Abstract
Recent studies have shown that increased lymphocyte apoptosis contributes to sepsis-induced mortality. Furthermore, studies have demonstrated that IL-10 can suppress lymphocyte apoptosis, in part, by upregulating Bcl-2 expression and interfering with activation induced cell death. We have previously shown that intrathymic delivery of IL-10 with an adenoviral vector in wild-type mice significantly improves outcome to sepsis. Presently, we investigated the role of endogenous IL-10 expression on thymocyte apoptosis and outcome in IL-10 null mice subject to induction of generalized polymicrobial peritonitis via cecal ligation and puncture. Compared to wild-type C57BL/6 mice, IL-10 null mice demonstrated increased mortality and enhanced lymphocyte apoptosis. Intrathymic injection with an adenoviral vector expressing human IL-10 prior to cecal ligation and puncture in IL-10 null mice significantly improved outcome and decreased thymic caspase-3 activity. Furthermore, plasma concentrations of IL-6 were also significantly reduced in IL-10 null mice treated with the IL-10 expressing adenovirus. In contrast, injection of a control adenovirus did not improve outcome in IL-10 null mice, nor was caspase-3 activity reduced. Thus, local thymic expression of IL-10 not only improves outcome but also reduces local tissue apoptosis and caspase-3 activity, and appears to attenuate the systemic proinflammatory cytokine response.
Introduction
Sepsis remains a major cause of morbidity and mortality in intensive care units worldwide with its incidence increasing [1]. Despite various attempts to counterregulate the subsequent overproduction of proinflammatory cytokines with respective anti-cytokine therapies, patients suffering from sepsis have yet failed to benefit from these therapeutic strategies [2]. The initial excess of proinflammatory mediators is associated with increased production of anti-inflammatory cytokines, monocyte deactivation, and apoptosis of lymphocytes, dendritic and diverse parenchymal cells [3–5]. Prevention of this increased lymphocyte apoptosis by caspase or protease inhibitors, has been shown to improve outcome in animal models of sepsis (cecal ligation and puncture) [6, 7]. Furthermore, overexpression of the anti-apoptotic protein Bcl-2 has also been demonstrated to improve survival in septic mice [6].
Considering the multitude of biological properties of IL-10, it is not surprising that IL-10 therapies in experimental models of sepsis and septic patients have not been entirely successful. A number of investigations have suggested endogenous IL-10 production and systemic administration to rather exacerbate T-cell dysfunction, reduce anti-microbial function, and thus increase mortality in other less acute bacterial models of sepsis or thermal injury [8–12]. Previously, we were able to demonstrate potential benefits in targeted delivery of IL-10 to individual tissues [13, 14]. For example, thymic expression of human IL-10 by means of gene therapy lead to an upregulation of the anti-apoptotic Bcl-2 protein in this organ. Consequently, septic animals overexpressing human IL- 10 demonstrated a significant decrease in thymic lymphocyte apoptosis with improved outcome [14].
In this study we hypothesized that a lack of endogenous IL-10 would increase thymic apoptosis, and thus, increase sepsis associated mortality. Our aim was therefore to evaluate whether local expression of human IL-10 (hIL-10) in mice otherwise incapable of expressing IL-10 (mIL-10−/−) could correct the exaggerated mortality when given intrathymically.
In this report we demonstrate that local thymic hIL-10 expression in transgenic mice expressing a null form of mIL-10 reduces thymic apoptosis and improves overall survival in a murine model of sepsis.
Materials and methods
Mice
Specific pathogen-free female C57BL/6 mice and C57BL/6 animals deficient in mIL-10 expression (mIL-10−/−) were purchased from The Jackson7 Laboratory (Bar Harbor, ME, USA). Animals were maintained on standard rodent chow and water ad libitum. All experiments were performed on animals between the ages of five to eight weeks. The studies were approved by the Institutional Animal Care and Use Committee at the University of Florida, College of Medicine prior to initiation of experiments.
Murine model of polymicrobial sepsis (cecal ligation and puncture)
Surgery for induction of a polymicrobial sepsis (cecal ligation and puncture) was performed as previously described [15]. In brief, a laparotomy incision was performed in the abdominal midline, the cecum explored, isolated, ligated at one centimeter proximal to the tip and punctured through and through with a 21 gauge needle. Depending on the experimental aim, mice were either euthanized at 24 h after surgery or were observed for 10 days to determine outcome. Sham-treated mice received anesthesia and a laparotomy, but the cecum was neither ligated nor punctured.
Caspase-3 activity assay
Protein extracts were prepared by tissue homogenization, and caspase-3 activities determined by a fluorogenic assay (ApoFluor, Enzyme Systems Products, Livermore, CA, USA). Harvested organs were homogenized in 25 mm HEPES buffer (pH 7.5) containing 5 mm MgCl2, 1 mm EGTA, 1 mm phenylmethylsulphonyl fluoride (PMSF), 1 µg/ml leupeptin and aprotinin. Supernatants were collected after centrifugation at 50,000 g for 15 min. Protein concentrations in the supernatant were assayed using the Bio-Rad Protein Assay kit (Bio-Rad Laboratories, Hercules, CA, USA). Fifty micrograms of the extracted proteins were incubated with synthetic fluorescent substrates and benzylooxycarbonyl-Asp-Glu-Val-Asp-7-amino-4-trifluoromethylcoumarin (Z-DEVD-AFC) for the caspase-3 activity assay at a concentration of 30 mm in 0.1 M HEPES buffer (pH 7.4) containing 2 mm dithiothreitol, 0.1% CHAPS (3-[93-cholamidopropyl0 dimethylammonio]-1-propane-sulfonate) and 10% sucrose. The kinetics of the proteolytic cleavage of the substrates were monitored in a fluorescence microplate reader using an excitation wave length of 360 nm and an emission wave length of 535 nm. The fluorescence intensity was calibrated with a standard concentration of AFC; caspase activity was calculated from the slope of the recorded fluorescence and expressed as relative fluorescence intensity (RFI).
Intrathymic gene therapy
Following anesthesia, a 1–2 cm midline incision was performed from the angle of the mandible to the level of the fourth rib with a subsequent upper median sternotomy incision to display the thymus. Intrathymic injections of 20 µl volume were performed under direct visualization using a 50 µl Hamilton syringe and a 30-gauge needle. Mice received injections of either 105 particles of a recombinant adenovirus construct expressing human interleukin- 10 (Adv/hIL-10) or an identical recombinant adenovirus vector (E1a,E1b,E3 deleted) containing an empty cassette (Adv/empty) 24 h prior to inducing polymicrobial sepsis via cecal ligation and puncture (CLP), respectively.
Construction of a recombinant adenovirus expressing human IL-10
Human IL-10 (hIL-10) exhibits approximately 73% homology with murine IL-10 (mIL-10) and has proven its efficacy in past gene therapeutic experimental studies investigating diverse inflammatory conditions [14, 16, 17]. With respect to the potential transfer to a clinical therapeutic approach and recent progress in vector design and construction, we chose to apply hIL-10 with an adenoviral vector. Hence, a derivative of human adenovirus serotype 5 was used as the source of viral DNA backbone. The construct was deleted in early region 1, polypeptide IX and early region 3, as previously described [18]. Recombinant adenoviruses were constructed using standard homologous recombination methods as described by Graham and Prevec [19]. Expression of hIL-10 and gfp was driven using a CMV early promoter enhancer.
Cytokine measurements
On the basis of our past experience in determining cytokine concentrations for early prediction of clinical outcome with sepsis [20], interleukin- 6 (IL-6) was specified to serve as immunological marker of treatment efficacy. Therefore, plasma concentrations of murine IL-6 (mIL-6), as well as murine (mIL-10) and human IL-10 (hIL-10) were measured by specific ELISA using commercially available reagents (mouse IL-6 and human IL-10 by Endogen, Woburn, MA, USA; mouse IL-10 by R&D Systems, Minneapolis, MN, USA). The detection limit of the mIL-6 assay was 15 pg/ml and 5 pg/ml for mIL-10 and hIL-10, respectively.
Presentation of data and statistics
Results are presented as the mean and standard error of the mean (mean ± SEM). Differences between experimental groups were considered significant at P < 0.05 as determined by a one way ANOVA. Post hoc analyses were performed with Tukey’s multiple range test. Differences in the survival study were determined with Kaplan-Meier log-transformed survival analysis.
Results
Lack of endogenous IL-10 increases mortality
As IL-10 can protect septic mice from death, we wanted to confirm that mice lacking the IL-10 gene are more susceptible to sepsis-induced mortality. To initially verify that IL-10 null (mIL-10−/−) mice did not produce any murine IL-10, wild type C57BL/(B6) mice and mice deficient in murine IL-10 (mIL-10−/−) were subject to either sham laparotomy or induced polymicrobial peritonitis by cecal ligation and puncture (CLP) and sacrificed 24 h later (n = 8 animals per group). Respectively, plasma mIL-10 levels were below the assay sensitivity in both sham groups as well as in septic IL-10−/− animals. In contrast, plasma mIL-10 levels of septic wildtype animals showed expected increases of 7271 ± 3243 pg/ml.
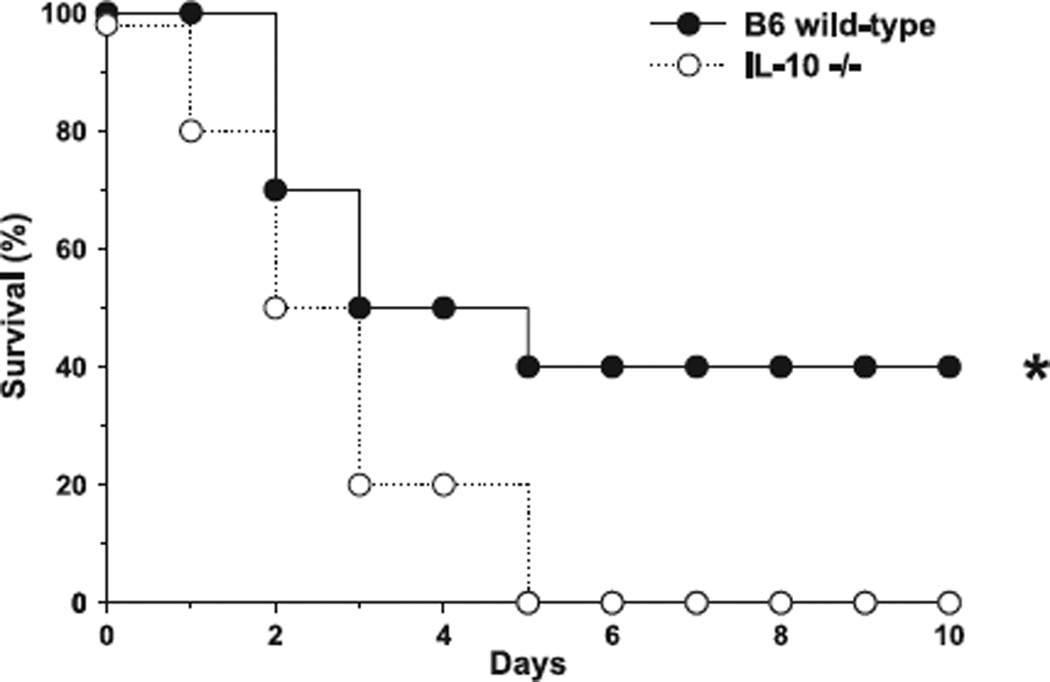
Furthermore, B6 and IL-10 null mice were observed throughout a period of 10 days to evaluate the overall clinical outcome in polymicrobial peritonitis (n = 20 animals per group). Although the mortality rate within the initial five days was distributed fairly equal among both groups, total mortality reached 100% after 10 days in the group of animals lacking the mIL-10 gene (Fig. 1).
Figure 1.
IL-10−/− mice show increased susceptibility to sepsis. Animals lacking the IL-10 gene (closed circles ●) challenged with CLP demonstrated 100% mortality after the 10-day observation period when compared to naïve wild-type C57BL6 controls (open circles ○).
Thymic apoptosis, caspase-3 activity and plasma concentrations of murine IL-6 (mIL-6) increase in the absence of IL-10 (mIL-10−/−)
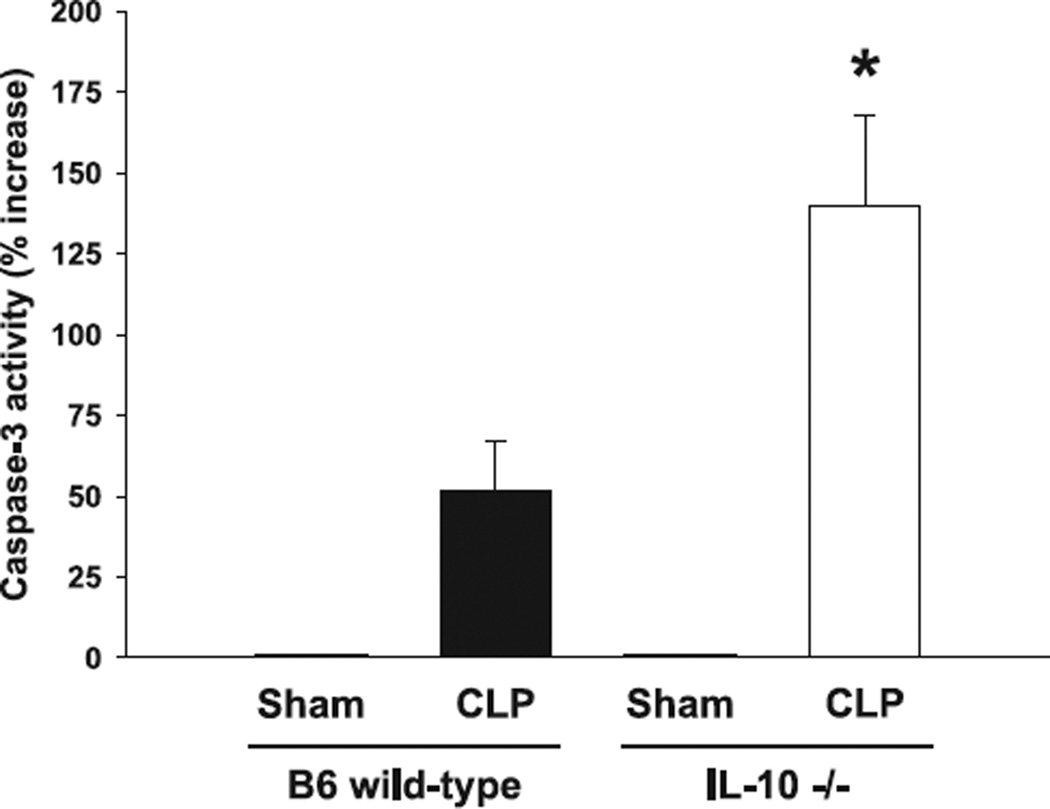
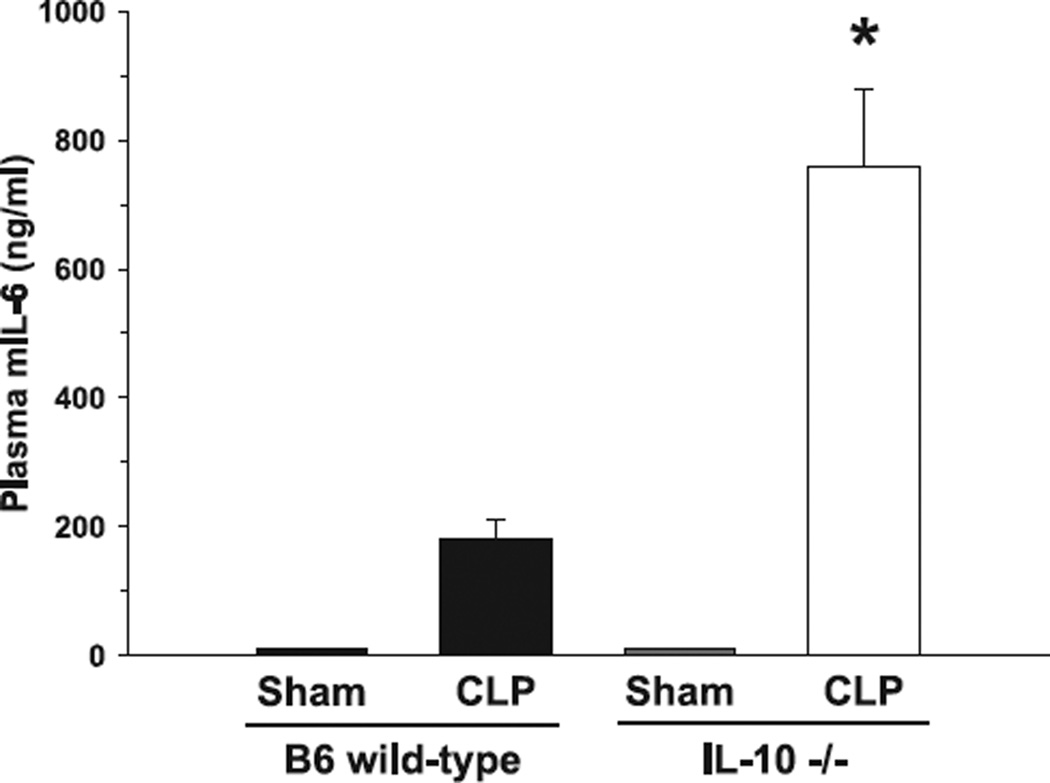
As lymphoid apoptosis is typically increased during sepsis, we hypothesized that IL-10−/− mice would deductively show an increase in thymic caspase-3 activity. Thus, analyses of caspase-3 activity 24 h after cecal ligation and pucture demonstrated an almost 3-fold increase in animals lacking the IL-10 gene compared to septic wild-type control animals (Fig. 2). In the analyses of proinflammatory mIL-6 plasma concentrations, C57BL6 wild-type mice subject to cecal ligation and puncture demonstrated significantly lower levels when compared to septic mIL-10−/− mice 24 h post-surgery (Fig. 3). Thymic caspase-3 activity was not detected in either of the sham groups, respectively.
Figure 2.
Caspase-3 activity is significantly increased in septic IL-10−/− mice. Caspase-3 activity in IL-10−/− animals was increased nearly threefold after CLP when compared to control wild-type litter mates. In SHAM animals, respective values were below the assay detection level. *P < 0.05 as determined with Student’s t-test (IL-10−/− versus control wild-type).
Figure 3.
Murine IL-6 (mIL-6) plasma concentrations in septic IL-10−/− mice exceed levels from septic wild-type controls. C57BL6 mice subject to CLP demonstrated significantly lower plasma concentrations of mIL-6 when compared to animals lacking the IL-10 gene (IL-10−/−). In Sham animals, respective values were below the assay detection level. *P < 0.05 as determined with Student’s t-test (IL-10−/− versus control wild-type).
Pretreatment with Adv/hIL-10 improves outcome of septic mIL-10−/− mice
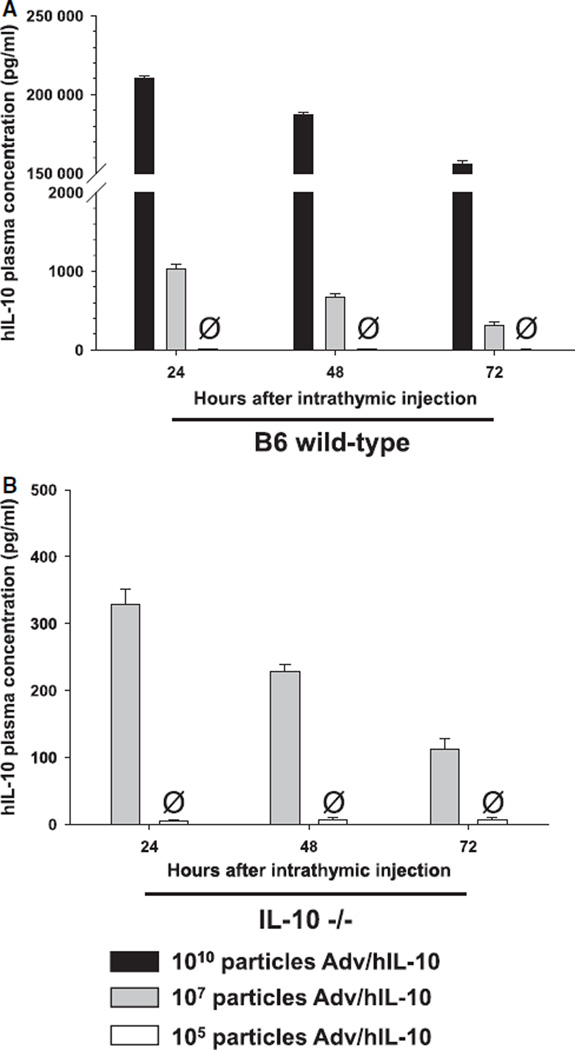
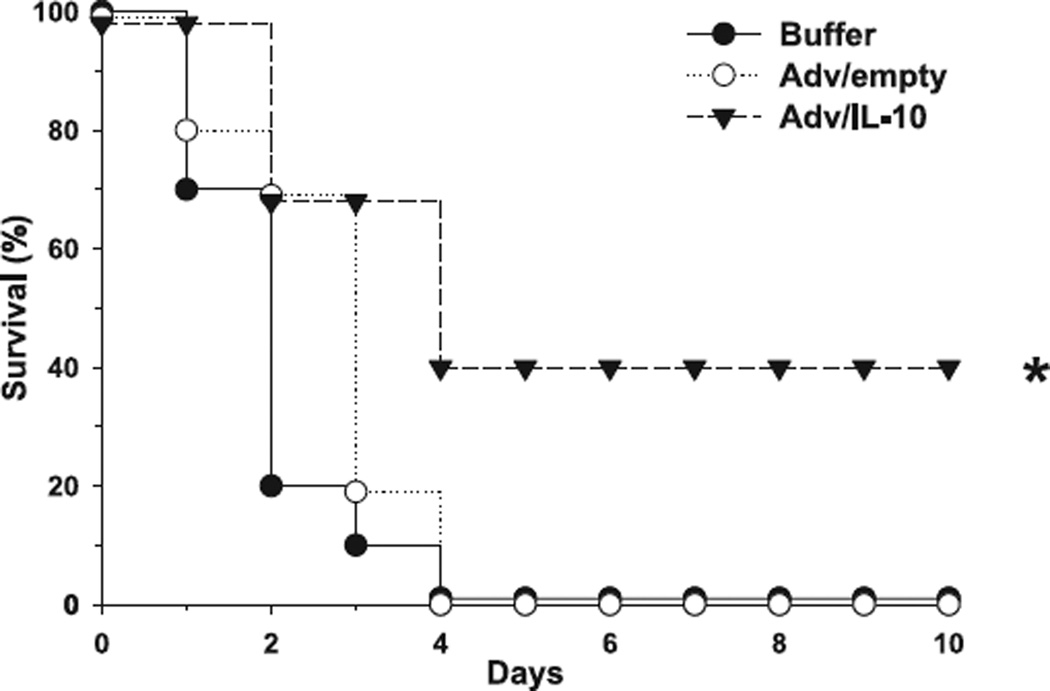
To assess the adequate particle dose of Adv/hIL-10 and minimize the probability of systemic hIL-10 transfer we performed titration experiments and analysed plasma concentrations of hIL-10 in both C57B6 wild-type and IL-10 null mice at 24, 48 and 72 h after intrathymic injection (Fig. 4). On the basis of these observations, we chose to treat animals 24 h prior to CLP with adenoviral recombinants at a dose of 105 particles. As confirmed earlier [14], intrathymic injection of adenovirus expressing human IL-10 at a dose of 105 particles did not result in systemic release of hIL-10. Respectively, mIL-10−/− mice were injected intrathymically with either adenovirus expressing human IL-10 (Adv/hIL-10), adenovirus with an empty cassette (Adv/empty) or virus buffer. 24 h after intrathymic injection, all animals underwent CLP and were observed for time-period of 10 days thereafter. The mortality rate of animals injected with the control (Adv/empty and buffer, respectively) was 100% by the end of the specified observation period, whereas 40% of the rodents pretreated with Adv/hIL-10 survived (P < 0.05) (Fig. 5).
Figure 4.
Plasma hIL-10 concentrations after intrathymic injections of Adv/hIL-10. Time and dose dependent decreases of systemic hIL-10 in A) C57BL6 wild-type and B) IL-10 null (IL-10−/−) mice after intrathymic injection of Adv/hIL-10.Ø Plasma concentrations of hIL-10 in animals treated with Adv/hIL-10 at a dose of 105 particles were below the detection level of the assay throughout all three time-points after injection.
Figure 5.
Therapeutic endogenous expression of human IL-10 in murine thymus increases survival in septic IL-10 knockout mice. IL-10−/− mice receiving the human IL-10 vector prior to CLP (▼) demonstrated a significant survival benefit compared to IL-10−/− mice pretreated with an empty vector (○) or buffer solution only (●). *P < 0.05 as determined by log-rank test in Kaplan–Meier survival analyses.
Pretreatment with Adv/hIL-10 reduces thymic apoptosis and caspase-3 activity in septic mIL-10−/− mice
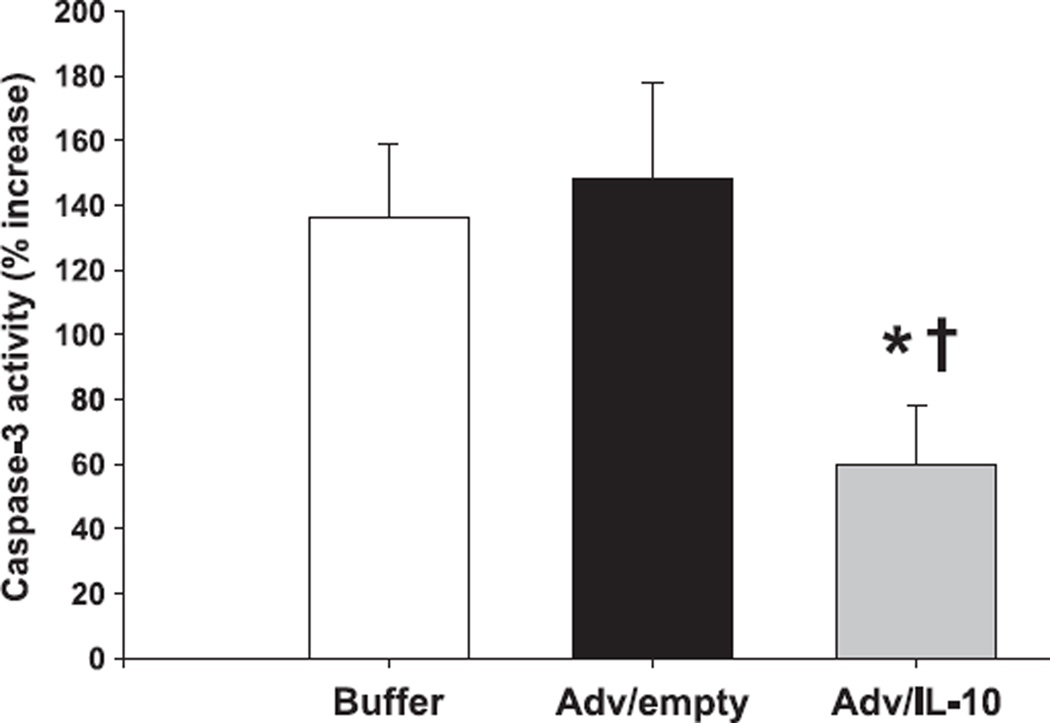
After observing an improved outcome in septic mIL-10−/− mice pretreated with the Adv/hIL-10 vector, it was of interest whether the divergence of mortality was in accordance with reductions of thymic apoptosis and the caspase-3 activity. Respectively, mIL-10−/− mice were pretreated 24 h prior to CLP with thymic instillations of either Adv/hIL-10, Adv/empty or virus buffer (as described above), and subsequently subjected to a CLP. Animals were sacrificed at day 3 (maximum divergence in mortality between Adv/hIL-10 treated animals and those receiving the empty cassette or buffer only, see Fig. 5) and the thymus harvested for further analyses. Consequently, thymic caspase-3-like activity in animals treated with the Adv/hIL-10 demonstrated significantly reduced activity levels when compared to both IL-10 null animals receiving either the empty vector (Adv/empty) or buffer only (P < 0.05) (Fig. 6).
Figure 6.
Caspase-3 activity is significantly reduced by therapeutic endogenous expression of human IL-10 in thymus of septic IL-10 knockout mice. Compared to animals pretreated with either an empty vector or buffer only, IL-10−/− mice receiving an intrathymic injection with Adv/hIL-10 prior to CLP demonstrated a significant reduction of thymic caspase-3 activity. *P < 0.05 as determined by Student’s t-test (Adv/hIL-10 versus Adv/empty). *P < 0.05 as determined by Student’s t-test (Adv/hIL-10 versus buffer).
Local hIL-10 expression decreases plasma mIL-6 in septic mIL-10−/− mice
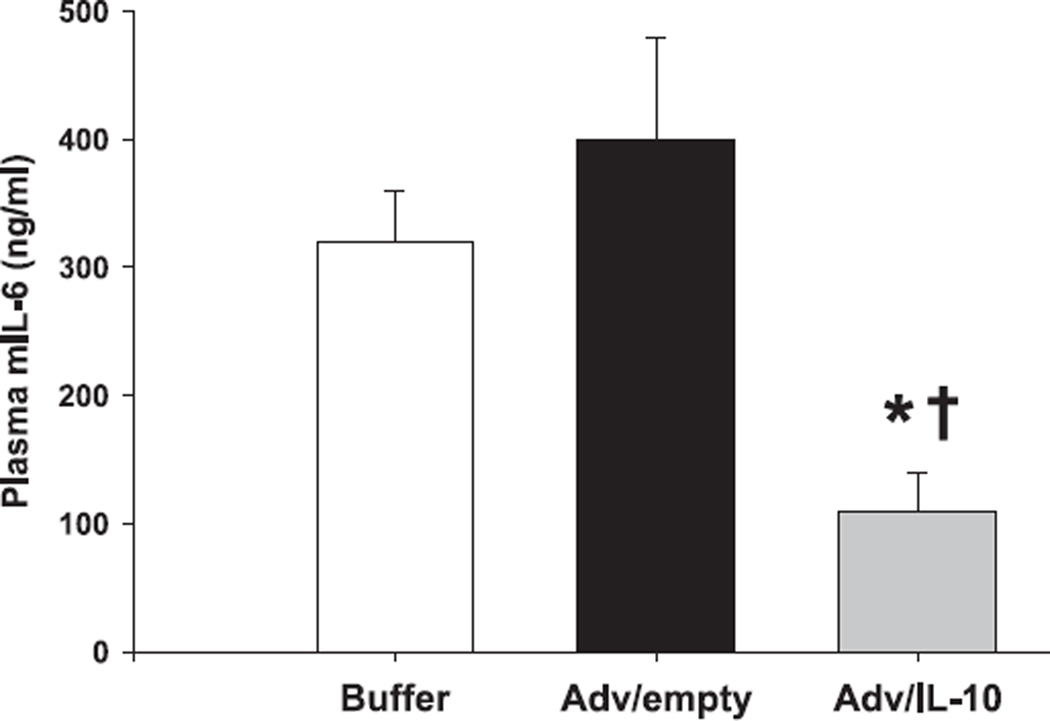
In addition to reducing thymic caspase-3 activity it was of further interest to evaluate the potential of endogenous thymic hIL-10 expression to influence systemic mIL-6 plasma concentrations. Hence, plasma mIL-6 concentrations in animals pretreated with the Adv/hIL-10 vector prior to CLP were significantly decreased by day 3 when compared to animals treated with the Adv/empty or buffer only (P < 0.05) (Fig. 7).
Figure 7.
Intrathymic expression of human IL-10 in septic IL-10 knockout mice reduces plasma concentrations of murine IL-6. IL-10−/− mice receiving intrathymic injections of Adv/hIL-10 prior to CLP showed significantly reduced plasma concentrations of mruine IL-6 when compared to knockout mice treated with the empty vector or buffer only. *P < 0.05 as determined by Student’s t-test (Adv/hIL-10 versus Adv/empty). †P < 0.05 as determined by Student’s t-test (Adv/hIL-10 versus buffer).
Discussion
Increased concentrations of systemically circulating pro-and anti-inflammatory mediators have been commonly acknowledged to closely correlate with the clinical course and final outcome in a variety of inflammation associated diseases [21, 22]. In this regard, targeted therapies to improve and benefit the host’s immune response have shown promising results in clinical trials with activated protein C [23] and experimental models of sepsis by early augmentation of anti-inflammatory immune functions [24]. More recently, studies investigating the influence of increased tissue specific concentrations of IL-10 have reported survival advantages in polymicrobial sepsis [13, 14], yet without coherent changes of local injury parameters, including gut epithelial apoptosis and intestinal permeability [25]. CD4+ T cell effector dysfunction has been frequently discussed as a key component within the pathophysiology of sepsis [26] and other inflammation-associated states of immunoparalysis [27]. Moreover, the majority of protective and immune stabilizing effects amount to IL-10 dependent mechanisms [28, 29]. In contrast, reduced IL-10 concentrations and its neutralization have shown to rather increase the host’s susceptibility to septic and endotoxic shock [30, 31]. Among the pluripotent regulatory properties of IL-10 are the mitigation of various proinflammatory mediators and suppression of NK cell activation via decreased IL-12 [12]. Furthermore, IL-10 has shown to protect from premature and injury-induced apoptosis by reconstituting specific immune effector cell functions, particularly T lymphocytes, and promoting proliferation of diverse parenchymal cells to maintain residential immune properties [13, 32–34]. With regard to the inefficient means of systemically administrating anti-inflammatory agents or cytokine inhibitors to target excessive production in individual tissue compartments, our study intended to evaluate the potential of local thymic endogenous IL-10 to regulate distant immune functions in complete absence of its natural production. Interestingly, IL-10−/− mice not only showed an increase in overall mortality after CLP, but also demonstrated concomitant increases of caspase-3 activity in thymus tissue along with an almost 4-fold increase in plasma IL-6 when compared to wild-type controls. Similar results were shown by Latifi and colleagues, who reported the effect of improving survival by delayed treatment with recombinant human IL-10 in mice undergoing cecal ligation and puncture [35]. In addition, IL-10 was postulated to play a critical role in regulating the transition of reversible sepsis to irreversible septic shock. In previous reports, we demonstrated that compartmental modulation of adenovirally transduced dendritic cells with human IL-10 positively effects outcome in the CLP model via local routes of T cell regulation [13]. Considering the suppressive effects of IL-10 on Th1 cell differentiation and activation, including T cells with regulatory functions (e.g. CD4+ CD25+ Treg cells) [36, 37] it was therefore interesting to observe a significant survival benefit in mIL-10−/− animals by gene therapeutic administration of human IL-10 to the site of T cell origin. Furthermore, pro-apoptotic molecules generated throughout the course of sepsis, along with injury-induced apoptosis of predominantly lymphoid tissues, are known to hallmark the development of organ dysfunction and subsequent failure. In a previous report, we were able to show a survival benefit by local inhibition of caspase-9 induced thymic apoptosis in septic mice [38]. These results further complement other studies demonstrating that inhibition of lymphocyte apoptosis shows efficacy in improving septic survival [7]. Yet, these rather tissue-specific observations remain debatable when considering IL-10’s two-edged role in acute inflammation as beneficial Th1 suppressor, or conversely, as Th2 promoter, eventually leading to a consecutive state of immunoparalysis. Furthermore, we demonstrated that targeted adenoviral-induced thymic expression of IL-10 resulted in a decrease in thymocyte apoptosis, apparent reduction of caspase-3 activity and an increase in Bcl-2 expression with the ultimate effect of improving outcome after CLP [14]. To our surprise, the effect of adenoviral transduction of human IL-10 into thymus of IL-10 deficient animals resulted in a similarly significant reduction of thymic caspase-3 activity and a survival benefit in comparison to IL-10 null mice without treatment. Moreover, although adenovirally transduced expression of hIL-10 was locally applied to the thymus and not detected in the circulation, it appeared to show a significant systemic antiinflammatory effect by decreasing plasma IL-6 levels. In complete absence of exogenous and circulating IL-10, this observation supposes a triggering mechanism by which initially activated local T cells may emerge from thymic tissue to restitute potentially impaired intercellular communication in distant locations. Although our investigations so far do not furnish definitive proof, these data suggest that IL-10 activated T cells in thymic tissue may thus be capable of reestablishing appropriate cellular immune responses by circulatory distribution. Moreover, locally activated and emerging T cells could omit the detrimental immunosuppressive Th2 type effect seen in continuous production or persistent circulation of exogenous IL-10. Although distant parenchymal tissue (e.g. liver, spleen and peripheral lymph nodes) and systemic immunocompetent cell types were not included in the analyses reported here, it may be hypothesized that local IL-10 gene therapy may have primed activation of cytotoxic T- and NK cells and subsequent immune regulatory properties (e.g. antigen presentation and phagocytosis by monocytes or dendritic cells) known to be of critical importance to the early phase of sepsis. Similar immune modulating effects with outcome improvement have recently been described for adenovirally transduced dendritic cells [17, 39].
In conclusion, our data provide further evidence that local expression of IL-10 significantly alters the overall immune response to sepsis towards a beneficial outcome. Moreover, therapeutically limited and locally restricted expression of IL-10 appears to prime adequate immune functions, even in the state of complete deficiency. Further investigations shall evaluate the mechanism by which transduced thymocytes affect these favorable systemic immunoregulatory functions in sepsis.
Acknowledgment
These studies were supported, in part, by R01 GM-63041, awarded by the National Institute of General Medical Sciences. SKT was supported, in part, by TS 174/1-1, awarded by the German Research Foundation (DFG).
Footnotes
Conflicts of interest
The authors declare that they have no financial, commercial or competing conflicts.
References
- 1.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 2.Dinarello CA. Anti-cytokine therapies in response to systemic infection. J Investig Dermatol Symp Proc. 2001;6:244–250. doi: 10.1046/j.0022-202x.2001.00046.x. [DOI] [PubMed] [Google Scholar]
- 3.Docke WD, Randow F, Syrbe U, et al. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med. 1997;3:678–681. doi: 10.1038/nm0697-678. [DOI] [PubMed] [Google Scholar]
- 4.Hotchkiss RS, Swanson PE, Freeman BD, et al. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Oberholzer C, Oberholzer A, Clare-Salzler M, Moldawer LL. Apoptosis in sepsis: a new target for therapeutic exploration. FASEB J. 2001;15:879–892. doi: 10.1096/fj.00-058rev. [DOI] [PubMed] [Google Scholar]
- 6.Hotchkiss RS, Swanson PE, Knudson CM, et al. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J Immunol. 1999;162:4148–4156. [PubMed] [Google Scholar]
- 7.Weaver JG, Rouse MS, Steckelberg JM, Badley AD. Improved survival in experimental sepsis with an orally administered inhibitor of apoptosis. FASEB J. 2004;18:1185–1191. doi: 10.1096/fj.03-1230com. [DOI] [PubMed] [Google Scholar]
- 8.Oberholzer A, Oberholzer C, Moldawer LL. Interleukin-10: a complex role in the pathogenesis of sepsis syndromes and its potential as an anti-inflammatory drug. Crit Care Med. 2002;30:S58–S63. [PubMed] [Google Scholar]
- 9.Berlato C, Cassatella MA, Kinjyo I, Gatto L, Yoshimura A, Bazzoni F. Involvement of suppressor of cytokine signaling-3 as a mediator of the inhibitory effects of IL-10 on lipopolysaccharide-induced macrophage activation. J Immunol. 2002;168:6404–6411. doi: 10.4049/jimmunol.168.12.6404. [DOI] [PubMed] [Google Scholar]
- 10.Kumar A, Zanotti S, Bunnell G, et al. Interleukin-10 blunts the human inflammatory response to lipopolysaccharide without affecting the cardiovascular response. Crit Care Med. 2005;33:331–340. doi: 10.1097/01.ccm.0000152229.69180.2. [DOI] [PubMed] [Google Scholar]
- 11.Lauw FN, Pajkrt D, Hack CE, Kurimoto M, van Deventer SJ, van der Poll T. Proinflammatory effects of IL-10 during human endotoxemia. J Immunol. 2000;165:2783–2789. doi: 10.4049/jimmunol.165.5.2783. [DOI] [PubMed] [Google Scholar]
- 12.Scott MJ, Hoth JJ, Turina M, Woods DR, Cheadle WG. Interleukin-10 suppresses natural killer cell but not natural killer T cell activation during bacterial infection. Cytokine. 2006;33:79–86. doi: 10.1016/j.cyto.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Oberholzer A, Oberholzer C, Bahjat KS, et al. Increased survival in sepsis by in vivo adenovirus-induced expression of IL-10 in dendritic cells. J Immunol. 2002;168:3412–3418. doi: 10.4049/jimmunol.168.7.3412. [DOI] [PubMed] [Google Scholar]
- 14.Oberholzer C, Oberholzer A, Bahjat FR, et al. Targeted adenovirusinduced expression of IL-10 decreases thymic apoptosis and improves survival in murine sepsis. Proc Natl Acad Sci USA. 2001;98:11503–11508. doi: 10.1073/pnas.181338198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker CC, Chaudry IH, Gaines HO, Baue AE. Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. Surgery. 1983;94:331–335. [PubMed] [Google Scholar]
- 16.Keravala A, Lechman ER, Nash J, Mi Z, Robbins PD. Human, viral or mutant human IL-10 expressed after local adenovirus-mediated gene transfer are equally effective in ameliorating disease pathology in a rabbit knee model of antigen-induced arthritis. Arthritis Res Ther. 2006;8:R91. doi: 10.1186/ar1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oberholzer A, Oberholzer C, Efron PA, et al. Functional modification of dendritic cells with recombinant adenovirus encoding interleukin 10 for the treatment of sepsis. Shock. 2005;23:507–515. [PubMed] [Google Scholar]
- 18.Minter RM, Rectenwald JE, Fukuzuka K, et al. TNF-alpha receptor signaling and IL-10 gene therapy regulate the innate and humoral immune responses to recombinant adenovirus in the lung. J Immunol. 2000;164:443–451. doi: 10.4049/jimmunol.164.1.443. [DOI] [PubMed] [Google Scholar]
- 19.Graham FL, Prevec L. Methods for construction of adenovirus vectors. Mol Biotechnol. 1995;3:207–220. doi: 10.1007/BF02789331. [DOI] [PubMed] [Google Scholar]
- 20.Oberholzer A, Souza SM, Tschoeke SK, et al. Plasma cytokine measurements augment prognostic scores as indicators of outcome in patients with severe sepsis. Shock. 2005;23:488–493. [PubMed] [Google Scholar]
- 21.Dimopoulou I, Orfanos S, Kotanidou A, et al. Plasma pro- and antiinflammatory cytokine levels and outcome prediction in unselected critically ill patients. Cytokine. 2008;41:263–267. doi: 10.1016/j.cyto.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 22.Ikonomidis I, Stamatelopoulos K, Lekakis J, Vamvakou GD, Kremastinos DT. Inflammatory and non-invasive vascular markers: the multimarker approach for risk stratification in coronary artery disease. Atherosclerosis. 2008;199:3–11. doi: 10.1016/j.atherosclerosis.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 23.Laterre PF. Clinical trials in severe sepsis with drotrecogin alfa (activated) Crit Care. 2007;11(Suppl. 5):S5. doi: 10.1186/cc6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mees ST, Dohm C, Broetzmann K, et al. Age- and gender-related differences of the immune function in a murine model of hemorrhagic shock: IL-10 restores immunodepression in aged females without reduction of mortality. Langenbecks Arch Surg. 2007;392:629–638. doi: 10.1007/s00423-007-0152-y. [DOI] [PubMed] [Google Scholar]
- 25.Rajan S, Vyas D, Clark AT, et al. Intestine-specific overexpression of IL-10 improves survival in polymicrobial sepsis. Shock. 2008;29:483–489. doi: 10.1097/shk.0b013e31815bbb26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scumpia PO, Delano MJ, Kelly-Scumpia KM, et al. Treatment with GITR agonistic antibody corrects adaptive immune dysfunction in sepsis. Blood. 2007;110:3673–3681. doi: 10.1182/blood-2007-04-087171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tschoeke SK, Ertel W. Immunoparalysis after multiple trauma. Injury. 2007;38:1346–1357. doi: 10.1016/j.injury.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 28.Buras JA, Holt D, Orlow D, Belikoff B, Pavlides S, Reenstra WR. Hyperbaric oxygen protects from sepsis mortality via an interleukin- 10-dependent mechanism. Crit Care Med. 2006;34:2624–2629. doi: 10.1097/01.CCM.0000239438.22758.E0. [DOI] [PubMed] [Google Scholar]
- 29.Tamion F, Richard V, Renet S, Thuillez C. Protective effects of heme-oxygenase expression against endotoxic shock: inhibition of tumor necrosis factor-alpha and augmentation of interleukin-10. J Trauma. 2006;61:1078–1084. doi: 10.1097/01.ta.0000239359.41464.ef. [DOI] [PubMed] [Google Scholar]
- 30.Gomes RN, Figueiredo RT, Bozza FA, et al. Increased susceptibility to septic and endotoxic shock in monocyte chemoattractant protein 1/cc chemokine ligand 2-deficient mice correlates with reduced interleukin 10 and enhanced macrophage migration inhibitory factor production. Shock. 2006;26:457–463. doi: 10.1097/01.shk.0000228801.56223.92. [DOI] [PubMed] [Google Scholar]
- 31.Murphey ED, Sherwood ER. Bacterial clearance and mortality are not improved by a combination of IL-10 neutralization and IFNgamma administration in a murine model of post-CLP immunosuppression. Shock. 2006;26:417–424. doi: 10.1097/01.shk.0000226343.70904.4f. [DOI] [PubMed] [Google Scholar]
- 32.Dinant S, Vetelainen RL, Florquin S, van Vliet AK, van Gulik TM. IL-10 attenuates hepatic I/R injury and promotes hepatocyte proliferation. J Surg Res. 2007;141:176–182. doi: 10.1016/j.jss.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 33.John T, Muller RD, Oberholzer A, et al. Interleukin-10 modulates pro-apoptotic effects of TNF-alpha in human articular chondrocytes in vitro. Cytokine. 2007;40:226–234. doi: 10.1016/j.cyto.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Werner T, Shkoda A, Haller D. Intestinal epithelial cell proteome in IL-10 deficient mice and IL-10 receptor reconstituted epithelial cells: impact on chronic inflammation. J Proteome Res. 2007;6:3691–3704. doi: 10.1021/pr070222x. [DOI] [PubMed] [Google Scholar]
- 35.Latifi SQ, O’Riordan MA, Levine AD. Interleukin-10 controls the onset of irreversible septic shock. Infect Immun. 2002;70:4441–4446. doi: 10.1128/IAI.70.8.4441-4446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marra LE, Zhang ZX, Joe B, et al. IL-10 induces regulatory T cell apoptosis by up-regulation of the membrane form of TNF-alpha. J Immunol. 2004;172:1028–1035. doi: 10.4049/jimmunol.172.2.1028. [DOI] [PubMed] [Google Scholar]
- 37.Roers A, Siewe L, Strittmatter E, et al. T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. J Exp Med. 2004;200:1289–1297. doi: 10.1084/jem.20041789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oberholzer C, Tschoeke SK, Moldawer LL, Oberholzer A. Local thymic caspase-9 inhibition improves survival during polymicrobial sepsis in mice. J Mol Med. 2006;84:389–395. doi: 10.1007/s00109-005-0017-1. [DOI] [PubMed] [Google Scholar]
- 39.Oberholzer C, Tschoeke SK, Bahjat K, et al. In vivo transduction of thymic dendritic cells with adenovirus and its potential use in acute inflammatory diseases. Scand J Immunol. 2005;61:309–315. doi: 10.1111/j.1365-3083.2005.01574.x. [DOI] [PubMed] [Google Scholar]