and sixth postpartum months are presented in Table 1. As seen, OT levels are consistent with previous studies in adults using enzyme-linked immunosorbent assay methodology (27,28,30). Figure 1 illustrates frequency distributions of OT levels in mothers and fathers during the first and second visits. Prior to data analysis, OT levels were log transformed and outliers higher than three standard deviations of the mean in both time points were omitted (n = 6).
Table 1.
Plasma OT Concentrations in First-Time Mothers and Fathers During the First Postpartum Weeks and at 6 Months Postpartum
| OT Levels | Means (SD) (pg/mL) |
Maximum (pg/mL) |
Minimum (pg/mL) |
n |
|---|---|---|---|---|
| Mothers (time 1) | 337.35 (195.64) | 1351 | 147.8 | 76 |
| Fathers (time 1) | 401.98 (360.28) | 2752.3 | 51.4 | 76 |
| Mothers (time 2) | 357.79 (207.72) | 1351 | 125.2 | 59 |
| Fathers (time 2) | 434.12 (401.88) | 2233.8 | 184 | 56 |
OT, oxytocin.
Figure 1.
Distributions of oxytocin concentrations in first-time mothers and fathers in the postpartum weeks and at 6 months postpartum.
Pearson correlations indicated that OT was unrelated to demographic variables, including parent age, height, weight, smoking, use of medications, and time of last meal. In addition, maternal OT was not related to menstrual cycle phase, contraceptive intake, mode of delivery (vaginal vs. caesarean section), feeding style (breastfeeding vs. bottle feeding), postpartum interval (weeks from birth to date of blood draw), or the interval from prior breastfeeding.
To examine our first study question, a repeated measures analysis of variance was conducted with parent gender as the between-subject factor. No main effect was found for parent, F(1,108) = .04, p = .83, effect size = .00, indicating that mothers and fathers show similar levels of OT across the first 6 months of parenthood. A significant main effect emerged for time: F (1,108) = 6.27, p < .005, effect size = .06. Overall, OT levels increased in mothers and fathers from the first postpartum weeks to 6 months after the birth of their first child.
To further examine our first and second study questions on the associations between OT across time in each parent and between partners, Pearson correlations were computed (Table 2).
Table 2.
Correlations Between Maternal and Paternal OT in the First and Second Assessments
| Fathers |
Mothers |
|||
|---|---|---|---|---|
| Postpartum | 6 Months | Postpartum | 6 Months | |
| Fathers | ||||
| Postpartum | — | .790b | .372b | .317a |
| 6 months | .790b | — | .423b | .317a |
| Mothers | ||||
| Postpartum | .372b | .423b | — | .756b |
| 6 months | .317a | .345a | .756b | — |
OT, oxytocin.
p < .05.
p < .01.
The data presented in Table 2 indicate that OT levels showed high individual stability in both mothers and fathers. Results also demonstrate that OT levels of husband and wife were interrelated at both time points. Furthermore, maternal OT at the first visit was related to paternal OT at the second visit and vice versa, pointing to a close interdependence between the OT levels of cohabitating parents during the transition to parenthood.
Parenting Behavior
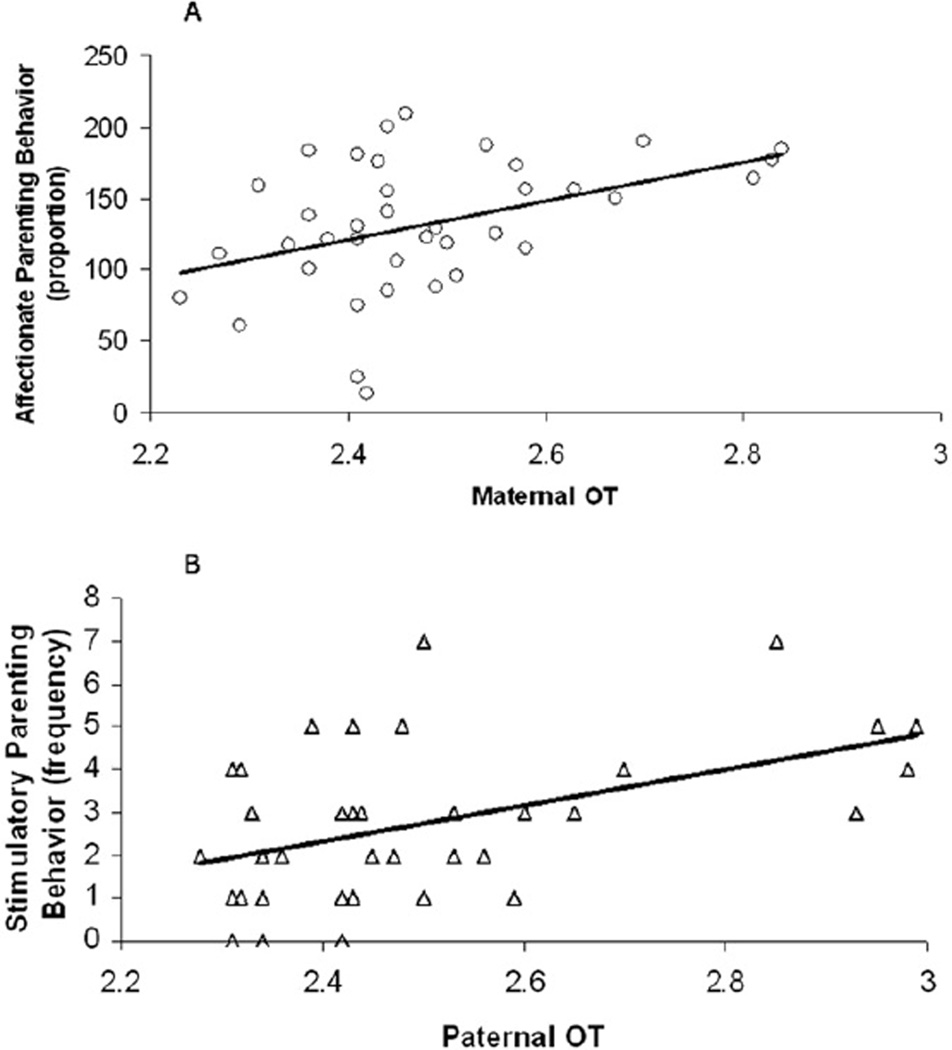
Mean proportions of affectionate play was .66 (SE = .05) and the frequency was 4.79 (SE = .20). Mean proportions of stimulatory play behavior was .37 (SE = .02) and the frequency was 2.65 (SE = .15). Pearson correlations showed that among both fathers and mothers the proportion of affectionate behavior and stimulatory play was negatively related (r = −.51, p < .005, and r = −.41, p < .005 for fathers and mothers, respectively). To examine the differences between mothers and fathers in the distributions of parenting behavior, paired sample t tests were preformed. No mean-level differences were found in the proportion of time mothers and fathers engaged in affectionate and stimulatory parenting behaviors. However, maternal OT correlated with the amount of time mothers spent in affectionate parenting behavior, r = .33, p < .05, but not with the time mothers engaged in stimulatory parenting behaviour, r = −.22, p > .10. On the other hand, paternal OT levels were related to the amount of time fathers spent in stimulatory parenting behavior, r = .30, p < .05, but not with the proportions of paternal affectionate parenting behavior, r = .08, p > .10. Figures 2A and 2B present the scatter plots for these correlations.
Figure 2.
Correlation between maternal oxytocin and mother’s affectionate play A and between paternal oxytocin and father’s stimulatory play B. OT, oxytocin.
Discussion
Results of the present study provide the first normative data on the distributions of OT in human mothers and fathers across the first months of parenthood. The data point to similarities between the OT levels of mothers and fathers and show that during the early phases of parenthood, peripheral OT levels in cohabitating parents are interrelated. Finally, the findings point to gender-specific associations between parental OT and parenting behaviors during dyadic interactions in the first postpartum weeks: affectionate parenting behavior was associated with maternal OT and stimulatory parenting behavior was related to paternal OT.
Although OT has typically been considered a maternal hormone associated with birth and lactation (3,36), our findings show similar basal concentrations of OT in maternal and paternal plasma. The present findings are consistent with recent research assessing plasma (30) and cerebrospinal fluid OT (37), which showed similar concentrations of OT in men and women. These gender similarities are also consistent with studies on the intranasal effects of OT (18,24,38).
The high level of stability found in basal OT levels may suggest that baseline OT levels are relatively stable during the postpartum period, except for moments of specific physiological processes such as breastfeeding or sexual intercourse that lead to considerable rise in OT but are short in duration (26,39–41). When longitudinal changes in basal OT concentrations have been reported, such changes appear to be relatively small in magnitude (27,42). Future research is required to assess whether breastfeeding has a prolonged effect on basal OT levels and whether such effect may explain the rise in OT levels from the second to the sixth postpartum month.
The significant rise in OT during the first 6 months of parenting may suggest that OT increases in parents as their relationship with the infant evolves. Parents derive a greater reward from interacting with an infant who is a more active and reciprocal social partner at 6 months (43–46) and this may lead to greater release of OT, similar to the role of positive interactions in increasing brain OT (13,47). In addition, as parents experience higher stress over the first 6 months of parenting (48,49), OT activity may increase to mediate such parenting stress (16,49,50).
Maternal OT was associated with parenting behavior that incorporates maternal gaze, positive affect, and affectionate touch, a behavioral constellation that defines the maternal postpartum repertoire in human mothers and has shown to predict infants’ social and emotional growth (28,31,32,34). On the other hand, paternal OT correlated with stimulatory, proprioceptive, and object-oriented play, a style that is typical of the human father’s interactions with his infant (51–55). The links between maternal OT and maternal affectionate contact are consistent with studies in animal models (13,14,47) that pointed to the involvement of central OT in regulating the degree of “licking-and-grooming” maternal behaviors in female rats. Future genetic research is required to shed light on the contribution of OT receptor gene polymorphisms to individual differences in parenting behavior and to potential susceptibilities to neglectful parenting.
The affectionate play and stimulatory play composites possibly represent two modes of parenting behavior that may be underlay by distinct genetic and hormonal constellations. For instance, two parenting styles—social and didactic—have been described in the interactions of mothers and their infants, which were stable over time and unrelated to each other (56). It is also possible that OT is related to the type of behaviors from which mothers and fathers derive the most reward. Infants tend to prefer fathers as playmates when they are positive and choose mothers for comfort when distressed (52,53,57). The infant’s preference may be of high reward value for the parent, and thus, although mothers and fathers displayed similar levels of affectionate and stimulatory play, OT may be linked to the behaviors each parent found the most rewarding. These findings are consistent with the associations reported between central OT functioning and the mesolimbic dopamine reward pathways in animals (7,58–61). Future research is thus needed to follow the parent’s interactive style in relation to hormonal levels and the developing attachment across the first years of life.
The present study is the first to demonstrate associations between OT levels in cohabitating partners; yet, due to the study design, it is not possible to determine the mechanisms through which such hormonal synchronization occurs. Similar interdependence between attachment partners has been reported for hormones such as cortisol, vasopressin, epinephrine, adrenocorticotropic hormone, growth hormones, and prolactin (62–64). Similarly, a couples’ intervention program including warm and affectionate touch was found to induce an “endocrine fit” between partners (65). Further longitudinal research is required to follow individuals throughout adulthood and examine changes in OT levels during periods of falling in love with a romantic partner or during the first months of pregnancy to asses whether the correlation in OT levels described here is specific to the postpartum period or represents a more general trend.
Among the limitations of the study is the reliance on basal and peripheral OT without the inclusion of central or reactive measures. Although the relationship between central and peripheral OT levels is not fully understood, both animal (29,66) and recent human (39) studies suggest that central and peripheral activity of the oxytocinergic system is coordinated. The high stability in OT levels across a period of several months and the links reported between plasma OT with socioemotional and behavioral indicators of affiliation (27,28,30,39,67–69) support the validity of peripheral OT as a biomarker of affiliation processes. In addition to the actual level of OT in the periphery, the distribution and density of central OT receptors may be another key factor in understanding the role of OT and related neuropeptides in affiliative behavior (70,71). For example, in rodents there is a major shift in the location and density of OT receptors following parturition in the dams and after weaning in the pups (72,73). Future studies may incorporate measures of OT reactivity to the study of the transition to parenthood.
Our findings highlight the involvement of OT in adaptive human parental bonding. As the functioning of the OT system is disrupted in a variety of psychopathological conditions, including depression and schizophrenia (68,74), assessing the transition to parenthood under risk conditions for the development of parenting is required to provide a broader perspective on the biological underpinning of healthy and high-risk parenting.
Acknowledgments
This study was supported by the United States-Israel Bi-National Science Foundation (#2005-273).
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Ainsworth MD. Attachments beyond infancy. Am Psychol. 1989;44:709–716. doi: 10.1037//0003-066x.44.4.709. [DOI] [PubMed] [Google Scholar]
- 2.Bowlby J. Attachment and Loss. London: Hogarth Press and Institute of Psycho-Analysis; 1969. [Google Scholar]
- 3.Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- 4.Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- 5.Insel TR. A neurobiological basis of social attachment. Am J Psychiatry. 1997;154:726–735. doi: 10.1176/ajp.154.6.726. [DOI] [PubMed] [Google Scholar]
- 6.Insel TR, Fernald RD. How the brain processes social information: Searching for the social brain. Annu Rev Neurosci. 2004;27:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- 7.Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- 8.Bales K, Carter CS. Oxytocin facilitates parental care in female prairie voles (but not in males) Horm Behav. 2002;41:456. [Google Scholar]
- 9.Holman SD, Goy RW. Experiential and hormonal correlates of care-giving in rhesus macaques. In: Pryce CR, Martin RD, Skuse D, editors. Motherhood in Human and Nonhuman Primates: Biosocial Determinants. Basel, Switzerland: Karger; 1995. pp. 87–93. [Google Scholar]
- 10.Ivell R, Russell JA. Oxytocin: Cellular and molecular approaches in medicine and research. Rev Reprod. 1996;1:13–18. doi: 10.1530/ror.0.0010013. [DOI] [PubMed] [Google Scholar]
- 11.Kendrick KM, Keverne EB, Baldwin BA. Intracerebroventricular oxytocin stimulates maternal behaviour in the sheep. Neuroendocrinology. 1987;46:56–61. doi: 10.1159/000124796. [DOI] [PubMed] [Google Scholar]
- 12.Pedersen CA. Oxytocin control of maternal behavior. Regulation by sex steroids and offspring stimuli. Ann N Y Acad Sci. 1997;807:126–145. doi: 10.1111/j.1749-6632.1997.tb51916.x. [DOI] [PubMed] [Google Scholar]
- 13.Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci U S A. 2001;98:12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Champagne FA. Epigenetic mechanisms and the transgenerational effects of maternal care. Front Neuroendocrinol. 2008;29:386–397. doi: 10.1016/j.yfrne.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leckman JF, Herman AE. Maternal behavior and developmental psychopathology. Biol Psychiatry. 2002;51:27–43. doi: 10.1016/s0006-3223(01)01277-x. [DOI] [PubMed] [Google Scholar]
- 16.Leckman JF, Carter CS, Hennessy MB, Hrdy SB, Kervene EB, Klann-Delius G, et al. Biobehavioral processes in attachment and bonding. In: Carter CS, Ahnert L, editors. Dahlem Workshop Report. Cambridge, MA: MIT Press; 2005. pp. 303–349. [Google Scholar]
- 17.Maestripieri D, Hoffman CL, Anderson GM, Carter CS, Higley JD. Mother-infant interactions in free-ranging rhesus macaques: Relationships between physiological and behavioral variables. Physiol Behav. 2009;96:613–619. doi: 10.1016/j.physbeh.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- 19.Ditzen B, Hoppmann C, Klumb P. Positive couple interactions and daily cortisol: On the stress-protecting role of intimacy. Psychosom Med. 2008;70:883–889. doi: 10.1097/PSY.0b013e318185c4fc. [DOI] [PubMed] [Google Scholar]
- 20.Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biol Psychiatry. 2009;65:728–731. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Fischer-Shofty M, Shamay-Tsoory SG, Harari H, Levkovitz Y. The effect of intranasal administration of oxytocin on fear recognition. Neuropsychologia. 2010;48:179–184. doi: 10.1016/j.neuropsychologia.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007;62:1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 24.Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biol Psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 25.Shamay-Tsoory SG, Fischer M, Dvash J, Harari H, Perach-Bloom N, Levkovitz Y. Intranasal administration of oxytocin increases envy and Schadenfreude (gloating) Biol Psychiatry. 2009;66:864–870. doi: 10.1016/j.biopsych.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Light KC, Smith TE, Johns JM, Brownley KA, Hofheimer JA, Amico JA. Oxytocin responsivity in mothers of infants: A preliminary study of relationships with blood pressure during laboratory stress and normal ambulatory activity. Health Psychol. 2000;19:560–567. doi: 10.1037//0278-6133.19.6.560. [DOI] [PubMed] [Google Scholar]
- 27.Levine A, Zagoory-Sharon O, Feldman R, Weller A. Oxytocin during pregnancy and early postpartum: Individual patterns and maternalfetal attachment. Peptides. 2007;28:1162–1169. doi: 10.1016/j.peptides.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 28.Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation: Plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychol Sci. 2007;18:965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- 29.Carter CS. Sex differences in oxytocin and vasopressin: Implications for autism spectrum disorders? Behav Brain Res. 2007;176:170–186. doi: 10.1016/j.bbr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 30.Gordon I, Zagoory-Sharon O, Schneiderman I, Leckman JF, Weller A, Feldman R. Oxytocin and cortisol in romantically unattached young adults: Associations with bonding and psychological distress. Psychophysiology. 2008;45:349–352. doi: 10.1111/j.1469-8986.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- 31.Feldman R, Eidelman AI, Rotenberg N. Parenting stress, infant emotion regulation, maternal sensitivity, and the cognitive development of triplets: A model for parent and child influences in a unique ecology. Child Dev. 2004;75:1774–1791. doi: 10.1111/j.1467-8624.2004.00816.x. [DOI] [PubMed] [Google Scholar]
- 32.Feldman R, Eidelman AI. Direct and indirect effects of breast milk on the neurobehavioral and cognitive development of premature infants. Dev Psychobiol. 2003;43:109–119. doi: 10.1002/dev.10126. [DOI] [PubMed] [Google Scholar]
- 33.Feldman R, Eidelman AI. Parent-infant synchrony and the social-emotional development of triplets. Dev Psychol. 2004;40:1133–1147. doi: 10.1037/0012-1649.40.6.1133. [DOI] [PubMed] [Google Scholar]
- 34.Feldman R, Eidelman AI. Maternal postpartum behavior and the emergence of infant-mother and infant-father synchrony in preterm and full-term infants: The role of neonatal vagal tone. Dev Psychobiol. 2007;49:290–302. doi: 10.1002/dev.20220. [DOI] [PubMed] [Google Scholar]
- 35.Gordon I, Feldman R. Synchrony in the triad: A microlevel process model of coparenting and parent-child interactions. Fam Process. 2008;47:465–479. doi: 10.1111/j.1545-5300.2008.00266.x. [DOI] [PubMed] [Google Scholar]
- 36.Kendrick KM. Oxytocin, motherhood and bonding. Exp Physiol. 2000;85:111S–124S. doi: 10.1111/j.1469-445x.2000.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 37.Leckman JF, Goodman WK, North WG, Chappell PB, Price LH, Pauls DL, et al. Elevated cerebrospinal fluid levels of oxytocin in obsessive-compulsive disorder. Comparison with Tourette’s syndrome and healthy controls. Arch Gen Psychiatry. 1994;51:782–792. doi: 10.1001/archpsyc.1994.03950100030003. [DOI] [PubMed] [Google Scholar]
- 38.Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- 39.Burri A, Heinrichs M, Schedlowski M, Kruger TH. The acute effects of intranasal oxytocin administration on endocrine and sexual function in males. Psychoneuroendocrinology. 2008;33:591–600. doi: 10.1016/j.psyneuen.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 40.Heinrichs M, Meinlschmidt G, Neumann I, Wagner S, Kirschbaum C, Ehlert U, et al. Effects of suckling on hypothalamic-pituitary-adrenal axis responses to psychosocial stress in postpartum lactating women. J Clin Endocrinol Metab. 2001;86:4798–4804. doi: 10.1210/jcem.86.10.7919. [DOI] [PubMed] [Google Scholar]
- 41.Uvnäs-Moberg K, Widstrom AM, Nissen E, Bjorvell H. Personality traits inwomen4 days post partumandtheir correlation with plasma levels of oxytocin and prolactin. J Psychosom Obstet Gynaecol. 1990;11:261–273. [Google Scholar]
- 42.Chatterton RT, Jr, Hill PD, Aldag JC, Hodges KR, Belknap SM, Zinaman MJ. Relation of plasma oxytocin and prolactin concentrations to milk production in mothers of preterm infants: Influence of stress. J Clin Endocrinol Metab. 2000;85:3661–3668. doi: 10.1210/jcem.85.10.6912. [DOI] [PubMed] [Google Scholar]
- 43.Feldman R. Parent-infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. J Child Psychol Psychiatry. 2007;48:329–354. doi: 10.1111/j.1469-7610.2006.01701.x. [DOI] [PubMed] [Google Scholar]
- 44.Haith MM, Bergman T, Moore MJ. Eye contact and face scanning in early infancy. Science. 1977;198:853–855. doi: 10.1126/science.918670. [DOI] [PubMed] [Google Scholar]
- 45.Robson KS, Moss HA. Patterns and determinants of maternal attachment. J Pediatr. 1970;77:976–985. doi: 10.1016/s0022-3476(70)80080-4. [DOI] [PubMed] [Google Scholar]
- 46.Stern DN. The First Relationship: Infant and Mother. Cambridge, MA: Harvard University Press; 1977. [Google Scholar]
- 47.Francis DD, Champagne FC, Meaney MJ. Variations in maternal behavior are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol. 2000;12:1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- 48.Cowan CP, Cowan PA. When Partners Become Parents: The Big Life Change for Couples. New York: Basic Books; 1992. [Google Scholar]
- 49.Neumann ID. Brain oxytocin: A key regulator of emotional and social behaviours in both females and males. J Neuroendocrinol. 2008;20:858–865. doi: 10.1111/j.1365-2826.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- 50.Uvnas-Moberg K, Petersson M. [Oxytocin, a mediator of antistress, well-being, social interaction, growth and healing] Z Psychosom Med Psychother. 2005;51:57–80. doi: 10.13109/zptm.2005.51.1.57. [DOI] [PubMed] [Google Scholar]
- 51.Feldman R. Infant-mother and infant-father synchrony: The co-regulation of positive arousal. Infant Ment Health J. 2003;24:1–23. [Google Scholar]
- 52.Lamb ME. A re-examination of the infant social world. Hum Dev. 1977;20:65–85. doi: 10.1159/000271548. [DOI] [PubMed] [Google Scholar]
- 53.Lamb ME. The role of the father: Interactions between 8 month old children and their fathers and mothers. In: Lamb ME, editor. The Role of the Father in Child Development. New York: Wiley & Sons; 1976. [Google Scholar]
- 54.Parke RD, Sawin DB. The father’s role in infancy: A re-evaluation. Fam Coord. 1976;25:365–371. [Google Scholar]
- 55.Rodholm M. Early mother-infant and father-infant interaction [dissertation] Sweden: University of Göteborg; 1981. [Google Scholar]
- 56.Bornstein MH, Tamis-LeMonda CS. Activities and interactions of mothers and their firstborn infants in the first six months of life: Covariation, stability, continuity, correspondence, and prediction. Child Dev. 1990;61:1206–1217. doi: 10.1111/j.1467-8624.1990.tb02854.x. [DOI] [PubMed] [Google Scholar]
- 57.Kotelchuck M. The infant’s relationship to the father: Experimental evidence. In: Lamb ME, editor. The Role of the Father in Child Development. New York: Wiley & Sons; 1976. [Google Scholar]
- 58.Ahern TH, Young LJ. The impact of early life family structure on adult social attachment, alloparental behavior, and the neuropeptide systems regulating affiliative behaviors in the monogamous prairie vole (Microtus ochrogaster) Front Behav Neurosci. 2009;3:17. doi: 10.3389/neuro.08.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci. 2004;24:4113–4123. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- 61.Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm Behav. 2001;40:133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- 62.Schreiber JE, Shirtcliff E, Van Hulle C, Lemery-Chalfant K, Klein MH, Kalin NH, et al. Environmental influences on family similarity in afternoon cortisol levels: Twin and parent-offspring designs. Psychoneuroendocrinology. 2006;31:1131–1137. doi: 10.1016/j.psyneuen.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robles TF, Kiecolt-Glaser JK. The physiology of marriage: Pathways to health. Physiol Behav. 2003;79:409–416. doi: 10.1016/s0031-9384(03)00160-4. [DOI] [PubMed] [Google Scholar]
- 64.Kiecolt-Glaser JK, Bane C, Glaser R, Malarkey WB. Love, marriage, and divorce: Newlyweds’ stress hormones foreshadow relationship changes. J Consult Clin Psychol. 2003;71:176–188. doi: 10.1037//0022-006x.71.1.176. [DOI] [PubMed] [Google Scholar]
- 65.Holt-Lunstad J, Birmingham WA, Light KC. Influence of a “warm touch” support enhancement intervention among married couples on ambulatory blood pressure, oxytocin, alpha amylase, and cortisol. Psychosom Med. 2008;70:976–985. doi: 10.1097/PSY.0b013e318187aef7. [DOI] [PubMed] [Google Scholar]
- 66.Wotjak CT, Ganster J, Kohl G, Holsboer F, Landgraf R, Engelmann M. Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: New insights into the secretory capacities of peptidergic neurons. Neuroscience. 1998;85:1209–1222. doi: 10.1016/s0306-4522(97)00683-0. [DOI] [PubMed] [Google Scholar]
- 67.Barraza JA, Zak PJ. Empathy toward strangers triggers oxytocin release and subsequent generosity. Ann N Y Acad Sci. 2009;1167:182–189. doi: 10.1111/j.1749-6632.2009.04504.x. [DOI] [PubMed] [Google Scholar]
- 68.Cyranowski JM, Hofkens TL, Frank E, Seltman H, Cai HM, Amico JA. Evidence of dysregulated peripheral oxytocin releaseamongdepressed women. Psychosom Med. 2008;70:967–975. doi: 10.1097/PSY.0b013e318188ade4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dumont GJ, Sweep FC, van der Steen R, Hermsen R, Donders AR, Touw DJ, et al. Increased oxytocin concentrations and prosocial feelings in humans after ecstasy (3,4-methylenedioxymethamphetamine) administration. Soc Neurosci. 2009;4:359–366. doi: 10.1080/17470910802649470. [DOI] [PubMed] [Google Scholar]
- 70.Leckman JF, Goodman WK, North WG, Chappell PB, Price LH, Pauls DL, et al. The role of central oxytocin in obsessive compulsive disorder and related normal behavior. Psychoneuroendocrinology. 1994;19:723–749. doi: 10.1016/0306-4530(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 71.Insel TR, O’Brien DJ, Leckman JF. Oxytocin, vasopressin, and autism: Is there a connection? Biol Psychiatry. 1999;45:145–157. doi: 10.1016/s0006-3223(98)00142-5. [DOI] [PubMed] [Google Scholar]
- 72.Curley JP, Jordan ER, Swaney WT, Izraelit A, Kammel S, Champagne FA. The meaning of weaning: Influence of the weaning period on behavioral development in mice. Dev Neurosci. 2009;31:318–331. doi: 10.1159/000216543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meddle SL, Bishop VR, Gkoumassi E, van Leeuwen FW, Douglas AJ. Dynamic changes in oxytocin receptor expression and activation at parturition in the rat brain. Endocrinology. 2007;148:5095–5104. doi: 10.1210/en.2007-0615. [DOI] [PubMed] [Google Scholar]
- 74.Keri S, Kiss I, Kelemen O. Sharing secrets: Oxytocin and trust in schizophrenia. Soc Neurosci. 2009;4:287–293. doi: 10.1080/17470910802319710. [DOI] [PubMed] [Google Scholar]