Abstract
Sustained imatinib treatment in chronic myeloid leukemia patients can result in complete molecular response allowing discontinuation without relapse. We set out to evaluate the frequency of complete molecular response in imatinib de novo chronic phase chronic myeloid leukemia patients, to identify base-line and under-treatment predictive factors of complete molecular response in patients achieving complete cytogenetic response, and to assess if complete molecular response is associated with a better outcome. A random selection of patients on front-line imatinib therapy (n=266) were considered for inclusion. Complete molecular response was confirmed and defined as MR 4.5 with undetectable BCR-ABL transcript levels. Median follow up was 4.43 years (range 0.79–10.8 years). Sixty-five patients (24%) achieved complete molecular response within a median time of 32.7 months. Absence of spleen enlargement at diagnosis, achieving complete cytogenetic response before 12 months of therapy, and major molecular response during the year following complete cytogenetic response was predictive of achieving further complete molecular response. Patients who achieved complete molecular response had better event-free and failure-free survivals than those with complete cytogenetic response irrespective of major molecular response status (95.2% vs. 64.7% vs. 27.7%, P=0.00124; 98.4% vs. 82.3% vs. 56%, P=0.0335), respectively. Overall survival was identical in the 3 groups. In addition to complete cytogenetic response and major molecular response, further deeper molecular response is associated with better event-free and failure-free survivals, and complete molecular response confers the best outcome.
Introduction
Front-line therapy with imatinib (IM) has dramatically improved the outcome of chronic phase chronic myeloid leukemia (CP-CML) patients. A single institution study recently reported that the 8-year survival for CML patients was 15% or under before 1983, 42–65% from 1983 to 2000, and has been 87% since 2001.1 Estimates of long-term survival show that life expectancy is increasing to levels close to those observed in the general population.2
The best outcome is associated with a complete hematologic response (CHR) during the first three months of treatment and a complete cytogenetic response (CCyR) during the first year of treatment.3–6 Monitoring the BCR-ABL transcript level from peripheral blood is, therefore, essential to evaluate the response. Analysis of the molecular follow up of the phase III International Randomized Study of Interferon versus STI571 (IRIS) study suggests that patients achieving a CCyR with a 3-log reduction in BCR-ABL transcript level, defined as a major molecular response (MMR), have better progression-free survival (PFS) and event-free survival (EFS) compared to the other patients.7,8
Nevertheless, while the prognostic value of achieving MMR is well established, it remains difficult to assess, particularly in patients who have achieved a previous CCyR. Furthermore, not all studies correlate MMR with better EFS, PFS, and overall survival (OS).3–5,7,9–11
Whether or not patients achieve MMR, most have persistent detectable disease at a molecular level and will have to continue IM indefinitely. However, a few patients achieve a so-called complete molecular response (CMR) that is usually defined by a minimum of a 4.5 log reduction in BCR-ABL transcript levels from the base-line value.
Although discussion about the definition of CMR is still ongoing, the clinical impact of achieving such a level of residual disease remains unknown. Nevertheless, it has been reported that sustained CMR for more than two consecutive years can lead to cessation of IM treatment without molecular relapse in almost 40% of cases.12 We set out to assess the frequency of CMR in CP-CML patients treated with IM as front-line therapy, identify both base-line and under-treatment predictive factors of CMR in patients who achieved CCyR on IM therapy, and determine whether achieving a CMR was associated with a better outcome.
Methods
Patients with diagnosed Philadelphia chromosome-positive (Ph-positive) CML referred to the two participating centers (University Hospitals of Bordeaux and Lyon) from January 2000 to March 2010 who were in chronic phase at diagnosis, treated with front-line IM, were considered for inclusion in this study.
All patients provided informed consent to participate in the study. The study was approved by the local ethics committee and by the French Data Protection Authority (Commission Nationale Informatique et Libertés, CNIL, Paris, France).
Cytogenetic responses were evaluated at least every six months during the first year of therapy and then every 3–6 months until CCyR. During follow up and after a first CCyR documented on cytogenetic analysis, a BCR-ABL transcript level below 1% (IS) was considered as equivalent to CCyR.
Molecular response was assessed according to previously reported recommendations.13 MMR was defined as a reduction of BCR-ABL/ABL level of at least 3 logs from a standardized base-line value and confirmed on two consecutive analyses at least two months apart. In the current work, CMR was defined as MR4.5 with undetectable BCR-ABL transcript on two consecutive analyses at least two months apart. Loss of MMR and of CMR were defined as BCR-ABL/ABL transcripts of 0.1% or over and detectable BCR-ABL transcript, respectively, both on two consecutive analyses at least two months apart.
Univariate and multivariate analyses were performed with Cox regressions. Fine and Gray models were used to analyze the cumulative incidence of molecular responses.
EFS, failure-free survival (FFS) and OS were measured from the date of the first CCyR on therapy to the date of event or death. Survival differences, estimated by Kaplan-Meier analysis for patients of the different groups, were assessed using a log rank test.
Times to event were measured as the time between date of CCyR and the event of interest. For censored cases, it corresponded to time between date of CCyR and the last observational period under IM. EFS referred to survival without loss of complete hematologic response, loss of CCyR, detection of a BCR-ABL domain kinase point mutation associated with a high level of IM resistance, progression to accelerated or blastic phase, death from any cause on or off therapy, treatment cessation for toxicity. FFS referred to survival without events previously described with the exception of treatment cessation for toxicity according to the ELN recommendations.14 Only the first event for an individual patient was considered.
Patients who achieved a CCyR without CMR on IM therapy with a follow up shorter than the observed median time to CMR, and without any reported event during follow up, were excluded for the identification of CMR predictive factors and survival studies.
The clinical outcomes were analyzed at landmark time points. For those analyses, patients had to be still on IM and have available data regarding the molecular response status at specified time points.
The significant level of the statistical tests was set at 5%. All analyses were performed using the SPSS statistical package (Chicago, IL, USA), version 20.0.0, and R program, version 2.14.2.
Results
Patients
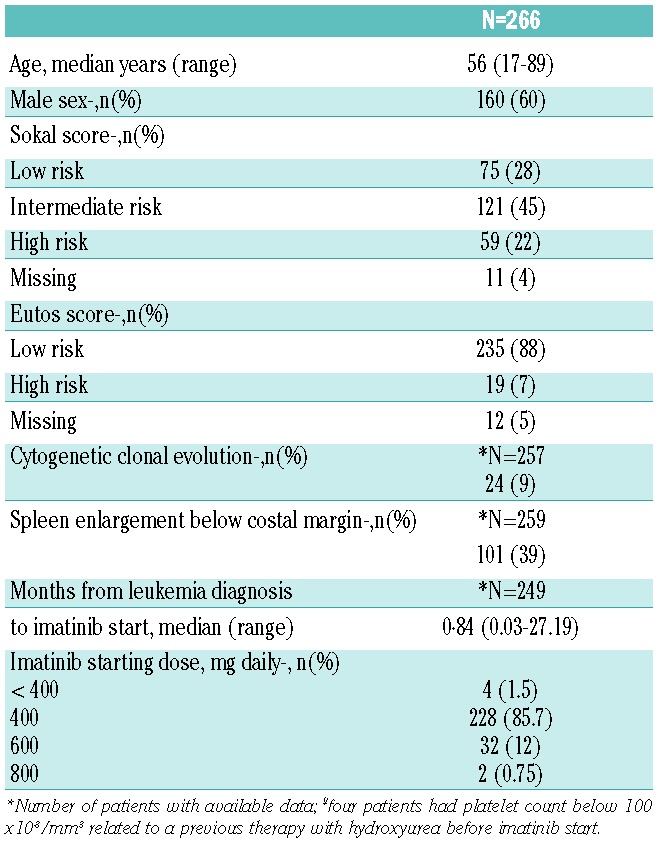
Two hundred and sixty-six adult patients diagnosed with Ph-positive CML-CP were treated by IM as front-line therapy during the study period in the two centers. Baseline characteristics of the population are presented in Table 1. The median follow up was 4.43 years (range 0.79–10.8 years). Seventeen patients died: 13 from CML progression and 4 from unrelated (n=2) or undetermined (n=2) causes. Initial IM daily dose was 400 mg in 85% of the patients. At the time of analysis, 178 (67%) patients were still on IM.
Table 1.
Base-line characteristics of the study population (n=266).
Thirty-three patients did not achieve CCyR on IM. Among them, 23 were considered to be in treatment failure according to the 2009 ELN criteria:15 lack of complete hematologic response at 3 months, n=4; lack of cytogenetic response at 6 months, n=7; lack of CCyR at 18 months, n=10; progression to accelerated phase (AP) or blast phase (BP), n=2. Ten patients had experienced a recurrent grade 3–4 toxicity leading to IM discontinuation.
Overall, 233 patients (88%) achieved CCyR on IM with a median time to the first CCyR of 6.4 months (range 2.6–39 months). Median duration of CCyR was 40.5 months (range 0.0–122.6 months). Seventeen patients had subsequent loss of a previous CCyR with a median CCyR duration of 10.3 months (range 1.5–77.7 months). One hundred and ninety-eight (74%) patients achieved MMR under IM therapy. Median time to the first MMR was 13.5 months (range 2.6–67.2 months).
Finally, 65 patients (24%) achieved CMR within a median time of 32.7 months (range 2.6–87.9 months). Forty-six patients (17%) were still in CMR at the last follow up with a median duration of 35.4 months (range 4.1–91.5 months) and 19 had a subsequent loss of a previous CMR with a median duration of CMR of nine months (range 4.1–37.3 months). The estimated probability of sustained CMR at two years was 68% (95%CI: 61.2–74.6).
Cumulative incidences of CCyR, MMR and CMR of the 266 patients are presented in Figure 1.
Figure 1.
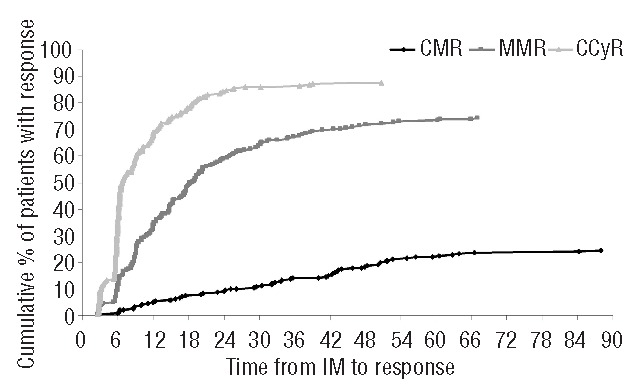
Cumulative incidence of complete cytogenetic response (CCyR), major molecular response (MMR) and complete molecular response (CMR) (n=266).
Base-line and under-treatment factors associated with CMR
Among the 233 patients who achieved CCyR on IM therapy, 53 had a shorter follow up than the median time to CMR (i.e. 32.7 months) without any reported event and were not considered for further analyses. The selection of the study population for CMR predictive factors and survival studies is presented in Figure 2. Among the 180 remaining patients, 23 (13%) achieved CCyR without MMR (CCyR+MMR-CMR− group), 92 (51%) achieved CCyR with MMR but without CMR (CCyR+MMR+CMR− group), and 65 (36%) achieved CCyR with CMR (CCyR+MMR+CMR+ group). The median follow up was 4.55 years (range 2.51–8.85 years) for the CCyR+MMR−CMR− group, 5.37 years (range 0.8–10.8 years) for the CCyR+MMR+CMR− group, and 6.16 years (range 1.01–10.78 years) for the CCyR+MMR+CMR+ group (P=0.055).
Figure 2.
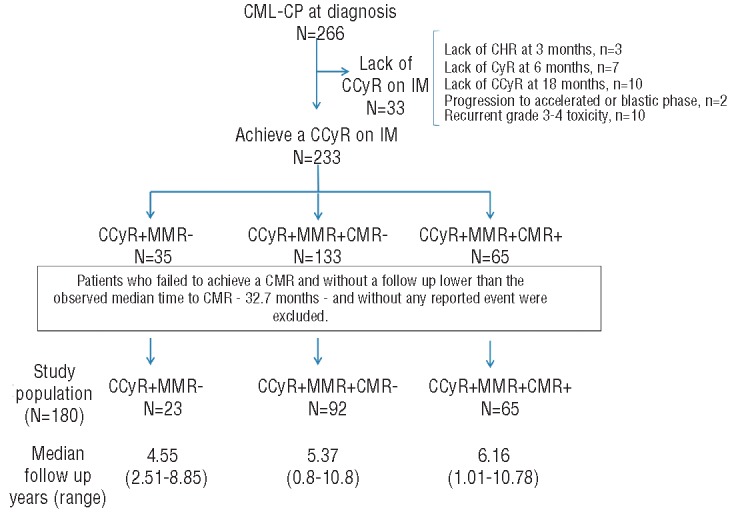
Selection of the study population for complete molecular response (CMR) predictive factors and survival studies.
Several base-line factors (age, gender, Sokal and Eutos scores, clonal evolution, spleen enlargement, white blood cell (WBC) and platelet counts, hemoglobin level, percent of peripheral basophils, percent of marrow blasts, time from diagnosis to IM start, IM starting dose) and under-treatment factors (time from IM start to first CCyR, time from CCyR to first MMR, IM dose increase to 600 mg daily or higher, average daily dose of IM during the first year of treatment, IM plasma level < or ≥ 1000 ng/mL) were analyzed to identify those associated with the achievement of CMR under IM therapy.
We redefined the “time from IM start to MMR” into “time from CCyR to MMR” to be able to analyze the impact of the time to reach MMR and CCyR in the same model. Analyses of this new variable in univariate and multivariate analyses were performed only in patients who reached MMR, and time to reach CMR was taken to be the time between first MMR and CMR.
Cytogenetic clonal evolution (yes vs. no), spleen enlargement (yes vs. no), WBC count (≤4×109/L G/L vs. >4×109/L, highest quartile), and platelet count (<60×109/L vs. ≥60×109/L, highest quartile) emerged as predictive factors among the base-line characteristics for achieving CMR in the univariate analysis. Patients with absence of clonal evolution, no spleen enlargement, with a WBC count of 4×109/L or under and a platelet count of 60×109/L or over, were more likely to achieve CMR in the univariate analysis.
Among the under-treatment characteristics, predictive factors identified by univariate analysis were time from IM initiation to first CCyR and time to first MMR.
As there was an association between platelet and WBC counts (i.e. patients with a platelet count less than 60×109/L had a higher WBC), only platelet count was entered into the final Cox regression model along with cytogenetic clonal evolution, spleen enlargement, time from IM start to first CCyR and time from CCyR to first MMR.
Multivariate analysis revealed that spleen enlargement, and time from IM initiation to CCyR and to MMR were strongly associated with achieving CMR: i) patients with spleen enlargement below the costal margin were 3 times less likely to reach CMR (HR: 0.3540; 95%CI: 0.192–0.654; P=0.0009); ii) patients who achieved CCyR after 12 months were half as likely to achieve a further CMR than those reaching CCyR before 12 months (HR: 0.5; 95%CI: 0.27–0.95; P=0.034); and iii) patients who achieved MMR after 12 months from CCyR 5 times less likely to reach CMR than those obtaining MMR within six months (HR: 0.2147; 95%CI: 0.092–0.5; P=0.00038).
To confirm the impact of achieving CCyR in the first 12 months and MMR in the first 18 months of IM therapy, the cumulative incidence of a further CMR was analyzed through the cytogenetic and molecular status at 12 and 18 months of therapy (Figures 3A–C). Patients achieving CCyR in the first twelve months of IM therapy had a significantly higher rate of CMR (P=0.0111) (Figure 3A). Interestingly, achieving CCyR and MMR in the first year of therapy was associated with a higher rate of further CMR when compared to patients who had achieved CCyR without MMR at 12 months and CCyR after 12 months (P<0.0001) (Figure 3B). The impact of achieving MMR at 18 months was also correlated with a higher rate of further CMR as illustrated in Figure 3C (P<0.0001).
Figure 3.
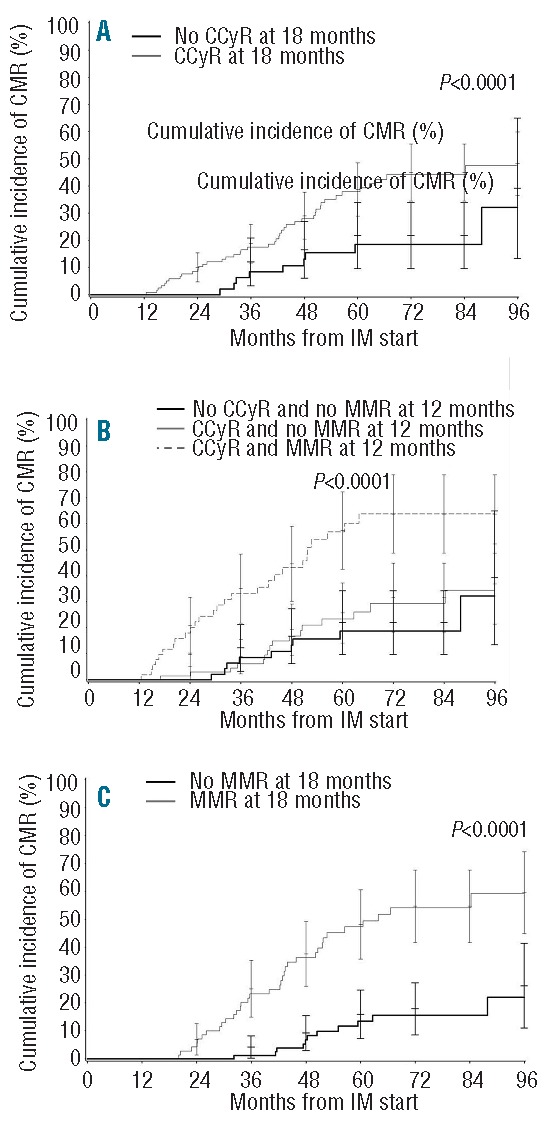
Cumulative incidence of complete molecular response (CMR) in patients who achieved complete cytogenetic response (CCyR) at 12 months of imatinib (IM) therapy or later (A), in patients who achieved CCyR and major molecular response (MMR), CCyR and no MMR at 12 months and CCyR after 12 months of IM therapy (B), and in patients who achieved MMR or not at 18 months of IM therapy (C).
Outcome
Thirty-five (19.4%) of the 180 patients who achieved CCyR on IM therapy presented an event as previously defined (Table 2).
Table 2.
Events of the patients who achieved CCyR without MMR (n=23), CCyR with MMR without CMR (n=92) and CCyR with CMR (n=65) on IM therapy.
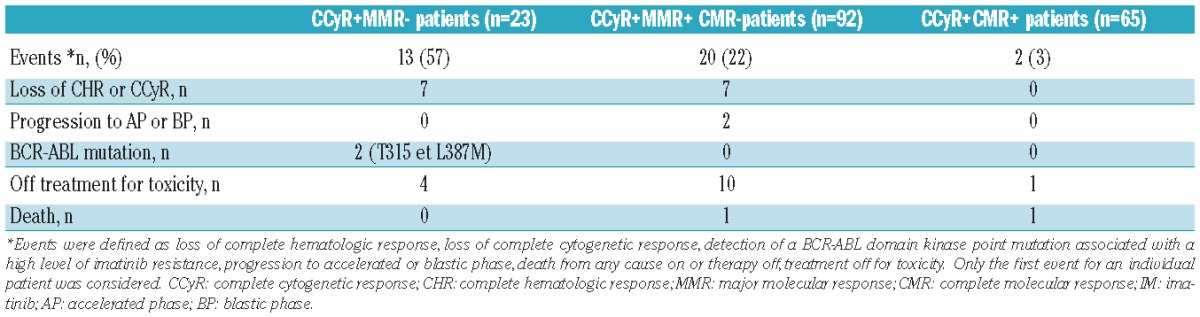
Fifty-seven percent of the patients in CCyR without MMR, 22% of the patients with CCyR and MMR without CMR, and 3% of the patients with CCyR and CMR presented an event. The event was death for 2 patients: one patient in CCyR and MMR died of an unknown cause while the other death was related to a concomitant aggressive non-Hodgkin lymphoma. Patients who achieved CMR had a better EFS and FFS than those with CCyR, irrespective of MMR status (95.2% vs. 64.7% vs. 27.7%, P=0.00124; 98.4% vs. 82.3% vs. 56%, P=0.0335) (Figure 4A and B).
Figure 4.
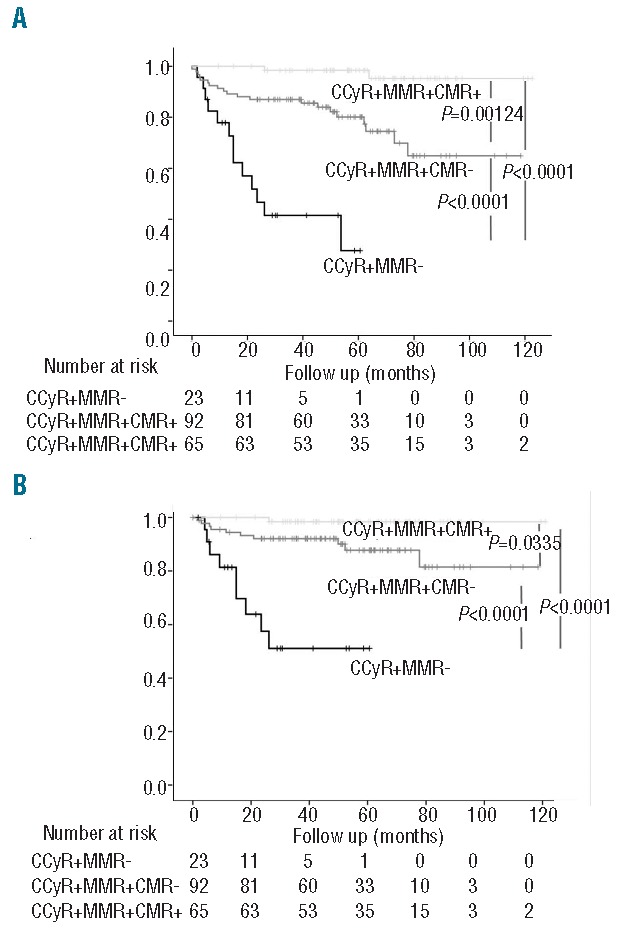
Event-free survival (A) and failure-free survival (B) of patients who achieved complete cytogenetic response (CCyR) without major molecular response (MMR) (n=23) (CCyR+MMR−), CCyR with MMR without complete molecular response (CMR) (n=92) (CCyR+MMR+CMR−) and CCyR with CMR (n=65) (CCyR+MMR+CMR+) on imatinib (IM) therapy. Event-free survival was measured from the date of the first CCyR on IM therapy to the date of the first event.
The estimated 5-year EFS and FFS were 98.4% and 98.4% in the CCyR+MMR+CMR+ patient group, 79.9% and 88.2% in the CCyR+MMR+CMR− patient group, and 27.7% and 56% in the CCyR+MMR− patient group. There was no difference in OS between the three groups of patients (P=0.465).
Survival analyses were also performed including the excluded patients, i.e. patients who had a follow up below the median observed time to CMR without event. EFS and FFS associated with CMR were still significantly better than CCyR with or without MMR (data not shown).
In order to also assess whether achieving CMR at specific time points had a clinical impact, EFS was analyzed by landmark analysis according to the molecular response status at 18 months from IM start. Among the 233 patients who had achieved a CCyR on IM treatment, 20 patients had a follow up of less than 18 months and 21 patients had achieved CCyR after more than 18 months and were not considered for subsequent analysis. Finally, among the 192 remaining patients, 19 (10%) were in CMR, 101 (53%) were in MMR and 72 (37%) were only in CCyR. There was no statistically significant difference in EFS between the 3 groups using landmark analysis at 18 months (P=0.11) (Figure 5). Among the 101 MMR patients, 42 achieved the CMR after 18 months. However, no significant differences were observed at the 24- and 30-month landmark analyses. FFS and OS were also subjected to landmark analyses at these time points but did not statistically demonstrate differences between the 3 groups of patients (data not shown).
Figure 5.
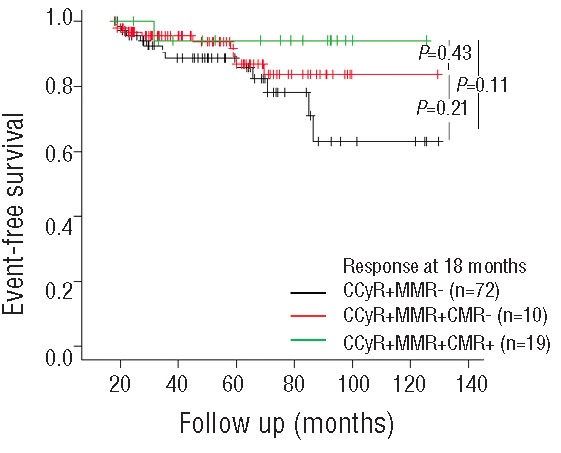
Event-free survival at 18-month landmark by molecular response (n=192).
Discussion
Imatinib has dramatically improved outcome for newly diagnosed CML-CP patients with most achieving a sustained CCyR and MMR that are considered to be the best surrogate markers of survival. In the current study, the cumulative incidence of CCyR and MMR (respectively 88% and 74%) is similar to that reported by other studies.3–5,11 By two and three years, 9% and 14% of the patients achieved a CMR that is also very similar to that reported in the IM arm in other randomized studies.16–18 Finally, 65 (24%) patients of the study cohort achieved CMR.
The second objective of the study was to identify baseline and under-treatment prognostic factors of achieving CMR at the time of the first CCyR on IM front-line therapy; the absence of spleen enlargement, achieving CCyR during the first 12 months of therapy, and achieving MMR within the year following CCyR were associated with a significantly increased probability of achieving a further CMR.
The negative prognostic value of spleen enlargement is, however, not very surprising. Spleen enlargement was already a strong predictive factor of survival as assessed by the Sokal and Hasford scores before the IM era and found to predict a lower probability of achieving a CCyR on IM by the recent EUTOS score.19–21 Our findings, highlighting the importance of a rapid MMR, are in accordance with those reported by Branford et al. who identified that achieving MMR during the first year of therapy was the only predictive factor of CMR.22
When taking into account the median time to CCyR observed in our patients who achieved at least MMR (6.92 months in the CCyR+MMR+CMR− patient group, 5.98 months in the CCyR+MMR+CMR+ patient group) and the impact of achieving MMR during the 12 months following CCyR, we hypothesize that obtaining MMR within 18 months of IM initiation may be associated with an increased probability of achieving CMR. Analyses of cumulative incidence of CMR through the achievement of MMR at 12 and 18 months of IM therapy confirms this hypothesis, as significantly higher rates of CMR were observed in patients in MMR at 12 and 18 months from IM start.
Our study focused on patients who achieved CCyR on IM therapy. To date, while most patients treated with front-line IM achieve CCyR, most of these will have persistent disease at the molecular level. In the subset of patients who achieved a previous CCyR, the prognostic value of MMR, defined as a 3-log reduction in BCR-ABL base-line transcript level, remains debatable.3,5,8,11 One of the reasons may be that CCyR and MMR thresholds are in close proximity for measuring residual disease: CCyR corresponds to a 2-log reduction of BCR-ABL transcript level from a base-line value and the standard deviation of quantitative BCR-ABL PCR can be around 0.5 log. Therefore, assessing the clinical impact of MMR in CCyR patients would require a long follow up and a large cohort of patients. However, MMR has been shown to be a strong reproducible surrogate marker that is useful to compare findings from different studies.
Significantly higher rates of CMR were observed in patients who had achieved CCyR and MMR at 12 months when compared to patients who had achieved CCyR at twelve months or later without MMR. Therefore, beyond its prognostic value, a faster MMR in CCyR patients has predictive impact on the probability of achieving a further CMR.
Finally, our study demonstrates that MMR and CMR in CCyR patients treated with front-line IM are associated with better EFS and FFS. We defined the events likely to influence clinical outcome of IM treatment as reported by Kantarjian et al.23 with slight modifications: we considered that detection of a BCR-ABL tyrosine kinase point mutation associated with a high level of IM resistance both in vitro and in IM resistant patients is a significant event leading to therapeutic change in case of failure or suboptimal response, as recently recommended.24 We also considered loss of a previous CCyR rather than loss of a major cytogenetic response at any time during treatment as a significant event leading to therapeutic change, as recommended by the ELN experts in case of failure.15
As molecular status for an individual patient may evolve during IM therapy, the usefulness of Kaplan-Meier analysis may be a subject of debate. However, the objective of the study was to assess whether achieving a deeper molecular response in CCyR patients at any time, rather than at defined milestones, was associated with a better outcome. Indeed, among patients who failed to achieve CMR, patients with a shorter follow up than the observed median time to CMR and without any reported event were excluded from the identification of predictive factors of CMR and survival analyses. Moreover, the 3 groups of patients defined by the best response achieved under IM therapy have now had a long follow up (4.55 years for the CCyR+MMR−, 5.37 years for the CCyR+MMR+CMR− and 6.16 years for the CCyR+MMR+CMR+).
Other studies used landmark methods to compare time-to-event outcome between groups determined during study follow up.8 However, the method used is sometimes limited by omission of events occurring before or after the landmark time point. In any case, we attempted to assess whether achieving a CMR at specific time points had a clinical impact with this method. So EFS was analyzed by landmark analysis according to the molecular response status at 18 months from IM start. Although a trend was observed, this 18-month landmark analysis did not demonstrate any significant difference in EFS according to the depth of molecular response. Clearly, for a time dependent variable, landmark analysis is an appropriate test to determine the prognostic value of the response, but it does not take into account what is happening after the time point. In the current study, the rate of CMR continues to increase after 18 months. In contrast, using the Kaplan-Meier method, the best molecular response achieved was taken into account independently of the time. So, the Kaplan-Meier analyses show that the achievement of CMR during the follow up is associated with a lower probability of clinically relevant events.
Until now, the relevance of deeper molecular response had been mainly linked to the strategies of IM discontinuation. The STIM study demonstrated that achievement of sustained CMR (for at least 2 years) was one of the first but essential criteria to be taken into account when considering IM discontinuation.12
However, besides discontinuation strategies, the impact of achieving CMR on clinical outcome and survival has not previously been demonstrated. Colombat et al. showed that failure to achieve a sustained CMR for patients in CCyR was correlated to the probability of loss of CCyR25 while Kantarjian et al. failed to correlate sustained CMR with PFS.5 More recently, Verma et al. suggested that patients who achieved CCyR with sustained CMR by 24 months of therapy had better EFS compared to those who achieved CCyR with or without MMR.26
In the current study, we demonstrated that once CCyR is achieved with front-line IM, achieving a deeper molecular response is associated with a lower probability of relevant clinical events.
Conclusion
Almost 25% patients treated with front-line IM therapy will achieve CMR with a 68% probability of remaining in CMR at two years. The absence of spleen enlargement, and not achieving a CCyR before 12 months and MMR in the first year following CCyR achievement, were predictive of achieving a further CMR. Our study demonstrates that once CCyR has been reached, achieving deeper molecular response is associated with a better EFS and FFS, and that CMR clearly confers the best outcome and should thus become the main objective of CML therapies.
Footnotes
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Kantarjian H, O’Brien S, Jabbour E, Garcia-Manero G, Quintas-Cardama A, Shan J, et al. Improved survival in chronic myeloid leukemia since the introduction of imatinib therapy: a single-institution historical experience. Blood. 2012;119(9):1981–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gambacorti-Passerini C, Antolini L, Mahon FX, Guilhot F, Deininger M, Fava C, et al. Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib. J Natl Cancer Inst. 2011;103(7):553–61 [DOI] [PubMed] [Google Scholar]
- 3.de Lavallade H, Apperley JF, Khorashad JS, Milojkovic D, Reid AG, Bua M, et al. Imatinib for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol. 2008;26(20):3358–63 [DOI] [PubMed] [Google Scholar]
- 4.Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–17 [DOI] [PubMed] [Google Scholar]
- 5.Kantarjian H, O’Brien S, Shan J, Huang X, Garcia-Manero G, Faderl S, et al. Cytogenetic and molecular responses and outcome in chronic myelogenous leukemia: need for new response definitions? Cancer. 2008;112(4):837–45 [DOI] [PubMed] [Google Scholar]
- 6.Marin D, Milojkovic D, Olavarria E, Khorashad JS, de Lavallade H, Reid AG, et al. European LeukemiaNet criteria for failure or suboptimal response reliably identify patients with CML in early chronic phase treated with imatinib whose eventual outcome is poor. Blood. 2008;112(12):4437–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes TP, Kaeda J, Branford S, Rudzki Z, Hochhaus A, Hensley ML, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003;349(15):1423–32 [DOI] [PubMed] [Google Scholar]
- 8.Hughes TP, Hochhaus A, Branford S, Muller MC, Kaeda JS, Foroni L, et al. Long-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: an analysis from the International Randomized Study of Interferon and STI571 (IRIS). Blood. 2010;116(19):3758–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jabbour E, Kantarjian H, O’Brien S, Shan J, Quintas-Cardama A, Faderl S, et al. The achievement of an early complete cytogenetic response is a major determinant for outcome in patients with early chronic phase chronic myeloid leukemia treated with tyrosine kinase inhibitors. Blood. 2011;118(17):4541–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Press RD, Love Z, Tronnes AA, Yang R, Tran T, Mongoue-Tchokote S, et al. BCR-ABL mRNA levels at and after the time of a complete cytogenetic response (CCR) predict the duration of CCR in imatinib mesylate-treated patients with CML. Blood. 2006;107 (11):4250–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hehlmann R, Lauseker M, Jung-Munkwitz S, Leitner A, Muller MC, Pletsch N, et al. Tolerability-adapted imatinib 800 mg/d versus 400 mg/d versus 400 mg/d plus interferon-alpha in newly diagnosed chronic myeloid leukemia. J Clin Oncol. 2011;29 (12):1634–42 [DOI] [PubMed] [Google Scholar]
- 12.Mahon FX, Rea D, Guilhot J, Guilhot F, Huguet F, Nicolini F, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11(11): 1029–35 [DOI] [PubMed] [Google Scholar]
- 13.Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108(1):28–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guilhot J, Baccarani M, Clark RE, Cervantes F, Guilhot F, Hochhaus A, et al. Definitions, methodological and statistical issues for phase 3 clinical trials in chronic myeloid leukemia: a proposal by the European LeukemiaNet. Blood. 2012; 119(25):5963–71 [DOI] [PubMed] [Google Scholar]
- 15.Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009; 27(35):6041–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kantarjian HM, Hochhaus A, Saglio G, De Souza C, Flinn IW, Stenke L, et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol. 2011;12(9): 841–51 [DOI] [PubMed] [Google Scholar]
- 17.Kantarjian HM, Shah NP, Cortes JE, Baccarani M, Agarwal MB, Undurraga MS, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION). Blood. 2012;119(5):1123–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larson RA, Hochhaus A, Hughes TP, Clark RE, Etienne G, Kim DW, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia. 2012;26(10):2197–203 [DOI] [PubMed] [Google Scholar]
- 19.Hasford J, Pfirrmann M, Hehlmann R, Allan NC, Baccarani M, Kluin-Nelemans JC, et al. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa. Writing Committee for the Collaborative CML Prognostic Factors Project Group J Natl Cancer Inst. 1998;90(11):850–8 [DOI] [PubMed] [Google Scholar]
- 20.Hasford J, Baccarani M, Hoffmann V, Guilhot J, Saussele S, Rosti G, et al. Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: the EUTOS score. Blood. 2011; 118(3):686–92 [DOI] [PubMed] [Google Scholar]
- 21.Sokal JE, Cox EB, Baccarani M, Tura S, Gomez GA, Robertson JE, et al. Prognostic discrimination in “good-risk” chronic granulocytic leukemia. Blood. 1984;63(4):789–99 [PubMed] [Google Scholar]
- 22.Branford S, Seymour JF, Grigg A, Arthur C, Rudzki Z, Lynch K, et al. BCR-ABL messenger RNA levels continue to decline in patients with chronic phase chronic myeloid leukemia treated with imatinib for more than 5 years and approximately half of all first-line treated patients have stable undetectable BCR-ABL using strict sensitivity criteria. Clin Cancer Res. 2007; 13(23):7080–5 [DOI] [PubMed] [Google Scholar]
- 23.Kantarjian H, O’Brien S, Jabbour E, Shan J, Ravandi F, Kadia T, et al. Impact of treatment end point definitions on perceived differences in long-term outcome with tyrosine kinase inhibitor therapy in chronic myeloid leukemia. J Clin Oncol. 2011; 29(23):3173–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soverini S, Hochhaus A, Nicolini FE, Gruber F, Lange T, Saglio G, et al. BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: recommendations from an expert panel on behalf of European LeukemiaNet. Blood. 2011;118 (5):1208–15 [DOI] [PubMed] [Google Scholar]
- 25.Colombat M, Fort MP, Chollet C, Marit G, Roche C, Preudhomme C, et al. Molecular remission in chronic myeloid leukemia patients with sustained complete cytogenetic remission after imatinib mesylate treatment. Haematologica. 2006;91(2):162–8 [PubMed] [Google Scholar]
- 26.Verma D, Kantarjian HM, Shan J, O’Brien S, Verma A, Jabbour E, et al. Sustained Complete Molecular Response to Imatinib in Chronic Myeloid Leukemia (CML): a Target Worth Aiming and Achieving? ASH Annual Meeting Abstracts. 2009;114(22): 505 [Google Scholar]


