Abstract
Lymphoma patients with persistent disease undergoing autologous transplantation have a very poor prognosis in the rituximab era. The addition of radioimmunotherapy to the conditioning regimen may improve the outcome for these patients. In a prospective, phase 2 study, we evaluated the safety and efficacy of the addition of 90Y-ibritumomab tiuxetan to the conditioning chemotherapy in patients with refractory diffuse large B-cell lymphoma. Thirty patients with induction failure (primary refractory; n=18) or refractory to salvage immunochemotherapy at relapse (n=12) were included in the study. The median age of the patients was 53 years (range, 25–67). All patients were given 90Y-ibritumomab tiuxetan at a fixed dose of 0.4 mCi/kg (maximum dose 32 mCi) 14 days prior to the preparative chemotherapy regimen. Histological examination showed that 22 patients had de novo diffuse large B-cell lymphoma and eight had transformed diffuse large B-cell lymphoma. All patients had persistent disease at the time of transplantation, with 25 patients considered to be chemorefractory. The median time to neutrophil recovery (>500 white blood cells/μL) was 11 days (range, 9–21), while the median time to platelet recovery (>20,000 platelets/μL) was 13 days (range, 11–35). The overall response rate at day +100 was 70% (95% CI, 53.6–86.4) with 60% (95% CI, 42.5–77.5) of patients obtaining a complete response. After a median follow-up of 31 months for alive patients (range, 16–54), the estimated 3-year overall and progression-free survival rates are 63% (95% CI, 48–82) and 61% (95% CI, 45–80), respectively. We conclude that autologous transplantation with conditioning including 90Y-ibritumomab tiuxetan is safe and results in a very high response rate with promising survival in this group of patients with refractory diffuse large B-cell lymphoma with a very poor prognosis. Study registered at European Union Drug Regulating Authorities Clinical Trials (EudraCT) N. 2007-003198-22.
Introduction
Despite the fact that rituximab in combination with anthra-cycline-containing chemotherapy as front-line treatment has significantly improved the survival of patients with diffuse large B-cell lymphoma (DBCL), a significant proportion of patients (20–50%) either fail to achieve a complete response or relapse.1–3 For these refractory/relapsing patients, salvage chemotherapy followed by high-dose chemotherapy with autologous stem cell transplantation (ASCT) continues to be the standard of care.4 However, a number of studies have shown that chemosensitivity is the main factor that determines the benefit derived from ASCT.5,6 Thus, patients with chemorefractory disease (either upfront or at relapse) have a very poor outcome, with little benefit from ASCT. Attention has been focused on this particular group of patients in order to develop more effective strategies for them. None of the different chemotherapy-based preparative regimens for ASCT has proven to be superior to the others and, since DLBCL is radiosensitive, total body irradiation has been incorporated into the conditioning regimens for ASCT. In the last years, to maximize the antitumor effect and reduce toxicities, total body irradiation has been replaced by radioimmunotherapy (RIT) in the conditioning regimen for ASCT.7
90Yttrium-ibritumomab tiuxetan is a radiolabeled anti-CD20 monoclonal antibody with significant activity against both indolent and aggressive B-cell non-Hodgkin lymphomas.8 In recent years, RIT has been incorporated into preparative regimens for ASCT in patients with relapsed DLBCL with the intention of enhancing the antitumor effect and of improving the outcomes of these patients, whose prognosis is poor.9–11 However, these studies have focused mainly on patients with relapsed, chemosensitive DLBCL, while little is known about the efficacy of this treatment in patients with chemorefractory disease.
Here, we present the data from a prospective, multicenter, clinical trial on the safety and efficacy of a standard dose of 90Y-ibritumomab tiuxetan combined with high-dose BEAM chemotherapy followed by ASCT in patients with DLBCL refractory to a rituximab-containing chemotherapy.
Methods
Eligibility
This study included patients with histologically confirmed DLBCL, either de novo or transformed from a previous indolent CD20+ B-cell lymphoma. Patients were eligible if they failed to achieve at least a partial response after front-line immunochemotherapy (induction failure), and were further unresponsive (i.e. failed to attain a partial response) to salvage immunochemotherapy. Patients with a relapse who failed to achieve a partial response to salvage immunochemotherapy were also eligible. Other eligibility criteria included age between 18 and 70 years old, a performance status of 0–1, and standard transplantation criteria (i.e. adequate cardiac, renal, and respiratory function). All patients had measurable disease by fluorine-18fluorodeoxyglucose positron emission tomography combined with computed tomography (PET-CT). Patients were excluded if they had central nervous system lymphoma at the time of enrollment, a history of human immunodeficiency virus infection, or had previously undergone ASCT.
Study design and treatment
This was a phase II study conducted at 17 centers within Spain, approved by the Ethics Committee of each center, and conducted in accordance with the Declaration of Helsinki. Patients were recruited from January 2008 to February 2010. Signed informed consent was obtained from all patients prior to any study-related procedure. The study was registered under European Union Drug Regulating Authorities Clinical Trials (EudraCT) N. 2007-003198-22.
On day -21, patients were given rituximab 250 mg/m2; on day -14, patients received 250 mg/m2 rituximab followed by 90Y-ibritumomab tiuxetan at a fixed dose of 0.4 mCi/kg (with a maximum total dose of 32 mCi) in an outpatient setting, with no dose adjustments for neutropenia or thrombocytopenia. One week later, patients were given high-dose BEAM chemotherapy (carmustine 300 mg/m2 on day −6, etoposide 200 mg/m2 on days −5 to −2, cytarabine 200 mg/m2 twice a day on days −5 to −2, and melphalan 140 mg/m2 on day −1). Autologous stem cells were reinfused on day 0. Granulocyte colony-stimulating factor 5 μg/kg/day was started on day +7 after ASCT and continued until neutrophil recovery. Acyclovir and trimethoprim sulfamethoxazole were used as prophylaxis 1 and 3 months after ASCT, respectively. Adverse events were assessed and graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events, version 3.0.
Response evaluation and follow-up
All patients were required to have a complete baseline evaluation before the treatment, including a physical examination, blood count and serum biochemistry determinations, bone marrow biopsy and a whole body evaluation with PET-CT. Response was evaluated 3 months after ASCT, according to the 2007 Cheson criteria.12 Follow-up procedures were done every 3 months for the first year after transplantation and every 6 months thereafter for 2 years.
Statistical analysis
The primary end-point of this study was response rate after transplantation. Secondary end-points included progression-free survival, overall survival, and toxicity. The results are reported on an intent-to treat basis. Probabilities of progression-free survival and overall survival were estimated using the Kaplan-Meier method. Differences between survival curves were assessed using the log-rank test. All calculations were analyzed using the SPSS statistical 19.0 package (SPSS Inc, Chicago, IL, USA).
Results
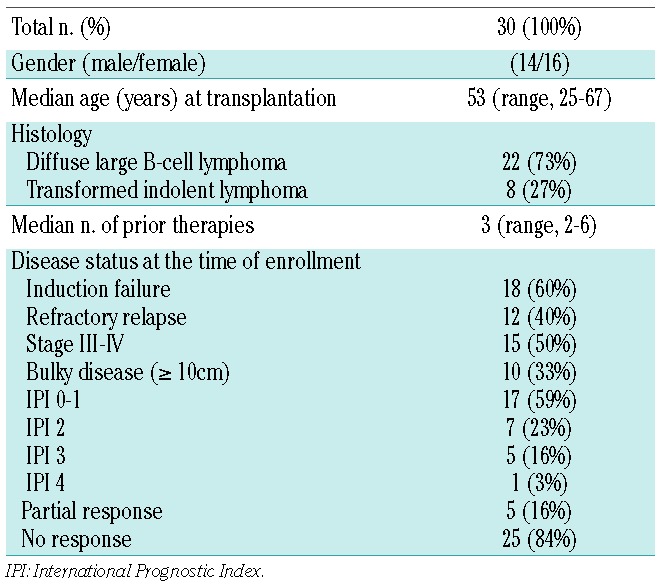
Patients’ characteristics
Thirty-one patients gave consent to participation in this study between January 2008 and February 2010. One patient was not evaluable since he experienced explosive progression of disease between consent and the planned start of the therapy and did not receive any therapy. Analyses were done on an intention-to treat basis in the remaining 30 patients. The clinical characteristics of the patients are listed in Table 1. The median age at transplantation was 53 years (range, 25–67). Of the 30 cases, 22 had de novo DLBCL and eight had transformed indolent lymphoma. At the time of inclusion, 15 patients had stage III/IV disease, 13 had an International Prognostic Index score of 2–4, and ten patients had bulky disease (maximum diameter ≥10 cm). Patients received a median of three rituximab-containing regimens (range, 2–6). Induction therapy consisted of conventional-dose R-CHOP (6 cycles, every 21 days) in 28 cases or high-dose R-CHOP (cyclophosphamide 1500 mg/m2/day 1 with mesna 150% of total cyclophosphamide dose; doxorubicin 65 mg/m2 day 1, vincristine 2 mg day 1, prednisone 60 mg/m2 days 1–5; at least 3 cycles every 21 days) in two cases; radiotherapy to bulky sites was given to six patients. Induction therapy failed in 18 patients and 12 patients had a relapse that was refractory to salvage therapy.
Table 1.
Patients’ characteristics.
Patients with induction failure received salvage therapy as follows: R-DHAP (rituximab, dexamethasone, cytarabine and cisplatin; 2 cycles) followed by R-IFE (rituximab, ifosfamide, etoposide; 2 cycles) (n=3), R-IFE (3 cycles) (n=3), R-ICE (rituximab, ifosfamide, carboplatin, etoposide, 3 cycles) (n=4), R-ESHAP (rituximab, etoposide, prednisone, cytarabine, cisplatin; 2–3 cycles) (n=3), R-DHAP (rituximab, dexamethasone, cytarabine and cisplatin; 2–3 cycles) (n=1), R-DHAP (2 cycles) followed by RESHAP (2 cycles) (n=1), R-GemOx (rituximab, dexamethasone, gemcitabine, oxaliplatin every 14 days, 6 cycles) (n=1), R-ESHAP (2 cycles) followed by R-GemOx (4 cycles) (n=1), and R-IFE (2 cycles) followed by R-DHAP (2 cycles) (n=1).
For relapsed patients, salvage chemotherapy was R-MINE (rituximab, ifosfamide, etoposide and mitoxantrone; 3 cycles) (n=1), R-ESHAP (2–3 cycles) (n=6), RESHAP (2 cycles) followed by R-GemOx (n=1), R-ICE (3 cycles) (n=1), R-ICE (2 cycles) followed by R-GemOx (4 cycles) (n=1), R-IFE (3 cycles) (n=1), and one patient received R-DHAP (2 cycles) followed by R-IFE (1 cycle) and R-GemOx (3 cycles).
All patients had active disease, as defined by PET-CT, at the time of inclusion in the trial. At the time of ASCT, 25 patients were chemorefractory and five patients had chemosensitive disease (3 patients with induction failure had a partial response to salvage treatment - 2 of them after additional radiotherapy - and 2 relapsed patients did not respond to salvage chemotherapy but did achieve a partial response after radiotherapy).
Peripheral blood progenitor cells were mobilized with granulocyte colony-stimulating factor alone in eight patients and with chemotherapy plus granulocyte colony-stimulating factor in the other 22 patients. The chemotherapy was ESHAP for eight patients, ICE for four, IFE for nine, and one patient received DHAP. All patients had a successful peripheral stem cell collection prior to ASCT, with a median yield of 3.9×106/kg CD34+ cells (range, 2–18.3).
Early toxicity and engraftment
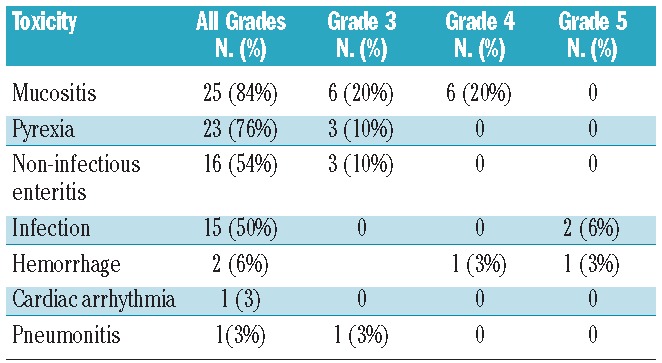
One patient had a grade 1 reaction to 90Y-ibritumomab tiuxetan infusion, and three patients developed grade 4 hematologic toxicities related to 90Y-ibritumomab tiuxetan (neutropenia in 2 patients and thrombocytopenia in 1) before ASCT. One patient had bacterial sepsis after an infusion of 90Y-ibritumomab tiuxetan and recovered fully with antibiotic treatment. Two patients died during the conditioning treatment, one with a cerebral hemorrhage and one due to disease progression. The remaining 28 patients proceeded to ASCT. All 28 patients engrafted. The median time from ASCT to neutrophil engraftment was 11 days (range, 9–21), and the median time to platelet engraftment was 13 days (range, 11–35). Fever was documented in 23 patients, with a median duration of 6 days (range, 1–16). Mucositis was present in 25 patients; it was grade 3 in six cases and grade 4 in six other cases. Three patients developed grade 3 non-infectious enteritis. One patient died early after transplantation due to Gram-negative bacterial sepsis (Table 2). Transplantation-related mortality at 100 days was 3.5%.
Table 2.
Non-hematologic toxicities within 2 years after transplantation.
Response and survival
Response was evaluated on an intention-to-treat basis. The overall response rate was 70% (95% CI, 53.6%–86.4%). Eighteen patients (60%) achieved a complete response with negative PET-CT (95% CI, 42.5%–77.5%) and three patients had a partial response. Ten patients experienced progressive disease and nine of them died as a result of lymphoma. Among five patients with a partial response at the time of ASCT, four were alive and free of lymphoma at their last follow-up. Disease progression occurred within 18 months in all cases. With a median follow-up of 31 months among surviving patients (range, 16–54), 11 patients have died. The estimated 3-year overall and progression-free survival rates are 63% (95% CI, 48%–82%) and 61% (95% CI, 45%–80%), respectively (Figures 1,2). Patients with a partial response (n=5) at the time of ASCT had a 3-year overall and progression-free survival rate of 80% (95% CI, 65%–95%), while chemorefractory patients (n=25) had a 3-year overall and progression-free survival of 60% (95% CI 41%–79%) (P=0.48). Survival according to PET-CT status at 3 months after ASCT was different. Patients with a negative PET-CT (n=18) had a 3-year overall and progression-free survival of 70%, (95% CI, 52%–87%) while it was 0% for those patients (n=3) with a positive PET-CT (P=0.005) (Figure 3).
Figure 1.
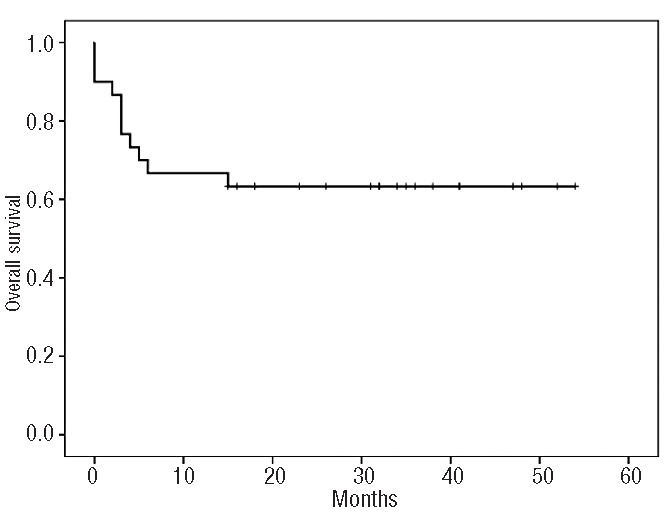
Overall survival for all patients (N=30).
Figure 2.
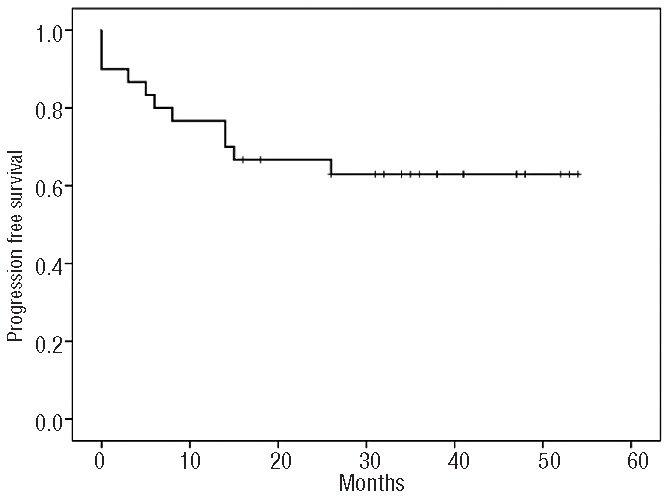
Progression-free survival for all patients (N=30).
Figure 3.
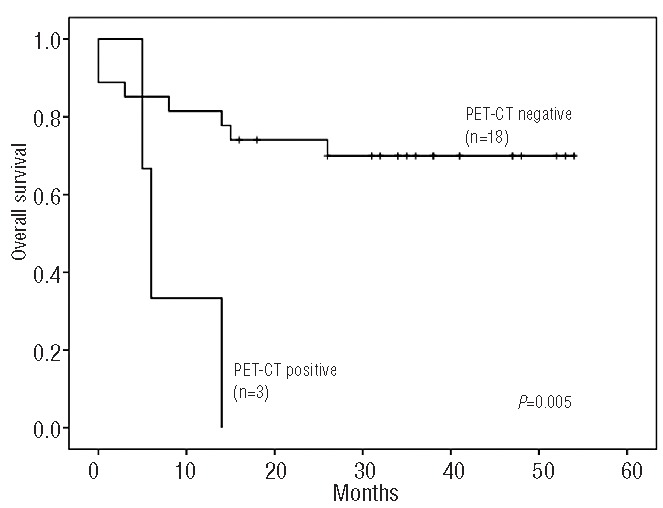
Overall survival according to PET-CT response at 3 months after autologous stem cell transplantation.
Concerning patients with transformed DLBCL (n=8), seven were chemorefractory to salvage therapy and one patient achieved a partial response at the time of ASCT. No differences in survival were found between patients with de novo DLBCL (overall/progression-free survival 63%; 95% CI, 43%–82%) and those with transformed DLBCL (overall/progression-free survival 66%; 95% CI, 48%–85%) (P=0.9).
Late effects
One patient died of septic shock due to pneumonia 14 months after ASCT, while in complete remission. One patient developed pneumococcal meningitis 11 months after ASCT and had a full recovery with antibiotics. One patient in remission of his lymphoma died 14 months after ASCT as a result of a secondary acute myeloid leukemia with a complex karyotype. One patient experienced pancytopenia 33 months after ASCT. A bone marrow biopsy showed a myelodysplastic syndrome consisting of refractory anemia with excess of blasts-2 with cytogenetic studies showing monosomy 7; at the last follow-up, the patient was receiving 5′-azacytidine treatment with no evidence of lymphoma.
Discussion
High-dose chemotherapy followed by ASCT remains a reasonable option in the rituximab era for relapsed patients with DLBCL.13 Patients with chemosensitive disease have better outcomes compared to those who are chemorefractory, whose 3-year progression-free survival rate is 20%.14 Nevertheless, relapsed DLBCL patients treated with rituximab-containing chemotherapy who have persistent disease (i.e. who are PET-positive) do very poorly after ASCT.15 To improve the outcome of this group of patients, RIT has been used as part of the transplantation preparative regimen. Phase II studies have shown promising results in relapsed DLBCL patients.8–11 Our study preferentially focused on chemoresistant patients, since the majority (84%) did not achieve a partial response to salvage chemotherapy, either after front-line therapy or at relapse. Our data are in agreement with those of previous studies suggesting an improved outcome for DLBCL patients receiving RIT as part of the conditioning treatment before ASCT. In our study, 61% of patients were alive and free of disease at 2.5 years. These numbers are similar to those reported by Shimoni et al. in a similar group of patients.16 Other phase II studies with the addition of RIT (90Y-ibritumomab tiuxetan) to the conditioning regimen for ASCT have also shown promising results in relapsed DLBCL patients.17 In a recent comparative study in patients with relapsed/refractory DLBCL, addition of RIT (90Y-ibritumomab tiuxetan) to BEAM followed by ASCT resulted in a superior overall survival but not progression-free survival.11 The low numbers of patients did, however, preclude definitive conclusions and the therapeutic benefit of RIT in the setting of ASCT remains unproven.
Very recently, Vose et al. showed that relapsed DLBCL patients treated with RIT (131I-tositumomab) plus BEAM and ASCT had a 2-year progression-free survival rate of 48%, similar to that of patients receiving rituximab plus BEAM as the transplantation regimen.18 However, the radiolabeled antibody used in that study (131I-tositumomab) was different from the one used in our study (90Y-ibritumomab tiuxetan) and our results, with longer follow-up, seem to be better in a more chemoresistant population of patients. Although both antibodies target the CD20 antigen and are β-emitters, the monoclonal antibodies are completely different, and their antitumor effect may not necessarily be identical. Besides killing targeted tumor cells, an additional mechanism of tumor killing is the crossfire effect that might eliminate non-targeted tumor cells. In line with this, 90Y-ibritumomab tiuxetan emits β radioactivity with a path length 6-fold larger than that of 131I-tositumomab,19 which could potentially contribute to better disease control. Unfortunately, there are no comparative data available on the efficacy of those radiolabeled antibodies. Nevertheless, the fact that the addition of RIT failed to significantly improve the outcome of patients with relapsed chemosensitive DLBCL raises concern about the clinical impact of addition of RIT to the preparative regimen for ASCT.
The 90Y-ibritumomab tiuxetan dose in this study was chosen based on previous phase I studies showing substantial myelosuppression at a fixed dose of 0.4 mCi/kg dose for a maximum of 32 mCi,20 and the fact that no dosimetry was needed when using this dose, which allows the treatment to be delivered in an outpatient setting. Since patients receive a transplant, myelosuppression is not longer a problem, and higher doses of RIT may be given, which could potentially improve the effectiveness of this treatment. Preliminary studies have shown that 90Y-ibritumomab tiuxetan can be given safely at a dose up to 0.95 mCi/kg followed by high-dose chemotherapy and ASCT, which represents at least twice the conventional 0.4 mCi/kg dose.21 Studies attempting to translate high-dose RIT followed by ASCT to patients with relapsed/refractory lymphoma have shown feasibility with no increased toxicities,10 but it has not been formally demonstrated that this approach improves the outcome of patients compared to that achieved with conventional-dose RIT. Our study shows that, even in this very poor-risk patient population, addition of RIT to the preparative regimen before ASCT is safe, and does not seem to add to the toxicity of the BEAM conditioning regimen. All patients had an adequate hematopoietic recovery with no late engraftments and, with perhaps the exception of mucositis, the remaining adverse events were similar to those expected with BEAM alone. A transplantation-related mortality of 3.5% is in keeping with current experience with other high-dose therapies.
An important issue regarding long-term toxicities after ASCT is the occurrence of treatment-related myelodysplasia (tMDS) or acute myeloid leukemia (tAML). With the increasing use of RIT, mostly as part of the preparative regimen for ASCT, the risk of developing tMDS/tAML is of concern and deserves careful attention. In our study, two patients developed tMDS/tAML between 1 and 3 years after RIT, which is a crude incidence of 7%. One patient, with a de novo DLBCL, had received three previous regimens before RIT, including alkylating agents and etoposide, and developed tAML with a complex karyotype. A second patient, with a transformed DLBCL, had a tMDS with cytogenetic changes involving chromosome 7. This patient had previously been treated with a combination of fludarabine and alkylating agents for an indolent non-Hodgkin lymphoma, before the transformation to a DLBCL. The true incidence of tMDS/tAML after RIT and ASCT is not known, since it has not been prospectively studied in a large series of patients with a long follow-up. In our study, bone marrow assessments during the follow-up were only done if additional abnormalities were detected on routine laboratory counts which may contribute to an underreported incidence tMDS/tAML.
In a recent randomized study assessing the use of RIT versus rituximab as part of the conditioning regimen before ASCT,18 the incidence of tMDS was less than 1% in each arm, although follow-up was short. In a recent retrospective survey of patients with non-Hodgkin lymphoma treated with 90Y-ibritumomab tiuxetan as a single agent, the crude incidence of tMDS/tAML was found to be 2.5%.22 Interestingly, an association between previous fludarabine treatment and an increased risk of tMDS/tAML was found in this study, with frequent cytogenetic changes involving chromosomes 5 and 7; this was the case for one of the patients in our study. Overall, the use of RIT seems not to be associated with an increased risk of tMDS/tAML, higher than the reported 2–10% for conventional high-dose chemo-radiotherapy and ASCT.23 Longer follow-up is needed to draw definitive conclusions, and a special focus on those patients with a history of previous fludarabine treatment is warranted. Of note, a very recent study with RIT and ASCT as front-line treatment in mantle cell lymphoma reported a 20% incidence of secondary malignancies, unusually higher than expected.24
In summary, the use of RIT as part of the preparative regimen for ASCT in patients with chemorefractory DLBCL who have been exposed to rituximab is safe and may be associated with a high response rate. Randomized clinical trials with either conventional or high-dose RIT are necessary to definitively confirm the therapeutic benefit of this approach.
Footnotes
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;23(116):2040–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cunningham D, Hawkes EA, Jack A, Qian W, Smith P, Mouncey P, et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet. 2013;381(9880): 1817–26 [DOI] [PubMed] [Google Scholar]
- 3.Schmitz N, Nickelsen M, Ziepert M, Haenel M, Borchmann P, Schmidt C, et al. Conventional chemotherapy (CHOEP-14) with rituximab or high-dose chemotherapy (MegaCHOEP) with rituximab for young, high-risk patients with aggressive B-cell lymphoma: an open-label, randomised, phase 3 trial (DSHNHL 2002-1). Lancet Oncol. 2012;13(12):1250–9 [DOI] [PubMed] [Google Scholar]
- 4.Gisselbrecht C, Glass B, Mounier N, Singh Gill D, Linch DC, Trneny M, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28(27): 4184–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vose JM, Zhang MJ, Rowlings PA, Lazarus HM, Bolwell BJ, Freytes CO, et al. Autologous transplantation for diffuse aggressive non-Hodgkin’s lymphoma in patients never achieving remission: a report from the Autologous Blood and Marrow Transplant Registry. J Clin Oncol. 2001;19 (2):406–13 [DOI] [PubMed] [Google Scholar]
- 6.Caballero MD, Pérez-Simón JA, Iriondo A, Lahuerta JJ, Sierra J, Marín J, et al. High-dose therapy in diffuse large cell lymphoma: results and prognostic factors in 452 patients from the GEL-TAMO Spanish Cooperative Group. Ann Oncol. 2003; 14(1):140–51 [DOI] [PubMed] [Google Scholar]
- 7.Shimoni A, Nagler A. Radioimmunotherapy and stem-cell transplantation in the treatment of aggressive B-cell lymphoma. Leuk Lymphoma. 2007; 48(11):2110–20 [DOI] [PubMed] [Google Scholar]
- 8.Witzig TE, White CA, Gordon LI, Wiseman GA, Emmanouilides C, Murray JL, et al. Safety of yttrium-90 ibritumomab tiuxetan radioimmunotherapy for relapsed low-grade, follicular, or transformed non- Hodgkin’s lymphoma. J Clin Oncol. 2003; 21 (7):1263–70 [DOI] [PubMed] [Google Scholar]
- 9.Krishnan A, Nademanee A, Fung HC, Raubitschek AA, Molina A, Yamauchi D, et al. Phase II trial of a transplantation regimen of yttrium-90 ibritumomab tiuxetan and high-dose chemotherapy in patients with non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26(1):90–5 [DOI] [PubMed] [Google Scholar]
- 10.Nademanee A, Forman S, Molina A, Fung H, Smith D, Dagis A, et al. A phase 1/2 trial of high-dose yttrium-90-ibritumomab tiuxetan in combination with high-dose etoposide and cyclophosphamide followed by autologous stem cell transplantation in patients with poor-risk or relapsed non-Hodgkin lymphoma. Blood. 2005;106(8): 2896–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimoni A, Avivi I, Rowe JM, Yeshurun M, Levi I, Or R, et al. A randomized study comparing yttrium-90 ibritumomab tiuxetan (Zevalin) and high-dose BEAM chemotherapy versus BEAM alone as the conditioning regimen before autologous stem cell transplantation in patients with aggressive lymphoma. Cancer. 2012;118 (19):4706–14 [DOI] [PubMed] [Google Scholar]
- 12.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–86 [DOI] [PubMed] [Google Scholar]
- 13.Mounier N, Canals C, Gisselbrecht C, Cornelissen J, Foa R, Conde E, et al. High-dose therapy and autologous stem cell transplantation in first relapse for diffuse large B cell lymphoma in the rituximab era: an analysis based on data from the European Blood and Marrow Transplantation Registry. Biol Blood Marrow Transplant. 2012;18(5):788–93 [DOI] [PubMed] [Google Scholar]
- 14.Kewalramani T, Zelenetz AD, Hedrick EE, Donnelly GB, Hunte S, Priovolos AC, et al. High-dose chemoradiotherapy and autologous stem cell transplantation for patients with primary refractory aggressive non-Hodgkin lymphoma: an intention-to-treat analysis. Blood. 2000;96(7):2399–404 [PubMed] [Google Scholar]
- 15.Derenzini E, Musuraca G, Fanti S, Stefoni V, Tani M, Alinari L, et al. Pretransplantation positron emission tomography scan is the main predictor of autologous stem cell transplantation outcome in aggressive B-cell non-Hodgkin lymphoma. Cancer. 2008;113(9):2496–503 [DOI] [PubMed] [Google Scholar]
- 16.Shimoni A, Zwas ST, Oksman Y, Hardan I, Shem-Tov N, Yerushalmi R, et al. Yttrium-90-ibritumomab tiuxetan (Zevalin) combined with high-dose BEAM chemotherapy and autologous stem cell transplantation for chemo-refractory aggressive non-Hodgkin’s lymphoma. Exp Hematol. 2007; 35(4):534–40 [DOI] [PubMed] [Google Scholar]
- 17.Krishnan A, Nademanee A, Fung HC, Raubitschek AA, Molina A, Yamauchi D, et al. Phase II trial of a transplantation regimen of yttrium-90 ibritumomab tiuxetan and high-dose chemotherapy in patients with non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26(1):90–5 [DOI] [PubMed] [Google Scholar]
- 18.Vose JM, Carter S, Burns LJ, Ayala E, Press OW, Moskowitz CH, et al. Phase III randomized study of rituximab/carmustine, etoposide, cytarabine, and melphalan (BEAM) compared with iodine-131 tositumomab/BEAM with autologous hematopoietic cell transplantation for relapsed diffuse large B-Cell lymphoma: results from the BMT CTN 0401 Trial. J Clin Oncol. 2013;31(13):1662–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witzig TE. Radioimmunotherapy with Yttrium-90-labelled ibritumomab tiuxetan (Zevalin) for B-cell non-Hodgkin’s lymphoma. In: Immunotherapy of Lymphoid Malignancies. Hillmen P, Witzig TE. eds. Clinical Publishing; (Oxford: ). 2005; 101–14 [Google Scholar]
- 20.Witzig TE, White CA, Wiseman GA, Gordon LI, Emmanouilides C, Raubitschek A, et al. Phase I/II trial of IDEC-Y2B8 radioimmunotherapy for treatment of relapsed or refractory CD20(+) B-cell non-Hodgkin’s lymphoma. J Clin Oncol. 1999;17(12):3793–803 [DOI] [PubMed] [Google Scholar]
- 21.Winter JN, Inwards DJ, Spies S, Wiseman G, Patton D, Erwin W, et al. Yttrium-90 ibritumomab tiuxetan doses calculated to deliver up to 15 Gy to critical organs may be safely combined with high-dose BEAM and autologous transplantation in relapsed or refractory B-cell non-Hodgkin’s lymphoma. J Clin Oncol. 2009; 27(10):1653–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Czuczman MS, Emmanouilides C, Darif M, Witzig TE, Gordon LI, Revell S, et al. Treatment-related myelodysplastic syndrome and acute myelogenous leukemia in patients treated with ibritumomab tiuxetan radioimmunotherapy. J Clin Oncol. 2007; 25(27):4285–92 [DOI] [PubMed] [Google Scholar]
- 23.Armitage JO, Carbone PP, Connors JM, Levine A, Bennett JM, Kroll S. Treatment-related myelodysplasia and acute leukemia in non-Hodgkin’s lymphoma patients. J Clin Oncol. 2003;21(5):897–906 [DOI] [PubMed] [Google Scholar]
- 24.Arranz R, Garcia-Noblejas A, Grande C, Cannata-Ortiz J, Sanchez J, Garcia-Marco JA, et al. First line treatment with rituximab-Hyper-CVAD alternating with rituximab-methotrexate-cytarabine and followed by consolidation with 90Y-ibritumomab-tiuxetan in patients with mantle cell lymphoma. Results of a phase 2 pilot multicenter trial from the GELTAMO group. Haematologica. 2013;98(10):1563–70 [DOI] [PMC free article] [PubMed] [Google Scholar]


