Abstract
African Americans and Latinos are underrepresented in clinical trials. The purpose of this study was to elicit solutions to participation barriers from African Americans and Latinos. Fifty-seven adults (32 African Americans, 25 Latinos) ages 50 years and older participated. The Institute of Medicine's Unequal Treatment conceptual framework was used. Six racially/ethnically homogenous focus groups were conducted at five sites in three counties. Themes within groups and cross-cutting themes were identified. The NVIVO program was used for data classification. The data were reviewed for final coding and consensus. Shared solutions included addressing costs, recruiting in community contexts, conducting community and individualized patient education, and sharing patient safety information. Participants were unanimously in favor of clinical trials navigation recruitment interventions. Solutions specific to African Americans included diversifying research teams, recognizing past research abuses, and increasing community trust. Solutions specific to Latinos included providing low-literacy materials, providing Spanish-speaking clinicians and advocates, and clarifying that immigration status would neither be documented nor prevent participation. Solutions from African Americans and Latinos reflect their cultural backgrounds and historical experiences. The results suggest the importance of developing a tailored, barriers-focused navigation intervention to improve participation among diverse racial and ethnic populations.
Keywords: African American, clinical trials, Latino, solutions, underrepresentation
Exciting new medical therapies for a number of diseases that disproportionately affect African Americans and Latinos are currently being developed and tested in clinical trials (Robinson & Trochim, 2007). Despite bearing an unequal burden of disease, African Americans and Latinos continue to be underrepresented in clinical trials research, even though the National Institutes of Health Revitalization Act of 1993 (P.L. 103-43) stipulating the participation of women and minority groups in research was created in 1993 and updated in 2001 (Pinsky et al., 2008). Insufficient representation of racially and ethnically diverse groups and women in clinical trials results in inequitable distribution of the risks and benefits of research participation and reduces the generalizability of trial results (Pinsky et al., 2008). Health disparities in the United States could be reduced if targeted therapies were discovered that work equally well in all populations or work especially well in members of affected racial and ethnic groups.
The purpose of this study was to use qualitative data obtained via focus groups with African American and Latino adults ages 50 years and older to elicit potential solutions to the problem of low rates of participation of such populations in clinical trials research. The conceptual framework of the study was based on the Institute of Medicine (IOM) report Unequal Treatment: Confronting Racial and Ethnic Disparities in Healthcare (Smedley & Nelson, 2003), which identified three factors as major sources of racial and ethnic disparities in health outcomes: (1) characteristics of health care systems, (2) perceptions of and actual interactions with health care providers, and (3) preferences and attitudes of patients.
We applied IOM's conceptual framework to the arena of disparities in recruitment of diverse populations to clinical trials research by revising the wording of the IOM framework to refer to clinical trials research instead of to health care disparities. For example, in the framework, we replaced “health care systems” with “health care systems and study processes,” “health care providers” with “researchers,” and “patients” with “potential trial participants.” The revised framework is depicted in Figure 1 and is described in the following sections. The conceptual framework is related to the systems approach in the field of social work, in which clients and their needs are related to a multilevel model of resources, systems, and institutions (Darnell, 2007; NASW, 2008).
Figure 1:
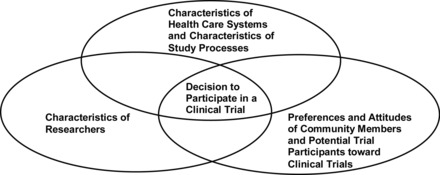
Model Framework of Multilevel Factors Affecting Decision to Participate in a Clinical Trial
CHARACTERISTICS OF HEALTH CARE SYSTEMS AND CHARACTERISTICS OF STUDY PROCESSES
Characteristics that influence participant recruitment include the extent to which clinical data collection occurs at times amenable to a working population, the content of the informed consent form being presented using language that is understandable to lay community members, and the ease of navigating the complex medical systems within which trial procedures often take place (Grunfeld, Zitzelsberger, Coristine, & Aspelund, 2009; Joseph & Dohan, 2009). Other characteristics of health care systems and study processes that affect trial participation include tight timelines for the amount of time that clinicians are expected to spend with each patient. For example, explaining complex trial procedures can take up to 45 minutes, which may have a negative impact on busy clinic schedules (Grunfeld et al., 2009; Joseph & Dohan, 2009).
CHARACTERISTICS OF RESEARCHERS
These characteristics affect participant recruitment. A study of clinician–researcher roles in the recruitment of underrepresented populations to clinical trials revealed that if clinician–researchers had negative perceptions of patients' ability to adhere to study protocols, they were less likely to refer those patients to clinical trials (Howerton et al., 2007). Doctor–patient communication barriers may be another reason why some clinician–researchers do not extend invitations to clinical trial enrollment to their underrepresented patients (Howerton et al., 2007). Physician recommendation is the primary reason cited by research participants for their decision to participate in a trial (Daugherty et al.,1995; Eggly et al., 2008; Grunfeld et al., 2009; Jenkins & Fallowfield, 2000). Less educated patients may not feel empowered to initiate discussions with their doctors about clinical trials (Ford et al., 2011).
PREFERENCES AND ATTITUDES OF POTENTIAL TRIAL PARTICIPANTS
An attitude of trust in the health care system and trust in the physician plays a major role in potential participants' decisions to take part in a clinical trial (Corbie-Smith, Thomas, Williams, & Moody-Ayers, 1999; Ford, Alford, Britton, McClary, & Gordon, 2007; Swanson & Ward, 1995). Other preferences and attitudes include a desire to avoid the burden of extra procedures, fear of exposure to investigational treatment with potentially toxic side effects, negative perceptions of clinical trials, and negative perceptions of physicians' expertise (Grunfeld et al., 2009).
METHOD
Setting
We chose five sites in three South Carolina counties (Richland, Florence, and Charleston) to conduct focus group sessions with African Americans and Latinos. Three focus groups were conducted with African Americans in Columbia, Florence, and North Charleston (in Richland, Florence, and Charleston counties, respectively), and three Spanish-language focus groups with Latinos were conducted in Charleston, Columbia, and Johns Island (in Charleston, Richland, and Charleston counties, respectively) (see Table 1). The rationale for the selection of sites was to include the perspectives of community members representing different regions of the state.
Table 1:
Number of Focus Group Participants at Each Study Site
| Focus Group Meeting Location | African American | Latino |
|---|---|---|
| Charleston, SC (Charleston County) | 9 | |
| Columbia, SC (Richland County) | 11 | 10 |
| Florence, SC (Florence County) | 10 | |
| Johns Island, SC (Charleston County) | 6 | |
| North Charleston, SC (Charleston County) | 11 | |
| Total | 32 | 25 |
Participants
Thirty-two African Americans and 25 Latinos participated in six racially and ethnically homogenous focus groups (three focus groups per racial or ethnic group). Participants were identified by a marketing firm that used magazine subscription lists, word-of-mouth referrals, and community advertisements to advertise the focus groups in each location to recruit participants. Marketing firm staff conducted a short eligibility screening interview with people who responded to the advertisements to ensure that they were African American or Latino and in the 50- to 80-year-old age range. Eligible and interested people were sent a written confirmation of their focus group date, time, and location. Participants received a reminder call the night before their scheduled focus group session.
Focus Group Methods and Guiding Questions
We developed a two-page focus group interview guide to assess participants' perceptions of potential solutions to commonly cited barriers to clinical trial participation (see the Appendix). The focus groups with African Americans were conducted by Marvella Ford, a female African American, and the focus groups with Latinos were conducted by Vanessa Diaz, a Latina.
The focus group structure followed Kohler et al.'s (1993) suggestion to include eight to 10 participants per group. Prior to each focus group, participants signed a consent form that explained the purpose of the focus group and encouraged participants to speak freely. Confidentiality ground rules were laid. The groups began with an icebreaker in which each participant was asked to describe his or her dream vacation. Each two-hour session was audiotaped (Sim, 1998). Following the completion of each session, participants signed receipts and received $50 gift cards as reimbursement for their time spent in the study.
Analysis
Content analysis centers on categorizing text to reduce and make sense of it. Our analysis used “manifest” content analysis, exploring usage of words or content by counting their frequencies, and “summative” content analysis, exploring the usage and underlying meanings of words or content by examining and interpreting the contexts in which they appear (Foreman & Damschroder, 2007). The NVIVO software program uses a systematic coding process to identify themes (Thom & Campbell, 1997). Statements were open-coded and grouped into conceptual categories, themes, or axial codes by authorial consensus (Bulmer, 1998; Nyamathi & Shuler, 1990; Thom & Campbell, 1997). Themes related to survey questions that were common across both racial and ethnic groups were identified, as were themes unique to a particular group. We identified both themes unique to a particular group and cross-cutting themes common in both racial and ethnic groups. NVIVO was used to classify the data. We iteratively reviewed the responses to develop the final codes and consensus. Laura A. Siminoff was the primary coder, with ambiguous responses discussed by all of us. We did not calculate percent reliability but used a consensus method.
The content analysis was divided into three phases: (1) immersion, (2) reduction, and (3) interpretation (Foreman & Damschroder, 2007; Weber, 2008). Laura A. Siminof and a graduate assistant read and coded the transcripts to provide reliability. In the first phase, the transcripts of the focus groups and individual interviews were read, and notes were taken to capture and record recurring themes. The reduction phase consisted of selected word counts and counts of repetitive language use and reduction of the data into unique themes and subdomains (consisting of unique codes). A color-coding scheme was used to organize and track the data. The interviewer guide was used to help in searching for study-relevant themes even as we remained open to discovering new and unanticipated themes and subdomains. The extracted and reduced data were used in the interpretation phase to answer the study research questions.
RESULTS
The study results were organized as solutions to barriers related to each component of the revised IOM framework. The solutions are presented according to shared themes from both racial and ethnic groups, themes from the African American groups only, and themes from the Latino groups only. Each group was fairly evenly divided in terms of male and female representation.
Themes Common across All Participants
Characteristics of Health Care Systems and Characteristics of Study Processes: Costs Associated with Participation in Research
Participants reported being concerned about costs associated with study participation, including gas and hotel room costs, and the risks associated with driving long distances to the research sites. As a potential solution to the cost issue, universally, participants believed that these costs could be alleviated through fair compensation. One participant suggested that institutions conducting clinical trials could provide shuttle service for participants. Overwhelmingly, participants believed that trial meetings and appointments should be held at night or on weekends to minimize interference with participants' work schedules.
Characteristics of Health Care Systems and Characteristics of Study Processes: Recruitment in Community Contexts Instead of in Health Care Systems, Emphasizing Word-of-Mouth Recruitment and Person-to-Person Contact
As a potential solution to recruitment challenges in underrepresented populations, members of both racial and ethnic groups advocated going outside of the health care system to recruit participants in community contexts such as churches, ethnic gathering places, and support groups. African Americans in the Columbia group offered a very interesting recommendation. They suggested that medical researchers conduct surveys in churches to learn which diseases people had and then target survey respondents (and their family members) with specific recruitment messages related to those diseases. Although mass media might be effective, one African American participant stated that participants recruited in churches might be of a “higher caliber” (and, thus, perhaps more adherent to study protocols). Along the same lines, other participants said that word-of-mouth or person-to-person contact was an invaluable recruitment strategy and one based in trust.
Characteristics of Researchers: Researchers Do Not Spend Enough Time with Potential Study Participants to Describe the Risks of a Trial
As a solution to this problem, participants recommended providing general community education about adverse effects and participant liability in clinical trials through mass media campaigns in addition to one-on-one education by clinicians. As a Latina from Charleston explained, “Studies have to be clearer and more specific about what is going to be researched … If you want to participate, that's fine. If you don't, that's fine too. But you are conscious of what you are going to do.”
Overwhelmingly, participants reported desire for their doctors to provide them with appropriate and accurate information about clinical trials. Participants said they wanted clinicians to reassure them that participation was in their best interest and not to feel pressured or have to worry that their doctors were recruiting them to “make money.” Participants recommended that physicians receive communication skills training to learn how to better present clinical trials information to diverse audiences. Many participants further stated that they would be more willing to participate in clinical trials if their own doctors administered the drugs or at least stayed involved in the process.
Preferences and Attitudes of Potential Participants: Fear of Adverse Effects, Participant Liability, and Being Exposed to Unsafe Treatments
Regardless of their race or ethnicity, participants often extrapolated from their experiences as health care consumers to their concepts of clinical trials. Concerns were most apparent when participants described their fears about participating in clinical trials because of possible adverse effects.
As a potential solution to fears related to adverse effects, participants reported that these fears could be alleviated if researchers took more responsibility for their studies. One female African American participant explained as follows:
I think what would help take some of the fear away is to claim some liability for after the research is done whatever. If there's major side effects and for the research people to accept some of the responsibility to what has happened to that person. It's my understanding that as it is now, once you've agreed to do the research, you're on your own.
Participants wanted personal guarantees about their safety. They understood researcher responsibility to include the following: sharing efficacy statistics for the drug under study, guaranteeing that free health care will be provided to victims of adverse effects, and reassuring participants that a drug will not cause harm or exacerbate other health conditions.
Preferences and Attitudes of Potential Participants: Conducting Clinical Trials with Healthy People
Participants were very resistant to trials that tested drugs on healthy people (phase I trials). However, even the focus group participants who were most unwilling to participate in clinical trials agreed that they were more likely to participate in a clinical trial if it would help with a chronic condition, especially one that had the potential to be life threatening. This theme was often expressed by participants as “if ain't broke, then don't fix it.” One male African American participant said,
If I'm dying and they got a cure that they're not sure but yeah, I'll try it. But if I'm fine, if it ain't broke, don't fix it. Leave it alone. But to research, be a part of the research that's going to cause illness—like right now, I got diabetes, I got high blood pressure. If they got research that is going to help cure, I'm going to get better, yeah. Something like that. But if it's something new, no, I ain't going to try it.
Preferences and Attitudes of Potential Participants: Willingness among Potential Participants to Take Part in Trials
Participants from both racial and ethnic groups spoke very positively about the importance of clinical research in advancing science in general and as a means of alleviating health disparities and improving the next generation's health outcomes. Quite poignantly, an African American man said,
That means that inherently, in all of us, we possess the ability to care, to want to do the right thing because of society and things that may have happened in one's personal life … it's not for an individual purposely. It's for the greater good, the larger group.
In a similar manner, a Latina participant said, “Yes, we Latinos are charitable. … We worry about the other person.” Although these statements do not indicate that the participants were high in altruism, they do indicate that, in general, participants perceived altruism as a positive attribute. Participants who stated that they trusted their physicians and had long-standing relationships with them tended to express more positive perceptions of the health care system and greater probability that they would consider clinical trial participation than did other participants.
Themes Unique to African American Participants
Characteristics of the Health Care System and Characteristics of Study Processes: Publicly Recognizing Past Abuses of the Health Care System
African Americans said that they would be positively influenced to participate in clinical trials at hospitals and in health systems that conducted patient satisfaction surveys, formally apologized for medical errors, and publically admitted mistakes they had made. These actions served to foster trust among African Americans in health care providers and medical researchers at these institutions.
Characteristics of Researchers: Lack of Diversity in Research Teams
Two useful recommendations for alleviating mistrust were (1) coaching clinicians in better patient communication and (2) developing diverse research teams.
Preferences and Attitudes of Potential Participants: Mistrust of Medical Research
Only one participant mentioned the Tuskegee syphilis study specifically. However, other participants expressed the historic mistrust of research in African American communities.
Themes Unique to Latino Participants
Characteristics of the Health Care System and Characteristics of Study Processes: Lack of Availability of Study Materials in Spanish
Latino participants overwhelmingly argued for the availability of study materials in Spanish. They also stressed that materials need to be translated so that people with low literacy or education could understand them. One Latino advised, “If that message is going to be translated from English to Spanish, you have to be sure to choose the right words.”
Characteristics of Researchers: Lack of Spanish-speaking Clinicians and Patient Advocates Involved with a Trial
Many participants stressed the importance of having Spanish-speaking clinicians and patient advocates to the successful recruitment of Latinos into clinical trials. In general, Latinos were much more likely to trust Spanish-speaking physicians and often complained that “American” doctors did not have their best interests at heart and might be “in it for the money.”
Preferences and Attitudes of Potential Participants: Fear of Participating because of Concerns about Immigration Status that Are Not Adequately Addressed by Researchers
Participants recommended that researchers take the time to clearly explain that immigration status will not be documented and will not prevent participation. They also suggested that study advertisements and informed consent forms make this point explicitly.
DISCUSSION
The goal of the present study was to use a revised IOM framework in a qualitative study to obtain solutions to clinical trials recruitment barriers from racially and ethnically diverse community members. The IOM framework was revised by changing its wording to reflect clinical trials research instead of health care disparities.
To accomplish the study goal, we conducted focus groups with African American and Latino residents of South Carolina. The study is unique in its inclusion of the perspectives of both of these racial and ethnic groups and in its focus on solutions rather than barriers to participation.
The revised IOM model was supported by the study results. The model provided a good fit for the themes that emerged from each group. It is, thus, a novel framework with broad translational applicability to recruitment of diverse populations to a variety of clinical trial types.
Summary of Findings
In the present study, many themes that emerged were shared by the African American and the Latino focus groups. These themes included solutions to characteristics of study processes such as addressing safety concerns and costs associated with research participation. Solutions to characteristics of researchers included increasing the amount of time they spend with study participants and building on the preferences and attitudes of potential trial participants, such as feelings of altruism as a motivator for trial participation. It was not surprising that many of the proposed solutions were related to the themes commonly found in research on racial and ethnic barriers to research participation (Pinsky et al., 2008).
Although a large amount of information is available on barriers to clinical trial participation, far fewer studies have highlighted solutions or facilitators to minority recruitment (Gadegbeku et al., 2008; Grunfeld et al., 2009). In our study, themes that were specific to African American or Latino participants were related to the unique cultural backgrounds and historical experiences of members of these groups in the United States, particularly in relation to health care. Themes that other investigators have found to be important for both groups—including increasing levels of trust between the community and the research team, making participation available at times that are convenient to participants, and incorporating meaningful incentives into the recruitment process (Gonzalez, Gardner, & Murasko, 2007)—were repeated in the solutions proposed by focus group participants.
Themes that were specific to African American participants focused mainly on solutions to characteristics of study processes, including a request for public recognition of past abuses. Solutions to characteristics of researchers included increasing the diversity of research teams and developing better communication skills among health care professionals.
Among Latino participants, proposed solutions were related to characteristics of study processes, including language-congruent care, which has been defined as a set of congruent behaviors, attitudes, and policies that influence awareness of the distinctive (and similar) characteristics of populations that receive treatment at medical centers and are recruited into clinical trials at those centers (IQ Solutions, Inc., 2000; Lindenberg, Solorzano, Vilaro, & Westbrook, 2001; O'Brien et al., 2006). For example, Lindenberg et al. (2001) conducted a prevention intervention trial to reduce substance abuse and risky sexual activities in young Latinas with low income and found that recruitment outcomes were most successful when conducted by recruiters who were bicultural and bilingual and who identified with the potential participants.
A potential clinical trial navigation approach was viewed favorably by the study participants. The ultimate goal of the present study was to use the proposed solutions generated by the focus group participants to create a multifaceted intervention to increase minority participation in medical research. Such a recruitment intervention could serve as a national model.
A Novel, Tailored, Barriers-focused Navigation Intervention to Enhance Enrollment into Clinical Trials
When questioned about the potential utility of a navigation intervention to enhance enrollment into clinical trials, African American and Latino participants in all of the focus groups were unanimously in favor of such an intervention. The findings, therefore, suggest the importance of the development of a clinical trials navigation intervention to enhance participation of racially and ethnically diverse groups in clinical trials.
We previously successfully applied such an approach in a randomized trial to retain older African American men in a longitudinal cancer screening trial (Ford et al., 2004; Ford, Havstad, Demers, & Cole Johnson, 2005; Ford et al., 2006). Patient navigators contacted trial participants at least once per month by telephone and provided information and referral services to community resources to address needs of the potential participants that, if not addressed, would have interfered with their trial enrollment. Referral services included referrals to food banks, agencies that helped to pay utility costs, and transportation services. The navigators also assisted the participants with scheduling their trial screening appointments and their regular medical care appointments. Over the three-year study period, the navigators made 14,978 calls to study the 351 participants in the intervention group (Ford et al., 2004). The intensive intervention had the greatest impact among participants with low income, who are often the most difficult to retain. Among participants with low income, those who were in the intervention group demonstrated significantly higher screening adherence rates than those in the control group for prostate cancer screening via prostate-specific antigen test (p = .001) and digital rectal exam (p = .011) and for lung cancer screening via chest X-ray (p = .012) (Ford et al. 2006).
A limitation of prior research is that although the use of a patient navigation intervention has been tested in overcoming barriers to retaining diverse study participants, few studies have used this approach in overcoming barriers to recruiting diverse participants. Two published studies described the design and implementation of a navigation-based trial focusing on recruitment of American Indians to clinical trials (Guadagnolo et al., 2009; Petereit & Burhansstipanov, 2008). The study results showed that even though clinical trial participation rates were low overall, they were three times higher in the navigated group than in the control group (Guadagnolo et al., 2009).
Navigation approaches are consistent with the core social work function of helping clients to obtain needed services (Darnell, 2007; Davis, Darby, Likes, & Bell, 2009). Similar to social workers, clinical trial navigators could be trained to understand the barriers to and facilitators of trial participation. As navigators begin to mobilize resources on behalf of the client to overcome barriers and capitalize on facilitators, their function will extend beyond an individual-level approach to use a multilevel approach addressing characteristics of the health care system and study processes, characteristics of researchers, and preferences and attitudes of potential participants (Darnell, 2007; NASW, 2008).
For example, Darnell (2007) pointed out that an essential feature of social work practice is the concurrent consideration of the individual and the social context in which the individual lives. In the case of a navigation intervention to improve clinical trial participation, many barriers that inhibit patients from considering participation in trials are grounded in the social context of the health care system and patients' community and personal histories of experiences within it. This is congruent with the multilevel approach of the IOM conceptual framework of characteristics of the health care system and study processes, characteristics of researchers, and preferences and attitudes of patients.
In the case of clinical trial recruitment, navigators could work with patients to understand multilevel barriers and to develop strategies to overcome them. This approach has great potential to lead to enhanced clinical trial enrollment and increased diversity of trial participants, making trial results more widely generalizable.
LIMITATIONS AND STRENGTHS
This article presents qualitative data from only 57 African American and Latino participants ages 55 to 80 years living in a small area of the United States. However, we made a significant effort to ensure that the focus group participants were from different geographic regions of South Carolina.
Although the study has some limitations, it also has a number of strengths, one of which is the broad application of the revised IOM model to clinical trials in many different disease areas. As such, the information described here has high translational research potential.
In addition, we deliberately held focus groups in nonacademic, community settings (for example, hotels, public libraries, a marketing research firm) in different areas of South Carolina to increase the likelihood of recruiting community-based focus group participants who might not have participated had the groups been held at an academic medical center. In future studies, investigators could conduct surveys with similar populations to ascertain whether similar results can be obtained.
CONCLUSION
This study provides an application of a conceptual framework focused on multiple levels of factors that contribute to disparities in clinical trial participation. The study results could be used to design future, culturally tailored clinical trial navigation recruitment interventions with African American and Latino potential participants.
Many patients who are eligible to enroll in clinical trials face a plethora of tangible and psychosocial barriers that could be addressed by navigators, who could use social work principles to provide navigation assistance. Such assistance would include identifying the following types of resources: transportation resources for patients who make return health care visits related to their trial participation, housing resources for patients who live a great distance from the trial site and for whom travel would have a prohibitive effect on their ability to participate, and sociocultural resources to help patients (with input from clinical trial investigators and staff) work through issues such as mistrust of the medical system in which the trial takes place (Ferrante, Cohen, & Crosson, 2010).
Navigators also have the potential to change systems to enhance trial recruitment. For example, if the language of consent forms is virtually incomprehensible to the average person, navigators could work with investigators and an institution's institutional review board to revise the language of the forms while retaining the needed content. As Parker et al. (2010) noted, whereas navigation is a barriers-focused approach, navigators also have the capacity to change the systems in which patients function to achieve desired outcomes.
Social workers, who are trained in systems-level approaches to meeting the needs of patients, could play a key role in conducting navigation recruitment interventions to enhance clinical trial participation among diverse population groups, leading to the ultimate goal of making trial results more broadly generalizable and, therefore, significantly more useful in developing new therapeutic interventions.
Appendix: Introduction and Verbal Informed Consent
“Hello. My name is Dr. [____]. I'm from the Medical University of South Carolina. Thank you for attending this session. Our purpose is to get your thoughts on overcoming barriers that may keep you from taking part in medical research studies. Since we want everyone's opinions and thoughts, we ask that everyone be given a chance to speak. We will ask a few questions and may follow up on what is said. Please know that everything we say to each other here is confidential and that we will not use your name in anything we report. We would like to tape this session, and we will erase the audiotapes after they are analyzed. You are taking part voluntarily. If there is anything you do not want to discuss, just say so. At the end of the session, you will receive a $50 grocery gift card to reimburse you for your time.
“Are there any questions so far?
“May I have your permission to continue?
“Before we start, it would be helpful to go around and say who you are just by first name.”
Discussion: Some people may have concerns about being in medical research studies. These concerns may be about travel costs, time, fear of being in an experiment, worries about safety, or lack of trust in medical researchers or the health care system in general.
QUESTION 1: “What can be done to overcome these concerns?”
Probe 1: “What would make you or your family or friends want to take part in medical research?”
Probe 2: “What could your doctor do to get you or your family or friends to want to take part?”
Probe 3: “What could the medical researchers do to get you or your family or friends to take part in research?”
Probe 4: “What would prevent you or your family or friends from taking part in medical research?”
QUESTION 2: “Imagine that someone was available to guide people through the process of taking part in clinical research. What kinds of things could that person help with?”
Probes: Health information
Transportation
Financial support
“Would you be interested in working with a person in this way?”
QUESTION 3: Participants were provided with a flyer in either Spanish with Latinos in the photos or in English with African Americans in the photos. They were then shown an online video about participation in clinical research. They were asked whether they would be more likely to participate in research after seeing the flyer and video. (The video can be viewed at the following address: http://www.ciscrp.org/downloads/medhero_ad.html.) [Stop tape. Give out gift cards.]
REFERENCES
- Bulmer C. Clinical decisions: Defining meaning through focus groups. Nursing Standards. 1998;12:34–36. doi: 10.7748/ns.12.20.34.s51. [DOI] [PubMed] [Google Scholar]
- Corbie-Smith G., Thomas S. B., Williams M. V., Moody-Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. Journal of General Internal Medicine. 1999;14:537–546. doi: 10.1046/j.1525-1497.1999.07048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. S. Patient navigation: A call to action [Commentary] Social Work. 2007;52:81–84. doi: 10.1093/sw/52.1.81. [DOI] [PubMed] [Google Scholar]
- Daugherty C., Ratain M. J., Grochowski E., Stocking C., Kodish E., Mick R., Siegler M. Perceptions of cancer patients and their physicians involved in phase I trials. Journal of Clinical Oncology. 1995;13:1062–1072. doi: 10.1200/JCO.1995.13.5.1062. [DOI] [PubMed] [Google Scholar]
- Davis C., Darby K, Likes W., Bell J. Social workers as patient navigators for breast cancer survivors: What do African-American medically underserved women think of this idea? Social Work in Health Care. 2009;48:561–578. doi: 10.1080/00981380902765212. [DOI] [PubMed] [Google Scholar]
- Eggly S., Albrecht T. L., Harper F. W., Foster T., Franks M. M., Ruckdeschel J. C. Oncologists' recommendations of clinical trial participation to patients. Patient Education and Counseling, 2008;70:143–148. doi: 10.1016/j.pec.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante J. M., Cohen D. J., Crosson J. C. Translating the patient navigator approach to meet the needs of primary care. Journal of the American Board of Family Medicine. 2010;23:736–744. doi: 10.3122/jabfm.2010.06.100085. [DOI] [PubMed] [Google Scholar]
- Ford M. E., Alford S. H., Britton D., McClary B., Gordon H. S. Factors influencing perceptions of breast cancer genetic counseling among women in an urban health care system. Journal of Genetic Counseling. 2007;16:735–753. doi: 10.1007/s10897-007-9106-3. [DOI] [PubMed] [Google Scholar]
- Ford M. E., Havstad S. L., Demers R., Cole Johnson C. Effects of false-positive prostate cancer screening results on subsequent prostate cancer screening behavior. Cancer Epidemiological Biomarkers and Prevention. 2005;14:190–194. [PubMed] [Google Scholar]
- Ford M. E., Havstad S., Vernon S. W., Davis S. D, Kroll D., Lamerato L., Swanson G. M. Enhancing adherence among older African American men enrolled in a longitudinal cancer screening trial. Gerontologist. 2006;46:545–550. doi: 10.1093/geront/46.4.545. [DOI] [PubMed] [Google Scholar]
- Ford M. E., Randolph V., Hopkins-Johnson L., Eason S. L., Havstad S., Jankowski M., et al. Design of a case management approach to enhance cancer screening trial retention among older African American men. Journal of Aging and Health. 2004;16, 39S–57S doi: 10.1177/0898264304268148. [DOI] [PubMed] [Google Scholar]
- Ford M. E., Wahlquist A. E., Ridgeway C, Streets J., Mitchum K. A., Harper R. R., Jr, et al. Evaluating an intervention to increase cancer knowledge in racially diverse communities in South Carolina. Patient Education and Counseling. 2011;83:256–280. doi: 10.1016/j.pec.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J., Damschroder L. Qualitative content analysis. In: Jacoby L., Siminoff L. A., editors. Empirical methods for bioethics: A primer. Oxford, England: JAI Press; 2007. pp. 39–62. [Google Scholar]
- Gadegbeku C. A., Stillman P. K., Huffman M. D., Jackson J. S., Kusek J. W., Jamerson K. A. Factors associated with enrollment of African Americans into a clinical trial: Results from the African American study of kidney disease and hypertension. Contemporary Clinical Trials. 2008;29:837–842. doi: 10.1016/j.cct.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez E. W., Gardner E. M., Murasko D. Recruitment and retention of older adults in influenza immunization study. Journal of Cultural Diversity. 2007;14:81–87. [PubMed] [Google Scholar]
- Grunfeld E., Zitzelsberger L., Coristine M., Aspelund F. Barriers and facilitators to enrollment in cancer clinical trials: Qualitative study of the perspectives of clinical research associates. Cancer. 2009;95:1577–1583. doi: 10.1002/cncr.10862. [DOI] [PubMed] [Google Scholar]
- Guadagnolo B. A., Petereit D. G., Helbig P., Koop D., Kussman P., Dunn E. F., Patnaik A. Involving American Indians and medically underserved rural populations in cancer clinical trials. Clinical Trials. 2009;6:610–617. doi: 10.1177/1740774509348526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howerton M. W., Gibbons M. C., Baffi C. R., Gary T. L., Lai G. Y., Bolen S., et al. Provider roles in the recruitment of underrepresented populations to cancer clinical trials. Cancer. 2007;109:465–476. doi: 10.1002/cncr.22436. [DOI] [PubMed] [Google Scholar]
- IQ Solutions, Inc. National standards on culturally and linguistically appropriate services in health care: Final report. Washington, DC: U.S: Department of Health and Human Services, Office of Minority Health; 2000, March. [Google Scholar]
- Jenkins V., Fallowfield L. Reasons for accepting or declining to participate in randomized clinical trials for cancer therapy. British Journal of Cancer. 2000;82:1783–1788. doi: 10.1054/bjoc.2000.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph G., Dohan D. Recruiting minorities where they receive care: Institutional barriers to cancer clinical trials recruitment in a safety-net hospital. Contemporary Clinical Trials. 2009;30:552–559. doi: 10.1016/j.cct.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Kohler C. L., Dolce J. J., Manzella B. A., Higgins D., Brooks C. M., Richards J. M., Jr., Bailey W. C. Use of focus group methodology to develop an asthma self-management program useful for community-based medical practices. Health Education Quarterly. 1993;20:421–429. doi: 10.1177/109019819302000311. [DOI] [PubMed] [Google Scholar]
- Lindenberg C. S., Solorzano R. M., Vilaro F. M., Westbrook L. O. Challenges and strategies for conducting intervention research with culturally diverse populations. Journal of Transcultural Nursing. 2001;12:132–139. doi: 10.1177/104365960101200207. [DOI] [PubMed] [Google Scholar]
- National Association of Social Workers. Code of ethics of the National Association of Social Workers. Washington, DC: NASW Press; 2008. [Google Scholar]
- National Institutes of Health Revitalization Act of. 1993;122(1993) P.L. 103-43, 107 Stat. [Google Scholar]
- Nyamathi A., Shuler P. Focus group interview: A research technique for informed nursing practice. Journal of Advanced Nursing. 1990;15:1281–1288. doi: 10.1111/j.1365-2648.1990.tb01743.x. [DOI] [PubMed] [Google Scholar]
- O'Brien R. L., Kosoko-Lasaki O., Cook C. T., Kissell J., Peak F., Williams E. H. Self-assessment of cultural attitudes and competence of clinical investigators to enhance recruitment and participation of minority populations in research. Journal of the National Medical Association. 2006;98:674–682. [PMC free article] [PubMed] [Google Scholar]
- Parker V. A., Clark J. A., Leyson J., Calhoun E., Carroll J. K., Freund K. M., Battaglia T. A. Patient navigation: Development of a protocol for describing what navigators do. Health Services Research. 2010;45:514–531. doi: 10.1111/j.1475-6773.2009.01079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petereit D. G., Burhansstipanov L. Establishing trusting partnerships for successful recruitment of American Indians to clinical trials. Cancer Control. 2008;15:260–268. doi: 10.1177/107327480801500310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsky P. F., Ford M., Gamito E., Higgins D., Jenkins V., Lamerato L., et al. Enrollment of racial and ethnic minorities in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Journal of the National Medical Association. 2008;100:291–298. doi: 10.1016/s0027-9684(15)31241-4. [DOI] [PubMed] [Google Scholar]
- Robinson J. M., Trochim W. M. An examination of community members', researchers' and health professionals' perceptions of barriers to minority participation in medical research: An application of concept mapping. Ethnicity & Health. 2007;12:521–539. doi: 10.1080/13557850701616987. [DOI] [PubMed] [Google Scholar]
- Sim J. Collecting and analysing qualitative data: Issues raised by the focus group. Journal of Advanced Nursing. 1998;28:345–352. doi: 10.1046/j.1365-2648.1998.00692.x. [DOI] [PubMed] [Google Scholar]
- Smedley B.S.A., Nelson A. R. Unequal treatment: Confronting racial and ethnic disparities in healthcare. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- Swanson G. M., Ward A. J. Recruiting minorities into clinical trials: Toward a participant-friendly system. Journal of the National Cancer Institute. 1995;87:1747–1759. doi: 10.1093/jnci/87.23.1747. [DOI] [PubMed] [Google Scholar]
- Thom D. H., Campbell B. Patient–physician trust: An exploratory study. Journal of Family Practice. 1997;44:169–176. [PubMed] [Google Scholar]
- Weber R. P. Basic content analysis. Newbury Park, CA: Sage Publications; 2008. [Google Scholar]


