Abstract
For more than half a century, cytotoxic agents have been investigated as a possible treatment for cancer. Research on animal venoms has revealed their high toxicity on tissues and cell cultures, both normal and tumoral. Snake venoms show the highest cytotoxic potential, since ophidian accidents cause a large amount of tissue damage, suggesting a promising utilization of these venoms or their components as antitumoral agents. Over the last few years, we have studied the effects of snake venoms and their isolated enzymes on tumor cell cultures. Some in vivo assays showed antineoplastic activity against induced tumors in mice. In human beings, both the crude venom and isolated enzymes revealed antitumor activities in preliminary assays, with measurable clinical responses in the advanced treatment phase. These enzymes include metalloproteases (MP), disintegrins, L-amino acid oxidases (LAAOs), C-type lectins, and phospholipases A2 (PLA2s). Their mechanisms of action include direct toxic action (PLA2s), free radical generation (LAAOs), apoptosis induction (PLA2s, MP, and LAAOs), and antiangiogenesis (disintegrins and lectins). Higher cytotoxic and cytostatic activities upon tumor cells than normal cells suggest the possibility for clinical applications. Further studies should be conducted to ensure the efficacy and safety of different snake venom compounds for cancer drug development.
1. Introduction
Cancer is a chronic degenerative disease considered to be the second most common cause of death in economically developing countries [1, 2]. According to a recent report by the International Agency for Research on Cancer (IARC), there are currently more than 10 million cases of cancer per year worldwide. In 2008 alone there were 12.7 million new cases of cancer worldwide and the WHO estimates that the disease will cause about 13.1 million deaths by 2030 [3].
Cancer is characterized by an accelerated and uncontrolled multiplication of a set of aberrant cells which lose their apoptotic ability. Research has been undertaken in order to find out the factors which promote uncontrolled multiplication of cells and how cancer genes affect cell signaling, chromatin, and epigenomic regulation and RNA splicing, protein homeostasis, metabolism, and lineage maturation [4–6].
Understanding the events that transform a normal cell into a cancer cell has caused new therapies to develop that are more precisely designed to treat a critical gene or biological pathway [7]. Based on their mechanism of action, antitumor drugs that target the cell cycle can be divided generally into three categories, namely, blocking DNA synthesis, causing DNA damage, and stopping mitosis [8]. However, cancer therapy continues involving invasive procedures, including catheter application of chemotherapy, surgery to remove the tumor(s), the use of radiation, and even nonselective cytotoxic drugs [9, 10]. Therefore, the search for new active drugs for cancer therapy is one of the goals of biotechnological research. The expansion of new drugs in oncology represents one of the most promising objectives of the pharmaceutical industry. Many of these compounds are derived from the extraction and purification of toxins and secondary metabolites originating from microorganisms, plants, and animals [11, 12].
Several compounds from venomous animals, such as snakes, spiders, scorpions, caterpillars, bees, insects, wasps, centipedes, ants, toads, and frogs, have largely shown biotechnological or pharmacological applications [13–17]. Numerous examples may be mentioned. Compound TM-601, a modified form of the peptide Chlorotoxin (CTX), isolated from Leiurus quinquestriatus scorpion venom, has been shown to bind specifically to glioma cell surfaces as a specific chloride channel blocker and is currently in phase II of human trials [18, 19]. Another example is the venom-derived drug Prialt (ziconotide) generated from the venom peptide of the marine snail Conus magus [20] and the drug Byetta (exenatide), a synthetic version of exendin-4 utilized in the treatment of Type 2 diabetes, from the saliva of the Gila monster lizard [21, 22].
The ability of some snake venom toxins to cause toxicity is associated with their high specificity and affinity for cell and tissues. In spite of their toxicological effects, several isolated snake venom proteins and peptides have practical applications as pharmaceutical agents [23]. For example, thrombolytic agents have been used in several cases of vascular disorder [24], antimicrobial activity against gram-positive and gram-negative bacteria [25, 26], antiviral activity against several types of viruses including the herpes simplex virus [27], yellow fever and dengue [28], antiparasitic activity against Leishmania [29] and Plasmodium falciparum [30], and antifungal activity [31], among other examples.
For cancer treatment, there is great interest in drug design, providing structural templates for the study of new molecules or cellular mechanisms. The use of snake venom in the treatment of some diseases began about sixty years ago in folk medicine. Thus, the biological and toxicological mechanisms involved in snakebites led physicians to study new methods on the isolation of venom constituents, as well as to understand how these compounds could help in medicine.
2. Antitumoral Activity of Snake Venoms
Snake venom is a complex mixture of different components that include peptides, proteins, enzymes, carbohydrates, and minerals. Inside a group of enzymes may be found acetylcholinesterases, L-amino acid oxidases, serineproteases, metalloproteases, and phospholipases A2 [32] (Figure 1). The cytotoxicity of snake venoms is related to cellular metabolism alterations with a major effect on tumor cells when compared with normal cells. This observation stimulated the development of most chemotherapeutic drugs based on toxins produced in animals, which have the capacity to be highly cytotoxic.
Figure 1.
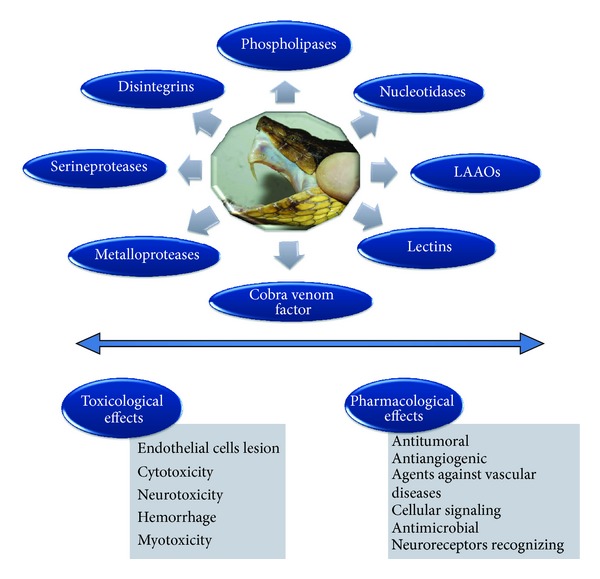
The wide spectrum of action and recent applications of snake venom toxins. The figure depicts the paradigms between toxicological and pharmacological effects of isolated toxins. Different cellular targets are related to different kinds of mechanisms.
The ability of snake venoms to act upon tumor cells has been known for a long time. The first reported studies on using snake venom against tumor cells were related to the defibrination process. It was suggested that Ancrod, a polypeptide from Agkistrodon rhodostoma, administered with cyclophosphamide, could produce defibrination, thus decreasing the tumor weight by fibrinolysis and contributing to both detachment and decreased spread of some tumors [33]. However, their results showed that, besides defibrination, other complex mechanisms including platelet aggregation could be involved in the process.
Braganca et al. [34, 35] assayed a small fraction of Naja naja venom on cell cultures of Yoshida sarcoma, calling it cobra venom factor (CVF). Kaneda et al. [36] studied the antitumoral potential of purified peptides (cardiotoxin and cytotoxin) from the Naja naja atra snake. Then, Chiam-Matyas and Ovadia [37] showed the cytotoxic properties of several crude venoms from terrestrial snakes with lytic effects on cultures of malignant melanoma tumor cells.
In the past, snake venoms were used to understand the molecular mechanism of some receptors, such as acetylcholine (ACh), and their involvement with some diseases. Two groups of toxins (α-BuTX and Erabutoxin and b—ETXa and ETXb) isolated from Bungarus multicinctus and Laticauda semifasciata, respectively, showed high affinity toward normal and tumor cells, displaying both cytolytic and cytotoxic effects. Interactions of such toxins with ACh led to the application of these compounds as probes not only to elucidate neurophysiology but also to study some tumor cells. Although α-BuTX inhibited neuroblastomas, it was too toxic for in vivo assays [38]. Moreover, no relationship was observed between a cytotoxic effect and ACh receptors [39].
Experiments have shown that cytotoxic effects displayed by snake venoms are specifically related to the species, genus, and tissue targets. Thus, snake venoms were grouped according to their pathophysiological activities as follows: (i) venoms which cause irreversible alterations on the cell, totally destroying it (this group includes Elapidae venoms); (ii) Crotalidae venoms which cause loss of the cell process viability; and (iii) Viperidae venoms, which cause alterations of cell aggregation. In vivo, it was demonstrated that the venom of Naja nigricollis inhibited the growth of melanoma through one of these mechanisms. Thus, these findings gave new direction and probable application of snake venom as well as isolated toxins for cancer treatment [40].
Snake venom toxins were also investigated as blockers of metastasis. Metastasis is one of the major causes of death in patients with cancer, being dependent on steps such as adhesion, migration, invasion of blood or lymph vessels, exiting the vessel (with the help of matrix metalloproteinases—MMPs), and finally interaction with the tissue target [41]. Yang et al. [42] studied an inhibitor of integrins that is an important class of cell surface receptors, critically involved in cell-cell and cell-matrix interactions. Particularly, the subfamilies β1 and β3 play a key role in tumor invasion and dissemination. The group isolated contortrostatin, a disintegrin from Agkistrodon contortrix contortrix venom, which is a potent inhibitor of β1-integrin-mediated adhesion in human metastatic melanoma cells. Cardiotoxin III (CTX-III) isolated from Naja naja atra in the study by Jokhio and Ansari [43] also demonstrated antimetastatic potential by decreasing the expression and activity of matrix metalloproteinase MMP-9, caused by the inactivation of p38 MAPK and PI3K/Akt signaling pathways and NF-κB activity. This suppressive effect assists in inhibiting the migration and invasion of cells causing breast cancer.
As of the last decade, a new strategy has been applied to research on snake venoms with antitumor action, with the focus not only on identifying components with this feature but also on understanding the mechanism of action of toxins that reduce cancer. Several mechanisms of action have been related, as in the study of a cardiotoxin that induces apoptosis in K562 cells through an ROS-independent mitochondrial dysfunction pathway and the caspase-dependent mechanism of Bax/Bcl-2 ratio in human colorectal Colo205 cancer [44]. Juhl et al. [45] described the feasibility of using snake venom in suppressing breast cancer tissue through the inhibition of nucleic acid synthesis. This study shows that snake venom strongly inhibited the formation of nucleic acids in breast cancer tissues. It may cause a decrease in cell proliferation.
The ability of snake venom toxins to destroy malignant cells or to share cytotoxic activity was interesting in areas such as immunology. The use of monoclonal antibodies as antitumor therapeutic agents has not been very promising. However, in coupling a nontoxic CVF isolated from Naja naja siamensis to monoclonal antitumor antibodies, several nontoxic antibodies were activated and converted into cytotoxic compounds [46, 47]. Thus, these antibody-CVF conjugates might be a promising therapeutic approach, mediating selective complement-dependent agents of human melanoma, leukemia, and neuroblastomas. Later, it was confirmed that CVF is an important factor for the synthesis of immunoconjugates, which are more specific towards carcinoma cells [48]. In another study using cytotoxin P4, isolated from the same snake, primary conclusions showed for the first time, in vitro and in vivo, that this peptide caused histopathological changes in leukemia cells and specifically in organelles such as mitochondria [47, 48]. Cytotoxins CT1 and CT2 from Naja oxiana, CT3 from Naja kaouthia, and CT1 from Naja haje were demonstrated to possess this property against human lung adenocarcinoma A549 and promyelocytic leukemia HL60 cells [49].
There are studies showing that Bothrops jararaca venom (BjV) induces inhibition of Ehrlich ascites tumor (EAT) growth, accompanied by an increase of mononuclear (MN) leukocytes in all groups inoculated with EAT and/or venom [50]. Different effects were reported with Crotalus durissus terrificus venom, one of which was analgesic activity. Zhang et al. [51] showed that the administration of crotoxin to cancer patients reduced the consumption of analgesics.
Several studies suggest the application of snake venom toxins for the treatment of animal tumors. Despite several findings and much evidence, there is much controversy regarding this subject. New advances in cellular and molecular biology, as well as biotechnology, focus on the need to understand new mechanisms displayed by snake venom toxins (Figure 2).
Figure 2.
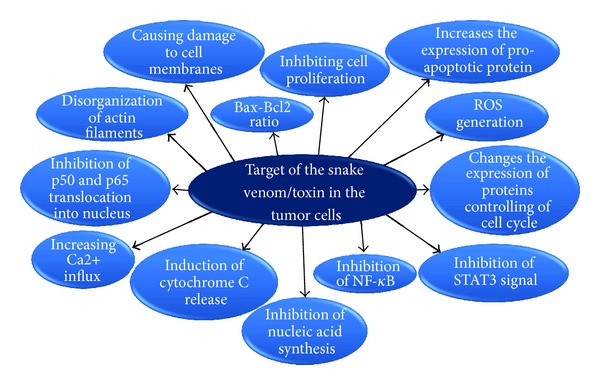
Actions triggered by venoms and/or snake toxins which cause an antitumor effect.
3. “Targets” in Tumor Cells
Understanding snake venom toxins not only helps relieve the healthcare burden of snakebites but also contributes significantly to the treatment of many other medical conditions. In the early 20th century, the idea of utilizing purified toxins as a source of therapeutics emerged [21]. Anticancer drug developments from natural biological resources are ventured throughout the world. The biodiversity of venoms or toxins makes them a tool from which new therapeutic agents may be developed. Snake venom has been shown to possess a wide spectrum of biological activities. Anticarcinogenic activities of snake crude venoms have been recognized, and their components, including cytotoxins, have been isolated and characterized. These components exhibit various physiological effects such as cytotoxicity, inhibition of platelet aggregation, cardiac arrest, and hemolysis [20].
One of the targets investigated is integrins. They are cell surface adhesion molecules coupling the extracellular environment to the cytoskeleton and are also receptors for transmitting important signals for cell migration, invasion, proliferation, and survival. At least six integrin inhibitors are being evaluated in clinical trials for cancer. The parallel development of integrin antagonists as imaging tools for patient selection may accelerate the discovery of new ways for their use [52].
Integrins play multiple important roles in cancer pathology including tumor cell proliferation, angiogenesis, invasion, and metastasis. The inhibition of angiogenesis is one of the most heavily explored treatment options for cancer, and snake venom disintegrins represent a library of molecules with different structures, potencies, and specificities and are good starting points for developing antiangiogenesis therapeutics [21, 53–55].
Recently, Bazaa et al. [56] characterized MVL-PLA2, a novel phospholipase A2 from Macrovipera lebetina venom, reporting that it exhibited anti-integrin activity. Chwetzoff [57] studied the cytotoxic activity of a basic phospholipase A2 from Naja nigricollis venom on different cell types and its esterase activity. The cytotoxicity observed was not due to a contaminant, since that would have been eliminated after immunoprecipitation of the basic phospholipase A2 by specific monoclonal antibodies. All eukaryotic cells tested were sensitive to the cytotoxic action of the basic phospholipase A2. In contrast, the Escherichia coli K-12 wild strain was resistant. Thus, the participation of cell membranes in whether the cell is sensitive or resistant to phospholipase A2's attack was investigated using E. coli K-12 membrane mutants, and some of them were sensitive. Whether or not esterase activity was required for phospholipase A2's cytotoxic attack was dependent on the cell line tested. Indeed, when the esterase activity of the basic PLA2 was eliminated by treatment with p-bromophenacyl bromide, the enzyme retained cytotoxic potency inducing necrosis of certain tumor cells grown in vitro, but not of other cells, such as erythrocytes, for which concomitant esterase activity was also necessary. In vivo toxicity studies showed that the loss of either cytotoxic potency or esterase activity eliminated the lethal character of the basic PLA2. This leads to the proposal that the in vivo phospholipase A2 toxicity depends on the simultaneous expression of esterase activity and a nonenzymatic property, manifested by the in vitro cytotoxic potency.
da Silva et al. [58] demonstrated that Bothrops jararaca venom (BjV) has an antitumoral effect on Ehrlich ascites tumor (EAT) cells and induces an increase of polymorphonuclear leukocytes in early stages of tumor growth. The study reported that this venom presents an important inflammatory effect when inoculated in animal models and in human snakebites, and that cytokine levels have been detected in these cases. To evaluate whether the cytokines are involved with the suppression of the tumor's growth, the authors evaluated the cytokine profile in the peritoneal cavity of mice inoculated with EAT cells and treated with BjV. It was observed that EAT implantation induces IL-6, IL-10, and tumor necrosis factor-alpha (TNF-α) production and that the treatment with BjV suppresses production of these cytokines. Furthermore, it was suggested that the IL-6 detected in the present study was produced by the EAT cells and the suppression of its production could be associated with the antitumoral effect of BjV.
Pituitary adenomas are neuroendocrine tumors that produce different endocrine and metabolic alterations, including hyperprolactinemia, acromegaly, and Cushing's disease. These different clinical features of pituitary tumors are the result of the overproduction of hormones by the different pituitary cell types. Recent advances in the understanding of the signaling pathways that control hormone production in pituitary cells provide a source of potential therapeutic targets. Therefore, the study of signaling pathways that control hormone production and proliferation is a good source of candidate targets in pituitary tumors [59].
Structural and functional investigations of these proteins and enzymes from snake venoms may contribute to the advancement of toxinology and to the elaboration of novel therapeutic agents [60].
4. Snake Venom L-Amino Acid Oxidases (svLAAOs)
L-amino acid oxidases (LAAO) are enzymes diffusely distributed in several organisms, such as bacteria, fungi, algae, and snakes. They are dimeric flavoenzymes, which catalyse the oxidative deamination of L-amino acids into ammonia, alpha-keto acids, and H2O2 through an intermediary amino acid. These glycoproteins are typically found in the homodimeric form accompanied by a cofactor, which can be flavin adenine dinucleotide or flavin mononucleotide. LAAOs are also found in venoms of several snake species [61]. They are purified generally in either acidic or basic form, with an isoelectric point between 4.4 and 8.5, having FMN and FAD as cofactors (approximately 2 mol/mol), with a relative molecular mass of 120.000–150,000 in native form and 55,000–66,000 in its monomeric form [61–68].
Until the nineties, researchers were restricted to the study of structural and functional characteristics of these enzymes [61]. From this decade, the correlation between the production of L-amino acid oxidases and their use in the metabolic pathways that involve nitrogen, as well as the production of hydrogen peroxide and ammonia, started to represent a horizon in the development of new biotechnological applications.
The high toxicity presented by this class of enzymes is not yet completely understood. Hypotheses have been studied through interaction with cell membrane receptors, which have the potential to produce high hydrogen peroxide concentrations [69]. The first function of LAAO is probably to promote hypotension in the victim by activating soluble guanylate cyclase in the presence of superoxide dismutase [70]. It has been demonstrated that the isolated enzymes of C. adamanteus and C. atrox can interact specifically with mammal endothelial cells possibly by increasing hydrogen peroxide production [61, 71]. Snake venom LAAOs and their studies in structural and molecular biology have been very important for pharmacology [61, 72]. They have been characterized through their different functions, such as substrate preference, apoptosis induction, cytotoxicity, hemolysis, activation or inhibition of platelet aggregation, hemorrhage induction, edema, and bactericidal activity [61, 68, 73, 74]. Hemorrhage is a common phenomenon caused by snake venom LAAOs, which unchains complex processes, such as apoptosis of endothelial or vascular cells [61].
Many research groups describe LAAOs as apoptosis inducers, in human embryonic cells (293T) [75], human promyelocytic leukemia cells (HL-60) [65, 76, 77], human monocytic cells (MM6) [68], rat lymphocytic leukemia cells (L1210), and human leukemia T cells [71].
In this review, we will focus on the results obtained through several assays regarding their cytotoxic effect upon cell cultures and animal models, as well as the mechanisms involved and reactions able to explain these effects.
Araki et al. [78] showed that cytotoxic substances in the venoms of C. atrox, T. flavoviridis, G. h. blomhoffii, V. ammodytes, and B. arietans cause apoptosis in cell lines in a selective way: being active on vascular endothelial cells of the human lung and inactive on the same cell line of rats, smooth muscle cells of bovines, and embryonic fibroblasts of human beings [70].
Interestingly, the assays that evaluated the cytotoxic activity of LAAOs attributed the apoptotic effect to H2O2 produced by the oxidative reaction, but other studies showed that different mechanisms might exist [71]. Several mice tumor cell lines were assayed for LAAO from Agkistrodon halys with high apoptosis induction, even at low concentrations. In the presence of the enzyme, cultured L1210 cell nuclei were split and showed the characteristic ladder-like pattern of DNA fragmentation. The enzyme binds directly to the cell surface, thereby increasing the local concentration of H2O2. However, experimental evidence suggests that the apoptotic mechanism induced by LAAO is distinguished from the one caused by exogenous H2O2 [71].
In 1997, the Korean group of Ahn et al. [79] published an interesting study investigating the LAAO from Ophiophagus hannah, starting from its purification, biochemical characterization up to its cytotoxic activity upon several tumor cell cultures, both human and murine, achieving around 74% of inhibition of tumor proliferation at a concentration of 2 μg/mL. A different mechanism for H2O2 production was also postulated, by inhibition of thymidine incorporation, with a consequent interaction with DNA. Markland [80] suggested that this enzyme probably prevents the adhesion of tumor cells and the formation of metastasis in the host by inhibition of platelet aggregation and activation of phagocytic cells from the immunological system.
In 1999, Souza et al. [67] showed the cytotoxicity level of an LAAO from Agkistrodon contortrix laticinctus through the fragmentation of DNA on HL-60 cultures hybrid cells. Results showed signs of induction of apoptosis after extraction of DNA. Apoptosis related to svLAAO activity is a polemic subject since some authors postulate that this activity derives from H2O2 action from the enzymatic reaction. Suhr and Kim [71] already showed that apoptosis induction was not related to H2O2 alone. Dipietrantonio et al. [81] detected an increase of caspase 3 activity in HL-60 cells exposed to H2O2. Caspases are proteases of the cysteine family that are commonly apoptosis markers.
Suhara et al. [82] found that H2O2 induces the regulation of the TNF receptor superfamily (FAS) in human endothelial cells and that the activation of the protein tyrosine kinase may be involved in the expression of FAS induced by H2O2. Thus, apoptosis mediated by FAS in human endothelial cells can contribute to the mechanism of H2O2 in inducing cellular damage.
Stábeli et al. [83] showed that inhibition of the toxic effect of LAAO from Bothrops moojeni was retained when catalase, an H2O2 scavenger, was added. This same enzyme showed a cytotoxic effect upon Ehrlich ascite tumor cells and showed efficiency as a bactericidal, trypanocidal, leishmanicidal and apoptotic agent through DNA fragmentation. The same observation was made regarding other isoforms of this enzyme, isolated from the same species, on leishmanicidal activity [84].
BjarLAAO-I, an L-amino acid oxidase from Bothrops jararaca snake venom, was purified by de Vieira Santos et al. [85]. This LAAO inhibited Ehrlich ascites tumor growth and induced an influx of polymorphonuclear cells, as well as spontaneous liberation of H2O2 from peritoneal macrophages. Later, BjarLAAO-I induced mononuclear influx and peritoneal macrophage spreading but the mechanisms that inhibit tumor growth have not been clarified. Animals treated with BjarLAAO-I showed higher survival time.
Zhang et al. [76] evaluated the activity of an LAAO from Trimeresurus stejnegeri as an antiviral agent, as well as the cytotoxic effect of this enzyme upon lymphocytic leukemia C8166 cells, discussing the role of H2O2 in cytotoxic activity. Using catalase, an H2O2 scavenger, these authors observed that LAAO greatly lost its activity, but even in the absence of H2O2 cytotoxicity was still significant, supporting the hypothesis that other mechanisms of action are probably involved. This was already postulated by the assay that evaluated the bactericidal activity of the mouse milk enzyme as a protecting agent of mastitis [86].
In investigating apoxin, an LAAO from Crotalus atrox, Torii et al. [75] concluded that H2O2 indeed played an important role in apoptosis induction. Thus Ali et al. [68] showed that the studies with LAAO from Eristicophis macmahoni reinforce the already proposed theory of the participation of H2O2, produced during the enzyme's activity, in biological and pharmacological effects, such as apoptosis, cytotoxicity, bactericidal activity, and platelet aggregation induction.
Sun et al. [87] showed the antitumoral effect on an LAAO from Trimeresurus flavoviridis at several concentrations upon human glioma cell cultures. They evaluated apoptosis using flow cytometry, showing the inhibitory effect of the enzyme in the presence of catalase. Once more it was shown that concentrations as low as 10 μg/mL were able to destroy 90% of the cells. However, in addition to inhibition by catalase, there was still apoptosis, probably related to the binding of the enzyme to the cell membrane.
Bp-LAAO, an L-amino acid oxidase from Bothrops pauloensis snake venom purified by Rodrigues et al. [60], showed dose-dependent leishmanicidal, bactericidal, and antitumoral activities. This antitumor activity was observed in human breast cancer cells (SKBR-3), acute T leukemia cell (JURKAT), and Ehrlich ascitic tumor (EAT) cell lines. Moreover Bp-LAAO induced platelet aggregation in platelet-rich plasma by inhibiting catalase.
In 2012, LAAOs isolated from Ophiophagus hannah venom decreased thymidine uptake in murine melanoma, fibrosarcoma, colorectal cancer, and Chinese hamster ovary cell line and also showed reduction in cellular proliferation [79]. In addition, an LAAO isolated from Agkistrodon acutus snake venom showed an accumulation of tumor cells at the sub-G1 phase of the cell cycle. It also induced apoptosis via the Fas pathway in A549 cells (human alveolar epithelial cell line) [88–90].
5. Snake Venom Phospholipases A2 (svPLA2s)
Phospholipases A2 (PLA2s) are enzymes of high medical-scientific interest due to their involvement in several inflammatory human diseases and in envenomation by snake and bee venoms. PLA2s also play an important role in diet lipid catabolism and in the general metabolism of lipid membranes. In addition, arachidonic acid, one of their hydrolysis products, is the precursor of important eicosanoids displaying prominent biological activities, namely, prostaglandins, prostacyclins, thromboxanes, and leucotrienes. PLA2s constitute a super-family of different enzymes belonging to four groups based on their source, amino acid sequences, and biochemical characteristics [91–93].
Altered lipid biosynthesis and deregulated lipogenesis are typical features of cancer. Consequently, these pathways have been investigated as novel therapeutic targets. Lipolytic phospholipase A2 (PLA2) enzymes have been explored as novel anticancer agents [94–96].
Different types of phospholipases have been shown to possess antitumor and antiangiogenic properties, such as acidic and basic PLA2s, and synthetic peptides derived from PLA2 homologues [97–100]. Recently, two phospholipases A2 from Cerastes cerastes venom, CC-PLA2-1 and CC-PLA2-2, were purified and characterized. They were able to inhibit cancerous cell adhesion and migration, along with angiogenesis, both in vitro and in vivo [101, 102]. Phospholipase A2 from Macrovipera lebetina transmediterranea venom (MVL-PLA2) inhibited tumor cell adhesion and migration, as well as angiogenesis. This process occurs through an increase in microtubule dynamics and disorganization of focal adhesions [56, 103].
Some PLA2s isolated from Viperidae venoms are capable of inducing antitumoral activity, suggesting that these molecules may be a new class of anticancer agents and provide new molecular and biological insights into cancer drug development [60, 102].
PLA2 activity is related to the metabolism of cell membranes. In 1989, Chwetzoff et al. reported that a Naja nigricollis PLA2, called nigexin, displays important cytotoxicity upon cell cultures of several tumors, such as epithelial, neuroblastoma, and leukemia tumors [104]. Most PLA2s do not show this profile and the authors suggest that the enzymatic activity is not responsible for the cytotoxic effect and other mechanisms must be involved.
VRCTC-310-Onco is a pharmaceutical product under development, composed of crotoxin (from Crotalus durissus terrificus) and cardiotoxin (from Naja naja atra) at an equimolar ratio. Crotoxin B is the main component, a 14 kDa neurotoxic secretory phospholipase A2, that, in addition to its classic enzymatic activity, binds and activates cell receptors located in the plasma membrane [105]. By these or other mechanisms, crotoxin interferes with the signaling of the epidermal growth factor receptor [106]. Addition of cardiotoxin dissociates cytotoxicity (required for antitumoral activity) and neurotoxicity (otherwise, its main side effect) and allows a useful concentration to be achieved in vivo [107]. Injection of crotoxin into mice has been reported to increase the in vivo production of tumor necrosis factor α (TNF- α) together with the stimulation of the hypothalamic-pituitary axis [108].
Preliminary data showed that a protein fraction of the venom could be a useful tool for cancer therapy. Costa et al. [108] evaluated the probable mechanism of action of this union and concluded that PLA2s act on the receptors of the epidermal growth factor. Another route of action might be a decreased production of tumor necrosis factor. In 2002, the same group suggested intravenous (i.v) administration of the drug, and that route did not show toxicity for the kidneys, heart, or lungs [109].
Roberto et al. [97] showed that the Bothrops jararacussu acidic PLA2, BthA-I-PLA2, displays antitumoral effects upon Ehrlich ascites tumor, leukemia (Jurkat), and breast cell lines, using several enzyme concentrations. At 100 μg/mL, the toxicity was close to that of the control drug (methotrexate). This activity seems to be related to apoptosis. It is postulated that PLA2s probably speed up the turnover of phospholipids, what may generate typical changes to apoptosis.
Gebrim et al. [110] evaluated both in vitro and in vivo antitumor activity of BPB-modified BthTX-I (PLA2 Lys49) and its cationic synthetic peptide derived from the 115–129 C-terminal region. BPB-BthTX-I presented 70 and 90% cytotoxicity upon Jurkat, B16F10, and S180 tumor cell lines, which were also susceptible to the lytic action of the synthetic peptide. BPB-BthTX-I showed dose-dependent cytotoxicity and this effect was shown to be inferior to that of the native toxin on all tumor cell lines and macrophages.
Several articles state the affinity of secretory PLA2s for different membrane receptors and lipids, but since those related to Bothrops myotoxins are still unknown, two mechanisms have been proposed to explain Lys49 myotoxin cytotoxicity: a fatty acid-dependent lysis by means of an interaction with a receptor able to activate the myotoxin and the activation of intracellular lipase unleashed by the binding of the myotoxin to the receptor [110–112].
A phospholipase B purified from Pseudechis colletti was assayed on rabdomiosarcoma A673 tumor cells. A cytotoxic effect on sarcoma cells was observed, but no lytic activity against fibroblasts was found. This effect was related to destructive action of the enzyme upon the striated muscles [113]. Another study with recombinant sea snake PLA2 (rSSBPLA2) from Lapemis hardwickii venom showed its in vivo and in vitro enzymatic activity on different tumor cell lines [114].
The synthetic peptides p-AppK and pEM-2 derived from Lys49 phospholipase A2 homologues from Agkistrodon piscivorus piscivorus snake venom were evaluated against different tumor cell lines (B16 melanoma, EMT6 mammary carcinoma, S-180 sarcoma, P3X myeloma, and tEnd endothelial cells) and showed a rapid cytotoxic effect. In general, peptide p-AppK was slightly more potent than pEM-2 against various tumor cell lines, except for the P3X myeloma cells, which were slightly more susceptible to pEM-2 [98].
Ferguson et al. [96] have proposed dextrin-PLA2 as a bioresponsive anticancer therapeutic polymer and a new example of polymer-enzyme liposome therapy (PELT). Cytotoxicity was assessed in MCF-7, B16F10, and HT29 tumor cell lines using an MTT assay. Therefore, prior to designing protocols for in vivo studies it was considered important to further investigate the dextrin-PLA2 action mechanism, particularly since this could potentially involve multiple cellular targets. Preliminary experiments show that the conjugate is internalized by endocytosis more readily than PLA2 alone. The resulting redistribution of intracellular vesicles suggests a multimodal mechanism involving both plasma and intracellular-vesicle membrane interactions.
Documented literature reported that the PLA2 from Macrovipera lebetina venom exhibits anti-integrin activity. In their study with HMEC-1 (human microvascular endothelial cells), MVL-PLA2 has shown inhibition of cell adhesion and migration, as well as disturbed the actin cytoskeleton and the distribution of αvβ3 integrin [56, 90].
Khunsap et al. [100] purified a PLA2 (Drs-PLA2) from Daboia russelii siamensis venom which exhibited indirect hemolytic, anticoagulant, and cytotoxic activities. Moreover, this PLA2 decreased human skin melanoma (SK-MEL-28) cell viability in a dose-dependent manner, as well as migration with an IC50 of 25.6 nM. Moreover, Drs-PLA2 inhibited the colonization of skin melanoma cells (B16F10) in BALB/c mice lungs by 65%.
6. Snake Venom Lectins
Lectins, proteins that bind to carbohydrates, are found in several animal and vegetal species. They interfere with tumor cell proliferation and the studies about their relationship with cancer are based on the characteristics of endogenous lectins from tumor cells. Some plant lectins showed an inhibitory effect on human prostatic tumor cells [115].
Lectins are polyvalent carbohydrate-binding proteins of nonimmune origin and have been used extensively as histochemical probes to describe changes in tumor cell surface. These glycoproteins are known to influence the growth of cancer cells. BJcuL, a lectin isolated from Bothrops jararacussu snake venom, was purified and functionally characterized, and its effect on the proliferation of a number of established human cancer cell lines was determined. The growth of eight cancer cell lines was inhibited in a dose-related manner in the presence of BJcuL. This lectin was a potent growth inhibitor in renal (Caki-1 and A-498) and pancreatic (CFPAC-1) cancer cell lines, with an inhibitory concentration of 50%. These results suggest that BJcuL lectin is an effective inhibitor of cell growth in some cancer cell lines [116].
In 2001, de Carvalho et al. [117] observed that human metastatic breast cancer (MDA-MB-435) and human ovarian carcinoma (OVCAR-5) cell lines adhere, although weakly, to BJcuL. However, the lectin did not inhibit the adhesion of cells to the extracellular matrix proteins fibronectin, laminin, and type I collagen. Importantly, the viability of these tumor cells, other human tumor cell lines, and bovine brain endothelial cell lines were suppressed by BJcuL. These findings suggest that the lectin BJcuL may serve as an interesting tool for combating tumor progression by inhibiting the growth of tumor and endothelial cells. The integrin family of adhesion receptors play an essential role during tumor progression and thus represent interesting potential targets for the development of new therapeutic agents. More recently, Sarray et al. [118] reinforced the cytotoxicity of BJcuL on tumor cells mainly by altering cell adhesion and inducing apoptosis in gastric carcinoma cells MKN45 and AGS. The authors suggested that BJcuL may compete for binding to the cell surface with extracellular matrix glycoproteins and promote actin disassembly and possibly accelerate cellular detachment from the extracellular matrix. After, it was demonstrated that lebecetin, a C-type lectin isolated from Macrovipera lebetina venom, displays anti-integrin activity. Lebecetin inhibited integrin-mediated attachment of various tumor cell lines to different adhesion substrates. This protein was able to inhibit adhesion, migration, and invasion of tumor cells. Apparently, the fact that lectin acts on the integrin domains and its antiproliferative activity was significant. At 10 μg/mL, melanoma and sarcoma cell lines had their proliferative profile strongly inhibited [118].
Sarray et al. (2001) demonstrated that lebecetin also interferes with the adhesion of IGR39 melanoma and HT29D4 adenocarcinoma cells. In fact, these two cancer cell lines tightly adhere to immobilized lebecetin. Lebecetin is also able to strongly reduce IGR39 and HT29D4 cell adhesion to fibrinogen and laminin but not to fibronectin and collagen types I and IV, respectively. Adhesion properties of lebecetin may thus involve integrin receptors [118]. Six years later, the same group [119] presented a second C-type lectin, isolated from the same venom which showed potent inhibition of platelet aggregation and seemingly affected cell adhesion, migration, invasion, and proliferation by inhibiting α5β1 and αv-containing integrins. Moreover, the inhibition of α5β1 and αv integrins is likely due to the binding of venom peptides, as both lebectin and lebecetin coimmunoprecipitate with these integrins. Lebectin and lebecetin were the first examples of venom C-type lectins inhibiting an integrin other than the collagen receptor α2β1.
In a short communication, Nunes et al. [120] showed the cytotoxic activity on tumor cells and apoptosis in K562 cells of BlL, a galactoside-binding lectin isolated from Bothrops leucurus venom. Antitumor activity was verified by phosphatidylserine externalization analysis and mitochondrial membrane potential determination.
Therefore, lectins have been proved to be prospects for potential use in cancer therapy.
7. Snake Venom Metalloproteases
Metalloproteases are important compounds of most viperid and crotalid venoms. They can trigger hemorrhage by causing changes in blood coagulation or interaction with the main components of the extracellular matrix such as collagen, laminin, and fibronectin [121–123]. Also known as zinc-proteases, snake venom metalloproteases (SVMPs) are multidomain proteins that, through autoproteolysis, can generate biologically active products [124, 125]. According to their structure, these proteins are classified as either part of the mature P-I class, which has only a metalloprotease domain, P-II, which contains a metalloprotease domain followed by a disintegrin domain, P-III, a metalloprotease, with disintegrin-like and cysteine-rich domains, or P-IV, the heterotrimeric class of SVMPs, which possesses an additional snake C-type lectin-like (snaclec) domain, found close to the carboxyl end of the protease which is now included in the P-III group as a subclass (P-IIId) [125–131].
High molecular weight SVMPs are classified as metalloprotease/disintegrin-like/cysteine-rich (MDC) proteins. The complex hemorrhage mechanism induced by these enzymes has led to the investigation of the relationship between the disintegrin domain and the main components of blood coagulation, especially platelets and integrins α, β [132]. In addition, the application of these enzymes in platelet physiology research contributed to elucidating other probable mechanisms involved in cellular adhesion, which was widely studied with jararhagin, a high molecular weight hemorrhagic metalloprotease isolated from Bothrops jararaca venom [128, 133–136].
Various groups of matrix metalloproteases/ADAMs have been shown to be involved in the formation of new vessels during tumor growth [137]. These multidomain proteins are involved in both cancerous cell proliferation and indeed in cell-cell/cell-ECM adhesion [138–144].
Molecular approaches have been performed with high molecular weight metalloproteases from a number of Viperidae species in order to elucidate the complex integrin-disintegrin interactions. SVMPs containing disintegrin-like domains (PIII/PIIIb class) may play a role in targeting the protein to a particular site in cells such as platelets, and endothelial cells, as well as in integrins, extracellular matrix and other substrates [122, 134, 145–148]. The structural similarity between mammalian MMPs (ADAM) and SVMPs (low and high MMPs), including disintegrins, reinforces the idea that snake venom components captivate medical interest as potential molecules for the treatment of animal tumors [149].
8. Disintegrins
Along with metalloproteases, disintegrins are important compounds in most viperid and crotalid venoms. Disintegrins represent a family of nontoxic and nonenzymatic low molecular weight (5–10 kDa) RGD-containing peptides naturally presented in snake venoms or synthetics. Originally, these compounds are characterized by their ability to interact with integrins αIIbβ3, α5β1, and αvβ3lls expressed by a number of cells including those involved in tumor development and proliferation [150, 151].
Based on binding experiments, αβ integrins and their subtypes have been identified as major functional adhesion receptors on tumor cells. Indeed, disintegrins from several snake venoms have revealed new possibilities of uses not only in cardiovascular diseases but also as potent inhibitors of tumor cells [128, 149, 152]. Thus, a number of toxins containing RGD-peptides or RGD-containing disintegrins isolated from snake venoms have also been used to elucidate target receptors in a wide variety of primary cultured tumor cells (Table 1).
Table 1.
Antitumor activity of snake venoms and isolated compounds.
| Protein name | Snakes | Cellular target/mechanism | Reference | |
|---|---|---|---|---|
| Phospholipase A2 (PLA2) | Nigexine | Naja nigricollis nigricollis | Cytotoxic, altered cell viability and prevented cell proliferation. | [104] |
| BthA-I-PLA2 | Bothrops jararacussu | Effect against breast adenocarcinoma, human leukemia T, and Ehrlich ascitic tumor | [97] | |
| rSSBPLA2 | Lapemis hardwickii (sea snake) | Antitumor effect | [114] | |
| PLB | Pseudechis colletti | Cytotoxicity | [113] | |
| PLA2 | Agkistrodon piscivorus piscivorus | Synergistic effects with antineoplastic drugs against S49 lymphoma cells | [98] | |
| BPB-BthTX-I | B. jararacussu | Cytotoxicity on S180 tumor cells | [189] | |
| CC-PLA2-1 and 2 | Cerastes cerastes | Antitumor and antiangiogenic activities | [101] | |
| Drs-PLA2 | Daboia russelii siamensis | Inhibition of SK-MEL-28 cell migration and inhibition of the colonization of B16F10 cells in lungs | [100] | |
| MVL-PLA2 | Macrovipera lebetina | Inhibits angiogenesis and induces changes in actin cytoskeleton | [56, 102] | |
|
| ||||
| L-Aminoacid oxidases (LAAOs) | LAAO | Ophiophagus hannah | Cytotoxicity in stomach cancer, murine melanoma, fibrosarcoma, and colorectal and ovary cell lines | [79] |
| LAAO | Agkistrodon halys | Apoptosis | [71] | |
| Apoxin-I | Crotalus atrox | Apoptosis | [75] | |
| LAAO | Eristicophis macmahoni | Apoptosis | [68] | |
| BmooLAAO-I | Bothrops moojeni | Cytotoxicity and apoptosis | [83] | |
| LAAO | Trimeresurus stejnegeri | Cytotoxicity | [76] | |
| LAAO | Trimeresurus flavoviridis | Antitumor activity | [87] | |
| AHP-LAAO | Agkistrodon halys pallas | Apoptosis | [190] | |
| LAAO | Vipera berus berus | Apoptosis | [77] | |
| ACTX-6 | Agkistrodon acutus | Induces apoptosis in human cervical cancer Hela cell line | [191] | |
| B1-LAAO | Bothrops leucurus | Cytotoxicity in the stomach cancer MKN-45, adenocarcinoma HUTU, colorectal RKO, and human fibroblast LL-24 cell lines. | [192] | |
|
| ||||
| Metalloproteases | Crovidisin | Crotalus viridis | Detachment of ROS 17/2.8 osteosarcoma cells. | [147] |
| Jararhagin | Bothrops jararaca | Inhibition of melanoma cells and proapoptotic effect selective, interfering with the adhesion mechanisms | [136] | |
| leucurolysin-B | Bothrops leucurus | Potent cytotoxic effect in a micromolar range against T98, U87 and RT2, MCF7, EAC, and UACC cancer cell lines | [193] | |
| TSV-DM | Trimeresurus stejnegeri | Apoptosis, inhibitor of cell proliferation and inducer cell morphologic changes. | [194] | |
|
| ||||
| Disintegrin | Contortrostatin | Agkistrodon contortrix contortrix | Anti-invasive and antiadhesive activities on tumor cells and endothelial cells. Antitumor, antiangiogenic activities and inhibitor of tumor growth. | [171–175] |
| Leucurogin | Bothrops leucurus | Anti-angiogenesis effect | [195] | |
| Saxatilin | Gloydius saxatilis | Inhibitor of tumor growth. | [181] | |
| Obtustatin | Vipera lebetina | Anti-angiogenesis effect | [165] | |
| Adinbitor | Agkistrodon halys stejneger | Inhibits angiogenesis (in vitro and in vivo) | [196] | |
| Albolabrin | Trimeresurus albolabris | Inhibits RGD-dependent integrins and metastasis | [169] | |
| Rhodocetin | Calloselasma rhodostoma | Inhibits the cell adhesion, migration, and collagen contraction | [197] | |
| Salmosin | Agkistrodon halys brevicaudus | Antiangiogenic and antitumorigenic | [88, 179, 180, 185] | |
| Trigramin | Trimeresurus gramineus | Inhibits the adhesion melanoma cells to fibronectin and fibrinogen. | [164, 166] | |
| Triflavin | Trimeresurus flavoviridis | Inhibits adhesion and migration cell and angiogenesis. | [168] | |
| Rhodostomin | Agkistrodon rhodostoma | Inhibits angiogenesis and grow tumor cell adhesion. | [163, 164, 177] | |
| Echistatin | Echis carinatus | Inhibits the adhesion of melanoma cells to extracellular matrix components | [170] | |
|
| ||||
| Serineproteases | Crotalase | Crotalus adamanteus | Inhibition of tumor growth | [153] |
| Batroxobin | B. moojeni | Antimetastatic effect | [183] | |
|
| ||||
| Lectins | BjcuL | B. jararacussu | Cytotoxic effects and inhibits cell adhesion | [117] |
| Lebectin and Lebecetin | Macrovipera lebetina | Inhibits adhesion, migration, and invasion of tumor cells; inhibits angiogenesis | [119] | |
| EM16 | Echis multisquamatus | Cytoskeleton disassembly; inhibits adhesion and migration of HUVEC cells | [198] | |
|
| ||||
| Peptides | Cardiotoxin III (CTX III) | Naja naja atra | Blocks migration and invasion of MDA-MB-231 breast cancer cells | [42, 108] |
| Cytotoxin P4 | Naja n. nigricollis | Cytotoxicity | [40, 47, 48] | |
| Cathelicidin-BF | Bungarus fasciatus | Inhibits B16F10 and B16 proliferation | [199] | |
|
| ||||
| Inhibitors | BJ46a | B. jararaca | Inhibits the invasion and metastasis of tumor cells B16F10, a melanoma cell line, and MHCC97H, a human hepatocellular carcinoma cell line | [200] |
| PIVL | Macrovipera lebetina transmediterranea | Serine protease inhibitor; exhibits an anti-tumor effect and displays integrin inhibitory activity without being cytotoxic. Inhibit the adhesion, migration, and invasion of human glioblastoma U87 cells. | [201] | |
|
| ||||
| Crude | Montivipera xanthina | Cytotoxic effect | [202] | |
| WEV—venom extracted | Walterinnesia aegyptia | Induction of apoptosis | [203] | |
| Vipera lebetina turanica | Inhibits the growth of human ovarian cancer through induction of apoptosis | [204] | ||
Studies with metalloproteases and respective peptides that contain the disintegrin domain called RGD have focused on their antitumoral effects. These peptides can act in the extracellular matrix and play an important role in the evolution of angiogenesis and metastatic dissemination of cancer [152–154].
Venoms of Trimeresurus and Agkistrodon genera contain peptides that potentially inhibit platelet aggregation [150, 155–161], frequently induced by tumor cells. In order to discover new natural compounds to inhibit tumor cells, Kang et al. [88] showed strong in vivo evidence that a disintegrin containing the RGD sequence from Agkistrodon halys brevicaudus could suppress tumor angiogenesis through αvβ3 integrin interactions. A number of isolated disintegrins demonstrated potential inhibition of tumor cells: Contortrostatin [162], Eritostatin [163], Rhodostomin [164, 165], Obtustatin [166], Trigramin [167], Salmosin [168], Triflavin [169], Albolabrin [170], and Echistatin [171].
Contortrostatin (CN), a disintegrin containing Arg-Gly-Asp, isolated from Agkistrodon contortrix contortrix venom, interacts with different epithelial carcinoma and endothelial cell surface receptors. The anticancer potential of CN, a 13.5 kDa protein, was demonstrated because CN recognizes integrins αIIbβ3; α5β1; α5β3; α5β5. Schmitmeier et al. [172] demonstrated that CN exerted antitumor activity along with tumor necrosis factor (TNF-α) on human glioblastoma cell lines. This activity may be related to the fact that CN induced the disruption and prevented the binding of integrins to the extracellular matrix [173]. It has been demonstrated that this peptide inhibits in vitro and in vivo ovarian cancer dissemination and the recruitment of blood vessels to tumors [162, 174, 175]. This first study reinforces that CN is a potent and important molecule not only for therapeutic use in cancer but also to elucidate the several steps involved in tumor progression and metastasis. In 2004, the pharmacological effectiveness of CN was investigated using a mouse model of human mammary cancer. Clinically, the peptide is nonimmunogenic and stable when submitted to a relevant method of drug delivery, such as the liposomal delivery system. This study also shows the effectiveness of the inhibitory effect of contortrostatin on breast cancer progression in orthotopic and xenographic models, as well as the importance of integrins in cellular migration, invasion, matrix degradation, proliferation, and angiogenesis. This protein binds and affects the function of some integrins expressed in cancer, platelet, and endothelial cell surfaces. Encapsulated liposomes were used to release the active protein at the tumor site, thus having a chemotherapeutic application [176].
Another study using the human metastatic breast cancer cell line MDA-MB-435 in mice revealed that contortrostatin from Agkistrodon contortrix contortrix has potent antitumoral and antiangiogenic activities and demonstrated that contortrostatin may have potential as a therapeutic agent for the treatment of malignant gliomas [177].
The exact mechanisms that cause tumor regression in experimental animals after treatment with disintegrins are not well established. However, in addition to cell detachment, antiangiogenic activity is an important characteristic of these groups of peptides. Obtustatin, a disintegrin isolated from the venom of Vipera lebetina obtusa, showed significant in vivo inhibition of Lewis lung carcinoma growth and interacted selectively with the integrin α1β1, promoting inhibition of angiogenesis [166]. Rhodostomin, a disintegrin isolated from Calloselasma rhodostoma venom, affected tumor formation and the angiogenic process in different ways. It was first demonstrated that rhodostomin caused inhibition of Saos-2 (human osteosarcoma cells), TCIPA (tumor cell-induced platelet aggregation), and adhesion of tumor cells to the ECM, suggesting that host platelets may act as causative agents in tumor formation and metastasis [164, 165]. Yang and Huang [178] also showed that rhodostomin strongly inhibits the adhesion activity of ROS 17/2.8 cells (osteosarcoma cells) by TGF-β1 (transforming growth factor-β1). In 2001, Huang et al. [179] reported that rhodostomin showed antimetastatic activity due to the fact that it is an antiangiogenic substance, which selectively inhibits basic fibroblast growth factor (bFGF-treated) and the viability of human umbilical vein endothelial cells (HUVEC). The interaction between tumor cells and microvasculature including cell-cell adhesion as well as their migratory activity and angiogenesis was also reported using albolabrin, a disintegrin isolated from Trimeresurus albolabris venom. The peptide inhibited the adhesion of melanoma cells to extracellular matrix proteins. Inhibition exerted by albolabrin was dose-dependent and reached 70% for fibronectin and 60% for laminin at 20 μg/mL [170]. At 30 μg/mouse, it inhibits the tumor alone in a dose-dependent manner, showing no damage to animal health. The effect of albolabrin on metastasis was evaluated using a B16-BL6 melanoma line and compared with data from the synthetic RGD peptides. Albolabrin was 2000-fold more effective on the mouse melanoma cells. In addition to its inhibitory effect on platelets, salmosin, a disintegrin isolated from Agkistrodon halys brevicaudus venom, acts on tumor growth through an antiangiogenic mechanism [88]. Some studies have reported salmosin activity on melanoma cell metastasis and proliferation. Kang et al. [180] and Chung et al. [181] investigated the action of salmosin on integrin receptors, which are mediators of human tumor cell proliferation. Salmosin significantly inhibited the proliferation of human melanoma cells (SK-Mel-2 and B16 cells) in a dose-dependent manner. These authors also observed that salmosin inhibits the adhesion process via two possible mechanisms: (i) it specifically blocks the adhesion of cells to the ECM, via αvβ3 or β1 or (ii) via induction of apoptosis, also blocking the αv receptor. A novel disintegrin purified from Gloydius saxatilis snake venom, named saxatilin, was able to strongly inhibit tumor growth and may be an alternative procedure for antiangiogenic cancer therapy [182].
In 2009, Colombistatin, a disintegrin that inhibited ADP-induced platelet aggregation, human urinary (T24) and skin melanoma (SK-Mel-28) cancer cell adhesion to fibronectin, and cell migration was isolated from the venom of Bothrops colombiensis [183].
Thus, along with metalloproteinases, disintegrins from snake venoms have shown a high potential for treatment against cancer.
9. Snake Venom Serineproteases
Serineproteases are enzymes found in microorganisms, plants, and many animals. These enzymes display several biological functions and may be involved with digestion, activation of the complement system, cell differentiation, hemostasis, and others. Serineproteases affect several steps of blood clotting, often not specifically, inactivating blood clotting factors involved with platelet aggregation, clotting, and fibrinolysis [184–186]. The antitumoral potential of these proteins is still not well explored, and there are few studies published on their effects and mechanisms of action.
Markland [153] evaluated the in vitro and in vivo effects of crotalase, a serine protease from Crotalus adamanteus, on the growth of B16 melanoma cells in C57BL/6 mice and concluded that the enzyme was able to inhibit the growth of B16 melanoma cells in vitro, did not show cytotoxic or cytostatic effects on cells in vivo, and did not significantly increase the survival time of the animals.
The effect of defibrinogenation of batroxobin, a thrombin-like enzyme found in Bothrops atrox moojeni, presently Bothrops moojeni snake venoms, was studied on artificial lung metastasis in mice. The role of natural killer (NK) cells in the inhibitory effects of defibrinogenation on metastasis was also investigated. The administration of batroxobin had no effect at all on spleen lymphocyte NK activity. These results indicated that defibrinogenation due to batroxobin inhibits lung metastasis, and these effects depend on the NK activity of the host [187].
SVSPs have shown great potential for therapeutic and diagnostic use of coagulant disorders, which has generated a positive outlook regarding their study, even though few studies have been published to date.
10. Concluding Remarks
An uncontrolled rush toward the development of cytotoxic agents, selective for tumor cells, has already started. Several classes of drugs were produced, as happened in the seventies with platinum derivatives able to inhibit the growth of bacterium cultures. In the sixties, we find the first reports of cytotoxic activity of snake venom PLA2s on Yoshida sarcoma cell cultures [188]. So far, the association of animal venoms with important tissue damages in ophidian accidents is known and the investigation of active fractions for clinical use started to stand out in the scientific literature.
Nowadays, some works have been published with emphasis on the evaluation of specific points of tumor metabolism, like its immunological aspects and the induction of apoptosis. Cellular proliferation is not the only event to be fought in cancer treatment; the capacity of the tumor to invade adjacent tissues and to create new blood vessels can also be used as targets for new treatments. These relationships between cells and components of the extracellular matrix are fundamental in the events that occur in cancer for the invasion of tumor cells and also for the mechanisms of angiogenesis.
In this review, we focused on the new paradigms of both cytotoxic and pharmacological effects of snake venom toxins. A number of in vivo and in vitro experiments have demonstrated that snake venoms can contribute to the development of new drugs to combat a number of diseases including cancer. Moreover, from 1980 to the 1990s, snake venom peptides isolated from Viperidae and Crotalidae species and containing RGD/ECD sequences in their structures proved to be invaluable tools to recognize specific structures/receptors of platelets and some kinds of cells, as well as to promote physiological and biochemical changes on a cellular level. To date, these compounds are also complements for new therapeutic strategies in mutagenesis-related diseases.
Disintegrins interact with integrins via glycoprotein receptors located on cellular surfaces, which are related to cell-cell and cell-matrix interactions. These complex mechanisms have led to several studies on the elucidation of events or factors that may affect focal adhesion in cancer. Enthusiastic studies using these promising “Leads” or templates have demonstrated that peptides containing the RGD sequence from several snake venom species have the ability either to inhibit angiogenic activity via subtypes of αβ receptors or to exhibit selective apoptotic effects. These observations were essential according to a wide variety of pharmacological targets that make snake venom toxins invaluable sources of binders for studying new drugs and a considerable number of inhibitors [140, 189].
Acknowledgments
The authors are grateful to the Ministry of Science and Technology (MCTI), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Financiadora de Estudos e Projetos (FINEP), Fundação de Tecnologia do Acre (FUNTAC/FDCT), Coordenação de Aperfeiçoamento de Nível Superior (CAPES)-Projeto NanoBiotec, Rede de Biodiversidade e Biotecnologia da Amazônia Legal (BIONORTE/CNPq/MCTI), Instituto Nacional para Pesquisa Translacional em Saúde e Ambiente na Região Amazônica (INCT-INPeTAm/CNPq/MCTI) e Instituto Nacional para Pesquisa em Toxinas (INCT-Tox), Secretary of Development of Rondonia State (SEPLAN/PRONEX/CNPq) for financial support.
Abbreviations
- svMP:
Snake venom metalloproteases
- svSP:
Snake venom serineproteases
- svPLA2s:
Snake venom phospholipases A2
- svLAAO:
Snake venom L-amino acid oxidases
- svCTL:
Snake venom C-type lectin-like
- CVF:
Cobra venom factor.
Conflict of Interests
The authors state that there is no conflict of interests.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: Cancer Journal for Clinicians. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Hidetomo N, David JR, David GLD, et al. Can anesthetic techniques or drugs affect cancer recurrence in patients undergoing cancer surgery? Journal of Anesthesia. 2013;27(5):731–741. doi: 10.1007/s00540-013-1615-7. [DOI] [PubMed] [Google Scholar]
- 3.Bray F, Ren JS, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Journal of Cancer. 2013;132:1133–1145. doi: 10.1002/ijc.27711. [DOI] [PubMed] [Google Scholar]
- 4.de Vita F, Orditura M, Martinelli E, Moraca L, de Chiara A, Catalano G. The new european gold standard treatment for rectum cancer. Tumori. 2000;86:S26–S29. [PubMed] [Google Scholar]
- 5.Boveri T. Concerning the origin of malignant tumours by Theodor Boveri. Translated and annotated by Henry Harris. Journal of Cell Science. 2008;121:1–84. doi: 10.1242/jcs.025742. [DOI] [PubMed] [Google Scholar]
- 6.Garraway LA, Lander ES. Lessons from the cancer genome. Cell. 2013;153:117–137. doi: 10.1016/j.cell.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Mcleod HL. Cancer pharmacogenomics: early promise, but concerted effort needed. Science. 2013;339(6127):1563–1566. doi: 10.1126/science.1234139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang N, Wang X, Yang Y, Dai W. Advances in mitotic inhibitors for cancer treatment. Mini-Reviews in Medicinal Chemistry. 2006;6(8):885–895. doi: 10.2174/138955706777934955. [DOI] [PubMed] [Google Scholar]
- 9.Brannon-Peppas L, Blanchette JO. Nanoparticles and systems, target for cancer therapy. Advanced Drug Delivery Reviews. 2012;64:206–212. doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Kamath S, Buolamwini JK. Targeting EGFR and HER-2 receptor tyrosine kinases for cancer drug discovery and development. Medicinal Research Reviews. 2006;26(5):569–594. doi: 10.1002/med.20070. [DOI] [PubMed] [Google Scholar]
- 11.Perry MC, Anderson CM, Donebwer RC. Chemotherapy. In: Abeloff MD, Armitage JO, Lichter AS, Niederbuber JE, editors. Clinical Oncology. 3rd edition. Philadelphia, Pa, USA: Churclhill-Livingstone; 2000. pp. 379–422. [Google Scholar]
- 12.Adkins I, Jana H, Kosova M, Sadilkova L. Bacteria and their toxins tamed is immunotherapy. Current Pharmaceutical Biotechnologyp. 2012;13:1446–1473. doi: 10.2174/138920112800784835. [DOI] [PubMed] [Google Scholar]
- 13.Lewis RJ, Garcia ML. Therapeutic potential of venom peptides. Nature Reviews Drug Discovery. 2003;2(10):790–802. doi: 10.1038/nrd1197. [DOI] [PubMed] [Google Scholar]
- 14.Fernandes-Pedrosa MF, Félix-Silva J, Menezes YAS. An Integrated View of the Molecular Recognition and Toxinology: From Analytical Procedures to Biomedical Applications. InTech; 2013. Toxins from venomous animals: gene cloning, protein expression and biotechnological applications. [Google Scholar]
- 15.Warner RL, McClintock SD, Barron AG, de la Iglesia F. Hemostatic properties of a venomic protein in rodent dermal injuries. Experimental and Molecular Pathology. 2007;83(2):241–248. doi: 10.1016/j.yexmp.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Warner RL, McClintock SD, Barron AG, de la Iglesia FA. Hemostatic properties of a venomic protein in rat organ trauma. Experimental and Molecular Pathology. 2009;87(3):204–211. doi: 10.1016/j.yexmp.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fenard D, Lambeau G, Valentin E, Lefebvre J, Lazdunski M, Doglio A. Secreted phospholipases A2, a new class of HIV inhibitors that block virus entry into host cells. The Journal of Clinical Investigation. 1999;104(5):611–618. doi: 10.1172/JCI6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Escoubas P, King GF. Venomics as a drug discovery platform. Expert Review of Proteomics. 2009;6(3):221–224. doi: 10.1586/epr.09.45. [DOI] [PubMed] [Google Scholar]
- 19.Fu Y, Na N, Li K, et al. Chlorotoxin-conjugated nanoparticles as potential glioma-targeted drugs. Journal of Neuro-Oncology. 2012;107:457–462. doi: 10.1007/s11060-011-0763-6. [DOI] [PubMed] [Google Scholar]
- 20.Vetter I, Lewis RJ. Therapeutic potential of cone snail venom peptides (conopeptides) Current Topics in Medicinal Chemistry. 2012;12:1546–1552. doi: 10.2174/156802612802652457. [DOI] [PubMed] [Google Scholar]
- 21.Bond A. Exenatide (Byetta) as a novel treatment option for type 2 diabetes mellitus. Proceedings (Baylor University. Medical Center) 2006;19(3):281–284. doi: 10.1080/08998280.2006.11928181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aramadhaka LR, Prorock A, Dragulev B, et al. Connectivity maps for biosimilar drug discovery in venoms: the case of Gila Monster Venom and the anti-diabetes drug Byetta. Toxicon. 2013;69:160–167. doi: 10.1016/j.toxicon.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 23.Vyas VK, Brahmbhatt K, Bhatt H, et al. Therapeutic potential of snake venom in cancer therapy: current perspectives. Asian Pacific Journal Tropical of Biomedicine. 2013;3(2):156–162. doi: 10.1016/S2221-1691(13)60042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koh CY, Kini RM. From snake venom toxins to therapeutics: cardiovascular examples. Toxicon. 2012;59(4):497–506. doi: 10.1016/j.toxicon.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Samy RP, Gopalakrishnakone P, Stiles BG, et al. Snake venom phospholipases A2: a novel tool against bacterial diseases. Current Medicinal Chemistry. 2012;19(36):6150–6162. doi: 10.2174/092986712804485791. [DOI] [PubMed] [Google Scholar]
- 26.Wen Y, Bao-Jueng WU, Pei-Hsiu K, et al. Antibacterial and membrane-damaging activities of b-bungarotoxin B chain. Journal of Peptide Science. 2013;19:1–8. doi: 10.1002/psc.2463. [DOI] [PubMed] [Google Scholar]
- 27.Hubbard S, Choudhary S, Maus E, et al. Contortrostatin, a homodimeric disintegrin isolated from snake venom inhibits herpes simplex virus entry and cell fusion. Antiviral Therapy. 2012;17(7):1319–1326. doi: 10.3851/IMP2291. [DOI] [PubMed] [Google Scholar]
- 28.Muller VDM, Russo RR, Cintra AC, et al. Crotoxin and phospholipases A2 from Crotalus durissus terrificus showed antiviral activity against dengue and yellow fever viruses. Toxicon. 2012;59(4):507–515. doi: 10.1016/j.toxicon.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 29.Passero LFD, Tomokane TY, Corbett CEP, Laurenti MD, Toyama MH. Comparative studies of the anti-leishmanial activity of three Crotalus durissus ssp. venoms. Parasitology Research. 2007;101(5):1365–1371. doi: 10.1007/s00436-007-0653-1. [DOI] [PubMed] [Google Scholar]
- 30.Castillo JC, Vargas LJ, Segura C, et al. In vitro antiplasmodial activity of phospholipases A2 and a phospholipase homologue isolated from the venom of the snake Bothrops asper . Toxins. 2012;4(12):1500–1516. doi: 10.3390/toxins4121500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamane ES, Fernando CBA, Oliveira EB, et al. Unraveling the antifungal activity of a South American rattlesnake toxin crotamine. Biochimie. 2013;95:231–240. doi: 10.1016/j.biochi.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 32.Calvete JJ, Juárez P, Sanz L. Snake venomics. Strategy and applications. Journal of Mass Spectrometry. 2007;42(11):1405–1414. doi: 10.1002/jms.1242. [DOI] [PubMed] [Google Scholar]
- 33.DeWys WD, Kwaan HC, Bathina S. Effect of defibrination on tumor growth and response to chemotherapy. Cancer Research. 1976;36(10):3584–3587. [PubMed] [Google Scholar]
- 34.Braganca BM. Biologically active components of cobra venom in relation to cancer research. Indian Journal of Medical Research. 1976;64(8):1197–1207. [PubMed] [Google Scholar]
- 35.Braganca BM, Hospattankar AV. Potentiating action of cobra venom cytotoxin on the antitumour effects of an alkylating agent (melphalan) European Journal of Cancer and Clinical Oncology. 1978;14(6):707–712. doi: 10.1016/0014-2964(78)90307-9. [DOI] [PubMed] [Google Scholar]
- 36.Kaneda N, Hamaguchi M, Kojima K. Action of cobra venom cardiotoxin on chick embryonal fibroblasts transformed with a temperature-sensitive mutant of Rous sarcoma virus. FEBS Letters. 1985;192(2):313–316. doi: 10.1016/0014-5793(85)80132-0. [DOI] [PubMed] [Google Scholar]
- 37.Chiam-Matyas A, Ovadia M. Cytotoxic activity of various snake venoms on melanoma, B16F10 and chondrosarcoma. Life Sciences. 1987;40(16):1601–1607. doi: 10.1016/0024-3205(87)90126-3. [DOI] [PubMed] [Google Scholar]
- 38.Chiou GCY, Martin MK. Chemotherapy of neuroblastoma in mice with anticancer agents. Journal of Pharmaceutical Sciences. 1980;69(5):592–594. doi: 10.1002/jps.2600690532. [DOI] [PubMed] [Google Scholar]
- 39.Kato E, Narahashi T. Low sensitivity of the neuroblastoma cell cholinergic receptors to erabutoxins and α-bungarotoxin. Brain Research. 1982;245(1):159–162. doi: 10.1016/0006-8993(82)90352-3. [DOI] [PubMed] [Google Scholar]
- 40.Borkow G, Chaim-Matyas A, Ovadia M. Binding of cytotoxin P4 from Naja nigricollis nigricollis to B16F10 melanoma and WEHI-3B leukemia cells. FEMS Microbiology Immunology. 1992;5(1–3):139–146. doi: 10.1111/j.1574-6968.1992.tb05896.x. [DOI] [PubMed] [Google Scholar]
- 41.Trikha M, de Clerck YA, Markland FS. Contortrostatin, a snake venom disintegrin, inhibits β1 integrin- mediated human metastatic melanoma cell adhesion and blocks experimental metastasis. Cancer Research. 1994;54(18):4993–4998. [PubMed] [Google Scholar]
- 42.Yang S, Lu M, Chien C, et al. Induction of apoptosis in human leukemia K562 cells by cardiotoxin III. Life Sciences. 2005;76(21):2513–2522. doi: 10.1016/j.lfs.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Jokhio R, Ansari AF. Cobra snake venom reduces significantly tissue nucleic acid levels in human breast cancer. Journal of the Pakistan Medical Association. 2005;55(2):71–73. [PubMed] [Google Scholar]
- 44.Juhl H, Petrella EC, Cheung N-KV, Bredehorst R, Vogel C-W. Complement killing of human neuroblastoma cells: a cytotoxic monoclonal antibody and its F(ab)’2-cobra venom factor conjugate are equally cytotoxic. Molecular Immunology. 1990;27(10):957–964. doi: 10.1016/0161-5890(90)90118-j. [DOI] [PubMed] [Google Scholar]
- 45.Juhl H, Petrella EC, Cheung NV, Bredehorst R, Vogel C. Additive cytotoxicity of different monoclonal antibody-cobra venom factor conjugates for human neuroblastoma cells. Immunobiology. 1997;197(5):444–459. doi: 10.1016/s0171-2985(97)80078-2. [DOI] [PubMed] [Google Scholar]
- 46.Wang X, Huang S. The selective cytotoxicity of cobra venom factor immunoconjugate on cultured human nasopharyngeal carcinoma cell line. Human and Experimental Toxicology. 1999;18(2):71–76. doi: 10.1177/096032719901800202. [DOI] [PubMed] [Google Scholar]
- 47.Chaim-Matyas A, Borkow G, Ovadia M. Isolation and characterization of a cytotoxin P4 from the venom of Naja nigricollis nigricollis preferentially active on tumor cells. Biochemistry International. 1991;24(3):415–421. [PubMed] [Google Scholar]
- 48.Oron U, Chaim-Matyas A, Ovadia M. Histopathological changes in WEHI-3B leukemia cells following intoxication by cytotoxin P4 from Naja nigricollis nigricollis venom. Toxicon. 1992;30(9):1122–1126. doi: 10.1016/0041-0101(92)90058-d. [DOI] [PubMed] [Google Scholar]
- 49.Feofanov AV, Sharonov GV, Astapova MV, Rodionov DI, Utkin YN, Arseniev AS. Cancer cell injury by cytotoxins from cobra venom is mediated through lysosomal damage. Biochemical Journal. 2005;390(1):11–18. doi: 10.1042/BJ20041892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Vieira Santos MM, da Silva RJ, da Silva MG, Fecchio D. Subpopulations of mononuclear leukocytes associated with inhibition of Ehrlich ascites tumor growth by treatment with Bothrops jararaca venom. Mediators of Inflammation. 2004;13(1):29–32. doi: 10.1080/09629350410001664770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang H, Han R, Chen Z, et al. Opiate and acetylcholine-independent analgesic actions of crotoxin isolated from Crotalus durissus terrificus venom. Toxicon. 2006;48(2):175–182. doi: 10.1016/j.toxicon.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 52.Gould RJ, Polokoff MA, Friedman PA, et al. Disintegrins: a family of integrin inhibitory proteins from viper venoms (43129B) Proceedings of the Society for Experimental Biology and Medicine. 1990;195(2):168–171. doi: 10.3181/00379727-195-43129b. [DOI] [PubMed] [Google Scholar]
- 53.Niewiarowski S, McLane MA, Kloczewiak M, Stewart GJ. Disintegrins and other naturally occurring antagonists of platelet fibrinogen receptors. Seminars in Hematology. 1994;31(4):289–300. [PubMed] [Google Scholar]
- 54.Yang R-S, Tang C-H, Chuang WJ, et al. Inhibition of tumor formation by snake venom disintegrin. Toxicon. 2005;45:661–669. doi: 10.1016/j.toxicon.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 55.Dennis MS, Henzel WJ, Pitti RM, et al. Platelet glycoprotein IIb-IIIa protein antagonists from snake venoms: evidence for a family of platelet-aggregation inhibitors. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(7):2471–2475. doi: 10.1073/pnas.87.7.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bazaa A, Pasquier E, Defilles C, et al. MVL-PLA2, a snake venom phospholipase A2, inhibits angiogenesis through an increase in microtubule dynamics and disorganization of focal adhesions. PLoS ONE. 2010;5(4) doi: 10.1371/journal.pone.0010124.e10124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chwetzoff S. On the mode of action of basic phospholipase A2 from Naja nigricollis venom. Biochimica et Biophysica Acta. 1990;1045(3):285–290. doi: 10.1016/0005-2760(90)90132-h. [DOI] [PubMed] [Google Scholar]
- 58.da Silva RJ, da Silva MG, Vilela LC, Fecchio D. Cytokine profile of Ehrlich ascites tumor treated with Bothrops jararaca venom. Mediators of Inflammation. 2002;11(4):197–201. doi: 10.1080/0962935029000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paez-Pereda M, Kuchenbauer F, Arzt E, Stalla GK. Regulation of pituitary hormones and cell proliferation by components of the extracellular matrix. Brazilian Journal of Medical and Biological Research. 2005;38(10):1487–1494. doi: 10.1590/s0100-879x2005001000005. [DOI] [PubMed] [Google Scholar]
- 60.Rodrigues RS, da Silva JF, França JB, et al. Structural and functional properties of Bp-LAAO, a new l-amino acid oxidase isolated from Bothrops pauloensis snake venom. Biochimie. 2008;91(4):490–501. doi: 10.1016/j.biochi.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 61.Du X, Clemetson KJ. Snake venom L-amino acid oxidases. Toxicon. 2002;40(6):659–665. doi: 10.1016/s0041-0101(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 62.Tan NH, Saifuddin MN. Isolation and characterization of an unusual form of L-amino acid oxidase from King cobra (Ophiophagus hannah) venom. Biochemistry International. 1989;19(4):937–944. [PubMed] [Google Scholar]
- 63.Tan N-H, Swaminathan S. Purification and properties of the L-amino acid oxidase from monocellate cobra (Naja naja kaouthia) venom. International Journal of Biochemistry. 1992;24(6):967–973. doi: 10.1016/0020-711x(92)90105-a. [DOI] [PubMed] [Google Scholar]
- 64.Ponnudurai G, Chung MCM, Tan N-H. Purification and properties of the L-amino acid oxidase from Malayan pit viper (Calloselasma rhodostoma) venom. Archives of Biochemistry and Biophysics. 1994;313(2):373–378. doi: 10.1006/abbi.1994.1401. [DOI] [PubMed] [Google Scholar]
- 65.Torii S, Naito M, Tsuruo T. Apoxin I, a novel apoptosis-inducing factor with L-amino acid oxidase activity purified from western diamondback rattlesnake venom. The Journal of Biological Chemistry. 1997;272(14):9539–9542. doi: 10.1074/jbc.272.14.9539. [DOI] [PubMed] [Google Scholar]
- 66.Abe Y, Shimoyama Y, Munakata H, Ito J, Nagata N, Ohtsuki K. Characterization of an apoptosis-inducing factor in Habu snake venom as a glycyrrhizin (GL)-binding protein potently inhibited by GL in vitro . Biological and Pharmaceutical Bulletin. 1998;21(9):924–927. doi: 10.1248/bpb.21.924. [DOI] [PubMed] [Google Scholar]
- 67.Souza DHF, Eugenio LM, Fletcher JE, et al. Isolation and structural characterization of a cytotoxic L-amino acid oxidase from Agkistrodon contortrix laticinctus snake venom: preliminary crystallographic data. Archives of Biochemistry and Biophysics. 1999;368(2):285–290. doi: 10.1006/abbi.1999.1287. [DOI] [PubMed] [Google Scholar]
- 68.Ali SA, Stoeva S, Abbasi A, et al. Isolation, structural, and functional characterization of an apoptosis-inducing L-amino acid oxidase from leaf-nosed viper (Eristocophis macmahoni) snake venom. Archives of Biochemistry and Biophysics. 2000;384(2):216–226. doi: 10.1006/abbi.2000.2130. [DOI] [PubMed] [Google Scholar]
- 69.Iijima R, Kisugi J, Yamazaki M. L-amino acid oxidase activity of an antineoplastic factor of a marine mollusk and its relationship to cytotoxicity. Developmental and Comparative Immunology. 2003;27(6-7):505–512. doi: 10.1016/s0145-305x(02)00140-4. [DOI] [PubMed] [Google Scholar]
- 70.Aird SD. Ophidian envenomation strategies and the role of purines. Toxicon. 2002;40(4):335–393. doi: 10.1016/s0041-0101(01)00232-x. [DOI] [PubMed] [Google Scholar]
- 71.Suhr S, Kim D. Identification of the snake venom substance that induces apoptosis. Biochemical and Biophysical Research Communications. 1996;224(1):134–139. doi: 10.1006/bbrc.1996.0996. [DOI] [PubMed] [Google Scholar]
- 72.Stábeli RG, Marcussi S, Carlos GB, et al. Platelet aggregation and antibacterial effects of an L-amino acid oxidase purified from Bothrops alternatus snake venom. Bioorganic and Medicinal Chemistry. 2004;12(11):2881–2886. doi: 10.1016/j.bmc.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 73.Izidoro LFM, Ribeiro MC, Souza GRL, et al. Biochemical and functional characterization of an l-amino acid oxidase isolated from Bothrops pirajai snake venom. Bioorganic and Medicinal Chemistry. 2006;14(20):7034–7043. doi: 10.1016/j.bmc.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 74.Campos LD, Pucca MD, Roncolato EC, et al. Analysis of phospholipase A2, L-amino acid oxidase, and proteinase enzymatic activities of the Lachesis muta rhombeata venom. Journal of Biochemical and Molecular Toxicology. 2012;26(8):308–314. doi: 10.1002/jbt.21422. [DOI] [PubMed] [Google Scholar]
- 75.Torii S, Naito M, Tsuruo T. Apoxin I, a novel apoptosis-inducing factor with L-amino acid oxidase activity purified from western diamondback rattlesnake venom. Biochemistry. 2000;39:3197–3205. doi: 10.1074/jbc.272.14.9539. [DOI] [PubMed] [Google Scholar]
- 76.Zhang Y, Wang J, Lee W, et al. Molecular characterization of Trimeresurus stejnegeri venom L-amino acid oxidase with potential anti-HIV activity. Biochemical and Biophysical Research Communications. 2003;309(3):598–604. doi: 10.1016/j.bbrc.2003.08.044. [DOI] [PubMed] [Google Scholar]
- 77.Samel M, Vija H, Rönnholm G, Siigur J, Kalkkinen N, Siigur E. Isolation and characterization of an apoptotic and platelet aggregation inhibiting l-amino acid oxidase from Vipera berus berus (common viper) venom. Biochimica et Biophysica Acta. 2006;1764(4):707–714. doi: 10.1016/j.bbapap.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 78.Araki S, Ishida T, Yamamoto T, Kaji K, Hayashi H. Induction of apoptosis by hemorrhagic snake venom in vascular endothelial cells. Biochemical and Biophysical Research Communications. 1993;190(1):148–153. doi: 10.1006/bbrc.1993.1023. [DOI] [PubMed] [Google Scholar]
- 79.Ahn MY, Lee BM, Kim YS. Characterization and cytotoxicity of L-amino acid oxidase from the venom of king cobra (Ophiophagus hannah) International Journal of Biochemistry and Cell Biology. 1997;29(6):911–919. doi: 10.1016/s1357-2725(97)00024-1. [DOI] [PubMed] [Google Scholar]
- 80.Markland FS. Snake venoms and the hemostatic system. Toxicon. 1998;36(12):1749–1800. doi: 10.1016/s0041-0101(98)00126-3. [DOI] [PubMed] [Google Scholar]
- 81.Dipietrantonio AM, Hsieh T, Wu JM. Activation of caspase 3 in HL-60 cells exposed to hydrogen peroxide. Biochemical and Biophysical Research Communications. 1999;255(2):477–482. doi: 10.1006/bbrc.1999.0208. [DOI] [PubMed] [Google Scholar]
- 82.Suhara T, Fukuo K, Sugimoto T, et al. Hydrogen peroxide induces up-regulation of Fas in human endothelial cells. Journal of Immunology. 1998;160(8):4042–4047. [PubMed] [Google Scholar]
- 83.Stábeli RG, Pimenta Magalhães LM, Selistre-de-Araujo HS, Oliveira EB. Antibodies to a fragment of the Bothrops moojeni L-amino acid oxidase cross-react with snake venom components unrelated to the parent protein. Toxicon. 2005;46(3):308–317. doi: 10.1016/j.toxicon.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 84.Tempone AG, Andrade HF, Jr., Spencer PJ, Lourenço CO, Rogero JR, Nascimento N. Bothrops moojeni venom kills Leishmania spp. with hydrogen peroxide generated by its L-amino acid oxidase. Biochemical and Biophysical Research Communications. 2001;280(3):620–624. doi: 10.1006/bbrc.2000.4175. [DOI] [PubMed] [Google Scholar]
- 85.de Vieira Santos MM, Sant’Ana CD, Giglio JR, et al. Antitumoural effect of an L-amino acid oxidase isolated from Bothrops jararaca snake venom. Basic and Clinical Pharmacology and Toxicology. 2008;102(6):533–542. doi: 10.1111/j.1742-7843.2008.00229.x. [DOI] [PubMed] [Google Scholar]
- 86.Sun Y, Nonobe E, Kobayashi Y, et al. Characterization and expression of L-amino acid oxidase of mouse milk. The Journal of Biological Chemistry. 2002;277(21):19080–19086. doi: 10.1074/jbc.M200936200. [DOI] [PubMed] [Google Scholar]
- 87.Sun L, Yoshii Y, Hyodo A, et al. Apoptotic effect in the glioma cells induced by specific protein extracted from Okinawa Habu (Trimeresurus flavoviridis) venom in relation to oxidative stress. Toxicology in Vitro. 2003;17(2):169–177. doi: 10.1016/s0887-2333(03)00010-9. [DOI] [PubMed] [Google Scholar]
- 88.Kang I, Lee Y, Kim D. A novel disintegrin salmosin inhibits tumor angiogenesis. Cancer Research. 1999;59(15):3754–3760. [PubMed] [Google Scholar]
- 89.Cura JE, Blanzaco DP, Brisson C, et al. Phase I and pharmacokinetics study of crotoxin (cytotoxic PLA2, NSC-624244) in patients with advanced cancer. Clinical Cancer Research. 2002;8(4):1033–1041. [PubMed] [Google Scholar]
- 90.Jain D, Kumar S. Snake venom: a potent anticancer agent. Asian Pacific Journal of Cancer Prevention. 2012;13(10):4855–4860. doi: 10.7314/apjcp.2012.13.10.4855. [DOI] [PubMed] [Google Scholar]
- 91.Gutiérrez J, Lomonte B. Phospholipase A2 myotoxins from Bothrops snake venoms. Toxicon. 1995;33(11):1405–1424. doi: 10.1016/0041-0101(95)00085-z. [DOI] [PubMed] [Google Scholar]
- 92.Soares AM, Giglio JR. Chemical modifications of phospholipases A2 from snake venoms: effects on catalytic and pharmacological properties. Toxicon. 2003;42(8):855–868. doi: 10.1016/j.toxicon.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 93.Teixeira CFP, Landucci ECT, Antunes E, Chacur M, Cury Y. Inflammatory effects of snake venom myotoxic phospholipases A2 . Toxicon. 2003;42(8):947–962. doi: 10.1016/j.toxicon.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 94.Cummings BS. Phospholipase A2 as targets for anti-cancer drugs. Biochemical Pharmacology. 2007;74(7):949–959. doi: 10.1016/j.bcp.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 95.Mashima T, Seimiya H, Tsuruo T. De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. British Journal of Cancer. 2009;100(9):1369–1372. doi: 10.1038/sj.bjc.6605007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ferguson EL, Richardson SCW, Duncan R. Studies on the mechanism of action of dextrin-phospholipase A2 and its suitability for use in combination therapy. Molecular Pharmaceutics. 2010;7(2):510–521. doi: 10.1021/mp900232a. [DOI] [PubMed] [Google Scholar]
- 97.Roberto PG, Kashima S, Marcussi S, et al. Cloning and identification of a complete cDNA coding for a bactericidal and antitumoral acidic phospholipase A2 from Bothrops jararacussu venom. Protein Journal. 2004;23(4):273–285. doi: 10.1023/b:jopc.0000027852.92208.60. [DOI] [PubMed] [Google Scholar]
- 98.Araya C, Lomonte B. Antitumor effects of cationic synthetic peptides derived from Lys49 phospholipase A2 homologues of snake venoms. Cell Biology International. 2007;31(3):263–268. doi: 10.1016/j.cellbi.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 99.Maity G, Mandal S, Chatterjee A, Bhattacharyya D. Purification and characterization of a low molecular weight multifunctional cytotoxic phospholipase A2 from Russell’s viper venom. Journal of Chromatography B. 2007;845(2):232–243. doi: 10.1016/j.jchromb.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 100.Khunsap S, Pakmanee N, Khow O, et al. Purification of a phospholipase A2 from Daboia russelii siamensis venom with anticancer effects. Journal of Venom Research. 2011;2:42–51. [PMC free article] [PubMed] [Google Scholar]
- 101.Zouari-Kessentini R, Luis J, Karray A, et al. Two purified and characterized phospholipases A2 from Cerastes cerastes venom, that inhibit cancerous cell adhesion and migration. Toxicon. 2009;53(4):444–453. doi: 10.1016/j.toxicon.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 102.Kessentini-Zouari R, Jebali J, Taboubi S, et al. CC-PLA2-1 and CC-PLA2-2, two Cerastes cerastes venom-derived phospholipases A2, inhibit angiogenesis both in vitro and in vivo . Laboratory Investigation. 2010;90(4):510–519. doi: 10.1038/labinvest.2009.137. [DOI] [PubMed] [Google Scholar]
- 103.Bazaa A, Luis J, Srairi-Abid N, et al. MVL-PLA2, a phospholipase A2 from Macrovipera lebetina transmediterranea venom, inhibits tumor cells adhesion and migration. Matrix Biology. 2009;28(4):188–193. doi: 10.1016/j.matbio.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 104.Chwetzoff S, Tsunasawa S, Sakiyama F, Menez A. Nigexine, a phospholipase A2 from cobra venom with cytotoxic properties not related to esterase activity. Purification, amino acid sequence, and biological properties. The Journal of Biological Chemistry. 1989;264(22):13289–13297. [PubMed] [Google Scholar]
- 105.Costa LA, Miles H, Araujo CE, González S, Villarrubia VG. Tumor regression of advanced carcinomas following intra-and/or peri-tumoral inoculation with VRCTC-310 in humans: preliminary report of two cases. Immunopharmacology and Immunotoxicology. 1998;20(1):15–25. doi: 10.3109/08923979809034806. [DOI] [PubMed] [Google Scholar]
- 106.Donato NJ, Martin CA, Perez M, Newman RA, Vidal JC, Etcheverry M. Regulation of epidermal growth factor receptor activity by crotoxin, a snake venom phospholipase A2 toxin: a novel growth inhibitory mechanism. Biochemical Pharmacology. 1996;51(11):1535–1543. doi: 10.1016/0006-2952(96)00097-4. [DOI] [PubMed] [Google Scholar]
- 107.Corin RE, Viskatis LJ, Vidal JC, Etcheverry MA. Cytotoxicity of crotoxin on murine erythroleukemia cells in vitro . Investigational New Drugs. 1993;11(1):11–15. doi: 10.1007/BF00873905. [DOI] [PubMed] [Google Scholar]
- 108.Costa LA, Fornari MC, Berardi VE, Miles HA, Diez RA. In vivo effect of snake phospholipase A2 (crotoxin + cardiotoxin) on serum IL-1α, TNF-α and IL-1ra level in humans. Immunology Letters. 2001;75(2):137–141. doi: 10.1016/s0165-2478(00)00293-5. [DOI] [PubMed] [Google Scholar]
- 109.Stanchi NO, Arias D, Martino PE, Diez RA, Costa LA. 30-day intravenous administration of VRCTC-310-ONCO in rabbits. Farmaco. 2002;57(2):167–170. doi: 10.1016/s0014-827x(01)01177-6. [DOI] [PubMed] [Google Scholar]
- 110.Gebrim LC, Marcussi S, Menaldo DL, et al. Antitumor effects of snake venom chemically modified Lys49 phospholipase A2-like BthTX-I and a synthetic peptide derived from its C-terminal region. Biologicals. 2009;37(4):222–229. doi: 10.1016/j.biologicals.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 111.Ownby CL, Selistre-de-Araujo HS, White SP, Fletcher JE. Lysine 49 phospholipase A2 proteins. Toxicon. 1999;37(3):411–445. doi: 10.1016/s0041-0101(98)00188-3. [DOI] [PubMed] [Google Scholar]
- 112.Hanasaki K, Arita H. Biological and pathological functions of phospholipase A2 receptor. Archives of Biochemistry and Biophysics. 1999;372(2):215–223. doi: 10.1006/abbi.1999.1511. [DOI] [PubMed] [Google Scholar]
- 113.Bernheimer AW, Linder R, Weinstein SA, Kim K-S. Isolation and characterization of a phospholipase B from venom of collett’s snake, Pseudechis colletti . Toxicon. 1987;25(5):547–554. doi: 10.1016/0041-0101(87)90290-x. [DOI] [PubMed] [Google Scholar]
- 114.Liang Y, Yang X, Wei J, et al. Correlation of antitumor effect of recombinant sea snake basic phospholipase A2 to its enzymatic activity. Ai Zheng. 2005;24(12):1474–1478. [PubMed] [Google Scholar]
- 115.Camby I, Salmon I, Rombaut K, Pasteels J, Kiss R, Danguy A. Influence of culture media and multidrug resistance on the wheat germ agglutinin (WGA) glycocytochemical expression of two human glioblastoma cell lines. Anticancer Research. 1996;16(4 A):1719–1725. [PubMed] [Google Scholar]
- 116.Pereira-Bittencourt M, Carvalho DD, Gagliardi AR, Collins DC. The effect of a lectin from the venom of the snake, Bothrops jararacussu, on tumor cell proliferation. Anticancer Research B. 1999;19:4023–4025. [PubMed] [Google Scholar]
- 117.de Carvalho DD, Schmitmeier S, Novello JC, Markland FS. Effect of BJcuL (a lectin from the venom of the snake Bothrops jararacussu) on adhesion and growth of tumor and endothelial cells. Toxicon. 2001;39(10):1471–1476. doi: 10.1016/s0041-0101(01)00106-4. [DOI] [PubMed] [Google Scholar]
- 118.Sarray S, Srairi N, Luis J, Marvaldi J, El Ayeb M, Marrakchi N. Lebecetin, a C-lectin protein from the venom of Macrovipera lebetina that inhibits platelet aggregation and adhesion of cancerous cells. Haemostasis. 2001;31(3–6):177–182. doi: 10.1159/000048060. [DOI] [PubMed] [Google Scholar]
- 119.Sarray S, Delamarre E, Marvaldi J, Ayeb ME, Marrakchi N, Luis J. Lebectin and lebecetin, two C-type lectins from snake venom, inhibit α5β1 and αv-containing integrins. Matrix Biology. 2007;26(4):306–313. doi: 10.1016/j.matbio.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 120.Nunes ES, Souza MAA, Vaz AFM, et al. Cytotoxic effect and apoptosis induction by Bothrops leucurus venom lectin on tumor cell lines. Toxicon. 2012;59(7-8):667–671. doi: 10.1016/j.toxicon.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 121.Kamiguti AS, Hay CRM, Theakston RDG, Zuzel M. Insights into the mechanism of haemorrhage caused by snake venom metalloproteinases. Toxicon. 1996;34(6):627–642. doi: 10.1016/0041-0101(96)00017-7. [DOI] [PubMed] [Google Scholar]
- 122.Estêvão-Costa MI, Diniz CR, Magalhães A, Markland FS, Sanchez EF. Action of metalloproteinases mutalysin I and II on several components of the hemostatic and fibrinolytic systems. Thrombosis Research. 2000;99(4):363–376. doi: 10.1016/s0049-3848(00)00259-0. [DOI] [PubMed] [Google Scholar]
- 123.Loría GD, Rucavado A, Kamiguti AS, et al. Characterization of “basparin A”, a prothrombin-activating metalloproteinase, from the venom of the snake Bothrops asper that inhibits platelet aggregation and induces defibrination and thrombosis. Archives of Biochemistry and Biophysics. 2003;418(1):13–24. doi: 10.1016/s0003-9861(03)00385-0. [DOI] [PubMed] [Google Scholar]
- 124.Jia L, Shimokawa K, Bjarnason JB, Fox JW. Snake venom metalloproteinaes: structure, function and relationship to the adams family of proteins. Toxicon. 1996;34(11-12):1269–1276. doi: 10.1016/s0041-0101(96)00108-0. [DOI] [PubMed] [Google Scholar]
- 125.Matsui T, Fujimura Y, Titani K. Snake venom proteases affecting hemostasis and thrombosis. Biochimica et Biophysica Acta. 2000;1477(1-2):146–156. doi: 10.1016/s0167-4838(99)00268-x. [DOI] [PubMed] [Google Scholar]
- 126.Gutiérrez JM, Rucavado A. Snake venom metalloproteinases: their role in the pathogenesis of local tissue damage. Biochimie. 2000;82(9-10):841–850. doi: 10.1016/s0300-9084(00)01163-9. [DOI] [PubMed] [Google Scholar]
- 127.Gutiérrez JM. Comprendiendo los venenos de serpientes: 50 Años de investigaciones en América Latina. Revista de Biologia Tropical. 2002;50(2):377–394. [PubMed] [Google Scholar]
- 128.Kamiguti AS, Zuzel M, Theakston RDG. Snake venom metalloproteinases and disintegrins: interactions with cells. Brazilian Journal of Medical and Biological Research. 1998;31(7):853–862. doi: 10.1590/s0100-879x1998000700001. [DOI] [PubMed] [Google Scholar]
- 129.Bjarnason JB, Fox JW. Hemorrhagic metalloproteinases from snake venoms. Pharmacology and Therapeutics. 1994;62(3):325–372. doi: 10.1016/0163-7258(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 130.Clemetson KJ, Morita T, Manjunatha KR. Scientific and standardization committee communications: classification and nomenclature of snake venom C-type lectins and related proteins. Journal of Thrombosis and Haemostasis. 2009;7(2):p. 360. doi: 10.1111/j.1538-7836.2008.03233.x. [DOI] [PubMed] [Google Scholar]
- 131.Sarray S, Luis EL, Ayeb M, Marrakchi N. Snake venom peptides: promising molecules with anti-tumor effects. In: Hernández-Ledesma B, Hsieh CC, editors. Bioactive Food Peptides in Health and Disease. InTech; 2013. [Google Scholar]
- 132.Andrews RK, Berndt MC. Snake venom modulators of platelet adhesion receptors and their ligands. Toxicon. 2000;38(6):775–791. doi: 10.1016/s0041-0101(99)00187-7. [DOI] [PubMed] [Google Scholar]
- 133.Kamiguti AS, Moura-da-Silva AM, Laing GD, et al. Collagen-induced secretion-dependent phase of platelet aggregation is inhibited by the snake venom metalloproteinase jararhagin. Biochimica et Biophysica Acta. 1997;1335(1-2):209–217. doi: 10.1016/s0304-4165(96)00140-7. [DOI] [PubMed] [Google Scholar]
- 134.Kamiguti AS, Markland FS, Zhou Q, Laing GD, Theakston RDG, Zuzel M. Proteolytic cleavage of the β1 subunit of platelet α2β1 integrin by the metalloproteinase jararhagin compromises collagen-stimulated phosphorylation of pp72(syk) The Journal of Biological Chemistry. 1997;272(51):32599–32605. doi: 10.1074/jbc.272.51.32599. [DOI] [PubMed] [Google Scholar]
- 135.Moura-da-Silva AM, Marcinkiewicz C, Marcinkiewicz M, Niewiarowski S. Selective recognition of α2β1 integrin by jararhagin, a metalloproteinase/disintegrin from Bothrops jararaca venom. Thrombosis Research. 2001;102(2):153–169. doi: 10.1016/s0049-3848(01)00216-x. [DOI] [PubMed] [Google Scholar]
- 136.Corrêa MC, Maria DA, Moura-da-Silva AM, et al. Inhibition of melanoma cells tumorigenicity by the snake venom toxin jararhagin. Toxicon. 2002;40:739–748. doi: 10.1016/s0041-0101(01)00275-6. [DOI] [PubMed] [Google Scholar]
- 137.Kraut EH, Rojko JL, Olsen RG, Tuomari DL. Effects of treatment with cobra venom factor on experimentally induced feline leukemia. American Journal of Veterinary Research. 1987;48(7):1063–1066. [PubMed] [Google Scholar]
- 138.DeClerck YA. Interactions between tumour cells and stromal cells and proteolytic modification of the extracellular matrix by metalloproteinases in cancer. European Journal of Cancer. 2000;36(10):1258–1268. doi: 10.1016/s0959-8049(00)00094-0. [DOI] [PubMed] [Google Scholar]
- 139.Howard L, Zheng Y, Horrocks M, Maciewicz RA, Blobel C. Catalytic activity of ADAM28. FEBS Letters. 2001;498(1):82–86. doi: 10.1016/s0014-5793(01)02506-6. [DOI] [PubMed] [Google Scholar]
- 140.Peterson JT. Matrix metalloproteinase inhibitor development and the remodeling of drug discovery. Heart Failure Reviews. 2004;9(1):63–79. doi: 10.1023/B:HREV.0000011395.11179.af. [DOI] [PubMed] [Google Scholar]
- 141.Poindexter K, Nelson N, Dubose RF, Black RA, Cerretti DP. The identification of seven metalloproteinase-disintegrin (ADAM) genes from genomic libraries. Gene. 1999;237(1):61–70. doi: 10.1016/s0378-1119(99)00302-9. [DOI] [PubMed] [Google Scholar]
- 142.Primakoff P, Myles DG. The ADAM gene family: surface proteins with adhesion and protease activity. Trends in Genetics. 2000;16(2):83–87. doi: 10.1016/s0168-9525(99)01926-5. [DOI] [PubMed] [Google Scholar]
- 143.Tang BL. ADAMTS: a novel family of extracellular matrix proteases. International Journal of Biochemistry and Cell Biology. 2001;33(1):33–44. doi: 10.1016/s1357-2725(00)00061-3. [DOI] [PubMed] [Google Scholar]
- 144.Eto K, Huet C, Tarui T, et al. Functional classification of ADAMs based on a conserved motif for binding to integrin α9β1. Implications for sperm-egg binding and other cell interactions. The Journal of Biological Chemistry. 2002;277(20):17804–17810. doi: 10.1074/jbc.M200086200. [DOI] [PubMed] [Google Scholar]
- 145.Smith JB, Dangelmaier C, Selak M. Identification of 50 kDa snake venom proteins which specifically inhibit platelet adhesion to collagen. FEBS Letters. 1991;283(2):307–310. doi: 10.1016/0014-5793(91)80615-a. [DOI] [PubMed] [Google Scholar]
- 146.Sanchez EF, Bush LR, Swenson S, Markland FS. Chimeric fibrolase: covalent attachment of an RGD-like peptide to create a potentially more effective thrombolytic agent. Thrombosis Research. 1997;87(3):289–302. doi: 10.1016/s0049-3848(97)00131-x. [DOI] [PubMed] [Google Scholar]
- 147.Liu C, Huang T. Crovidisin, a collagen-binding protein isolated from snake venom of Crotalus viridis, prevents platelet-collagen interaction. Archives of Biochemistry and Biophysics. 1997;337(2):291–299. doi: 10.1006/abbi.1996.9787. [DOI] [PubMed] [Google Scholar]
- 148.Swenson S, Bush LR, Markland FS., Jr. Chimeric derivative of fibrolase, a fibrinolytic enzyme from southern copperhead venom, possesses inhibitory activity on platelet aggregation. Archives of Biochemistry and Biophysics. 2000;384(2):227–237. doi: 10.1006/abbi.2000.2129. [DOI] [PubMed] [Google Scholar]
- 149.Tang C, Yang R, Liu C, Huang T, Fu W. Differential susceptibility of osteosarcoma cells and primary osteoblasts to cell detachment caused by snake venom metalloproteinase protein. Toxicon. 2004;43(1):11–20. doi: 10.1016/j.toxicon.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 150.Gould RJ, Polokoff MA, Friedman PA, et al. Disintegrins: a family of integrin inhibitory proteins from viper venoms (43129B) Proceedings of the Society for Experimental Biology and Medicine. 1990;195(2):168–171. doi: 10.3181/00379727-195-43129b. [DOI] [PubMed] [Google Scholar]
- 151.Niewiarowski S, McLane MA, Kloczewiak M, Stewart GJ. Disintegrins and other naturally occurring antagonists of platelet fibrinogen receptors. Seminars in Hematology. 1994;31(4):289–300. [PubMed] [Google Scholar]
- 152.Brooks SA, Lomax-Browne HJ, Carter TM, Kinch CE, Hall DMS. Molecular interactions in cancer cell metastasis. Acta Histochemica. 2010;112(1):3–25. doi: 10.1016/j.acthis.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 153.Markland FS., Jr. Antitumor action of crotalase, a defibrinogenating snake venom enzyme. Seminars in Thrombosis and Hemostasis. 1986;12(4):284–290. doi: 10.1055/s-2007-1003568. [DOI] [PubMed] [Google Scholar]
- 154.Selistre-de-Araujo HS, Pontes CLS, Montenegro CF, Martin ACBM. Snake venom disintegrins and cell migration. Toxins. 2010;2(11):2606–2621. doi: 10.3390/toxins2112606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dennis MS, Henzel WJ, Pitti RM, et al. Platelet glycoprotein IIb-IIIa protein antagonists from snake venoms: evidence for a family of platelet-aggregation inhibitors. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(7):2471–2475. doi: 10.1073/pnas.87.7.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gan Z-R, Gould RJ, Jacobs JW, Friedman PA, Polokoff MA. Echistatin. A potent platelet aggregation inhibitor from the venom of the viper, Echis carinatus . The Journal of Biological Chemistry. 1988;263(36):19827–19832. [PubMed] [Google Scholar]
- 157.Huang TF, Ouyang C. Action mechanism of the potent platelet aggregation inhibitor from Trimeresurus gramineus snake venom. Thrombosis Research. 1984;33(2):125–138. doi: 10.1016/0049-3848(84)90173-7. [DOI] [PubMed] [Google Scholar]
- 158.Huang T-F, Holt JC, Lukasiewicz H, Niewiarowski S. Trigramin: a low molecular weight peptide inhibiting fibrinogen interaction with platelet receptors expressed on glycoprotein IIb-IIIa complex. The Journal of Biological Chemistry. 1987;262(33):16157–16163. [PubMed] [Google Scholar]
- 159.Neeper MP, Jacobson MA. Sequence of a cDNA encoding the platelet aggregation inhibitor trigramin. Nucleic Acids Research. 1990;18(14):p. 4255. doi: 10.1093/nar/18.14.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ouyang C, Huang TF. Potent platelet aggregation inhibitor from Trimeresurus gramineus snake venom. Biochimica et Biophysica Acta. 1983;757(3):332–341. doi: 10.1016/0304-4165(83)90059-4. [DOI] [PubMed] [Google Scholar]
- 161.Ouyang C, Yeh H, Haung TF. A potent platelet aggregation inhibitor purified from Agkistrodon halys (mamushi) snake venom. Toxicon. 1983;21(6):797–804. doi: 10.1016/0041-0101(83)90068-5. [DOI] [PubMed] [Google Scholar]
- 162.Markland FS, Jr., Shieh K, Zhou Q, et al. A novel snake venom disintegrin that inhibits human ovarian cancer dissemination and angiogenesis in an orthotopic nude mouse model. Haemostasis. 2001;31(3–6):183–191. doi: 10.1159/000048062. [DOI] [PubMed] [Google Scholar]
- 163.Danen EHJ, Marcinkiewicz C, Cornelissen IMHA, et al. The disintegrin eristostatin interferes with integrin α4β1 function and with experimental metastasis of human melanoma cells. Experimental Cell Research. 1998;238(1):188–196. doi: 10.1006/excr.1997.3821. [DOI] [PubMed] [Google Scholar]
- 164.Chiang H-S, Yang R-S, Huang T-F. The Arg-Gly-Asp-containing peptide, rhodostomin, inhibits in vitro cell adhesion to extracellular matrices and platelet aggregation caused by Saos-2 human osteosarcoma cells. British Journal of Cancer. 1995;71(2):265–270. doi: 10.1038/bjc.1995.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Swaim MW, Chiang H-S, Huang T-F. Characterization of platelet aggregation induced by PC-3 human prostate adenocarcinoma cells and inhibited by venom peptides, trigramin and rhodostomin. European Journal of Cancer. 1996;32(4):715–721. doi: 10.1016/0959-8049(95)00648-6. [DOI] [PubMed] [Google Scholar]
- 166.Marcinkiewicz C, Weinreb PH, Calvete JJ, et al. Obtustatin: a potent selective inhibitor of α1β1 integrin in vitro and angiogenesis in vivo . Cancer Research. 2003;63(9):2020–2023. [PubMed] [Google Scholar]
- 167.Knudsen KA, Tuszynski GP, Huang T-F, Niewiarowski S. Trigramin, an RGD-containing peptide from snake venom, inhibits cell-substratum adhesion of human melanoma cells. Experimental Cell Research. 1988;179(1):42–49. doi: 10.1016/0014-4827(88)90346-1. [DOI] [PubMed] [Google Scholar]
- 168.Kim SI, Kim KS, Kim HS, et al. Inhibition of angiogenesis by salmosin expressed in vitro . Oncology Research. 2003;14(4-5):227–233. doi: 10.3727/000000003772505353. [DOI] [PubMed] [Google Scholar]
- 169.Sheu JR, Lin CH, Huang TF. Triflavin, an antiplatelet peptide, inhibits tumor cell-extracellular matrix adhesion through an arginine-glycine-aspartic acid-dependent mechanism. Journal of Laboratory and Clinical Medicine. 1994;123(2):256–263. [PubMed] [Google Scholar]
- 170.Soszka T, Knudsen KA, Beviglia L, Rossi C, Poggi A, Niewiarowski S. Inhibition of murine melanoma cell-matrix adhesion and experimental metastasis by albolabrin, an RGD-containing peptide isolated from the venom of Trimeresurus albolabris . Experimental Cell Research. 1991;196(1):6–12. doi: 10.1016/0014-4827(91)90449-5. [DOI] [PubMed] [Google Scholar]
- 171.Staiano N, Villani GRD, Di Martino E, Squillacioti C, Vuotto P, Di Natale P. Echistatin inhibits the adhesion of murine melanoma cells to extracellular matrix components. Biochemistry and Molecular Biology International. 1995;35(1):11–19. [PubMed] [Google Scholar]
- 172.Schmitmeier S, Markland FS, Jr., Chen TC. Anti-invasive effect of contortrostatin, a snake venom disintegrin, and TNF-α on malignant glioma cells. Anticancer Research. 2000;20(6 B):4227–4233. [PubMed] [Google Scholar]
- 173.Schmitmeier S, Markland FS, Schönthal AH, Chen TC. Potent mimicry of fibronectin-induced intracellular signaling in glioma cells by the homodimeric snake venom disintegrin contortrostatin. Neurosurgery. 2005;57(1):141–153. doi: 10.1227/01.neu.0000163426.25227.56. [DOI] [PubMed] [Google Scholar]
- 174.Golubkov V, Hawes D, Markland FS., Jr. Anti-angiogenic activity of contortrostatin, a disintegrin from Agkistrodon contortrix contortrix snake venom. Angiogenesis. 2003;6(3):213–224. doi: 10.1023/B:AGEN.0000021396.47009.b0. [DOI] [PubMed] [Google Scholar]
- 175.Zhou Q, Nakada MT, Arnold C, Shieh KY, Markland FS., Jr. Contortrostatin, a dimeric disintegrin from Agkistrodon contortrix contortrix, inhibits angiogenesis. Angiogenesis. 1999;3(3):259–269. doi: 10.1023/a:1009059210733. [DOI] [PubMed] [Google Scholar]
- 176.Swenson S, Costa F, Minea R, et al. Intravenous liposomal delivery of the snake venom disintegrin contortrostatin limits breast cancer progression. Molecular Cancer Therapeutics. 2004;3(4):499–511. [PubMed] [Google Scholar]
- 177.Radu I, Nicola A, Micu D, Minea D. A rare case of glioblastoma multiforme. Romanian Journal of Morphology and Embryology. 2005;46(2):109–112. [PubMed] [Google Scholar]
- 178.Yang R, Huang T. Rhodostomin inhibits the transforming growth factor-β1-enhanced adhesion activity of ROS 17/2.8 osteosarcoma cells. Tohoku Journal of Experimental Medicine. 2000;191(3):145–155. doi: 10.1620/tjem.191.145. [DOI] [PubMed] [Google Scholar]
- 179.Huang TF, Yeh CH, Wu WB. Viper venom components affecting angiogenesis. Haemostasis. 2001;31(3–6):192–206. doi: 10.1159/000048063. [DOI] [PubMed] [Google Scholar]
- 180.Kang I, Kim D, Jang Y, Chung K. Suppressive mechanism of salmosin, a novel disintegrin in B16 melanoma cell metastasis. Biochemical and Biophysical Research Communications. 2000;275(1):169–173. doi: 10.1006/bbrc.2000.3130. [DOI] [PubMed] [Google Scholar]
- 181.Chung K, Kim S, Han K, et al. Inhibitory effect of salmosin, a Korean snake venom-derived disintegrin, on the integrin αv-mediated proliferation of SK-Mel-2 human melanoma cells. Journal of Pharmacy and Pharmacology. 2003;55(11):1577–1582. doi: 10.1211/0022357022160. [DOI] [PubMed] [Google Scholar]
- 182.Kim KS, Kim DS, Chung KH, Park YS. Inhibition of angiogenesis and tumor progression by hydrodynamic cotransfection of angiostatin K1-3, endostatin, and saxatilin genes. Cancer Gene Therapy. 2006;13(6):563–571. doi: 10.1038/sj.cgt.7700924. [DOI] [PubMed] [Google Scholar]
- 183.Sánchez EE, Rodríguez-Acosta A, Palomar R, et al. Colombistatin: a disintegrin isolated from the venom of the South American snake (Bothrops colombiensis) that effectively inhibits platelet aggregation and SK-Mel-28 cell adhesion. Archives of Toxicology. 2009;83(3):271–279. doi: 10.1007/s00204-008-0358-y. [DOI] [PubMed] [Google Scholar]
- 184.Braud S, Bon C, Wisner A. Snake venom proteins acting on hemostasis. Biochimie. 2000;82(9-10):851–859. doi: 10.1016/s0300-9084(00)01178-0. [DOI] [PubMed] [Google Scholar]
- 185.White CM. Thrombin-directed inhibitors: pharmacology and clinical use. American Heart Journal. 2005;149:S54–S60. doi: 10.1016/j.ahj.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 186.Costa JDO, Fonseca KC, Garrote-Filho MS, et al. Structural and functional comparison of proteolytic enzymes from plant latex and snake venoms. Biochimie. 2010;92(12):1760–1765. doi: 10.1016/j.biochi.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 187.Shibuya M, Niitani H, Aoyama A, et al. Antimetastatic effect of defibrinogenation with batroxobin depends on the natural killer activity of host in mice. Journal of Cancer Research and Clinical Oncology. 1990;116(2):168–172. doi: 10.1007/BF01612672. [DOI] [PubMed] [Google Scholar]
- 188.Yoshida BM, Patel NT, Badrinath PG. Isolation and properties of a cobravenom factor selectively cytotoxic to yoshida sarcoma cells. Biochimica et Biophysica Acta. 1967;136(3):508–520. doi: 10.1016/0304-4165(67)90009-8. [DOI] [PubMed] [Google Scholar]
- 189.Kennedy AR. Chemopreventive agents: protease inhibitors. Pharmacology and Therapeutics. 1998;78(3):167–209. doi: 10.1016/s0163-7258(98)00010-2. [DOI] [PubMed] [Google Scholar]
- 190.Gebrim LC, Marcussi S, Menaldo DL, et al. Antitumor effects of snake venom chemically modified Lys49 phospholipase A2-like BthTX-I and a synthetic peptide derived from its C-terminal region. Biologicals. 2009;37(4):222–229. doi: 10.1016/j.biologicals.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 191.Zhang L, Wu WT. Isolation and characterization of ACTX-6: a cytotoxic L-amino acid oxidase from Agkistrodon acutus snake venom. Natural Product Research. 2008;22(6):554–563. doi: 10.1080/14786410701592679. [DOI] [PubMed] [Google Scholar]
- 192.Zhang L, Wei LJ. ACTX-8, a cytotoxic L-amino acid oxidase isolated from Agkistrodon acutus snake venom, induces apoptosis in Hela cervical cancer cells. Life Sciences. 2007;80(13):1189–1197. doi: 10.1016/j.lfs.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 193.Naumann GB, Silva LF, Silva L, et al. Cytotoxicity and inhibition of platelet aggregation caused by an L-amino acid oxidase from Bothrops leucurus venom. Biochimica et Biophysica Acta. 2011;1810(7):683–694. doi: 10.1016/j.bbagen.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 194.Gabriel LM, Sanchez EF, Silva SG, et al. Tumor cytotoxicity of leucurolysin-B, a P-III snake venom metalloproteinase from Bothrops leucurus . Journal of Venomous Animals and Toxins including Tropical Diseases. 2012;18(1):24–33. [Google Scholar]
- 195.Wan SG, Jin Y, Lee WH, et al. A snake venom metalloproteinase that inhibited cell proliferation and induced morphological changes of ECV304 cells. Toxicon. 2006;47(4):480–489. doi: 10.1016/j.toxicon.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 196.Higuchi DA, Almeida MC, Barros CC, et al. Leucurogin, a new recombinant disintegrin cloned from Bothrops leucurus (white-tailed-jararaca) with potent activity upon platelet aggregation and tumor growth. Toxicon. 2011;58(1):123–129. doi: 10.1016/j.toxicon.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 197.Wang JH, Wu Y, Ren F, et al. Cloning and characterization of Adinbitor, a novel disintegrin from the snake venom of Agkistrodon halys brevicaudus stejneger. Acta Biochimica et Biophysica Sinica. 2004;36(6):425–429. doi: 10.1093/abbs/36.6.425. [DOI] [PubMed] [Google Scholar]
- 198.Eble JA, Niland S, Dennes A, et al. Rhodocetin antagonizes stromal tumor invasion in vitro and other alpha 2 beta 1 integrin-mediated cell functions. Matrix Biology. 2002;21(7):547–558. doi: 10.1016/s0945-053x(02)00068-9. [DOI] [PubMed] [Google Scholar]
- 199.Marcinkiewicz C, Lobb RR, Marcinkiewicz MM, et al. Isolation and characterization of EMS16, a C-lectin type protein from Echis multisquamatus venom, a potent and selective inhibitor of the alpha 2 beta 1 integrin. Biochemistry. 2000;39(32):9859–9867. doi: 10.1021/bi000428a. [DOI] [PubMed] [Google Scholar]
- 200.Tian Y, Wang H, Li B, et al. The cathelicidin-BF Lys16 mutant Cbf-K16 selectively inhibits non-small cell lung cancer proliferation in vitro . Oncology Reports. 2013;30(5):2502–2510. doi: 10.3892/or.2013.2693. [DOI] [PubMed] [Google Scholar]
- 201.Ji MK, Shi Y, Xu JW, et al. Recombinant snake venom metalloproteinase inhibitor BJ46A inhibits invasion and metastasis of B16F10 andMHCC97H cells through reductions of matrix metalloproteinases 2 and 9 activities. Anticancer Drugs. 2013;24(5):461–472. doi: 10.1097/CAD.0b013e32835f258d. [DOI] [PubMed] [Google Scholar]
- 202.Morjen M, Kallech-Ziri O, Bazaa A, et al. Matrix PIVL, a new serine protease inhibitor from Macrovipera lebetina transmediterranea venom, impairs motility of human glioblastoma cells. Biology. 2013;32(1):52–62. doi: 10.1016/j.matbio.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 203.Yalcın HT, Ozen MO, Gocmen B, et al. Effect of ottoman viper (Montivipera xanthina (Gray, 1849)) venom on various cancer cells and on microorganisms. Cytotechnology. 2014;66(1):87–94. doi: 10.1007/s10616-013-9540-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Al-Sadoon MK, Abdel-Maksoud MA, Rabah DM, et al. Induction of apoptosis and growth arrest in human breast carcinoma cells by a snake (Walterinnesia aegyptia) venom combined with silica nanoparticles: crosstalk between Bcl2 and caspase 3. Cellular Physiology and Biochemistry. 2012;30(3):653–665. doi: 10.1159/000341446. [DOI] [PubMed] [Google Scholar]


