Abstract
The treatment of advanced stage primary cutaneous T-cell lymphomas remains challenging. In particular, large-cell transformation of mycosis fungoides is associated with a median overall survival of two years for all stages taken together. Little is known regarding allogeneic hematopoietic stem cell transplantation in this context. We performed a multicenter retrospective analysis of 37 cases of advanced stage primary cutaneous T-cell lymphomas treated with allogeneic stem cell transplantation, including 20 (54%) transformed mycosis fungoides. Twenty-four patients (65%) had stage IV disease (for mycosis fungoides and Sézary syndrome) or disseminated nodal or visceral involvement (for non-epidermotropic primary cutaneous T-cell lymphomas). After a median follow up of 29 months, 19 patients experienced a relapse, leading to a 2-year cumulative incidence of relapse of 56% (95%CI: 0.38–0.74). Estimated 2-year overall survival was 57% (95%CI: 0.41–0.77) and progression-free survival 31% (95%CI: 0.19–0.53). Six of 19 patients with a post-transplant relapse achieved a subsequent complete remission after salvage therapy, with a median duration of 41 months. A weak residual tumor burden before transplantation was associated with increased progression-free survival (HR=0.3, 95%CI: 0.1–0.8; P=0.01). The use of antithymocyte globulin significantly reduced progression-free survival (HR=2.9, 95%CI: 1.3–6.2; P=0.01) but also transplant-related mortality (HR=10−7, 95%CI: 4.10−8–2.10−7; P<0.001) in univariate analysis. In multivariate analysis, the use of antithymocyte globulin was the only factor significantly associated with decreased progression-free survival (P=0.04). Allogeneic stem cell transplantation should be considered in advanced stage primary cutaneous T-cell lymphomas, including transformed mycosis fungoides.
Introduction
Primary cutaneous T-cell lymphomas are a group of peripheral T-cell lymphomas that primarily involve the skin and often have different clinical behavior and prognosis from histologically similar systemic lymphomas. Epidermotropic T-cell lymphomas, i.e. mycosis fungoides and its leukemic variant, Sézary syndrome, are the most frequent subtypes of primary cutaneous T-cell lymphomas.1 Clinical management of epidermotropic T-cell lymphomas is primarily dependent on their clinical presentation and disease stage2 that strongly correlate with overall prognosis.3 Large-cell transformation of mycosis fungoides is a rare event that represents a turning point in the history of the disease and is associated with a very poor prognosis.4,5 Advanced stage mycosis fungoides, and especially transformed mycosis fungoides, usually show early relapses after chemotherapy, or even after autologous hematopoietic stem cell transplantation (HSCT),6 and prolonged complete remissions rarely occur.5 Primary cutaneous CD30-positive anaplastic large cell lymphoma is a less frequent subtype of primary cutaneous T-cell lymphoma that, in its disseminated form, shares an aggressive clinical course.7 Allogeneic HSCT has been reported in advanced mycosis fungoides and Sézary syndrome in retrospective cases or case series,8–13 the 2 largest including 1910 and 6011 patients. Allogeneic HSCT for transformed mycosis fungoides has only been reported in case reports (n=11).8–10,13,14 Even less is known regarding allogeneic HSCT in other less frequent subtypes of advanced primary cutaneous T-cell lymphomas (γδ cutaneous T-cell lymphoma, n=3;13,15 subcutaneous panniculitic T-cell lymphoma, n=2;16,17 CD30-negative primary cutaneous T-cell lymphoma, n=1;18 primary cutaneous follicular helper T-cell lymphoma, n=119). We report on 37 cases of allogeneic HSCT for advanced stage, relapsed or refractory primary cutaneous T-cell lymphomas, including 20 cases of transformed mycosis fungoides.
Methods
Patients
Data collection and inclusion criteria
This study was approved by the scientific board of the Société Française de Greffes de Moelle et Thérapie Cellulaire (SFGM-TC), and was performed according to institutional guidelines in agreement with the principles of the Declaration of Helsinki.
We retrieved all patients with diagnosis of primary cutaneous T-cell lymphoma as defined in the World Health Organization (WHO)-European Organisation for Research and Treatment of Cancer (EORTC) classification of cutaneous lymphomas1 who underwent allogeneic HSCT from the French national registry of the Société Française de Greffe de Moëlle et Thérapie Cellulaire from July 1st 2002 to February 7th 2013. Diagnostic criteria are detailed in the Online Supplementary Methods. Patients were included if they displayed advanced clinical stage, i.e. IIB or greater, for mycosis fungoides and Sézary syndrome, as defined by the revised International Society for Cutaneous Lymphomas (ISCL)-EORTC classification,2 and N2, N3 and/or M1 for primary cutaneous T-cell lymphomas other than mycosis fungoides and Sézary syndrome, as defined by the ISCL/EORTC classification.20 Written informed consent for data registration was obtained for each patient.
Base-line, pre-transplant and post-transplant assessments
Evaluations at baseline and at least monthly during the follow up included complete history, physical examination, and assessment of the body-surface area involved with patches, plaques, and tumors. A cytomorphological and immunophenotypical examination of the peripheral blood lymphocytes to identify circulating Sézary cells were performed in each case of epidermotropic T-cell lymphoma.21–23 At baseline (at the time of diagnosis, or of any large-cell transformation) and three months after allogeneic HSCT, all patients underwent a staging computed tomography or positron emission tomography/computed tomography scan, and any abnormal lymph node was characterized histologically by an excisional biopsy. Pre-transplant global disease response was defined by comparing the disease status immediately prior to HSCT to the disease status before the onset of the last systemic treatment line before HSCT. This global disease response was defined as complete response (CR), very good partial response (VGPR), partial response (PR), stable disease (SD), progressive disease (PD) according to the international criteria.24 Current status at last follow up was defined as the disease status at last follow up compared to the disease status just before allogeneic HSCT, and assessed with the same criteria (CR, PR, SD and PD).
End points and definitions
Outcome analysis focused on engraftment, transplant-related mortality, relapse or progression, progression-free survival (PFS), overall survival (OS), acute and chronic graft-versus-host disease (GVHD), and disease response at last follow up (CR, PR, SD or PD). Engraftment was defined as an absolute neutrophil count greater than 0.5×109/L for three consecutive days.
Statistical analysis
The database was closed for analysis in April 2013. Probabilities of PFS and OS were estimated from the time of HSCT using Kaplan-Meier estimates. The occurrences of engraftment, acute and chronic GVHD, transplant-related mortality, and progression were calculated using cumulative incidence estimates taking into consideration the competing events.25 Factors were tested for their association with progression, transplant-related mortality, PFS and OS by Cox regression uni- and multivariate analysis as detailed in the Online Supplementary Methods.
Results
Demographics, stage and prior therapies
Demographics and disease stage at diagnosis, prior therapies, and transplantation regimens are listed in Table 1. Thirty-one (84%) patients had mycosis fungoides or Sézary syndrome, including 20 (54%) with transformed mycosis fungoides. Half of the patients (n=18, 49%) had stage IV disease. In patients with transformed mycosis fungoides, large T lymphocytes were CD4-positive in all 20 cases and CD30-positive in 10 cases. Two patients with mycosis fungoides had a past history of associated primary systemic lymphoma (1 primary nodal diffuse large B-cell lymphoma, and 1 Hodgkin disease); both systemic lymphomas were in CR at the time of allogeneic HSCT. Five patients had primary cutaneous anaplastic large-cell lymphoma of which 3 were ALK (anaplastic lymphoma kinase)-1-negative and 1 was ALK-1 positive. ALK-1 immunophenotyping was not performed in the last case. Out of 6 patients with non-epidermotropic T-cell lymphoma, 4 had nodal involvement (N2 or N3) and 3 had visceral involvement (M1). Patients had received a median of 5 prior systemic therapy regimens (range 2–11 regimens). All patients with epidermotropic T-cell lymphomas had failed skin-directed therapies (i.e. steroids, nitrogen mustard, or phototherapy) and methotrexate, retinoids, interferon-alpha and/or monochemotherapy. Twenty-two patients (59%) had received combination chemotherapy regimens.
Table 1.
Patients’ and transplant characteristics (n=37).
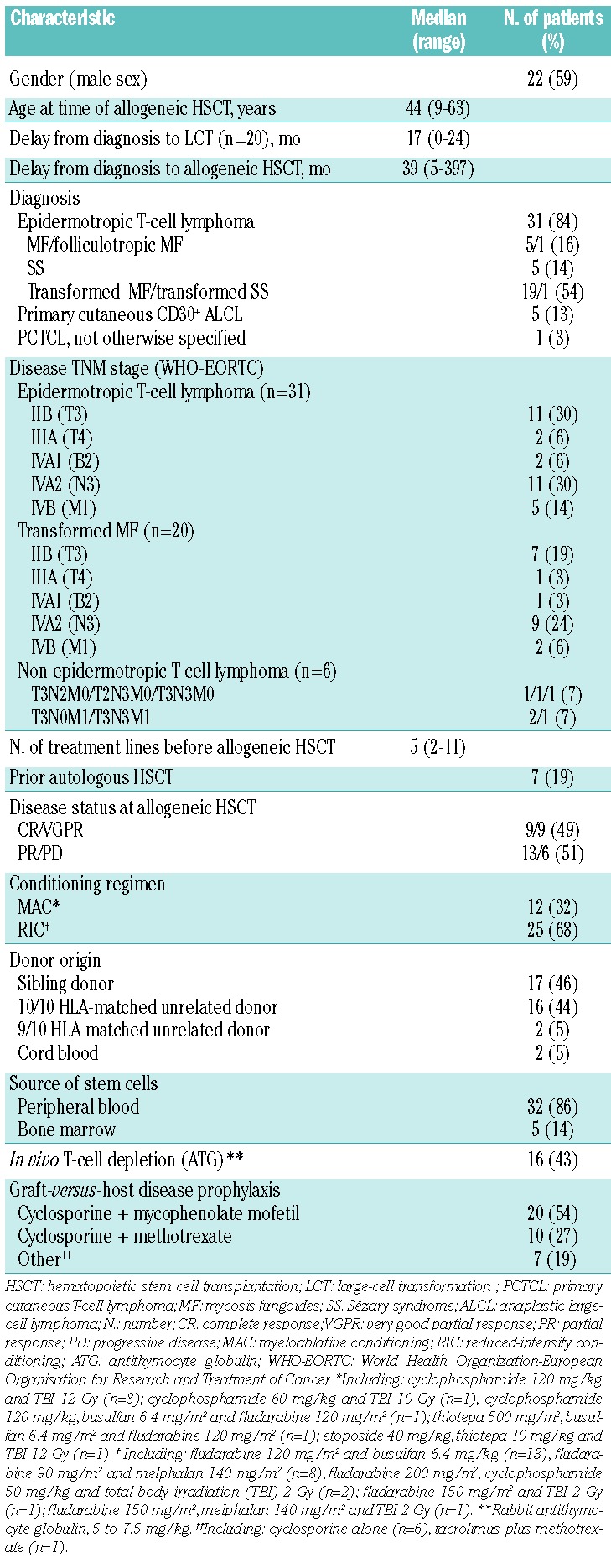
Donor selection, source of stem cells, conditioning regimens, and graft-versus-host disease prophylaxis
Class I HLA-A, HLA-B, and HLA-C loci and class II HLA-DRB1 and HLA-DQB1 (molecular typing) were performed in recipients and potential donors. Unrelated donors were selected with no more than 1 antigen mismatch (10 of 10 or 9 of 10 HLA match) except in cord blood transplants in which HLA-match was 4–6/6 (HLA-A, HLA-B, HLA-DRB1). Seventeen patients (46%) had sibling donors, 20 (54%) received allogeneic transplant from unrelated donors, of which 2 were cord blood units. Thirty-two patients (86%) received G-CSF-stimulated hematopoietic stem cells obtained from the peripheral blood via apheresis and 5 (14%) received bone marrow grafts. None of the hematopoietic products was depleted of T cells in vitro. Sixteen patients (43%) received in vivo T-cell depletion with rabbit antithymocyte globulin. The use of antithymocyte globulin was mainly dependent on the local protocol. It was not associated with any donor type: 7 of 17 (41%) patients had antithymocyte globulin among those who had a sibling donor versus 9 of 20 (45%) among those who had a matched unrelated donor (P=1, Fisher’s exact test). Neither was use of antithymocyte globulin associated with any conditioning regimen: 12 of 25 (48%) patients had antithymocyte globulin among those who received a reduced intensity conditioning versus 4 of 12 (33%) among those who received a myeloablative conditioning (P=0.49). Conditioning regimens, sources of stem cells and GVHD prophylaxis are summarized in Table 1. Total body irradiation used at 2 Gy (n=4) was well tolerated in all patients. Total body irradiation used at 10 or 12 Gy (n=10) was associated with local side-effects in 5 patients. Three patients with pre-transplant residual skin lesions developed erythema and erosive, painful lesions at the sites of mycosis fungoides lesions. In one case, a skin biopsy was performed that revealed superficial radionecrosis. In a fourth case, a dermohypodermitis occurred at the site of residual mycosis fungoides lesions (no microbiological documentation, resolution on empirical antibiotic and antifungal systemic treatments). In the last case, a superficial candidiasis was noted in the perineal and perioral areas.
Engraftment
After transplantation, the median time to engraftment was 17 days (range 12–30 days) and the 30-day cumulative incidence of engraftment was 91% (95%CI: 81–100%). Chimerism evaluated on peripheral blood cells at three months was available in 27 patients. Full donor chimerism was demonstrated in 23 patients. Three patients had mixed chimerism, and experienced disease relapse two, nine and 18 months after HSCT. One patient had autologous reconstitution with evidence of disease relapse three months after HSCT.
Graft-versus-host disease
Twenty-six (70%) patients developed biopsy-proven acute GVHD after a median time of 24 days (range 7–68) after allogeneic HSCT, of which 18 (49%) had grade 2 or higher acute GVHD. Twenty-two (59%) had acute cutaneous GVHD, among them 14 (38%) had stage 1 or 2, and 8 (22%) had stage 3 to 4. The 100-day cumulative incidence of acute GVHD was 76% (95%CI: 61–92%). Chronic GVHD developed in 15 patients, leading to a 2-year cumulative incidence of chronic GVHD of 44% (95%CI: 26–62%). Thirteen patients developed cutaneous chronic GVHD, of which 8 were extensive and 3 were sclerodermiform. The conditioning regimen, the use of antithymocyte globulin and the donor origin had no statistically significant impact on acute and chronic GVHD in univariate analysis.
Transplant-related mortality
Six patients (16%) died from transplant-related mortality, leading to cumulative incidences of 18% (95%CI: 5–31%) at one and two years. The cumulative incidence of transplant-related mortality was 22% (95%CI: 2–42%) in patients with transformed mycosis fungoides (n=20). Two had thrombotic microangiopathy, 1 unspecified pneumonia and vascular stroke, 1 invasive fungal infection, 2 disseminated adenovirus infection. The use of antithymocyte globulin as in vivo T-cell depletion was the only factor significantly associated with a decreased transplant-related mortality in univariate analysis (HR=1.10–7, 95%CI: 4.10-8-2.10-7; P<0.001) (Table 2 and Figure 1A). Indeed, no patient with in vivo T-cell depletion experienced transplant-related mortality. All patients with transplant-related mortality had acute GVHD, including 4 patients with grade 3 or 4 acute GVHD. The type of conditioning regimen (reduced intensity conditioning vs. myeloablative conditioning) had no significant impact on transplant-related mortality in univariate analysis. Of note, among 12 patients who received a myeloablative conditioning, only 1 died from transplant-related mortality. It must be noted that patients who received a myeloablative conditioning tended to be younger (mean age 36 years, range 17–56) than patients who had a reduced intensity conditioning (mean age 45 years, range 9–63; P=0.05). Patients who underwent a myeloablative conditioning had received a mean number of 4 (range 2–7) systemic treatments before transplant versus 5 (range 2–11) in patients who received a reduced intensity conditioning (P=0.22).
Table 2.
Overall outcomes, univariate (and *multivariate) analysis according to patients’ and transplantation characteristics.
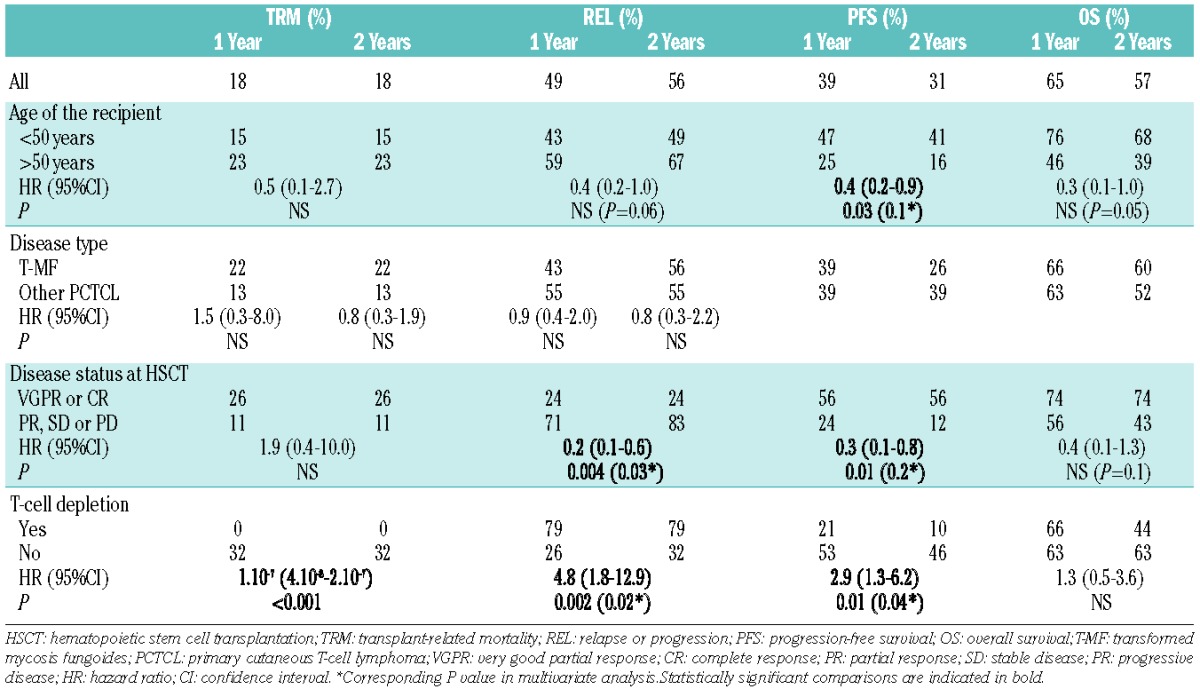
Figure 1.
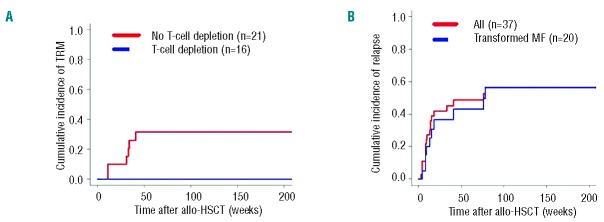
Cumulative incidence curves of transplant-related mortality (A) and relapse or progression (B) of 37 patients with advanced primary cutaneous T-cell lymphoma after allogeneic HSCT. (A) Incidence of transplant-related mortality in 37 patients with advanced primary cutaneous T-cell lymphoma, according to the use of T-cell depletion, yes versus no. (B) Incidence of relapse or progression in 37 patients with advanced primary cutaneous T-cell lymphoma, including 20 patients with transformed mycosis fungoides
Relapse or progression and treatment of disease relapse
Nineteen out of 37 patients (51%) experienced progression after transplantation: extracutaneous progression (n=4), including lymph node (n=3), blood tumor burden (n=1, patient with SS), isolated skin involvement progression (n=15) including localized skin disease (n=5), disseminated plaques or nodules (n=10). Among the 10 patients with transformed mycosis fungoides who experienced progression after HSCT, 5 patients relapsed with classical mycosis fungoides lesions and 5 patients relapsed with transformed mycosis fungoides lesions. In patients with post-transplant progression, median time to progression was ten weeks (range 3–78 weeks). In all cases, an attempt was made to reduce immunosuppressive drugs. Six patients achieved CR after: local radiotherapy (n=1), interferon and donor lymphocyte infusions (n=1), donor lymphocyte infusions (n=1), carmustine (n=1), bortezomib (n=1), bortezomib, donor lymphocyte infusions and local radiotherapy (n=1). Five patients achieved PR after gemcitabine and alemtuzumab (n=1), pentostatin and brentuximab vedotine (n=1), local radiotherapy (n=1), pegylated doxorubicin (n=1), donor lymphocyte infusions (n=1). Eight experienced disease-associated death. The cumulative incidence of progression was 49% (95%CI: 31–66%) at 1 year and 56% (95%CI: 38–74%) at 2 years, 43% (95%CI: 19–67%) at 1 year, and 56% (95%CI: 31–81%) in patients with transformed mycosis fungoides. The only factor associated with progression was the use of antithymocyte globulin (HR=4.8, 95%CI: 1.8–12.9; P=0.002) whereas the presence of pre-transplant CR or VGPR of the lymphoma disease (HR=0.3, 95%CI: 0.1–0.8; P=0.01) was associated with a decreased risk of progression in univariate analysis. Chronic GVHD (HR=0.6, 95%CI: 0.2–1.6; P=0.32) tended to be associated with a protective effect on the risk of progression in univariate analysis, although this did not reach statistical significance. The use of antithymocyte globulin and the pre-transplant disease status were still significantly associated with progression in multivariate analysis (Table 2 and Figure 1B).
Progression-free survival
Fourteen patients (38%) remain alive and progression-free since allogeneic HSCT. The estimated PFS was 39% (95%CI: 26–60%) at one year and 31% (95%CI: 19–53%) at two years in 37 patients, 39% (95%CI: 22–71%) at one year and 26% (95%CI: 12–60%) at two years in patients with transformed mycosis fungoides (n=20). The use of antithymocyte globulin as in vivo T-cell depletion (HR=2.9, 95%CI: 1.3–6.2; P=0.01) led to decreased PFS, whereas age of the recipient under 50 years (HR=0.4, 95%CI: 0.2–0.9; P=0.03) was associated with increased PFS. The existence of a VGPR or CR at HSCT was the most important prognostic factor for increased PFS in univariate analysis (HR=0.3, 95%CI: 0.1–0.8; P=0.01). In multivariate analysis, the use of antithymocyte globulin and the pre-transplant disease status were the only factors significantly influencing PFS (Table 2 and Figure 2A–C).
Figure 2.
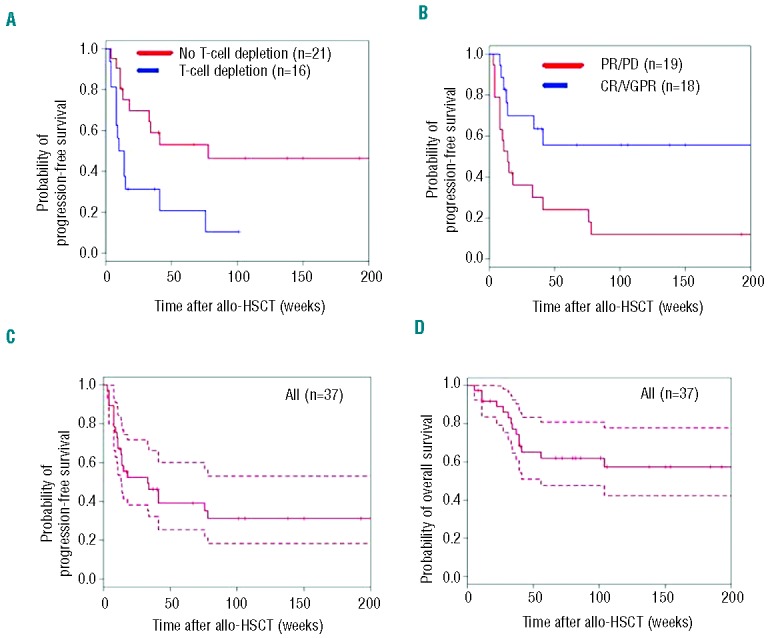
Kaplan-Meier plots of progression-free survival (A–C) and overall survival (D) of 37 patients with advanced primary cutaneous T-cell lymphoma after allogeneic HSCT. (A) Progression-free survival probabilities according to the use of T-cell depletion with antithymocyte globulin, yes versus no. (B) Progression-free survival probabilities according to the existence of a complete response (CR), very good partial response (VGPR), partial response (PR) or progressive disease (PD) of the lymphoma at time of allogeneic HSCT, CR/VGPR versus PR/PD. (C) Progression-free survival probability in all patients (plain lines, estimated probabilities of progression-free survival; dashed lines, 95% confidence intervals). (D) Overall survival probability in all patients (plain lines, estimated probabilities of overall survival; dashed lines, 95% confidence intervals).
Overall survival
After a median follow up for the surviving patients of 29 months (range 3–120 months), 23 patients were alive (62%) and 14 had died (38%), 8 from disease-associated death (22%) and 6 from transplant-related mortality (16%). The estimated OS rate was 65% (95%CI: 50–83%) and 57% (95%CI: 41–77%) at one and two years, respectively. In patients with transformed mycosis fungoides (n=20), the estimated OS probability was 66% (95%CI: 47–93%) at one year and 60% (95%CI: 41–88%) at two years. The disease type and the maximal stage before HSCT had no significant impact on OS in univariate analysis. Age of the recipient under 50 years tended to be associated with increased OS, but this did not reach statistical significance in univariate analysis (HR=0.3, 95%CI: 0.1–1; P=0.05) (Table 2 and Figure 2D).
Follow up after allogeneic HSCT
The median follow up after HSCT was 29 months (range 3–120 months). Out of 19 patients who experienced a relapse after allogeneic HSCT, 6 achieved subsequent CR. In all patients with disease relapse, a rapid decrease of immunosuppressive treatments was attempted. Five of the 6 patients with secondary CR after a first disease relapse did not experience a second relapse. They were all alive in CR at the last follow up, respectively 16 months after the disease relapse (after having received topical carmustine for the mycosis fungoides relapse), 33 months (after local radiotherapy), 34 months (after donor lymphocyte infusions), 4 years (after bortezomib, donor lymphocyte infusions and radiotherapy), and 4.5 years (after bortezomib). One of the patients with disease relapse and subsequent CR experienced a second disease relapse five years after the first one, and then a second CR; he was alive in CR at last follow up three years after the second relapse. Overall, 20 patients are alive and in CR at the last follow up. Of 2 patients who received cord blood transplants, one with erythrodermic mycosis fungoides (T4N1M0B0) died from disseminated adenovirus infection 33 weeks after transplant, and the other one with disseminated (T3N3M1) primary cutaneous anaplastic large-cell lymphoma is still alive with no evidence of disease relapse seven years after transplant.
Discussion
To the best of our knowledge, the present study represents the largest multicenter analysis of allogeneic HSCT for transformed mycosis fungoides. Most of the patients (54%) in this study had transformed mycosis fungoides and 24 (65%) had stage IV disease (for mycosis fungoides and Sézary syndrome) or disseminated nodal or visceral involvement (for other types of primary cutaneous T-cell lymphomas). Transformed mycosis fungoides, all stages taken together, is associated with a 2-year OS rate of 50%.5 Therefore, the estimated 2-year OS rate of 57% in our study confirms that allogeneic HSCT is suitable in advanced stage primary cutaneous T-cell lymphomas, including transformed mycosis fungoides. Nineteen patients (51%) experienced progression, leading to a 2-year cumulative incidence of progression of 56%. The relapse rate was higher than in the studies from Duvic et al. (39% of 19 patients experienced progression)10 and from Duarte et al. (3-year cumulative incidence of progression was 47%)11 which could be explained by a higher median follow up in our study than in the Duvic et al. study10 or by the fact that most of our patients had transformed mycosis fungoides, which is associated with a higher risk of relapse.5 In our study, most of the patients who relapsed had an isolated cutaneous progression (15 of 19 relapses) and half of the patients with transformed mycosis fungoides who relapsed had a non-transformed disease relapse. The median time to progression was ten weeks, and 90% of all relapses occurred within the first year after allogeneic HSCT. Six of the 19 patients who relapsed achieved a secondary CR after donor lymphocyte infusions or radio/chemotherapy, with a median duration of 41 months. These findings indicate that patients who underwent allogeneic HSCT for advanced primary cutaneous T-cell lymphomas can experience prolonged disease-free survival after a first disease progression. Overall, after a median follow up of 29 months, 20 patients were alive and in CR at the last follow up. In a recent clinical trial investigating pegylated doxorubicin in advanced stage refractory or recurrent mycosis fungoides, 72% of the patients had relapsed after a median follow up of 10.6 months.26 In our study, 51% of the patients had relapsed after a median follow up of 29 months. These findings could indicate that allogeneic HSCT leads to a delayed time to progression, by a possible graft-versus-malignancy effect. Consistent with this graft-versus-malignancy effect, the relapse rate was mostly and independently influenced by the residual tumor burden before HSCT and by the use of antithymocyte globulin as in vivo T-cell depletion. These findings are in line with the study from Duarte et al.11 who showed that refractory disease at allogeneic HSCT, and the use of alemtuzumab were independent prognostic factors of relapse. Therefore, the achievement of a complete or very good partial response just before transplant is critical for lowering the risk of post-transplant relapse. Should this not be the case, at least the use of antithymocyte globulin should be avoided in patients with no pre-transplant CR or VGPR.
We found high rates of acute cutaneous GVHD (59%). This could suggest antigenic stimulation by residual tumor cells in the skin. Regardless of the hematologic malignancy, GVHD is usually associated with prolonged PFS, providing evidence for a simultaneously present graft-versus-malignancy effect.27 As GVHD frequently occurs in the skin, the major tumor site of primary cutaneous T-cell lymphomas, this phenomenon may also contribute to the efficacy of allogeneic HSCT in these specific diseases. The 2-year cumulative incidence of transplant-related mortality of 18% could be regarded as lower than expected, since the patients were heavily pre-treated. We did not find any difference in transplant-related mortality associated with the use of a sibling versus unrelated donor in this study, which could either suggest that it lacks power, or that the use of a matched unrelated donor is a suitable option in allogeneic HSCT for advanced primary cutaneous T-cell lymphomas. Neither was the use of myeloablative conditioning associated with an increased transplant-related mortality. However, total body irradiation 10–12 Gy led to skin complications (infection or superficial radionecrosis) in some patients with pre-transplant residual skin lesions in the present study. As the use of reduced intensity conditioning (vs. myeloablative conditioning) has been associated with an increased OS in patients with advanced cutaneous T-cell lymphomas in a previous study,11 reduced intensity conditioning should probably be preferred. In this context, the use of total-skin electron beam therapy 1–2 months before transplant as described by Duvic et al.10 could be interesting to lower the pre-transplant disease burden while allowing sufficient time for skin healing before HSCT.
The 6 patients who experienced transplant-related mortality had not received antithymocyte globulin. The use of antithymocyte globulin was the only factor associated with a decreased transplant-related mortality, but also with a reduced PFS. As previously reported by Duarte et al.,11 the use of T-cell depletion tended to be associated with a decreased OS, although this was not statistically significant. T-cell depletion should probably be avoided in cases with high pre-transplant tumor burden, and limited to cases at high risk of GVHD.
Our work has limitations, among which a relatively small number of patients and heterogeneity in the presentations. The retrospective setting and the heterogeneity of the conditioning regimens and donor origins also preclude the generalization of our findings. However, owing to the extreme scarcity of these diseases, there are very few data regarding allogeneic HSCT in transformed mycosis fungoides.8–10,13,14 We are unaware of published data regarding allogeneic HSCT in advanced primary cutaneous anaplastic large-cell lymphomas, as this disease rarely leads to visceral involvement.7 Moreover, we found similar outcomes after allogeneic HSCT regardless of the subtype of primary cutaneous T-cell lymphoma, suggesting that our cohort was quite uniform in terms of prognosis.
In any case, our results indicate that allogeneic HSCT remains an interesting treatment option in advanced stage transformed mycosis fungoides and rare subtypes of primary cutaneous T-cell lymphomas. Prospective clinical trials will be the only way to confirm the efficacy of allogeneic HSCT in this setting, and to ascertain its optimal timing in the course of the disease.
Supplementary Material
Acknowledgments
The authors would like to thank Dr Raphaël Itzykson, Dr Marie Robin, Dr Aliénor Xhaard, Dr Flore Sicre de Fontbrune, Dr Mathieu Meunier (Greffes de Moëlle, Hôpital Saint-Louis, Paris), Prof Mauricette Michallet (Hématologie, CHU Lyon-Sud), Prof Dominique Bordessoule (Hématologie, CHU de Limoges), Dr Cécile Morice (Dermatologie, CHU de Caen), Dr Caroline Ram-Wolff (Dermatologie, Hôpital Saint-Louis, Paris), Dr Mathieu Wemeau (Hématologie, CHU de Lille), Dr Thomas Prebet (Hématologie, Institut Paoli Calmettes, Marseille), Dr Luc-Matthieu Fornecker (Hématologie, CHU de Strasbourg), Dr Jean-Valère Malfuson (Hématologie, Hôpital Percy), Nicole Raus, Sylvie Lengay, Malika Otmane-Cherif (data managers, SFGM-TC) for valuable assistance in the data collection; Prof Norbert Ifrah (Hématologie, CHU d’Angers), Prof Catherine Cordonnier (CHU Mondor), Prof Noël Milpied and members of the Hematology departments of the Hôpital Haut-Lévêque (Bordeaux), Hôpital Jean-Minjoz (Besançon), CHU de Montpellier, CHU Lyon-Sud, CHU de Caen, CHU Pontchaillou (Rennes), Hôpital Jean-Bernard (Poitiers), CHU de Liège and CHU de Rouen for taking care of the patients and providing clinical information, and Dr Raphaël Porcher (Université Paris VII, UMR-S717 et Service de Biostatistique et Informatique Médicale, Hôpital Saint-Louis, Paris) for his assistance in the statistical analysis.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105(10):3768–85 [DOI] [PubMed] [Google Scholar]
- 2.Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R, et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110(6):1713–22 [DOI] [PubMed] [Google Scholar]
- 3.Agar NS, Wedgeworth E, Crichton S, Mitchell TJ, Cox M, Ferreira S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28(31):4730–9 [DOI] [PubMed] [Google Scholar]
- 4.Vergier B, de Muret A, Beylot-Barry M, Vaillant L, Ekouevi D, Chene G, et al. Transformation of mycosis fungoides: clinicopathological and prognostic features of 45 cases. French Study Group of Cutaneious Lymphomas Blood. 2000;95 (7):2212–8 [PubMed] [Google Scholar]
- 5.Arulogun SO, Prince HM, Ng J, Lade S, Ryan GF, Blewitt O, et al. Long-term outcomes of patients with advanced-stage cutaneous T-cell lymphoma and large cell transformation. Blood. 2008;112(8):3082–7 [DOI] [PubMed] [Google Scholar]
- 6.Ingen-Housz-Oro S, Bachelez H, Verola O, Lebbé C, Marolleau JP, Hennequin C, et al. High-dose therapy and autologous stem cell transplantation in relapsing cutaneous lymphoma. Bone Marrow Transplant. 2004;33(6):629–34 [DOI] [PubMed] [Google Scholar]
- 7.Bekkenk MW, Geelen FA, van Voorst Vader PC, Heule F, Geerts ML, van Vloten WA, et al. Primary and secondary cutaneous CD30(+) lymphoproliferative disorders: a report from the Dutch Cutaneous Lymphoma Group on the long-term follow-up data of 219 patients and guidelines for diagnosis and treatment. Blood. 2000;95(12):3653–61 [PubMed] [Google Scholar]
- 8.Herbert KE, Spencer A, Grigg A, Ryan G, McCormack C, Prince HM. Graft-versus-lymphoma effect in refractory cutaneous T-cell lymphoma after reduced-intensity HLA-matched sibling allogeneic stem cell transplantation. Bone Marrow Transplant. 2004;34(6):521–5 [DOI] [PubMed] [Google Scholar]
- 9.Molina A, Zain J, Arber DA, Angelopolou M, O’Donnell M, Murata-Collins J, et al. Durable clinical, cytogenetic, and molecular remissions after allogeneic hematopoietic cell transplantation for refractory Sezary syndrome and mycosis fungoides. J Clin Oncol. 2005;23(25):6163–71 [DOI] [PubMed] [Google Scholar]
- 10.Duvic M, Donato M, Dabaja B, Richmond H, Singh L, Wei W, et al. Total skin electron beam and non-myeloablative allogeneic hematopoietic stem-cell transplantation in advanced mycosis fungoides and Sezary syndrome. J Clin Oncol. 2010;28(14):2365–72 [DOI] [PubMed] [Google Scholar]
- 11.Duarte RF, Canals C, Onida F, Gabriel IH, Arranz R, Arcese W, et al. Allogeneic hematopoietic cell transplantation for patients with mycosis fungoides and Sézary syndrome: a retrospective analysis of the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2010;28(29):4492–9 [DOI] [PubMed] [Google Scholar]
- 12.Duarte RF, Schmitz N, Servitje O, Sureda A. Haematopoietic stem cell transplantation for patients with primary cutaneous T-cell lymphoma. Bone Marrow Transplant. 2008;41(7):597–604 [DOI] [PubMed] [Google Scholar]
- 13.Paralkar VR, Nasta SD, Morrissey K, Smith J, Vassilev P, Martin ME, et al. Allogeneic hematopoietic SCT for primary cutaneous T cell lymphomas. Bone Marrow Transplant. 2012;47(7):940–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carrié E, Buzyn A, Fraitag S, Hermine O, Bodemer C. [Transformed juvenile-onset mycosis fungoides: treatment by bone marrow transplantation with graft-versus-lymphoma effect]. Ann Dermatol Venereol. 2007;134(5 Pt 1):471–6 [DOI] [PubMed] [Google Scholar]
- 15.Terras S, Moritz RKC, Ditschkowski M, Beelen DW, Altmeyer P, Stücker M, et al. Allogeneic Haematopoietic Stem Cell Transplantation in a Patient with Cutaneous γ/δ-T-cell Lymphoma. Acta Derm Venereol. 2013;93(3):360–1 [DOI] [PubMed] [Google Scholar]
- 16.Yuan L, Sun L, Bo J, Zhou Y, Li H, Yu L, et al. Durable remission in a patient with refractory subcutaneous panniculitis-like T-cell lymphoma relapse after allogeneic hematopoietic stem cell transplantation through withdrawal of cyclosporine. Ann Transplant. 2011;16(3):135–8 [DOI] [PubMed] [Google Scholar]
- 17.Pérez-Persona E, Mateos-Mazón JJ, López-Villar O, Arcos MJ, Encinas C, Graciani IF, et al. Complete remission of subcutaneous panniculitic T-cell lymphoma after allogeneic transplantation. Bone Marrow Transplant. 2006;38(12):821–2 [DOI] [PubMed] [Google Scholar]
- 18.Fijnheer R, Sanders CJG, Canninga MR, de Weger RA, Verdonck LF. Complete remission of a radiochemotherapy-resistant cutaneous T-cell lymphoma with allogeneic non-myeloablative stem cell transplantation. Bone Marrow Transplant. 2003;32(3):345–7 [DOI] [PubMed] [Google Scholar]
- 19.Ohmatsu H, Sugaya M, Fujita H, Kadono T, Sato S. Primary Cutaneous Follicular Helper T-cell Lymphoma Treated with Allogeneic Bone Marrow Transplantation: Immunohistochemical Comparison with Angioimmunoblastic T-cell Lymphoma. Acta Derm Venereol. 2013. June. 5 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 20.Kim YH, Willemze R, Pimpinelli N, Whittaker S, Olsen EA, Ranki A, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110(2):479–84 [DOI] [PubMed] [Google Scholar]
- 21.Poszepczynska-Guigné E, Schiavon V, D’Incan M, Echchakir H, Musette P, Ortonne N, et al. CD158k/KIR3DL2 is a new phenotypic marker of Sezary cells: relevance for the diagnosis and follow-up of Sezary syndrome. J Invest Dermatol. 2004; 122(3):820–3 [DOI] [PubMed] [Google Scholar]
- 22.Bouaziz J-D, Remtoula N, Bensussan A, Marie-Cardine A, Bagot M. Absolute CD3+ CD158k+ lymphocyte count is reliable and more sensitive than cytomorphology to evaluate blood tumour burden in Sézary syndrome. Br J Dermatol. 2010;162(1):123–8 [DOI] [PubMed] [Google Scholar]
- 23.Bensussan A, Remtoula N, Sivori S, Bagot M, Moretta A, Marie-Cardine A. Expression and function of the natural cytotoxicity receptor NKp46 on circulating malignant CD4+ T lymphocytes of Sézary syndrome patients. J Invest Dermatol. 2011;131(4):969–76 [DOI] [PubMed] [Google Scholar]
- 24.Olsen EA, Whittaker S, Kim YH, Duvic M, Prince HM, Lessin SR, et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29(18):2598–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007; 40(4):381–7 [DOI] [PubMed] [Google Scholar]
- 26.Dummer R, Quaglino P, Becker JC, Hasan B, Karrasch M, Whittaker S, et al. Prospective international multicenter phase II trial of intravenous pegylated liposomal doxorubicin monochemotherapy in patients with stage IIB, IVA, or IVB advanced mycosis fungoides: final results from EORTC 21012. J Clin Oncol. 2012;30 (33):4091–7 [DOI] [PubMed] [Google Scholar]
- 27.Storb R, Gyurkocza B, Storer BE, Sorror ML, Blume K, Niederwieser D, et al. Graft-versus-host disease and graft-versus-tumor effects after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2013;31(12): 1530–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


