Abstract
Aging is a gradual, complex process in which cells, tissues, organs, and the whole organism itself deteriorate in a progressive and irreversible manner that, in the majority of cases, implies pathological conditions that affect the individual's Quality of Life (QOL). Although extensive research efforts in recent years have been made, the anticipation of aging and prophylactic or treatment strategies continue to experience major limitations. In this review, the focus is essentially on the compilation of the advances generated by cellular expression profile analysis through proteomics studies (two-dimensional [2D] electrophoresis and mass spectrometry [MS]), which are currently used as an integral approach to study the aging process. Additionally, the relevance of the oxidative stress factors is discussed. Emphasis is placed on postmitotic tissues, such as neuronal, muscular, and red blood cells, which appear to be those most frequently studied with respect to aging. Additionally, models for the study of aging are discussed in a number of organisms, such as Caenorhabditis elegans, senescence-accelerated probe-8 mice (SAMP8), naked mole-rat (Heterocephalus glaber), and the beagle canine. Proteomic studies in specific tissues and organisms have revealed the extensive involvement of reactive oxygen species (ROS) and oxidative stress in aging.
1. Introduction
Despite the great research efforts performed since molecular techniques have emerged, it is unquestionable that the step-by-step approach of studying one gene or one protein at a time, even if their partners could eventually be unveiled, is a parsimonious endeavor. Therefore, a more integral and holistic approach is required. Within this context, genomic and proteomic studies, mainly by microarray for messenger RNA (mRNA) and two-dimensional (2D) electrophoresis for protein expression profiles, would eventually elicit the comprehension of the entire process of cell function, and also that at tissue and organ levels, which in turn will provide us with a wider panorama and lead us to a more comprehensive understanding of the aging mechanisms and the intrinsic role of reactive oxygen species (ROS) at a molecular level.
Both approaches yield a large amount of information from every group of cells, tissue, or organ condition, both in vivo and in vitro, under certain circumstances, and at a particular developmental time. In this review, we present a compilation of advances of the influence of oxidative stress during aging, obtained by means of a proteomic approach in different cellular types, tissue types, or animal models. The second major global approach for expression analysis, by gene expression profile by microarrays, will be reviewed elsewhere.
The existence of free radicals, such as chemical entities, was inferred 100 years ago, but their importance in biological systems was not recognized until the mid-1950s; nonetheless, for the majority of the remaining 20th century, ROS were considered “a type of biochemical rusting agent that caused stochastic tissue damage and disease” [1]. Now, in the 21st century, reactive oxygen biochemistry is quite relevant among the biomedical sciences, and it is currently recognized that nearly every disease involves some degree of oxidative stress. Additionally, it is recognized that ROS are produced in a well-regulated manner to help maintain homeostasis at the cellular level in normal, healthy tissue [1]; but, when ROS concentrations exceed the cell's antioxidant capacity, severe damage can occur and the organism experiences oxidative stress [2]. The damaging effects of these concentrations have been implicated in aging [3, 4], and also in neurodegenerative diseases such as Alzheimer's disease and Parkinson's disease, and in other chronic and degenerative diseases such as cancer, atherosclerosis, diabetes, and heart disease [5].
As early as 1956, Denham Harman proposed the “Free Radical Theory of Aging” [6]; additionally, other studies from Gilbert, Chance, and Commoner can be considered as the “founders” of reactive oxygen biochemistry. However, by 1980, oxyradicals were accepted biological entities, though their significance for aging and disease remained generally unappreciated [1]. Around 1980, the Stadtman group began to investigate the nature and consequences of protein oxidation in vitro and in vivo. These authors measured protein carbonyl groups as indices of oxidative damage and applied these techniques to the study of protein oxidation in aging tissue, describing the first, detailed determinations of protein oxidation in models of human aging [7, 8] and giving rise to serious consideration of oxidative stress as a pathological factor.
The application of genomics, proteomics, and even metabolomics to the research on aging, as well as the study of epigenetic influences that are able to induce histone and DNA modifications and influence enzyme activity, would increase our understanding of the origin and development of the different process that contributes to this unavoidable life consequence, senescence. Epigenetic mechanisms are typically associated with the aging process and age-related diseases and may have significant roles to play in the presence of oxidative stress during aging, thereby enabling the establishment of specific diagnostic profiles and therapeutic templates that could aid in improving Quality of Life (QOL) at advanced ages (for a striking revision regarding the relationship between epigenetic factors and aging, see [9]).
2. Global Approaches to the Study of Oxidative Stress in Aging at the Molecular Level
One of the major goals of Gerontology is to understand the complex mechanisms involved in aging at the molecular, cellular, and organ levels that would also make the understanding of age-related diseases possible. Because of practical limitations in studying the aging process in humans in vivo, animal models are frequently utilized [10]; however, differences in longevity between the majority of experimental animal models and humans make analysis somewhat difficult. Despite these limitations, research in this area has accelerated with the application of high-throughput technologies such as microarrays and mass spectrometry (MS). Once applied, the information generated must be analyzed accurately; thus, the use of bioinformatics is highly relevant [11]. One of the great advantages of the study of the proteomic is that the huge amount of information generated by a particular set of experiments, as occurs in genomics studies also, can be deposited in large databases, which in turn would accelerate, in the near future, experimental work and improve the results obtained, particularly in the study of aging as a comprehensive biological phenomenon (Figure 1). While aging has been considered a stochastic process, widespread opinion at present takes into account the existence of a strictly regulated system that fine-tunes the lifespan of an organism through modulation of its responses to oxidative stress [12].
Figure 1.
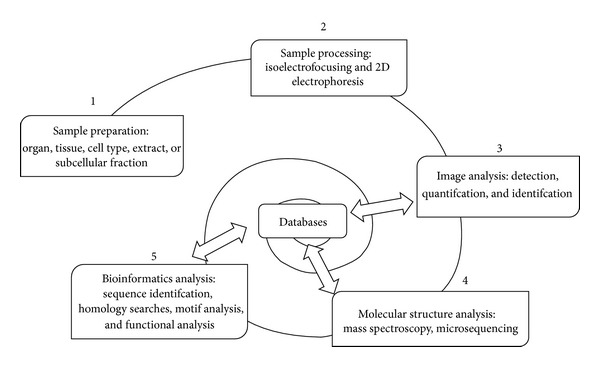
General stages of the proteomic analysis.
Research on ROS and oxidative stress has become a rapidly growing and evolving subject of study. Specifically in the field of aging studies, a review of the PubMed database (http://www.ncbi.nlm.nih.gov/) under the terms (“oxidative stress” OR “reactive oxygen species”) AND aging AND microarray or (“oxidative stress” OR “reactive oxygen species”) AND aging AND proteomic (both restricted to title/abstract search fields) shows the increasing interest in these integral molecular approaches during the last decade.
It is relevant to bear in mind the relevance of posttranslational modifications that regulate the activity of proteins inside the cell. Thus, the number of distinct protein functionalities exceeds the number of protein sequences and concentrations; therefore, one must establish the relative concentration, location, and posttranslational modification of each isoform in order to completely characterize protein function.
3. Accumulation of Altered Posttranslational Modifications and Lack of Adequate Protein Degradation Are Involved in Aging
During the normal aging process and also in age-related diseases such as atherosclerosis, cataracts, type 2 diabetes, and neurodegenerative diseases, proteins are the targets of several posttranslational deleterious modifications that alter their biological functions (Table 1). ROS increase oxidative stress [13–15] and reactive nitrogen species (RNS) yield nitrosative stress [15]. Together, as well as in conjunction with other toxic compounds, such as dicarbonyl and reactive aldehydes, which are mainly responsible for this damage [16, 17], which in several cases translates into clinical pathology.
Table 1.
Major posttranslational protein oxidative modifications during oxidative stress mediated aging.
| Type of modification | Consists of |
|---|---|
| Irreversible | |
| Carbonylation | Covalent adduction of lipid aldehydes, often six, nine, or 12 carbons, to the side chains of lysine, histidine, and cysteine residues |
| 3-Nitrotyrosilation | Formed between reactive nitrogen species and a protein's tyrosine residue |
| Reversible | |
| S-Sulfenation | Generation of sulfur-hydroxylation product (P-SOH) may be a prelude to sulfination, sulfonation, disulfide bond formation, and sulfenyl-amide bond formation |
| S-Nitrosylation | Covalent incorporation of a nitric oxide moiety into thiol groups to form S-nitrosothiol (SNO) |
| S-Glutathionylation | Covalent attachment of glutathione (GSH) to protein thiol groups |
| Disulfide formation | Disulfide bonds are usually formed from the oxidation of sulfhydryl (–SH) groups |
| 4-Hydroxy-2-nonenal (HNE) modification |
Is a major lipid peroxidation product formed during oxidative stress |
Additionally, protein degradation is integral for maintaining a healthy and functional proteome, particularly for turning over misfolded and damaged proteins. Removal of oxidized proteins first involves selective recognition of the modification and afterward, either their repair or their degradation [18]. Protein concentration increases with both decreased degradation and increased synthesis; yet, while decreased degradation results in the accumulation of “old” proteins, increased synthesis does not [19]. Thus, the age-related accumulation of damaged proteins is thought to result from both the increased occurrence of damage, which is due, at least in part, to alterations in the detoxification of the damaging agents, and from the decreased efficiency of the different systems involved in the elimination of damaged proteins [20]. This relationship is illustrated in Figure 2.
Figure 2.
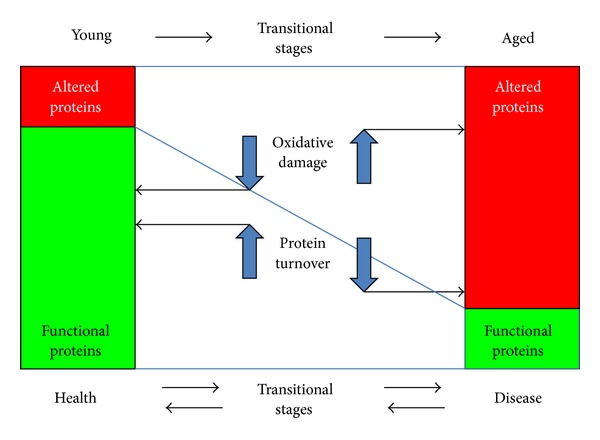
Alteration of proteins by oxidative damage and their turnover. The balance between functional proteins, present in young or healthy organisms, and detrimental or altered proteins, present in a large proportion of aged or diseased organisms, depends mainly on their modification and turnover. If proteins are affected by an increase in oxidative damage or by a low protein turnover, altered proteins accumulate, in contrast to when oxidative damage diminishes and protein turnover increases, when functional proteins increase their proportion and the organism transits to a healthy stage.
Protein quality, but not necessarily quantity, is altered in a disease state and can be reversed by appropriate treatment. This has been demonstrated, for example, in the case of apolipoprotein A1 (ApoA1) in type 1 diabetes, in which ApoA-1 proteins acutely acquired damage during insulin deprivation, providing a feasible mechanism for the association between chronically poor glycemic control and higher levels of protein oxidation in diabetes [21]. In fact, it may be that this rapid aging of ApoA-1, a key protein in lipoprotein metabolism, causes a higher risk of macrovascular disease in persons with type 1 diabetes [22]. Therefore, it has been proposed that aging, as well as certain pathologies such as diabetes, insulin resistance, and metabolic syndrome, are caused in part by the disproportionate accumulation of damaged and dysfunctional proteins, rather than by their increased concentration per se, by the impairing of cellular degradation systems, whereby oxidized proteins that would normally be targeted for degradation accumulate due to age-related slowing of degradation pathways, which are easily overwhelmed by an excess of posttranslational stress [19, 20, 23–28]. Accordingly, observational studies in animals suggest that enhancing protein degradation by caloric restriction and aerobic exercise may retard aging and reverse age-associated pathologies, and it has been hypothesized that caloric restriction helps to modulate the inflammatory process, subsequently leading to the reduction of chronic diseases known to compromise the functional longevity of humans [29]. Notwithstanding this, whether these strategies can promote similar improvements in humans remains to be shown [19].
Oxidation of proteins targets them for degradation; however, extensive oxidation acts against their recycling because it inhibits proteolysis, leading to pathological accumulation and deposition [28, 30]. Modification of proteins also can modify an active site, block a phosphorylation site, or disrupt a binding site for substrates, cofactors, or partner proteins. Furthermore, it can create new epitopes for antibody recognition and induce autoimmune disorders [31, 32].
Although it is widely recognized that cellular aging causes changes in the proteome, the nature and targets of these changes and their consequences have not yet been completely identified. It is noteworthy that accumulative oxidative posttranslational modifications are relevant only if these detected modifications are connected to functional consequences [33]. But in addition, it is relevant to consider that a slight modification in low abundance proteins may be of physiological importance; therefore, many proteomic studies have been undertaken to identify modified proteins. Distinguishing between inconsequential modifications and functionally significant ones requires careful biochemical and biophysical analysis of target proteins. Thus, proteomic approaches represent powerful tools to address these questions by identifying the targeted proteins and the extent of their modifications.
4. Oxidation of Proteins Directly Affects Energetic and Metabolic Pathways and Is Related to Longevity
Oxidation-reduction (Redox) regulatory control and oxidative stress are two sides of the same coin: oxidation of proteins can modify proteins under reversible Redox regulatory control or, alternatively, can result in reversible or irreversible oxidative damage. Proteins are very sensitive to the action of ROS [16] and represent nearly 70% of their targeted entities [34]; in addition, ROS can oxidize membrane lipids, generating intermediary compounds that have a longer lifespan than ROS and that can diffuse into the cell, acting as a “toxic second messenger,” amplifying the damage of free radicals [35]. Recently, systemic Redox regulation has been recognized as a highly important element for longevity in a group of Japanese semisuper centenarians (>105 years of age), who probably escape from many serious chronic and age-related diseases by their ability to deal with a variety of stresses, including oxidative stress [36].
Therefore, recent proposals tend to prevent, rather than counteract, ROS production, particularly at the mitochondrial level, during oxidative metabolism (the mitochondrial free radical theory of aging) [37]. Although there is evidence that ROS production in the human skeletal muscle is lower in mitochondria from older subjects [38], adenosine triphosphate (ATP) synthesis was significantly decreased, supporting the concept that aging is associated with a decrease in mitochondrial function, although in this case, ROS production appears to be reduced. In the same study, when older subjects were under a regimen of physical exercise, an improvement was found in their mitochondrial function, as well as a concomitant increase in ROS production [38], which apparently counteracted the effect of aging at the molecular level with respect to mitochondrial function.
Therefore, these apparently contradictory results can be conciliated on the basis of equilibrium between the amount of ROS generated, which can be beneficial or defective, and the amount of oxidized proteins, depending on the tissue and its metabolic status. As a result of the analysis of cumulative evidences, two general characteristics responsible for the degree of high maintenance of long-lived animals emerge: a low generation rate of endogenous damage and the possession of macromolecules that are highly resistant to oxidative modification [37].
To detect protein oxidation, carbonyls are the most commonly employed marker, and the use of 2D gel electrophoresis has provided very useful results for the study of specific carbonylated protein spots during oxidative stress and replicative senescence [39, 40]. Although MS is currently the most versatile technology in proteomics for the identification of proteins and their carbonylated residues, some limitations have led to the development of alternative strategies, such as fluorescent probes, which are able to detect lower abundance carbonylated proteins [20]. Additionally, cysteine oxidation became important because these lie precisely at the interface between Redox-sensitive and oxidative damage by ROS or nitrogen species during stress [41]. Interestingly, a highly significant inverse correlation between long lifespan and the percentage of mitochondrial cysteine is found in metaexaminations of genomic sequences (mitochondrial DNA) from 218 animal species (chordates and arthropods) [42], supporting the previously mentioned free radical theory of aging and point out the relevance of the vulnerability of the proteins in the organism to oxidative stress in terms of its lifespan.
Taking into account the alterations of metabolism in humans, the Cornelia de Lange syndrome is a rare multisystem disorder characterized by distinctive craniofacial dysmorphia, upper limb malformations, hirsutism, microcephaly, cardiac defects, gastroesophageal dysfunction, growth retardation, and neurodevelopment impairment ranging from moderate to severe, with a wide range of variability [43]. Patients present a premature aging process, and it is likely that a reduction in energy and the downregulation of proteins involved in antioxidant and detoxification pathways could lead to premature physiological aging and genome instability [44].
On the other hand, a possible model of longevity has been described; it is based on the knocking out of type 5 adenylyl cyclase (AC5) [45]. Adenylyl cyclase (AC) is a key enzyme that catalyzes the synthesis of cyclic adenosine monophosphate (cAMP) from ATP and it plays a pivotal role in β-adrenergic receptor signaling. In these AC5 KO mice, activation of the Raf/Mitogen-activated protein kinase/MAP kinase kinase (Raf/MEK/ERK) signaling pathway is present, which in turn promotes the upregulation of Mn-superoxide dismutase (Mn-SOD) and results in protection from oxidative stress and apoptosis, retarding aging phenotypes in the heart and bone and increasing resistance to stress, which leads to longevity in the lifespan, suggesting that retarding aging in an individual organ could be a fundamental therapy to prevent age-related diseases [46].
Delving deeper into the identification of proteins involved or affected by aging, postmitotic tissues have received more attention. Neurons, myocytes, and red blood cells have been studied during the aging process, and certain particular and some common factors have been discovered. In the following sections, the main advances in proteomics studies of these post-mitotic cells are presented.
5. Proteomic Studies Further Support Parallelism between Aging and Neurodegenerative Diseases
An increase in oxidative stress in the brain is part of normal aging and is related directly to decreased neurological activities and inversely to lifespan [47]. Common pathological pathways that are implicated both in aging and in the development of neurodegenerative disease include free radical damage and decreased energy production as characteristic hallmarks [48]. Consistent with this idea, proteins that increase with aging in the mice hippocampus are mainly enzymes that mediate energy production and oxidative stress [49]. In addition, one of the cellular processes that are altered mainly in the aging hippocampus is that of oxidative stress, as well as that of protein processing [50].
Consistent with previous ideas, wide proteomic analysis of brains during aging in mice identifies 40 proteins that exhibit changes in their natural pattern during the mouse lifespan (from 4 days to 15 months of age), showing that six proteins increased and 27 decreased in various ways. When analyzed together, the biological processes in which those proteins are involved correspond mainly to the following: protein metabolic processes (more than one third); transport (one third); nucleotide and nucleic acid metabolic process (one fourth); intracellular signal cascade (nearly one fifth), and response to stress proteins (more than one sixth). Additionally, about one fifth of the identified proteins can be localized in the mitochondria and the majority of these are related to energy metabolism [51], providing a broad panorama of proteomic changes in the brain during aging.
In addition, there is increasing evidence that protein oxidation is involved in the pathogenesis of Alzheimer's disease (AD), a neurodegenerative disorder associated with cognitive decline, oxidative stress, and aging [52–56]. Two initial studies using the proteomic approach have identified proteins that are specifically oxidized in AD [54, 55]. In the first study, three key enzymes in cellular metabolism, creatine kinase BB (CK BB), glutamine synthase (GS), and ubiquitin carboxy-terminal hydrolase L-1 (UCH L1), resulted as specific targets of protein oxidation in the brain of AD patients. In the second, a couple of additional targets were detected: dihydropyridine-related protein-2 (DRP-2), involved in axonal growth, and α-enolase, involved in glycolysis for energy metabolism and therefore related with the cerebral decrease of energy metabolism. These results strongly suggest that the process of free radical-mediated protein modification may be a crucial event in AD and that protein oxidation is a relevant part of the mechanism of neurodegeneration in AD brain.
Although in a pilot study of abundant carbonylated proteins in the cerebrospinal fluid of probable AD patients the extent of carbonylation did not vary in general, two proteins were detected that were highly carbonylated when compared to controls: immunoglobulin λ light chains and one unidentified protein [57], which suggests that further studies could be performed focusing on less abundant proteins, modified by oxidative stress, to detect some probable markers of early pathological stages. Therefore, the establishment of differences in protein oxidation state may provide a diagnostic tool for neurodegenerative diseases.
Additionally, Weinreb et al. found a significant parallelism in the protein profile affected between aging and neurodegenerative diseases in the hippocampus of rats [58]. They found that in the aged hippocampus, oxidative stress and mitochondrial dysfunction are important and that in treatment with an anti-AD drug, Ladostigil, or with an anti-Parkinson drug, Rasagiline, both drugs reversed the effect of aging on various mitochondrial and key regulator genes involved in neurodegeneration, cell survival, synaptogenesis, oxidation, and metabolism. Another consequence of oxidative stress in the brain includes the generation of RNS. The cerebellum is especially vulnerable to oxidative stress and exhibits an age-dependent increase of total 3-nitrotyrosine (3-NT) [59, 60], and some proteins have been identified as targets for nitration [59].
Additionally, in cultured neurons exposed to amyloid beta (Aβ) (1–42), two proteins are significantly oxidized: 14-3-3ξ and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and pretreatment with γ-glutamylcysteine ethyl ester, a compound that supplies the limiting substrate for antioxidant glutathione synthesis, protects both proteins from oxidation by Aβ 1–42 [61], which is consistent with the notion that antioxidant therapies may potentially be effective in slowing or ameliorating the neurodegenerative disease process [56, 62].
Conversely, the other cell type that is more abundant in the central nervous system, the glial cells, are more resistant than neurons to oxidative stress and are able to respond to protect them [63–66]. Surprisingly, proteomic studies that focus on the role of glial cells during aging, or in neurodegenerative diseases, in response to oxidative stress are very scarce. Miura et al. proposed that aging does not suppress the astrocytic capability to respond to oxidative stress. The authors found that α-tubulin was subjected to tyrosyl phosphorylation by H2O2-exposure and that aging enhanced this phosphorylation and prevented the formation of microtubules, but aging does not suppress the responses aimed at cell protection against severe oxidative stress [67]. Therefore, proteomic studies on the response of glial cells to oxidative stress during aging and in neurodegenerative diseases, focusing on their helping role for neuron metabolism, represent a promising avenue that is yet to be explored.
6. Aging in Cardiac and Skeletal Muscles Altered Energy Metabolism and Mitochondria
Cardiac performance declines with age [68] in a clear association with oxidative stress [3]. Accordingly, aging is a factor for cardiovascular disease. It appears that the protein signature or proteomic phenotype that changes during aging in the rodent heart renders the tissue more sensitive to oxidative stress damage with age by modifying the energy metabolism, particularly carbohydrate metabolism, fatty acid oxidation, cellular respiration, and energy production and their capacity to respond to oxidative stress [69].
In the aged rat heart, several proteins have been identified as differentially expressed when compared with those of young hearts [69–71], although there are many differences among studies, probably derived from methodologically different approaches and species particularities (mouse or rat), also due to the fact that many proteins change consistently during aging. Additionally, a study of a heart failure model, transverse aortic constriction in mice, demonstrated a differentially expressed protein expression of structural, signaling, and redox proteins [72]. Thus, it is possible to establish parallelism between aging heart dynamics and heart failure, both altering structural proteins as well as the oxidative metabolic profile and affecting the heart's capacity to respond adequately to oxidative stress during aging or under pathological distress.
Among the functional consequences of oxidative stress induced by ROS and RNS on cardiac and skeletal muscle tissue during aging, we find protein nitration [73], which may affect protein structure, function, and turnover. Thus, the accumulation of nitrated proteins in cardiac and skeletal muscle tissue [74, 75] may define the progress of biological aging or of any pathology.
7. During Red Blood Cell Aging, Hemoglobin-Generated Oxidants That Affect Cellular Membrane and Cytoskeleton
Many of the cell processes associated with the physiological removal of red blood cells (RBC) involve oxidative stress, which is generated by both endogenous hemoglobin (Hb) auto-oxidation and exogenous oxidants, which can result in functional impairment and in cellular aging [76]. The predominant factor that determines oxidative stress in RBC is Hb. The superoxide, H2O2, hydroxyl radicals, ferrylHb, oxoferrylHb, and peroxynitrite generated by redox reactions near the membrane can damage RBC membrane proteins, lipids, and the cytoskeleton, which are responsible for maintaining the RBC shape and deformability, thus being able to damage and promote cellular aging [77]. Consequently, instead of required large concentrations of antioxidants to neutralize the ROS species formed, blocking Hb interaction with the membrane will make it possible to eliminate Hb-generated oxidants, preventing oxidative stress in RBC [76]. In RBC, and due to their extensive use for blood transfusions, their oxidative stress is also highly relevant during long-term storage [78]. Consequently, the proteomic approach has been useful to identify molecular markers, such as Prx2, as a candidate biomarker for RBC oxidative injuries under blood bank conditions [79], possibly to be utilized in future blood component programs to improve the quality of stored RBC and to limit or avoid the risk of posttransfusional complications.
In brief, and beyond the cell type affected, on the basis of extensive experimental results, the main cellular dysfunctions directly caused by oxidative stress imbalance and by uncontrolled generation of ROS can be summarized as follows (Figure 3). Whether from self-cell metabolism or from extracellular sources generating an uncontrolled increase of ROS and RNS, both of these produce cell damage by modifying proteins (mainly by carbonylation, sulfoxidation, hydroxylation, nitration, and S-thiolation) and avoiding the recycling of these by the proteasome, yielding impaired protein function. Additionally, oxidative stress can directly affect the cellular membrane, causing lipid peroxidation, thus instability, and can also modify cytoskeletal proteins, causing structural damage and moreover altering signaling pathways. Furthermore, oxidative stress directly affects mitochondria and peroxisomes, thereby altering cell metabolism and energy production. Taken together, these alterations would be able, in some manner, to distress nuclear transcription and generate altered gene expression during normal aging, or leading to disease. Although this is a general panorama, there are more specific alterations depending on the cellular type affected or on the tissue or organ altered. Therefore, it is a priority to establish more models to study the whole effect of oxidative stress during aging and disease.
Figure 3.
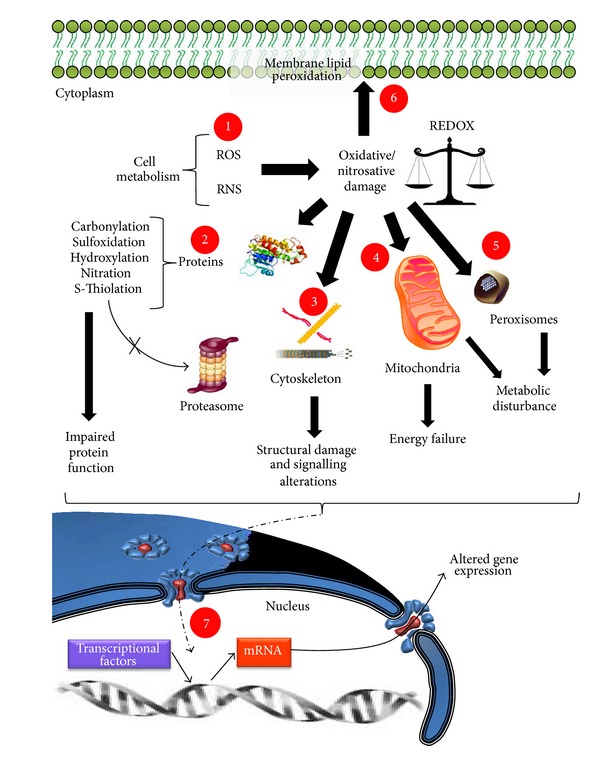
Cellular dysfunctions in aging or in age-related diseases by oxidative stress imbalance. (1) Cell metabolism generates reactive oxygen species (ROS) and reactive nitrogen species (RNS), which in turn causes oxidative/nitrosative damage. (2) Proteins are the most affected macromolecules by oxidative stress, undergoing several modifications that avoid their being correctly degraded and recycled by the proteasome, thus generating impaired protein function. (3) Oxidative stress also directly affects cytoskeletal proteins, causing structural damage and signaling alterations. (4) On affecting the mitochondria, oxidative stress alters energy production and (5) on affecting peroxisomes, oxidative stress alters correct metabolic functioning. (6) Oxidative stress also affects the cellular membrane. (7) Finally, all of the previously mentioned affections cause an alteration in the transcriptional activity of the cell, leading to an altered gene expression that in turn leads the cell to the aging process or to degenerative disease.
8. Particular Organisms as Alternative Models for Studying the Proteomics of Oxidative Stress during Aging
In addition to the more widely used models employed to study aging (such as rodents like mice or rats, cultured line cells, or even tissue fragments, blood, or serum), other particular organisms represent suitable alternatives for approaching the problem. In Table 2, we summarize some of the main works that, based on their wide proteomic approach, deal with the aging process, selecting those that have obtained results that are clearly related with the involvement of oxidative stress and ROS in the aging process. The following is a brief summary of some particular models and their contribution to elucidating the participation of oxidative stress during aging.
Table 2.
Comprehensive summary of proteomic studies focused on aging that involves oxidative stress-related proteins.
| Animal model (specie) and tissue | Sample and age | Results related to oxidative stress or ROS influence on aging | Main proteins altered in aging* | References |
|---|---|---|---|---|
| Invertebrate Caenorhabditis elegans (worm) | In vitro knocked down by RNAi | Ten iRNA tested caused substantial reduction in adult lifespan. When these genes are disturbed defensive mechanisms against oxidative stress become altered. | UBH-1, UBH-3, PRDX2, PRDX3, AMPK-β1, AMPK-β2, LBP-4, LBP-6, LBP-9, RHI-1 | [80] |
| In vitro knocked down by RNAi for K10C2.4 | K10C2.4 RNAi activates oxidative stress and endoplasmic reticulum stress response in the worm intestine by accumulation of tyrosine metabolites. | Enzymes in the tyrosine degradation pathway | [81] | |
| Long-lived daf-2(e1370) strain | Components of the enhanced longevity system identified in daf-2 deficient mutant include the alpha-crystallin family of small heat shock proteins, anti-ROS defense systems, and cellular phase II detoxification. GSTP were significantly URg, which detoxify and/or bind short-chain aldehydic natural toxic products of lipid peroxidation and long-chained fatty-acids at physiologically relevant concentrations, indicating a role in longevity. | GPX, SOD1, NME, RPS12, STK, LBP-6, HSP-12.6, HSP-12.3 | [82] | |
| Exposure of prdx-2 defective worms under H2O2-induced OS | Identified oxidation-sensitive cysteins in 40 different proteins involved in mobility (muscle contraction), feeding, protein translation, homeostasis, and ATP regeneration. | MYO-2, LET-75, EFT-1, HSP1, NME | [83] | |
| xpa-1 mutant UV-sensitive with shortened lifespan | Proteome changes in xpa-1 mutants correspond to transcriptome modulation by suffering oxidative stress and inducing antioxidative defenses. Polyubiquinated proteins accumulate, cyclopurine levels are reduced, and lesion-detection enzymes play active roles to generate a genomic stress signal. | NTH-1, XPC-1, DDB-1 | [84] | |
|
| ||||
| Rodents Mus musculus (mice) |
KO mice for 5 adenylyl cyclase (AC5) | AC5 KO mice are protected from aging-induced cardiomyopathy and their fibroblasts exhibited ERK-dependent resistance to oxidative stress. AC5 KO leads to upregulation of the Raf/MEK/ERK signaling pathway, which in turn mediates upregulation of SOD, an important mechanism mediating lifespan extension and stress resistance. | Increased: RSK, p-Bad, Bcl-xl, XIAP, HSP70, p-ERK, p-Raf-1 | [46] |
| SAMP8 | Brain and liver from SAMP8 | Progressive accumulation of oxidative damage to Cu, Zn-SOD may cause a dysfunction of defense systems against oxidative stress in SAMP8, with a higher oxidative stress and leading to the acceleration of aging. | SOD1, HCNP-pp | [85] |
| Hippocampus and cortex from 5 to 15 month old SAMP8 | 7 protein are related to age rather than strain and might be associated with brain aging process. One protein might be specifically associated with pathologically accelerated aging in SAMP8 mice; HEBP1. | NDRG2, enolase 2, SOD1, myosin, two unnamed protein (gi∣74214304; gi∣74178239), HEBP1 | [86] | |
| Brain | Brain tissue from 3 weeks to 18 months old C57B mice | Carbonylated proteins increased with aging are involved in cytoskeletal organization, mitochondrial energy metabolism, redox regulation (oxidative damage), and signal transduction. | Approximately 100 carbonylated proteins | [87] |
| Brain tissue from 3-, 6-, 12- to 15-month-old male Kunming mice | 60 proteins vary their expression on aging; 27 of them decrease, may be responsible for brain aging. Related with decline of protein quality control, shortage of energy and reducing agent, increase of DNA damage and transcription detuning, and disturbance of synaptic transport and ion signals. 6 proteins increase, may be involved in antiaging processes. | PSMA6, PSMA3, CALR, UCHL3, VCP, GLUD1, IDH1, UQCRC2, UBE2N, CALB1, HNRPA2/B1, AMPH, TKT, CKMT1, MRPL37, TPI1 | [49] | |
| Brain | Adult NPCs from brain of C57BL/6 mice from 3, 15–18 months of age | Aging is correlated with a loss of mitochondria and oxidative metabolism in NPCs. A coordinated shift in protein expression, subcellular structure, and metabolic physiology in aging NPCs, allowing resistance to hypoxia and mitochondrial inhibition. 124 proteins result as age-related. | Increased: PGK1, SEPT9. Decreased: ATP5 α and β | [88] |
| Liver | Livers from male C57BL6/J mice of 10-week-old and 18-month-old. Peroxisome enriched fraction | Most of the proteins identified are related to ROS production/breakdown; however, high biological variability between individuals is even more pronounced than changes induced by aging. | EPHX2, Acaa1, Pipox, Amy2a, Decr2, Phb2, COX6c, UQCRC2 | [89] |
| Kidney | Male and female mice CD1-Swiss outbred strain of 28, 52, 76-week-old | Differential protein expression of 8 aging related proteins (both genders). Increase in oxidative and proteolytic proteins and decrease in glycolytic proteins, and antioxidant enzymes with aging. | ATP syntase, Transferrin, HSP9A, Hibadh, IDH1 | [90] |
| Cardiac muscle | Hearts from male CB6F1 mice from 3, 15 to 23 months old | Detected age-related alterations in the levels of 73 proteins. Mithocondrial metabolism is affected and a net loss in antioxidants occurs with aging. | Mortalin, PRDX3, EPHX, SOD1, SOD2 | [70] |
| Adipose tissue | Male mutant mice deficient in Zmpste24 metalloproteinase | Zmpste24 deficiency causes premature aging. It enhanced lipolysis, fatty acid biogenesis, and β-oxidation as well as decreased fatty acid reesterification. Also URg protein networks related to tricarboxylic acid cycle and oxidative phosphorylation and increased mitochondrial response to oxidative stress and cytoskeleton. 37 proteins URg and 9 DRg. | Increased: ME1, PRDX3, HMGB1, CPT1, UCP1; Decreased: PCK1, vimentin isoforms | [91] |
| Macrophages | Peritoneal macrophages from male Balb/c mice (3-4 and 14-15 months) | An age-dependent increase in the extent of recruitment of macrophages into the peritoneum, as well as ex vivo functional changes involving enhanced nitric oxide production under resting conditions. Identified age-dependent increases in levels of proteins linked to immune cell pathways under basal conditions and following LPS activation. Immune pathways URg in macrophages isolated from aged mice include proteins critical to the formation of the immunoproteasome. | Hundreds of proteins | [92] |
|
| ||||
| Rattus novergicus (rat) | Male Wistar rats weighing 80–90 g, 6 and 24 months old | A beneficial role for virgin olive oil in modulating inflammation, homeostasis, oxidative stress, and cardiovascular risk during aging. Diet diminishes in general the changes that occurred with age. | Decreased: HPX, HP, AHSG, PRDX2, FGg, T-KNG, APO H, APO E, APO A-IV Increased: APO A-1 | [93] |
| Serum from young and old Fischer 344 rats | 16 of the modified proteins by peroxynitrite and 4-hydroxy-2-nonenal are involved in blood coagulation, lipid transport, blood pressure regulation, and protease inhibition. | 16 modified proteins | [94] | |
| Brain | Hippocampus from 8 to 27-month-old Wistar rats, and also treated with the anti-Parkinson drug; rasagiline or the anti-Alzheimer's disease drug; ladostigil | Significant molecular changes related to neurodegeneration were identified in aged rat hippocampus. Both drugs reversed the effect of aging on the expression of various mitochondrial and key regulator genes involved in neurodegeneration, cell survival, synaptogenesis, oxidation, and metabolism. Changes in proteins related to the iron-mediated oxidative stress pathway, including reduction in antioxidant enzymes. Oxidative stress and mitochondrial dysfunction may play a pivotal role in aging and age-associated neurodegenerative diseases. | Aprox. 200 proteins showed differential expression. NEFL, FTH1, TUFM, PEA15, PEBP, PFN1, CCT2, IDH3A, COX5A, COX5B, PRDX2 | [58] |
| cerebellum from Fisher 344/Brown Norway rats from 5-, 22- and 34-month-old rats | Genes encoding proteins of stress response and inflammatory processes show a significantly higher age dependent upregulation in the cerebellum suggesting higher levels of oxidative stress. Identification of nitrated proteins. | Ryr3, Lrp2, Nrap, Cnp | [95] | |
| Brain | Brain from male Wistar rats from 12 to 28 months old (hippocampus, cortex, striatum, and cerebellum) | Senescent animals showed significantly higher levels of oxidation. 11 proteins carbonylated in hippocampus, 15 in cortex, 10 in striatum, 11 in cerebellum, associated with significant changes in both cytosolic and mitochondrial redox status in all brain regions analyzed. | Decreased: PK, ATP5a1, ALDOC, CKB, a-enolase. Activity of PK and GAPDH diminished | [48] |
| Hypotalamus and hypophysis from male Wistar rats from 3, 12 to 24 months old treated with an antioxidant | Alterations of eEF-2 levels, secondary to lipid peroxidation and adduct formation with aldehydes could contribute to the suboptimal hormone production from these tissues during aging | eEF-2, ALDOA, GSTA, CKB, PPIA, PK, GADPH, INA, CFL1 | [96] | |
| MSC cultures from the tibial and femoral BM of 83-week- and 12-month-old Sprague-Dawley rats | Number of MSCs is reduced in aged animals. Aged MSCs are more susceptible toward senescence and display a lower migratory capacity. Aging affects MSCs antioxidant defense and cytoskeleton turnover. | Several proteins as members of the actin-binding protein family of calponins, galectin-3 | [97] | |
| Retina | Fisher 344/Brown Norway F1 rats from 3-4 to 24-25 months old | Decrease of antioxidant enzymes was detected in the old F344BN retina sections and increased presence of ROS and oxidative stress. | Increased: CD46, GABA2, DJ-1, EBP50, Ezrin, Cathepsin D. Decreased: NG, DDAH1, DPPX | [98] |
| Primary cell cultures from retinas of newborn (PD 1 or 2) Sprague-Dawley rats under H2O2-induced OS | Retinal pigmentary epithelium (RPE) and retina have higher O2 tension and ROS concentration with aging; this environment may contribute to the pathogenesis and progression of eye diseases. Decreased prohibitin in H2O2 treated RPE cells may indicate an antioxidative role. | Prohibitin | [99] | |
| Adipose tissue | White adipose tissue from male Wistar rats from 6 and 24-month-old under caloric restriction (CR) | Caloric restriction (CR) improves oxidative stress and prevents age-associated changes in several antioxidant enzymes. Metabolic enzymes involved in energy metabolism and transduction (glucose and lipid), oxidative stress response, cytoskeleton, and iron homeostasis were also modulated by age and/or CR. Several enzymes involved in cell protection against oxidative stress are increased by CR, whereas these protein levels decrease or do not change with age. | 133 differentially expressed spots, 57 of which were identified | [100] |
| Skeletal muscle | Skeletal muscles from Fisher 344/Brown Norway F1 rats, 34 months old | 11 nitrated proteins were identified as age-related. | CKM, TPM1, GAPDH, MYL2, ALDOA, PKM, PYGM, NOTCH1, ACTN1, ACTC1, RYR3 | [74] |
| Gastrocnemius muscle from Lou/c/jall male rats from 7, 18, to 30 months old | Aging is associated with differential expression of myofibrillar regulatory proteins, up-regulation of cytoskeletal proteins, perturbations in the energy metabolism, and detoxification of cytotoxic products. | 40 proteins differentially expressed | [101] | |
| Gastrocnemius muscle of 26-month-old Wistar rats | Mitochondria-enriched fraction revealed an age-related change in 39 protein species. An age-related increase in mitochondrial enzyme activity belonging to the inner membrane system, matrix, outer membrane, and intermembrane space, increasing aerobic-oxidative metabolism, involved in oxidative phosphorylation, ATP formation, and fatty acid oxidation. | Increased: NADH-DH, Immt mitofilin, PRDX3, F1-ATPase, SDH, Fis1, SUCLA2, ACAD, porin VDAC2, UQCRC1, prohibitin | [102] | |
| Cardiac muscle | Left ventricle from Fisher 344 rats from 4 to 24-month-old | 117 proteins differentially expressed: 23 signalling proteins, 25 metabolic proteins, 7 fatty acid metabolism, 19 energy metabolism, 13 oxidative stress related (antioxidant proteins and chaperones). First network describing proteins affecting cellular organization and morphology is presented. | αβ-Crystallin, GST π isoform, GST μ type, GST Ω1, C1qbp, HSP90b1, GPX1, DJ-1, SOD2, PRDX5, PRDX3, HSP8 | [69] |
| Heart from Fisher 344/Brown Norway F1 rats, 5 and 26 months old | 48 differentially nitrated proteins were identified that undergo an age-dependent protein tyrosine nitration. | α-Enolase, Aldolase, Desmin, ACO1, Aldh6a1, Acaa1a, GAPDH, MDH1, CKM, ETF, SOD2, F1-ATPase, VDAC | [103] | |
| Heart from Fisher 344/Brown Norway F1 rats, 5 and 34 months old | Abundance of 10 nitrated proteins identified in cardiac tissue increase with age. | N-RAP, neurofibromin, tropomyosin, MYO-HC | [73] | |
| Heterocephalus glaber (Naked mole-rat) | Liver, heart, and kidney tissues from naked mole-rats (NMRs; 2 years) and wild-type C57BL/6 mice (0.3 year) | Global protein carbonylation in citosolic fraction was elevated in all three tissues. NMRs have a protective cellular environment which restores enzyme function and prevents formation of oligomers during oxidative stress, modulating structure and function of structural proteins and enzymes. Activation of NRF2 pathway, which increases the transcription of antioxidant response elements, proteasome, antioxidants, and autophagy, could be a potential mechanism for these processes. | TPI, PRDX1 | [104] |
|
| ||||
| Porcine (Sus scrofa) | Porcine oocyte and effect of caffeine | 38 proteins were identified, 23 URg and 3 DRg by aging. Involved in metabolism, stress response, ROS, and cell cycle regulation. | CDK5, PCNA, AHCY, SLC25A6 | [105] |
|
| ||||
| Canine (Canis domesticus) | Brain from beagle dogs from 8.05 to 12.35 years old. Feeded with antioxidant-fortified food and growth in an enriched environment | Combined treatment (food and environment) significantly decreases protein cabonylation, nitrosylation, and lipid peroxidation, reducing the levels of oxidative damage and improving the antioxidant reserve systems in the brain. Propose a diagram of a functional interacteome of all parietal cortex proteins identified to be significantly less oxidatively modified following the combined treatment. | Decreased: GLUD1, GAPDH, a-Enolase, GST, FSCN1, NF-L. Increased: SOD1, ALDOC, CKB, GLUD1 (P), GAPDH (P) | [106] |
|
| ||||
| Primate (Homo sapiens) | Human female of 20–39, 100, and 106–109 years old | Results suggest that systemic redox regulation is important for the longevity of supercentenarians in humans. | Decreased: PON1, APO E. Increased: Hp-b, AMBP, CLU | [36] |
| Brain | Inferior parietal lobule tissue samples from AD patients autopsy | Protein modification by ROS occurs to a greater extent in AD suggesting a possible role for oxidation-related decrease in protein function in the process of neurodegeneration. Oxidative damage to proteins, assessed by measuring the protein carbonyl content, is involved in several events such as loss in specific protein function, abnormal protein clearance, depletion of the cellular redox-balance and interference with the cell cycle, and, ultimately, neuronal death. | Increased in AD: DRP-2, α-Enolase, HSC-71 | [54, 55] |
| CSF | Lumbar CSF samples from probable AD patients | Decreased concentrations of proteins in CSF may also be a secondary event to increased oxidative stress, since excessive carbonylation leads to an enhanced aggregation of proteins. Extent of protein carbonylation can vary between men and women, emphasizing the importance of sex-matched patients when studying carbonylation. | Decreased in AD: PTGDS, IgL, TTR. Increased carbonylation in AD: IgL and one unidentified protein | [57] |
| Blood | Whole blood from healthy volunteer donors. Stored for various periods | A progressive linkage of typical cytosolic proteins to the membrane was detected, including both antioxidant and metabolic enzymes. This phenomenon was unequivocally related to oxidative stress, since storage under anaerobic conditions suppresses it. | Prx2 | [79] |
| Skin | Fresh punch biopsies from the forearm of 21–30 and 75–92 years old donors | 22 proteins were consistently deregulated. Support that aging is linked with increased oxidative stress that could lead to apoptosis in vivo. | Mx-A, SOD1, WARS, PIK3r2, proteasomal PA28-α and SSP 0107 | [107] |
| Fibroblast | WI-38 human embryonic fibroblasts. Two stages PD < 25 and PD > 42 | Oxidized proteins accumulate with aging in vivo and during replicative senescence in vitro. 37 proteins were modified related to protein quality control, energy metabolism, and cytoskeleton. Impairment of glyoxal- and aldehyde-detoxification mitochondrial systems. | Decrease activity of proteasomal CT-L, PGPH and detoxification GLO1 | [39] |
| HCA3 human dermal fibroblasts under H2O2-induced OS | H2O2-induced senescent like human diploid fibroblasts increase the production of IGFBP-6 protein. | Increased: Collagen 1(VI), collagen 2(I), fibronectin, lumican, MMP-2, IGFBP-6 | [108] | |
| HCA3 human dermal fibroblasts under H2O2-induced OS | H2O2 treatment caused elevated levels of TXNRD1. Differences between mRNA versus proteins that vary under oxidative stress may be related to the regulatory mechanism of protein translation under oxidative stress. | Increased: TXNRD1, MMP-3, AURKA Decreased: Akap12, MDH1 | [109] | |
| Colon epithelial | Human normal colonic epithelial tissue from 25–30 to 60–65 years old | 35 differentially expressed proteins, 16 URg and 19 DRg. Involved in metabolism, energy generation, chaperone, antioxidation, signal transduction, protein folding, and apoptosis. | Increased: ATPB, ETFA, catalase, GPX1, annexin A2, HSP7C; decreased: FUBP1, NDK B, ERp6C, VDAC-2 | [110] |
| MSCs | Human BM-derived MSCs | Differentially expressed proteins under the low glucose condition may provide further information on the aging and differentiation of stem cells. | Increased: ALDH, neuropolypeptide h3, P4HA; Decreased: laminin-BP, actin, Sec 13, RPS12, PSMA1, SOD1, SNAP | [111] |
*Proteins based on their human homologue. ROS: reactive oxygen species, URg: upregulated, DRg: downregulated, PD: postnatal day, SAMP8: senescence-accelerated mouse prone 8, NPCs: neural precursor cells, MSCs: mesenquimal stem cells, BM: bone marrow, CSF: cerebrospinal fluid, KO: knock out, AD: Alzheimer's disease.
The invertebrate Caenorhabditis elegans has been successfully used to study the aging process. It has been described that some proteins are involved in both cellular senescence and the ROS-induced condition. By using interfering RNA (iRNA) against some genes, a substantial reduction can be caused in adult lifespan, and the defensive mechanism against external oxidative stress is also disturbed [80]. Therefore, some proteins whose expression is increased with cellular senescence and oxidative stress play a protective role against these processes. Additionally, when C. elegans is submitted to oxidative stress through sublethal short-treatment of peroxide (H2O2) stress, the majority of worms experience severe, yet fully reversible, behavioral changes that are highly reminiscent of well-known age-related changes [83], such as declines in body movement, pharyngeal pumping, and reproduction, as well as morphological changes and reduced metabolic activity [83], which supports the Harman free radical theory of aging, but would also include the potentially beneficial aspects of ROS as modulatory second messengers that affect stress resistance and longevity early in life [83]. In another example, C. elegans xpa-1 mutants, which are ultraviolet-light (UV)-sensitive and that have reduced capacity to repair UV-induced DNA damage [112], exhibit oxidative stress and its antioxidant defenses are induced, and they also show polyubiquitinated protein accumulation [84]. Obviously, there are differences between nematode (invertebrate) and mammalian (vertebrate) systems, but the fundamental mechanism of cellular senescence may be evolutionarily conserved.
Another very attractive model for studying the effect of oxidative stress during aging is the senescence-accelerated probe-8 (SAMP8) mouse, which exhibits age-related deterioration in memory and learning, along with an increase in oxidative markers and which is considered a useful model for the study of AD [113]. In AD, it has been demonstrated that treatment with α-lipoic acid, a coenzyme involved in the production of ATP in mitochondria and a potent antioxidant [114, 115], is able to reduce oxidative modification and increase the protein level of α-enolase, suggesting the possibility that the reduced glucose metabolism and neurochemical alterations in SAMP8 mouse brains can be reversed [116]. Additionally, it has been demonstrated that carbonyl modification of Cu, Zn-superoxide dismutase (Cu, Zn-SOD) in liver and hippocampal cholinergic neurostimulating peptide-precursor protein (HCNP-pp) in the brain were higher in SAMP8 compared with the control, SAMR1. Therefore, progressive accumulation of oxidative damage to Cu, Zn-SOD may cause dysfunction of the defense systems against oxidative stress in SAMP8 with higher oxidative states, leading to the acceleration of aging [85].
A different and very interesting organism for studying the effect of oxidative stress during aging is the naked mole-rat (H. glaber) because it has very low metabolic and respiratory rates and its protein structure and function is not apparently affected by either oxidative stress or carbonylation during aging, probably due to a particular characteristic of the cellular environment that maintains the functional structure of proteins [104]. As an example, activation of the Nuclear factor [(erythroid-derived 2)-like 2] (Nrf2) antioxidant response pathway, which increases the transcription of antioxidant response-element genes, proteasomes, and antioxidants and which affects the efficient maintenance of protein homeostasis, can protect proteins from misfolding or aggregation by oxidative stress [117, 118], being part of a protective cellular environment that efficiently maintains protein homeostasis as part of a potential plausible mechanism that explains the exceptional longevity of the naked mole-rat [104].
Finally, an attractive model for the aging human brain is the aging beagle (canine) brain, especially also as a model of AD [119–122]. In the aging canine brain, a proteomics study reveals that a combined treatment of antioxidant-fortified food and an enriched environment reduces the levels of oxidative damage, improves the antioxidant reserve systems, increases the activity and expression of key endogenous antioxidant enzymes, and may contribute to improvements in learning and memory [106].
9. Concluding Remarks
One limitation of some of the reports presented here is that details regarding animal ages, care, and behavior assessments/measures are limited, which impedes cross-study comparison and meta-analyses. Also, cell types and their particular characteristics rendered comparison of the effect of ROS on RBC or neurons difficult, for example, as well as under in vitro or in vivo conditions. Thus, therefore more and wider studies are needed.
In humans, it is difficult to compare among proteomic studies because of insufficient characterization of the study material, the small number of patients involved in studies, and variations in experimental designs. At present, basic aging research has arrived at a pharmaceutical phase, with the testing of novel drugs designed to extend a healthy life by targeting specific biochemical pathways, perhaps in specific organs [123]. In this respect, the National Institute on Aging Interventions Testing Program (ITP) experimentally evaluates chemical compounds with potential senescence-retarding effects that can be administered to mice in food or water [124]. While initial results are far from surprising, the experimental design is robust; therefore, it will be useful in order to develop a similar program in mouse genetics in aging.
It is evident that the sole fact of identifying the whole genome sequence of an organism, or to know the whole isoforms and modifications of its products (proteins), is not sufficient for complete elucidation of the aging process. It is necessary to integrate all of this information in a functional manner that reflects more precisely the real situation. Therefore, as important as the generation of all “omic” information is, the development of instruments to analyze and evaluate this efficiently is equally important. In this regard, bioinformatics and computational biology are devoted to performing these analyses, both based on systems biology, that is, the construction of gene, protein, and metabolic pathway networks that interact among them to constitute functional modules (Figure 1). In turn, they integrate design models for prediction from clinical phenotypes to diagnostic and therapeutic strategies after experimentation takes place. Albeit proteomics has already contributed relevant insights in the field of aging research and attempts have been made, in animal models such as mice to map aging-related brain proteins within the context of the biological processes involved [51]; a reference mapping of proteins in healthy aging human subjects has yet to be performed. Nonetheless, with the continued advances in proteomic technology, the study of the proteome during aging is entering a brand new phase of discovery.
Acknowledgments
This work was partially supported by Universidad de Guadalajara Grants P3E/2013/218202 and PIE-BKC 2013 (Visiting Lecturers of Excellence) from the University of Barcelona-Barcelona Knowlegde Campus to Daniel Ortuño-Sahagún. This work was also supported by Grants from CONACyT 2012-180268 and PROMEP/103.5/12/8143 to A. E. Rojas-Mayorquín and from Spanish Ministerio de Ciencia e Innovación (SAF2012-39852) to Mercè Pallàs. The authors apologize to authors whose works have not been reviewed and to those whose papers have not received the emphasis that they merit. The authors also apologize to authors whose work has not been appropriately cited due to space limitations and/or to limitations of our knowledge.
Conflict of Interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interests.
References
- 1.Hensley K, Floyd RA. Reactive oxygen species and protein oxidation in aging: a look back, a look ahead. Archives of Biochemistry and Biophysics. 2002;397(2):377–383. doi: 10.1006/abbi.2001.2630. [DOI] [PubMed] [Google Scholar]
- 2.Imlay JA. Pathways of oxidative damage. Annual Review of Microbiology. 2003;57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- 3.Sohal RS. Role of oxidative stress and protein oxidation in the aging process. Free Radical Biology and Medicine. 2002;33(1):37–44. doi: 10.1016/s0891-5849(02)00856-0. [DOI] [PubMed] [Google Scholar]
- 4.Merry BJ. Oxidative stress and mitochondrial function with aging—the effects of calorie restriction. Aging Cell. 2004;3(1):7–12. doi: 10.1046/j.1474-9728.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- 5.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 6.Harman D. Aging: a theory based on free radical and radiation chemistry. The Journal of Gerontology. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 7.Oliver CN, Ahn B-W, Moerman EJ. Age-related changes in oxidized proteins. The Journal of Biological Chemistry. 1987;262(12):5488–5491. [PubMed] [Google Scholar]
- 8.Smith CD, Carney JM, Starke-Reed PE, et al. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(23):10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaliman P, Párrizas M, Lalanza JF, Camins A, Escorihuela RM, Pallàs M. Neurophysiological and epigenetic effects of physical exercise on the aging process. Ageing Research Reviews. 2011;10(4):475–486. doi: 10.1016/j.arr.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Gershon H, Gershon D. Paradigms in aging research: a critical review and assessment. Mechanisms of Ageing and Development. 2000;117(1–3):21–28. doi: 10.1016/s0047-6374(00)00141-x. [DOI] [PubMed] [Google Scholar]
- 11.Raghothama C, Harsha HC, Prasad CK, Pandey A. Bioinformatics and proteomics approaches for aging research. Biogerontology. 2005;6(4):227–232. doi: 10.1007/s10522-005-2617-0. [DOI] [PubMed] [Google Scholar]
- 12.Colavitti R, Finkel T. Reactive oxygen species as mediators of cellular senescence. IUBMB Life. 2005;57(4-5):277–281. doi: 10.1080/15216540500091890. [DOI] [PubMed] [Google Scholar]
- 13.Stadtman ER. Role of oxidant species in aging. Current Medicinal Chemistry. 2004;11(9):1105–1112. doi: 10.2174/0929867043365341. [DOI] [PubMed] [Google Scholar]
- 14.Pérez VI, Buffenstein R, Masamsetti V, et al. Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(9):3059–3064. doi: 10.1073/pnas.0809620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nuss JE, Amaning JK, Bailey CE, et al. Oxidative modification and aggregation of creatine kinase from aged mouse skeletal muscle. Aging. 2009;1(6):557–572. doi: 10.18632/aging.100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. The Journal of Biological Chemistry. 1997;272(33):20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 17.Levine RL, Stadtman ER. Oxidative modification of proteins during aging. Experimental Gerontology. 2001;36(9):1495–1502. doi: 10.1016/s0531-5565(01)00135-8. [DOI] [PubMed] [Google Scholar]
- 18.Grune T, Catalgol B, Jung T. Protein Oxidation and Aging. Hoboken, NJ, USA: John Wiley & Sons; 2012. Removal of oxidized proteins. [Google Scholar]
- 19.McCoy RG, Nair KS. The 2010 ESPEN Sir David Cuthbertson lecture: new and old proteins: clinical implications. Clinical Nutrition. 2013;32(5):728–736. doi: 10.1016/j.clnu.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baraibar MA, Ladouce R, Friguet B. Proteomic quantification and identification of carbonylated proteins upon oxidative stress and during cellular aging. Journal of Proteomics. 2013;92:63–70. doi: 10.1016/j.jprot.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Jaleel A, Henderson GC, Madden BJ, et al. Identification of de novo synthesized and relatively older proteins: accelerated oxidative damage to de novo synthesized apolipoprotein A-1 in type 1 diabetes. Diabetes. 2010;59(10):2366–2374. doi: 10.2337/db10-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krolewski AS, Kosinski EJ, Warram JH, et al. Magnitude and determinants of coronary artery disease in juvenile-onset, insulin-dependent diabetes mellitus. The American Journal of Cardiology. 1987;59(8):750–755. doi: 10.1016/0002-9149(87)91086-1. [DOI] [PubMed] [Google Scholar]
- 23.Hipkiss AR, Carmichael PL, Zimmermann B. Metabolism of crystallin fragments in cell-free extracts of bovine lens: effects of ageing and oxygen free-radicals. Acta Biologica Hungarica. 1991;42(1–3):243–263. [PubMed] [Google Scholar]
- 24.Hipkiss AR. Accumulation of altered proteins and ageing: causes and effects. Experimental Gerontology. 2006;41(5):464–473. doi: 10.1016/j.exger.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Hipkiss AR. Error-protein metabolism and ageing. Biogerontology. 2009;10(4):523–529. doi: 10.1007/s10522-008-9188-9. [DOI] [PubMed] [Google Scholar]
- 26.Stadtman ER. Protein oxidation and aging. Science. 1992;257(5074):1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberger RF. Senescence and the accumulation of abnormal proteins. Mutation Research. 1991;256(2–6):255–262. doi: 10.1016/0921-8734(91)90016-5. [DOI] [PubMed] [Google Scholar]
- 28.Stadtman ER, Levine RL. Chemical modification of proteins by reactive oxygen species. In: Dalle-Donne I, Scaloni A, Butterfield DA, editors. Redox Proteomics: From Protein Modifications to Cellular Dysfunction and Diseases. Hoboken, NJ, USA: Wiley-Interscience; 2006. pp. 3–23. [Google Scholar]
- 29.Yu BP. Why calorie restriction would work for human longevity. Biogerontology. 2006;7(3):179–182. doi: 10.1007/s10522-006-9009-y. [DOI] [PubMed] [Google Scholar]
- 30.Dunlop RA, Dean RT, Rodgers KJ. The impact of specific oxidized amino acids on protein turnover in J774 cells. Biochemical Journal. 2008;410(1):131–140. doi: 10.1042/BJ20070161. [DOI] [PubMed] [Google Scholar]
- 31.Abello N, Kerstjens HAM, Postma DS, Bischoff R. Protein tyrosine nitration: selectivity, physicochemical and biological consequences, denitration, and proteomics methods for the identification of tyrosine-nitrated proteins. Journal of Proteome Research. 2009;8(7):3222–3238. doi: 10.1021/pr900039c. [DOI] [PubMed] [Google Scholar]
- 32.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiological Reviews. 2007;87(1):315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feeney MB, Schöneich C. Tyrosine modifications in aging. Antioxidants & Redox Signaling. 2012;17(11):1571–1579. doi: 10.1089/ars.2012.4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davies D. A quiet life with proteins. Annual Review of Biophysics and Biomolecular Structure. 2005;34:1–20. doi: 10.1146/annurev.biophys.34.040204.144531. [DOI] [PubMed] [Google Scholar]
- 35.Esterbauer H, Schaur RJ, Zollner H. Chemistry and Biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radical Biology and Medicine. 1991;11(1):81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 36.Miura Y, Sato Y, Arai Y, et al. Proteomic analysis of plasma proteins in Japanese semisuper centenarians. Experimental Gerontology. 2011;46(1):81–85. doi: 10.1016/j.exger.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Barja G. Updating the mitochondrial free radical theory of aging: an integrated view, key aspects, and confounding concepts. Antioxidants & Redox Signaling. 2013;19(12):1420–1445. doi: 10.1089/ars.2012.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghosh S, Lertwattanarak R, Lefort N, et al. Reduction in reactive oxygen species production by mitochondria from elderly subjects with normal and impaired glucose tolerance. Diabetes. 2011;60(8):2051–2060. doi: 10.2337/db11-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmed EK, Rogowska-Wrzesinska A, Roepstorff P, Bulteau A-L, Friguet B. Protein modification and replicative senescence of WI-38 human embryonic fibroblasts. Aging Cell. 2010;9(2):252–272. doi: 10.1111/j.1474-9726.2010.00555.x. [DOI] [PubMed] [Google Scholar]
- 40.Baraibar MA, Hyzewicz J, Rogowska-Wrzesinska A, et al. Oxidative stress-induced proteome alterations target different cellular pathways in human myoblasts. Free Radical Biology and Medicine. 2011;51(8):1522–1532. doi: 10.1016/j.freeradbiomed.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 41.Held JM, Gibson BW. Regulatory control or oxidative damage? Proteomic approaches to interrogate the role of cysteine oxidation status in biological processes. Molecular & Cellular Proteomics. 2012;11(4) doi: 10.1074/mcp.R111.013037.R111.013037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moosmann B, Behl C. Mitochondrially encoded cysteine predicts animal lifespan. Aging Cell. 2008;7(1):32–46. doi: 10.1111/j.1474-9726.2007.00349.x. [DOI] [PubMed] [Google Scholar]
- 43.Kline AD, Krantz ID, Sommer A, et al. Cornelia de lange syndrome: clinical review, diagnostic and scoring systems, and anticipatory guidance. American Journal of Medical Genetics A. 2008;146(20):1287–1296. doi: 10.1002/ajmg.a.31757. [DOI] [PubMed] [Google Scholar]
- 44.Gimigliano A, Mannini L, Bianchi L, et al. Proteomic profile identifies dysregulated pathways in Cornelia de Lange syndrome cells with distinct mutations in SMC1A and SMC3 genes. Journal of Proteome Research. 2012;11(12):6111–6123. doi: 10.1021/pr300760p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okumura S, Kawabe J-I, Yatani A, et al. Type 5 adenylyl cyclase disruption alters not only sympathetic but also parasympathetic and calcium-mediated cardiac regulation. Circulation Research. 2003;93(4):364–371. doi: 10.1161/01.RES.0000086986.35568.63. [DOI] [PubMed] [Google Scholar]
- 46.Yan L, Vatner DE, O’Connor JP, et al. Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell. 2007;130(2):247–258. doi: 10.1016/j.cell.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 47.Navarro A, Sánchez del Pino MJ, Gómez C, Peralta JL, Boveris A. Behavioral dysfunction, brain oxidative stress, and impaired mitochondrial electron transfer in aging mice. American Journal of Physiology. 2002;282(4):R985–R992. doi: 10.1152/ajpregu.00537.2001. [DOI] [PubMed] [Google Scholar]
- 48.Perluigi M, di Domenico F, Giorgi A, et al. Redox proteomics in aging rat brain: involvement of mitochondrial reduced glutathione status and mitochondrial protein oxidation in the aging process. Journal of Neuroscience Research. 2010;88(16):3498–3507. doi: 10.1002/jnr.22500. [DOI] [PubMed] [Google Scholar]
- 49.Yang S, Liu T, Li S, et al. Comparative proteomic analysis of brains of naturally aging mice. Neuroscience. 2008;154(3):1107–1120. doi: 10.1016/j.neuroscience.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 50.VanGuilder HD, Freeman WM. The hippocampal neuroproteome with aging and cognitive decline: past progress and future directions. Frontiers in Aging Neuroscience. 2011;3, article 8 doi: 10.3389/fnagi.2011.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang J-L, Weissman L, Bohr VA, Mattson MP. Mitochondrial DNA damage and repair in neurodegenerative disorders. DNA Repair. 2008;7(7):1110–1120. doi: 10.1016/j.dnarep.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Markesbery WR. Oxidative stress hypothesis in Alzheimer’s disease. Free Radical Biology and Medicine. 1997;23(1):134–147. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- 53.Korolainen MA, Goldsteins G, Alafuzoff I, Koistinaho J, Pirttilä T. Proteomic analysis of protein oxidation in Alzheimer’s disease brain. Electrophoresis. 2002;23(19):3428–3433. doi: 10.1002/1522-2683(200210)23:19<3428::AID-ELPS3428>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 54.Castegna A, Aksenov M, Aksenova M, et al. Proteomic identification of oxidatively modified proteins in Alzheimer’s disease brain. Part I: creatine kinase BB, glutamine synthase, and ubiquitin carboxy-terminal hydrolase L-1. Free Radical Biology and Medicine. 2002;33(4):562–571. doi: 10.1016/s0891-5849(02)00914-0. [DOI] [PubMed] [Google Scholar]
- 55.Castegna A, Aksenov M, Thongboonkerd V, et al. Proteomic identification of oxidatively modified proteins in Alzheimer’s disease brain. Part II: dihydropyrimidinase-related protein 2, α-enolase and heat shock cognate 71. Journal of Neurochemistry. 2002;82(6):1524–1532. doi: 10.1046/j.1471-4159.2002.01103.x. [DOI] [PubMed] [Google Scholar]
- 56.Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: potential causes and consequences involving amyloid β-peptide-associated free radical oxidative stress. Free Radical Biology and Medicine. 2002;32(11):1050–1060. doi: 10.1016/s0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- 57.Korolainen MA, Nyman TA, Nyyssönen P, Hartikainen ES, Pirttilä T. Multiplexed proteomic analysis of oxidation and concentrations of cerebrospinal fluid proteins in Alzheimer disease. Clinical Chemistry. 2007;53(4):657–665. doi: 10.1373/clinchem.2006.078014. [DOI] [PubMed] [Google Scholar]
- 58.Weinreb O, Drigues N, Sagi Y, Reznick AZ, Amit T, Youdim MBH. The application of proteomics and genomics to the study of age-related neurodegeneration and neuroprotection. Antioxidants & Redox Signaling. 2007;9(2):169–179. doi: 10.1089/ars.2007.9.169. [DOI] [PubMed] [Google Scholar]
- 59.Chung YH, Shin CM, Joo KM, Kim MJ, Cha CI. Immunohistochemical study on the distribution of nitrotyrosine and neuronal nitric oxide synthase in aged rat cerebellum. Brain Research. 2002;951(2):316–321. doi: 10.1016/s0006-8993(02)03261-4. [DOI] [PubMed] [Google Scholar]
- 60.Siles E, Martínez-Lara E, Cauelo A, et al. Age-related changes of the nitric oxide system in the rat brain. Brain Research. 2002;956(2):385–392. doi: 10.1016/s0006-8993(02)03575-8. [DOI] [PubMed] [Google Scholar]
- 61.Boyd-Kimball D, Sultana R, Poon HF, et al. γ-glutamylcysteine ethyl ester protection of proteins from Aβ(1–42)-mediated oxidative stress in neuronal cell culture: a proteomics approach. Journal of Neuroscience Research. 2005;79(5):707–713. doi: 10.1002/jnr.20393. [DOI] [PubMed] [Google Scholar]
- 62.Drake J, Kanski J, Varadarajan S, Tsoras M, Butterfield DA. Elevation of brain glutathione by γ-glutamylcysteine ethyl ester protects against peroxynitrite-induced oxidative stress. Journal of Neuroscience Research. 2002;68(6):776–784. doi: 10.1002/jnr.10266. [DOI] [PubMed] [Google Scholar]
- 63.Bolaños JP, Heales SJR, Land JM, Clark JB. Effect of peroxynitrite on the mitochondrial respiratory chain: differential susceptibility of neurones and astrocytes in primary culture. Journal of Neurochemistry. 1995;64(5):1965–1972. doi: 10.1046/j.1471-4159.1995.64051965.x. [DOI] [PubMed] [Google Scholar]
- 64.Desagher S, Glowinski J, Premont J. Astrocytes protect neurons from hydrogen peroxide toxicity. Journal of Neuroscience. 1996;16(8):2553–2562. doi: 10.1523/JNEUROSCI.16-08-02553.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanaka J, Toku K, Zhang B, Ishihara K, Sakanaka M, Maeda N. Astrocytes prevent neuronal death induced by reactive oxygen and nitrogen species. Glia. 1999;28(2):85–96. doi: 10.1002/(sici)1098-1136(199911)28:2<85::aid-glia1>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 66.Hollensworth SB, Shen C-C, Sim JE, Spitz DR, Wilson GL, Ledoux SP. Glial cell type-specific responses to menadione-induced oxidative stress. Free Radical Biology and Medicine. 2000;28(8):1161–1174. doi: 10.1016/s0891-5849(00)00214-8. [DOI] [PubMed] [Google Scholar]
- 67.Miura Y, Kano M, Abe K, Urano S, Suzuki S, Toda T. Age-dependent variations of cell response to oxidative stress: proteomic approach to protein expression and phosphorylation. Electrophoresis. 2005;26(14):2786–2796. doi: 10.1002/elps.200500172. [DOI] [PubMed] [Google Scholar]
- 68.Oxenham H, Sharpe N. Cardiovascular aging and heart failure. European Journal of Heart Failure. 2003;5(4):427–434. doi: 10.1016/s1388-9842(03)00011-4. [DOI] [PubMed] [Google Scholar]
- 69.Grant JE, Bradshaw AD, Schwacke JH, Baicu CF, Zile MR, Schey KL. Quantification of protein expression changes in the aging left ventricle of Rattus norvegicus . Journal of Proteome Research. 2009;8(9):4252–4263. doi: 10.1021/pr900297f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dai Q, Escobar GP, Hakala KW, Lambert JM, Weintraub ST, Lindsey ML. The left ventricle proteome differentiates middle-aged and old left ventricles in mice. Journal of Proteome Research. 2008;7(2):756–765. doi: 10.1021/pr700685e. [DOI] [PubMed] [Google Scholar]
- 71.Richardson MR, Lai X, Mason SB, Miller SJ, Witzmann FA. Differential protein expression during aging in ventricular myocardium of Fischer 344 × Brown Norway hybrid rats. Experimental Gerontology. 2008;43(10):909–918. doi: 10.1016/j.exger.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lindsey ML, Goshorn DK, Comte-Walters S, et al. A multidimensional proteomic approach to identify hypertrophy-associated proteins. Proteomics. 2006;6(7):2225–2235. doi: 10.1002/pmic.200500013. [DOI] [PubMed] [Google Scholar]
- 73.Hong SJ, Gokulrangan G, Schöneich C. Proteomic analysis of age dependent nitration of rat cardiac proteins by solution isoelectric focusing coupled to nanoHPLC tandem mass spectrometry. Experimental Gerontology. 2007;42(7):639–651. doi: 10.1016/j.exger.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kanski J, Alterman MA, Schöneich C. Proteomic identification of age-dependent protein nitration in rat skeletal muscle. Free Radical Biology and Medicine. 2003;35(10):1229–1239. doi: 10.1016/s0891-5849(03)00500-8. [DOI] [PubMed] [Google Scholar]
- 75.Kanski J, Behring A, Pelling J, Schöneich C. Proteomic identification of 3-nitrotyrosine-containing rat cardiac proteins: effects of biological aging. American Journal of Physiology. 2005;288(1):H371–H381. doi: 10.1152/ajpheart.01030.2003. [DOI] [PubMed] [Google Scholar]
- 76.Rifkind JM, Nagababu E. Hemoglobin redox reactions and red blood cell aging. Antioxidants & Redox Signaling. 2013;18(17):2274–2283. doi: 10.1089/ars.2012.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Romero N, Radi R. Hemoglobin and red blood cells as tools for studying peroxynitrite biochemistry. Methods in Enzymology. 2005;396:229–245. doi: 10.1016/S0076-6879(05)96021-7. [DOI] [PubMed] [Google Scholar]
- 78.Bosman GJCGM, Werre JM, Willekens FLA, Novotný VMJ. Erythrocyte ageing in vivo and in vitro: structural aspects and implications for transfusion. Transfusion Medicine. 2008;18(6):335–347. doi: 10.1111/j.1365-3148.2008.00892.x. [DOI] [PubMed] [Google Scholar]
- 79.Rinalducci S, D’Amici GM, Blasi B, Vaglio S, Grazzini G, Zolla L. Peroxiredoxin-2 as a candidate biomarker to test oxidative stress levels of stored red blood cells under blood bank conditions. Transfusion. 2011;51(7):1439–1449. doi: 10.1111/j.1537-2995.2010.03032.x. [DOI] [PubMed] [Google Scholar]
- 80.Ha MK, Cho JS, Baik O-R, Hoon LK, Koo H-S, Chung KY. Caenorhabditis elegans as a screening tool for the endothelial cell-derived putative aging-related proteins detected by proteomic analysis. Proteomics. 2006;6(11):3339–3351. doi: 10.1002/pmic.200500395. [DOI] [PubMed] [Google Scholar]
- 81.Fisher AL, Page KE, Lithgow GJ, Nash L. The Caenorhabditis elegans K10C2.4 gene encodes a member of the fumarylacetoacetate hydrolase family: a Caenorhabditis elegans model of type I tyrosinemia. The Journal of Biological Chemistry. 2008;283(14):9127–9135. doi: 10.1074/jbc.M708341200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jones LM, Staffa K, Perally S, LaCourse EJ, Brophy PM, Hamilton JV. Proteomic analyses of Caenorhabditis elegans dauer larvae and long-lived daf-2 mutants implicates a shared detoxification system in longevity assurance. Journal of Proteome Research. 2010;9(6):2871–2881. doi: 10.1021/pr9009639. [DOI] [PubMed] [Google Scholar]
- 83.Kumsta C, Thamsen M, Jakob U. Effects of oxidative stress on behavior, physiology, and the redox thiol proteome of Caenorhabditis elegans . Antioxidants & Redox Signaling. 2011;14(6):1023–1037. doi: 10.1089/ars.2010.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arczewska KD, Tomazella GG, Lindvall JM, et al. Active transcriptomic and proteomic reprogramming in the C. elegans nucleotide excision repair mutant xpa-1 . Nucleic Acids Research. 2013;41(10):5368–5381. doi: 10.1093/nar/gkt225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nabeshi H, Oikawa S, Inoue S, Nishino K, Kawanishi S. Proteomic analysis for protein carbonyl as an indicator of oxidative damage in senescence-accelerated mice. Free Radical Research. 2006;40(11):1173–1181. doi: 10.1080/10715760600847580. [DOI] [PubMed] [Google Scholar]
- 86.Zhu L, Yu J, Shi Q, et al. Strain-and age-related alteration of proteins in the brain of SAMP8 and SAMR1 mice. Journal of Alzheimer’s Disease. 2011;23(4):641–654. doi: 10.3233/JAD-2010-101389. [DOI] [PubMed] [Google Scholar]
- 87.Soreghan BA, Yang F, Thomas SN, Hsu J, Yang AJ. High-throughput proteomic-based identification of oxidatively induced protein carbonylation in mouse brain. Pharmaceutical Research. 2003;20(11):1713–1720. doi: 10.1023/b:pham.0000003366.25263.78. [DOI] [PubMed] [Google Scholar]
- 88.Stoll EA, Cheung W, Mikheev AM, et al. Aging neural progenitor cells have decreased mitochondrial content and lower oxidative metabolism. The Journal of Biological Chemistry. 2011;286(44):38592–38601. doi: 10.1074/jbc.M111.252171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Amelina H, Sjödin MOD, Bergquist J, Cristobal S. Quantitative subproteomic analysis of age-related changes in mouse liver peroxisomes by iTRAQ LC-MS/MS. Journal of Chromatography B. 2011;879(30):3393–3400. doi: 10.1016/j.jchromb.2011.08.044. [DOI] [PubMed] [Google Scholar]
- 90.Amelina H, Cristobal S. Proteomic study on gender differences in aging kidney of mice. Proteome Science. 2009;7, article 16 doi: 10.1186/1477-5956-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peinado JR, Quirós PM, Pulido MR, et al. Proteomic profiling of adipose tissue from Zmpste24−/− mice, a model of lipodystrophy and premature aging, reveals major changes in mitochondrial function and vimentin processing. Molecular & Cellular Proteomics. 2011;10(11) doi: 10.1074/mcp.M111.008094.M111.008094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smallwood HS, López-Ferrer D, Squier TC. Aging enhances the production of reactive oxygen species and bactericidal activity in peritoneal macrophages by upregulating classical activation pathways. Biochemistry. 2011;50(45):9911–9922. doi: 10.1021/bi2011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Santos-González M, López-Miranda J, Pérez-Jiménez F, Navas P, Villalba JM. Dietary oil modifies the plasma proteome during aging in the rat. Age. 2012;34(2):341–358. doi: 10.1007/s11357-011-9239-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim CH, Zou Y, Kim DH, Kim ND, Yu BP, Chung HY. Proteomic analysis of nitrated and 4-hydroxy-2-nonenal-modified serum proteins during aging. The Journals of Gerontology A. 2006;61(4):332–338. doi: 10.1093/gerona/61.4.332. [DOI] [PubMed] [Google Scholar]
- 95.Gokulrangan G, Zaidi A, Michaelis ML, Schöneich C. Proteomic analysis of protein nitration in rat cerebellum: effect of biological aging. Journal of Neurochemistry. 2007;100(6):1494–1504. doi: 10.1111/j.1471-4159.2006.04334.x. [DOI] [PubMed] [Google Scholar]
- 96.Argüelles S, Cano M, Machado A, Ayala A. Effect of aging and oxidative stress on elongation factor-2 in hypothalamus and hypophysis. Mechanisms of Ageing and Development. 2011;132(1-2):55–64. doi: 10.1016/j.mad.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 97.Kim H-J, Ji B-R, Kim J-S, Lee H-N, Ha D-H, Kim C-W. Proteomic analysis of proteins associated with cellular senescence by calorie restriction in mesenchymal stem cells. In Vitro Cellular & Developmental Biology. 2012;48(3):186–195. doi: 10.1007/s11626-012-9485-0. [DOI] [PubMed] [Google Scholar]
- 98.Gu X, Neric NJ, Crabb JS, et al. Age-related changes in the retinal pigment epithelium (RPE) PLoS ONE. 2012;7(6) doi: 10.1371/journal.pone.0038673.e38673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee H, Arnouk H, Sripathi S, et al. Prohibitin as an oxidative stress biomarker in the eye. International Journal of Biological Macromolecules. 2010;47(5):685–690. doi: 10.1016/j.ijbiomac.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Valle A, Sastre-Serra J, Roca P, Oliver J. Modulation of white adipose tissue proteome by aging and calorie restriction. Aging Cell. 2010;9(5):882–894. doi: 10.1111/j.1474-9726.2010.00613.x. [DOI] [PubMed] [Google Scholar]
- 101.Kanski J, Hong SJ, Schöneich C. Proteomic analysis of protein nitration in aging skeletal muscle and identification of nitrotyrosine-containing sequences in vivo by nanoelectrospray ionization tandem mass spectrometry. The Journal of Biological Chemistry. 2005;280(25):24261–24266. doi: 10.1074/jbc.M501773200. [DOI] [PubMed] [Google Scholar]
- 102.Piec I, Listrat A, Alliot J, Chambon C, Taylor RG, Bechet D. Differential proteome analysis of aging in rat skeletal muscle. FASEB Journal. 2005;19(9):1143–1145. doi: 10.1096/fj.04-3084fje. [DOI] [PubMed] [Google Scholar]
- 103.O’Connell K, Ohlendieck K. Proteomic DIGE analysis of the mitochondria-enriched fraction from aged rat skeletal muscle. Proteomics. 2009;9(24):5509–5524. doi: 10.1002/pmic.200900472. [DOI] [PubMed] [Google Scholar]
- 104.de Waal EM, Liang H, Pierce A, Hamilton RT, Buffenstein R, Chaudhuri AR. Elevated protein carbonylation and oxidative stress do not affect protein structure and function in the long-living naked-mole rat: a proteomic approach. Biochemical and Biophysical Research Communications. 2013;434(4):815–819. doi: 10.1016/j.bbrc.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 105.Jiang G-J, Wang K, Miao D-Q, et al. Protein profile changes during porcine oocyte aging and effects of caffeine on protein expression patterns. PLoS ONE. 2011;6(12) doi: 10.1371/journal.pone.0028996.e28996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Opii WO, Joshi G, Head E, et al. Proteomic identification of brain proteins in the canine model of human aging following a long-term treatment with antioxidants and a program of behavioral enrichment: relevance to Alzheimer’s disease. Neurobiology of Aging. 2008;29(1):51–70. doi: 10.1016/j.neurobiolaging.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gromov P, Skovgaard GL, Palsdottir H, Gromova I, Østergaard M, Celis JE. Protein profiling of the human epidermis from the elderly reveals up-regulation of a signature of interferon-γ-induced polypeptides that includes manganese-superoxide dismutase and the p85β subunit of phosphatidylinositol 3-kinase. Molecular & Cellular Proteomics. 2003;2(2):70–84. doi: 10.1074/mcp.M200051-MCP200. [DOI] [PubMed] [Google Scholar]
- 108.Xie L, Tsaprailis G, Chen QM. Proteomic identification of insulin-like growth factor-binding protein-6 induced by sublethal H2O2 stress from human diploid fibroblasts. Molecular & Cellular Proteomics. 2005;4(9):1273–1283. doi: 10.1074/mcp.M500032-MCP200. [DOI] [PubMed] [Google Scholar]
- 109.Xie L, Pandey R, Xu B, Tsaprailis G, Chen QM. Genomic and proteomic profiling of oxidative stress response in human diploid fibroblasts. Biogerontology. 2009;10(2):125–151. doi: 10.1007/s10522-008-9157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li M, Xiao Z-Q, Chen Z-C, et al. Proteomic analysis of the aging-related proteins in human normal colon epithelial tissue. Journal of Biochemistry and Molecular Biology. 2007;40(1):72–81. doi: 10.5483/bmbrep.2007.40.1.072. [DOI] [PubMed] [Google Scholar]
- 111.Kasper G, Mao L, Geissler S, et al. Insights into mesenchymal stem cell aging: involvement of antioxidant defense and actin cytoskeleton. Stem Cells. 2009;27(6):1288–1297. doi: 10.1002/stem.49. [DOI] [PubMed] [Google Scholar]
- 112.Lans H, Vermeulen W. Nucleotide excision repair in Caenorhabditis elegans . Molecular Biology International. 2011;2011:12 pages. doi: 10.4061/2011/542795.542795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pallas M, Camins A, Smith MA, Perry G, Lee H-G, Casadesus G. From aging to Alzheimer’s disease: unveiling “the switch” with the senescence-accelerated mouse model (SAMP8) Journal of Alzheimer’s Disease. 2008;15(4):615–624. doi: 10.3233/jad-2008-15408. [DOI] [PubMed] [Google Scholar]
- 114.Packer L, Roy S, Sen CK. Alpha-lipoic acid: a metabolic antioxidant and potential redox modulator of transcription. Advances in Pharmacology. 1997;38:79–101. doi: 10.1016/s1054-3589(08)60980-1. [DOI] [PubMed] [Google Scholar]
- 115.Packer L, Tritschler HJ, Wessel K. Neuroprotection by the metabolic antioxidant α-lipoic acid. Free Radical Biology and Medicine. 1997;22(1-2):359–378. doi: 10.1016/s0891-5849(96)00269-9. [DOI] [PubMed] [Google Scholar]
- 116.Poon HF, Farr SA, Thongboonkerd V, et al. Proteomic analysis of specific brain proteins in aged SAMP8 mice treated with alpha-lipoic acid: implications for aging and age-related neurodegenerative disorders. Neurochemistry International. 2005;46(2):159–168. doi: 10.1016/j.neuint.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 117.Lewis KN, Mele J, Hayes JD, Buffenstein R. Nrf2, a guardian of healthspan and gatekeeper of species longevity. Integrative & Comparative Biology. 2010;50(5):829–843. doi: 10.1093/icb/icq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rodriguez KA, Edrey YH, Osmulski P, Gaczynska M, Buffenstein R. Altered composition of liver proteasome assemblies contributes to enhanced proteasome activity in the exceptionally long-lived naked mole-rat. PLoS ONE. 2012;7(5) doi: 10.1371/journal.pone.0035890.e35890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Selkoe DJ, Bell DS, Podlisny MB, Cork LC. Conservation of brain amyloid proteins in aged mammals and humans with Alzheimer’s disease. Science. 1987;235(4791):873–877. doi: 10.1126/science.3544219. [DOI] [PubMed] [Google Scholar]
- 120.Cummings BJ, Head E, Afagh AJ, Milgram NW, Cotman CW. β-amyloid accumulation correlates with cognitive dysfunction in the aged canine. Neurobiology of Learning and Memory. 1996;66(1):11–23. doi: 10.1006/nlme.1996.0039. [DOI] [PubMed] [Google Scholar]
- 121.Head E, Torp R. Insights into Aβ and presenilin from a canine model of human brain aging. Neurobiology of Disease. 2002;9(1):1–10. doi: 10.1006/nbdi.2002.0476. [DOI] [PubMed] [Google Scholar]
- 122.Head E, McCleary R, Hahn FF, Milgram NW, Cotman CW. Region-specific age at onset of β-amyloid in dogs. Neurobiology of Aging. 2000;21(1):89–96. doi: 10.1016/s0197-4580(00)00093-2. [DOI] [PubMed] [Google Scholar]
- 123.Austad S. Advances in vertebrate aging research 2007. Aging Cell. 2008;7(2):119–124. doi: 10.1111/j.1474-9726.2008.00374.x. [DOI] [PubMed] [Google Scholar]
- 124.Miller RA, Harrison DE, Astle CM, et al. An aging interventions testing program: study design and interim report. Aging Cell. 2007;6(4):565–575. doi: 10.1111/j.1474-9726.2007.00311.x. [DOI] [PubMed] [Google Scholar]


