Abstract
During embryonic development, smooth muscle within the ascending aorta arises from two distinct sources: second heart field progenitors and the neural crest. It has recently been hypothesized that the boundary between smooth muscle from these distinct origins may be particularly susceptible to acute aortic dissection. While the contribution of second heart field progenitors to the ascending aorta is well established, detailed mapping of the anatomical distribution of second heart field-derived smooth muscle at this smooth muscle boundary has yet to be observed using a committed cardiac progenitor Cre-lineage. Using Nkx2-5-Cre knockin mice, the anatomical distribution of second heart field derived aortic smooth muscle was mapped in detail. Specifically, Nkx2-5-Cre-labeled cells constitute the entirety of the smooth muscle layer at the aortic base and then become restricted to the adventitial side of the ascending aortic media. This distribution pattern is present by E12.5 in the embryo and persists throughout embryonic development. These data reveal previously unappreciated details regarding the anatomical distribution of second heart field-derived smooth muscle within the aorta as well as the non-cardiomyocyte fates labeled by the Nkx2-5-Cre lineage.
Keywords: Second Heart Field, Aortic Media, Smooth Muscle Boundary, Outflow Tract, Nkx2-5-Cre, Lineage Tracing
Introduction
During embryogenesis, aortic smooth muscle originates from four major developmental sources: cardiac mesoderm, neural crest, somatic mesoderm and splanchnic mesoderm (Majesky, 2007). It has been hypothesized that boundaries between smooth muscle from distinct embryonic origins represent areas of particular vulnerability to aortic dissection (Waldo et al., 2005; Majesky et al., 2011; Cheung et al., 2012). Within the ascending aorta, such a boundary occurs where second heart field (SHF)-derived smooth muscle (Waldo et al., 2005) meets neural crest cell (NCC)-derived smooth muscle (Le Lièvre and Le Douarin, 1975; Jiang et al., 2000) at the base of the aorta.
Studies with markers for committed cardiac progenitors including Mef2c, Nkx2-5, FGF10 and Isl1 have traced the contribution of the SHF to aortic smooth muscle, however, the majority of these studies do not visualize the anatomical border between SHF- and NCC-derived smooth muscle within the ascending aorta. Mef2c-AHF-Cre and Nkx2-5-Cre are established markers of the SHF. However, previous aortic fate-mapping investigations with these lineages do not show the distribution of SHF-derived aortic smooth muscle with significant anatomic detail (Verzi et al., 2005; Ma et al., 2008). The results of these studies do not resolve the boundaries of SHF-derived cells with those of the NCC or within the specific layers of the aorta. Isl1-Cre has shown the distribution of SHF-derived aortic smooth muscle in more detail (Sun et al., 2007), however, studies with this marker are complicated by the expression of Isl1 in a subset of NCCs, which results in the labeling of both mesoderm and ectoderm-derived smooth muscle at the base of the aorta (Engleka et al., 2012).
To date the most detailed visualization of the boundary between SHF and NCC-derived smooth muscle has been with Mesp1-Cre (Choudhary et al., 2009). Mesp1 is transiently expressed in the pre-cardiac mesoderm during early gastrulation (Saga et al., 1999). Mesp1-Cre-derived smooth muscle is visible on the adventitial side of the aorta within the same region that Wnt1-labeled NCC-derived smooth muscle appears on the luminal side of the ascending aorta. However, this complimentary, but distinct, smooth muscle pattern has not been visualized using a committed cardiac progenitor lineage and the initiation of the smooth muscle boundary at the aortic base has not been directly observed.
Here we use Nkx2-5-Cre to provide the first detailed genetic tracing of the anatomic distribution of SHF-derived smooth muscle using a committed cardiac progenitor lineage. Using specific markers for the smooth muscle compartment and different developmental time points, we highlight the distinct anatomical distribution of SHF-derived cells as they recede to the adventitial side of the media layer creating a vertical boundary with NCC-derived tissue in the ascending aorta and pulmonary trunk.
Results
Distribution of SHF derived outflow tract smooth muscle during embryogenesis
To determine the anatomical distribution of SHF derived smooth muscle in the embryonic outflow tract, lineage tracing was performed using Nkx2-5-Cre knockin mice (Nkx2-5Cre/+) (Moses et al., 2001) bred with Rosa26-YFP reporter mice (R26YFP/YFP). The smooth muscle marker smMHC is not robustly expressed before E15.5, while early markers such as smooth muscle actin (SMA) and sm22α are additionally expressed in immature cardiomyocytes and the endocardial cushions. Therefore, we used two markers to differentiate between these cell types, scoring sm22α+/cTnT+ cells as immature cardiomyocytes and sm22α+/cTnT− cells within the developing aorta and pulmonary trunk as embryonic smooth muscle.
First, lineage tracing was conducted prior to outflow tract septation at E10.5 (Figure 1A). While the Nkx2-5 lineage labeled endothelial cells within both the outflow tract and the aortic sac (arrowheads), there were minimal YFP+/sm22α+/cTnT− cells surrounding the aortic sac (arrow). Analysis of the post-septation outflow tract at E12.5 showed that Nkx2-5-Cre visualizes primordial SHF-derived smooth muscle in the aorta and pulmonary trunk, which comprises the majority of smooth muscle proximal to the heart (Figure 1B). Co-staining with cTnT distinguishes the myocardial wall from the outflow media. Additionally, we analyzed transverse sections through the developing E12.5 aorta and pulmonary trunk (Figure 1C, 1D). At the base of the aorta (1C, bottom) and pulmonary trunk (1D, bottom) extensive YFP expression is seen in the sm22α+/cTnT− smooth muscle layer. Midway to the aortic root and arch (Figure 1C, middle) as well as where the pulmonary trunk branches to the lungs (Figure 1D, middle) YFP is expressed in a significant amount of smooth muscle, but markedly absent from the luminal side of the medial layer. Finally, within the distal ascending aorta (Figure 1C, top) and the ductus arteriosus (Figure 1D, top) YFP was absent from the medial layer. We observed YFP+ endothelial cells within the aorta and pulmonary trunk throughout the transverse levels we examined (1C & 1D, arrowheads). These data show that the unique pattern of SHF-derived smooth muscle receding to the adventitial side of the media, previously described in adult tissue (Choudhary et al., 2009), is established by E12.5 and persists throughout embryonic development.
Figure 1. Distribution of SHF-derived smooth muscle in the embryonic outflow tracts.
Frontal and transverse sections of E10.5 and E12.5 Nkx2-5Cre/+; Rosa26YFP/+ embryos were stained for YFP (green), sm22α (red) and cTnT (white). Sm22α+/cTnT− cells within the embryonic media were scored as developing smooth muscle, while Sm22α+/cTnT+ cells represented immature cardiomyocytes. (A) At E10.5, few YFP+/sm22α+/cTnT+ cells are seen surrounding the aortic sac (arrow), while a significant number of YFP+ endothelial cells are present in the developing outflow tract and aortic sac (arrowheads). (B) At E12.5, YFP+/sm22α+/cTnT− smooth muscle cells are visible within the majority of the developing media and intima of the aorta and pulmonary trunk. (C, D) Staining of transverse sections of the aorta and pulmonary trunk. YFP+ smooth muscle is present throughout the smooth muscle layer within the aorta and pulmonary trunk proximal to the heart (bottom). Further from the heart YFP expression recedes to the adventitial side of the vessel (middle) before becoming absent (top). YFP+ endothelial cells are seen throughout each transverse section, including distal sections (top, arrowheads). Ao – Aorta; AS – Aortic Sac; BCA – Brachiocephalic Artery; LA – Left Atrium; LCCA – Left Common Carotid Artery; PT – Pulmonary Trunk; RA – Right Atrium. Scale bars equal 100µm.
The Nkx2-5-Cre lineage visualizes the boundary between SHF and NCC-derived smooth muscle in the post-natal aorta
Sections of neonatal Nkx2-5Cre/+; R26RYFP/+ hearts were stained with the mature smooth muscle-specific contractile protein Smooth Muscle Myosin Heavy Chain (smMHC) and counterstained for YFP. Similarly to what was observed embryonically, YFP+ cells constituted the majority of the smooth muscle compartment at the base of the aorta proximal to the heart (Figure 2A, bracket) and then becomes restricted to the adventitial side of the media before becoming absent within the ascending aorta. YFP expression was also seen in the majority of endothelial cells of the aortic intima (arrows) and covering the aortic valve. In addition to labeling the majority of ventricular cardiomyocytes, our results showed YFP expression in the adventitia as well as a subset of valve mesenchyme (arrowhead), which originates from labeled cushion endocardium (Ma et al., 2008; Nakano et al., 2013).
Figure 2. Distribution of Nkx2-5-Cre+ SHF-derived smooth muscle in the post-natal aorta.
Lineage labeling was performed using Nkx2-5-, Wnt1- and Mlc2v-Cre lines crossed to a Rosa26YFP/YFP reporter line. Frontal sections of P0 neonatal hearts were stained for YFP (green) and the smooth muscle compartment (smMHC, Red). (A) The Nkx2-5-Cre labeled SHF lineage contributes substantially to smooth muscle at the base of the aorta (bracket). (A, A’) YFP expression recedes to the adventitial side of the media and becomes absent before the aortic arch. YFP+ cells are observed within the intima (arrows), aortic valve mesenchyme (arrowhead) and the endothelial lining of the aortic valve. (B) The Wnt1-Cre labeled neural crest lineage comprises the majority of valve mesenchyme (arrowhead) and is present on the luminal side of the media before constituting the majority of smooth muscle within the ascending aorta (bracket). (C) Lineage analysis using the committed cardiac lineage Mlc2v-Cre did not result in the labeling of aortic smooth muscle or endothelial cells. Ao - Aorta; PT – Pulmonary Trunk; AoV – Aortic Valve. Scale bars equal 100µm.
For comparison, we conducted lineage tracing using Wnt1-Cre transgenic mice (Danielian et al., 1998) to mark the neural crest lineage. Consistent with previous reports (Jiang et al., 2000), Wnt1-Cre, R26RYFP/+ lineage tracing shows the majority of the smooth muscle layer within the ascending aorta is YFP+ (Figure 2B, bracket). YFP expression is also present on the luminal side of the aortic media within the same region where Nkx2-5-Cre+ SHF-derived smooth muscle is restricted to the adventitial side. This suggests a complimentary distribution of cells from these lineages within the media, that when combined, constitutes the entirety of smooth muscle in the ascending aortic. Committed cardiomyocyte lineages such as Myosin Light Chain 2v (Mlc2v)-Cre showed a complete lack of YFP expression in both smooth muscle and endothelial compartments (Figure 2C).
To look in greater detail at the ability of the Nkx2-5 lineage to label the complimentary distribution of SHF and neural crest-derived aortic smooth muscle, transverse aortic sections of neonatal Nkx2-5Cre/+, R26YFP/+ animals were stained for YFP and the smooth muscle marker sm22α (Figure 3A, solid lines). Representative transverse sections were analyzed at distances from the aortic annulus greater than 500µm (Figure 3B), between 200–500µm (Figure 3C) and within 200µm (Figure 3D) and quantified for YFP expression within the smooth muscle layer (Figure 3E). Taken together with Figure 2A, these data show in detail that while SHF and NCC-derived smooth muscle occupy distinct regions of the aortic media, they form a complimentary vertical boundary within the ascending aorta.
Figure 3. Nkx2-5 visualizes the boundary between SHF and neural crest-derived smooth muscle in the ascending aorta.
Sections of P0 neonatal Nkx2-5Cre/+; Rosa26YFP/+ aortae were analyzed at representative distances from the aortic annulus. (A) Frontal section of the aorta stained for YFP (green) and the smooth muscle marker sm22α (red). Solid lines denote the level and plane of stained transverse sections in B, C and D. (B, C, D) Transverse aortic sections from Nkx2-5Cre/+; Rosa26YFP/+ neonates. (B) At 340 µm distal from the aortic annulus, the majority of the smooth muscle does not express YFP. (C) At 195 µm from the annulus more than half of aortic smooth muscle is YFP+, but this expression is restricted to the adventitial side of the smooth muscle layer. (D) A transverse section at the base of the aorta, 86µm from the annulus, shows nearly the entire smooth muscle is YFP+. (E) Quantification of Nkx2-5+ progenitor contribution to aortic smooth muscle at representative transverse levels, excluding signal within the intima and adventitia. Error bars represent the standard deviation of three measurements. Scale bars equal 100µm.
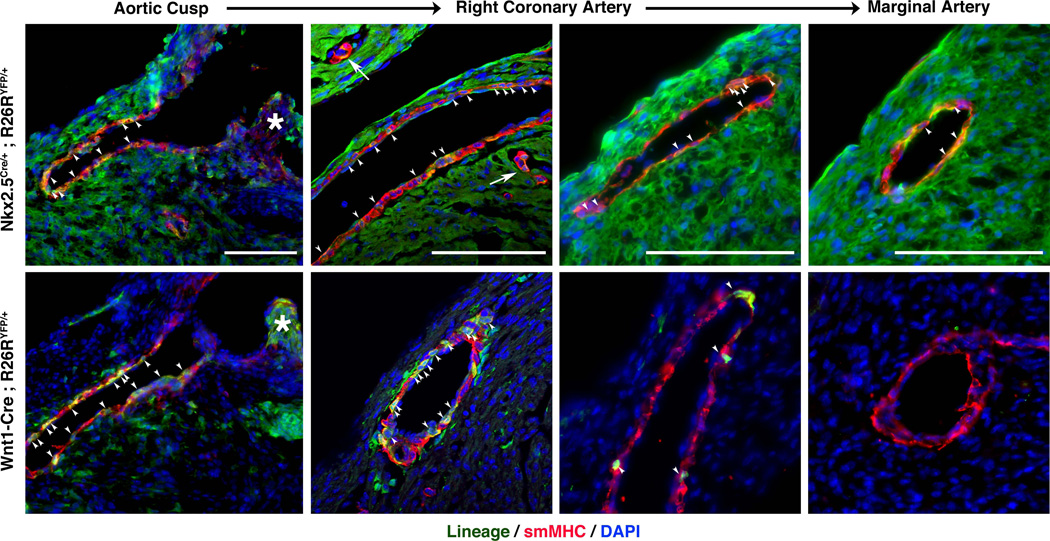
Nkx2-5-Cre visualizes coronary artery smooth muscle
In addition to examining the outflow tracts, we examined Nkx2-5-Cre labeling in epicardially-derived (Mikawa and Gourdie, 1996; Pérez-Pomares et al., 2002) coronary smooth muscle. Analysis of Nkx2-5Cre/+; Rosa26YFP/+ neonatal sections showed YFP expression in a mosaic pattern throughout the smooth muscle layer of the main coronary arteries (Figure 4, top, arrowheads) and small coronary branches (Figure 4, arrows). In addition, YFP was observed in a significant number of coronary endothelial cells. These results showed similar efficiency in visualizing Nkx2-5-Cre labeled coronary smooth muscle and endothelial cells as previous approaches (Ma et al., 2008), but without the necessity of a specialized reporter allele. Nkx2-5-Cre labeling was additionally compared to the neural crest lineage, which also contributes to coronary smooth muscle proximal to the aorta. As previously described (Jiang et al., 2000), NCC contribution is reduced within the descending coronary vessels and absent distal to the aorta (Fig. 4, bottom). Of note, Wnt1-Cre; Rosa26YFP/+ neonatal heart sections show mosaic YFP expression in the smooth muscle of the main coronary arteries, indicating that smooth muscle cells of this region are not exclusively derived from NCCs. The presence of Nkx2-5 and Wnt1-derived smooth muscle within the same region suggest that proximal to the aorta, coronary smooth muscle from different origins do not reside in mutually exclusive regions and do not form a clear boundary like within the aorta.
Figure 4. Nkx2-5-Cre lineage tracing in the coronary arteries.
Frontal sections of P0 neonatal Nkx2-5Cre/+; Rosa26YFP/+ and Wnt1-Cre; Rosa26YFP/+ hearts were stained for YFP (green), smMHC (red) and DAPI (blue). (Top) Nkx2-5-Cre labels a significant, but incomplete, portion of the coronary artery’s smooth muscle layer (arrowheads). Mosaic YFP expression is observed throughout the smooth muscle of the main right coronary artery from the arotic cusp (left) to within the right marginal artery (right), as well as in small coronary branches (arrows). YFP+ endothelial cells can also be observed luminal to the marked smooth muscle compartment. (Bottom) Lineage tracing using Wnt1-Cre labels a significant subset, but not all, of smooth muscle (arrowheads) within the coronary artery proximal to the aortic cusp (left two panels). YFP expression is limited within the main coronary artery (third panel) and is absent within the right marginal artery distal to the aorta (right). * - Aortic Valve, Scale Bars equal 100µm.
Discussion
Previous fate mapping studies using Nkx2-5-Cre knockin lines have reported limitations with standard Rosa26-LacZ reporter alleles, showing labeling that is restricted to a cardiomyocyte fate (Ma et al., 2008). Here we demonstrate sufficient sensitivity using the Rosa26-YFP reporter and antibody based fluorescent detection to visualize Nkx2-5-derived smooth muscle and endothelial cells in addition to cardiomyocytes.
Several studies have highlighted lineage-specific responses to extracellular stimuli from smooth muscle from different developmental origins (Topouzis and Majesky, 1996; Gadson et al., 1997; Cheung et al., 2012). These findings raise the hypothesis that an incoherent response from two different groups of adjacent smooth muscle may underlie pathologies associated with the aortic media such as dissection. As the creation of a false lumen during aortic dissection can involve splitting of the medial layer (Macura et al., 2003), a potential mechanism for the propagation of type 1 and type 2 dissection could be along the vertical seam between SHF and neural crest-derived smooth muscle (Figure 5).
Figure 5. Model of the second heart field-neural crest smooth muscle boundary.
Smooth muscle at the base of the aorta is derived from SHF progenitors. The SHF contribution to the media then forms a vertical seam complimentary with neural crest-derived smooth muscle before being replaced entirely within the ascending aorta. SHF-derived endothelial cells constitute the majority of the aortic intima within the base of the aorta and a portion of the ascending aorta, including the endothelial layer of the aortic valve. While the majority of aortic valve mesenchyme is derived from the neural crest, a subset of cells within this region come from a cardiac progenitor source.
One obstacle of directly testing these hypotheses is that aortic smooth muscle from different embryonic origins is histologically indistinguishable, requiring lineage based labeling to visualize smooth muscle borders. We use Nkx2-5-Cre lineage tracing to build on previous aortic fate-mapping studies, showing the distribution of SHF-derived smooth muscle within the embryonic and post-natal outflow tracts becomes restricted to the adventitial side forming a complimentary boundary with neural crest-derived smooth muscle. Use of a widely available cardiac progenitor lineage marker, such as Nkx2-5-Cre, within the genetic background of models of aortic dissection could be one approach for mapping dissection initiation in regards to the border of SHF-derived aortic smooth muscle.
Experimental Procedures
Mice and animal husbandry
This investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH Publication No. 85-23, revised 1996). All animal protocols, experiments, and housing were performed following Institutional Approval for Appropriate Care and use of Laboratory animals by the UCLA Institutional Animal Care and Use Committee (Chancellor's Animal Research Committee (ARC)), Animal Welfare assurance number A3196-01.
Cardiac and neural crest cell specific Cre recombinase lines were crossed to Rosa26YFP/YFP females as follows. The neural crest cell lineage was traced with using mice containing Cre under the control of Wnt1 enhancer elements (Wnt1-Cre, Rosa26YFP/+) (Danielian et al., 1998). The common cardiac / common second heart field progenitor lineage was traced with Cre knocked into one allele of the Nkx2-5 locus (Nkx2-5Cre/+, Rosa26YFP/+) (Moses et al., 2001). First heart field and anterior heart field derived cardiomyocytes were traced with Cre under the control of Mlc2v promoter elements (Mlc2v-Cre, Rosa26YFP/+) (Chen et al., 1998). Noon of the day a vaginal plug was detected was considered embryonic day (E) 0.5. The day when newly born pups were discovered was considered postnatal day (P) 0.
Histology and immunostaining
Embryonic dissections were performed in phosphate buffered saline supplemented with 1mM EDTA. The pericardial wall was removed and embryos E12.5 or older were beheaded before fixation. Embryos were fixed on ice in 4% paraformaldehyde for 1–2 hours depending on age. After washing, embryos were cryo-protected in 30% sucrose/PBS overnight. Embryos were transferred to a 1:1 mixture of 30% sucrose/PBS and OCT (Sakura, Torrance, CA) for 2 hours and then incubated in OCT for 1 hour and frozen in a bath of isopropanol and dry ice. Perinatal hearts were perfused with 4% PFA and removed with the assistance of a pin board. Embedding was then carried out similarly to embryonic dissections.
Immunofluorescent stainings were performed as follows: 8–10µm fixed frozen sections were blocked with 10% normal goat serum; 0.1% TritonX. Antibody reactions were carried out in 5% normal goat serum for one hour at room temperature or at 4 °C overnight. Fluorescent conjugated secondary antibody reactions were performed in 5% normal goat serum for one hour at room temperature. Slides were mounted with ProLong Gold DAPI media (Invitrogen). Alternatively, when primary antibodies raised in mice were used, staining was performed using M.O.M. reagents (Vector Labs) as described in the manufacturer’s protocol.
Antibodies used and their working concentrations are as follows: mouse anti-cTnT (1:200, Lab Vision Corp., Fremont, CA), rabbit anti-smMHC (1:200, Biomedical Technologies Inc., Stoughton, MA), rabbit anti-sm22α (1:200, Abcam, Cambridge, UK), chicken anti-GFP (1:1000, Aves Labs, Tigard, OR). For immunofluorescent detection the following Alexafluor conjugated secondary antibodies were used: goat anti-mouse-647, goat anti-chicken-488, goat anti-rabbit-594 (1:1000, Invitrogen, Grand Island, NY).
Image analysis
Immunofluorescent imaging was performed on a Zeiss AxioImager system using Axiovision release 4.8. Confocal microscopy was performed on a Zeiss Laser Scanning Microscope (LSM) 780 system using the 2010 release of Zen image acquisition software.
Key findings.
Nkx2-5-Cre labels the anatomical distribution of SHF-derived aortic smooth muscle in embryonic and post-natal tissue
In the ascending aorta, SHF-derived smooth muscle becomes restricted to the adventitial side of the media, complimentary to increasing NCC-derived smooth muscle on the luminal side
The distinct pattern of SHF-derived aortic smooth muscle is present before midgestation
Nkx2-5-Cre labels aortic and coronary smooth muscle without indirect use of specialized reporters
Acknowledgments
This manuscript is supported by the UCLA Broad Stem Cell Research Center, AHA (GRNT9420039) and NIH/NHLBI (R21HL109938) to AN. AWH is supported by the NIH (T32 GM007185). We thank the UCLA Broad Stem Cell Research Center for the use of their confocal microscope, Donna Crandall for the digital illustration of Figure 5 and Matthew Veldman, Xiaoqian Liu and Katrina Adams for feedback on the manuscript.
References
- Chen J, Kubalak SW, Chien KR. Ventricular muscle-restricted targeting of the RXRalpha gene reveals a non-cell-autonomous requirement in cardiac chamber morphogenesis. Development. 1998;125:1943–1949. doi: 10.1242/dev.125.10.1943. [DOI] [PubMed] [Google Scholar]
- Cheung C, Bernardo AS, Trotter MWB, Pedersen RA, Sinha S. Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility. Nat Biotechnol. 2012;30:165–173. doi: 10.1038/nbt.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary B, Zhou J, Li P, Thomas S, Kaartinen V, Sucov HM. Absence of TGFbeta signaling in embryonic vascular smooth muscle leads to reduced lysyl oxidase expression, impaired elastogenesis, and aneurysm. Genesis. 2009;47:115–121. doi: 10.1002/dvg.20466. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- Engleka KA, Manderfield LJ, Brust RD, Li L, Cohen A, Dymecki SM, Epstein JA. Islet1 derivatives in the heart are of both neural crest and second heart field origin. Circ Res. 2012;110:922–926. doi: 10.1161/CIRCRESAHA.112.266510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadson PF, Dalton ML, Patterson E, Svoboda DD, Hutchinson L, Schram D, Rosenquist TH. Differential response of mesoderm- and neural crest-derived smooth muscle to TGF-beta1: regulation of c-myb and alpha1 (I) procollagen genes. Exp Cell Res. 1997;230:169–180. doi: 10.1006/excr.1996.3398. [DOI] [PubMed] [Google Scholar]
- Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- Le Lièvre CS, Le Douarin NM. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. Journal of embryology and experimental morphology. 1975;34:125–154. [PubMed] [Google Scholar]
- Ma Q, Zhou B, Pu WT. Reassessment of Isl1 and Nkx2-5 cardiac fate maps using a Gata4-based reporter of Cre activity. Dev Biol. 2008;323:98–104. doi: 10.1016/j.ydbio.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macura KJ, Corl FM, Fishman EK, Bluemke DA. Pathogenesis in acute aortic syndromes: aortic dissection, intramural hematoma, and penetrating atherosclerotic aortic ulcer. American Journal of Roentgenology. 2003;181:309–316. doi: 10.2214/ajr.181.2.1810309. [DOI] [PubMed] [Google Scholar]
- Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol. 2007;27:1248–1258. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- Majesky MW, Dong XR, Hoglund VJ. Parsing aortic aneurysms: more surprises. Circ Res. 2011;108:528–530. doi: 10.1161/CIRCRESAHA.111.240861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikawa T, Gourdie RG. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev Biol. 1996;174:221–232. doi: 10.1006/dbio.1996.0068. [DOI] [PubMed] [Google Scholar]
- Moses KA, DeMayo F, Braun RM, Reecy JL, Schwartz RJ. Embryonic expression of an Nkx2-5/Cre gene using ROSA26 reporter mice. Genesis. 2001;31:176–180. doi: 10.1002/gene.10022. [DOI] [PubMed] [Google Scholar]
- Nakano H, Liu X, Arshi A, Nakashima Y, van Handel B, Sasidharan R, Harmon AW, Shin J-H, Schwartz RJ, Conway SJ, Harvey RP, Pashmforoush M, Mikkola HKA, Nakano A. Haemogenic endocardium contributes to transient definitive haematopoiesis. Nat Commun. 2013;4:1564. doi: 10.1038/ncomms2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Pomares JM, Carmona R, González-Iriarte M, Atencia G, Wessels A, Muñoz-Chápuli R. Origin of coronary endothelial cells from epicardial mesothelium in avian embryos. Int J Dev Biol. 2002;46:1005–1013. [PubMed] [Google Scholar]
- Saga Y, Miyagawa-Tomita S, Takagi A, Kitajima S, Miyazaki JI, Inoue T. MesP1 is expressed in the heart precursor cells and required for the formation of a single heart tube. Development. 1999;126:3437–3447. doi: 10.1242/dev.126.15.3437. [DOI] [PubMed] [Google Scholar]
- Sun Y, Liang X, Najafi N, Cass M, Lin L, Cai C-L, Chen J, Evans SM. Islet 1 is expressed in distinct cardiovascular lineages, including pacemaker and coronary vascular cells. Dev Biol. 2007;304:286–296. doi: 10.1016/j.ydbio.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topouzis S, Majesky MW. Smooth muscle lineage diversity in the chick embryo. Two types of aortic smooth muscle cell differ in growth and receptor-mediated transcriptional responses to transforming growth factor-beta. Dev Biol. 1996;178:430–445. doi: 10.1006/dbio.1996.0229. [DOI] [PubMed] [Google Scholar]
- Verzi MP, McCulley DJ, De Val S, Dodou E, Black BL. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev Biol. 2005;287:134–145. doi: 10.1016/j.ydbio.2005.08.041. [DOI] [PubMed] [Google Scholar]
- Waldo KL, Hutson MR, Ward CC, Zdanowicz M, Stadt HA, Kumiski D, Abu-Issa R, Kirby ML. Secondary heart field contributes myocardium and smooth muscle to the arterial pole of the developing heart. Dev Biol. 2005;281:78–90. doi: 10.1016/j.ydbio.2005.02.012. [DOI] [PubMed] [Google Scholar]