Abstract
Background
To observe the primary tumor (PT) regression speed after radiotherapy (RT) in nasopharyngeal carcinoma (NPC) and evaluate its prognostic significance.
Methods
One hundred and eighty-eight consecutive newly diagnosed NPC patients were reviewed retrospectively. All patients underwent magnetic resonance imaging and fiberscope examination of the nasopharynx before RT, during RT when the accumulated dose was 46–50 Gy, at the end of RT, and 3–4 months after RT.
Results
Of 188 patients, 40.4% had complete response of PT (CRPT), 44.7% had partial response of PT (PRPT), and 14.9% had stable disease of PT (SDPT) at the end of RT. The 5-year overall survival (OS) rates for patients with CRPT, PRPT, and SDPT at the end of RT were 84.0%, 70.7%, and 44.3%, respectively (P < 0.001, hazard ratio [HR] = 2.177, 95% confidence interval [CI] = 1.480-3.202). The 5-year failure-free survival (FFS) and distant metastasis-free survival (DMFS) rates also differed significantly (87.8% vs. 74.3% vs. 52.7%, P = 0.001, HR = 2.148, 95% CI, 1.384-3.333; 91.7% vs. 84.7% vs. 66.1%, P = 0.004, HR = 2.252, 95% CI = 1.296-3.912). The 5-year local relapse–free survival (LRFS) rates were not significantly different (95.8% vs. 86.0% vs. 81.8%, P = 0.137, HR = 1.975, 95% CI, 0.976-3.995). By multivariate analyses, the PT regression speed at the end of RT was the only independent prognostic factor of OS, FFS, and DMFS (P < 0.001, P = 0.001, and P = 0.004, respectively). The 5-year FFS rates for patients with CRPT during RT and CRPT only at the end of RT were 80.2% and 97.1%, respectively (P = 0.033). For patients with persistent PT at the end of RT, the 5-year LRFS rates of patients without and with boost irradiation were 87.1% and 84.6%, respectively (P = 0.812).
Conclusions
PT regression speed at the end of RT was an independent prognostic factor of OS, FFS, and DMFS in NPC patients. Immediate strengthening treatment may be provided to patients with poor tumor regression at the end of RT.
Keywords: Nasopharyngeal carcinoma, Radiotherapy, Tumor regression, Survival
Background
Radiotherapy (RT) is the first choice and main treatment for newly diagnosed and nonmetastatic nasopharyngeal carcinoma (NPC). Intensity-modulated RT (IMRT), an important milestone in RT development, can not only increase local control, but also reduce RT-related toxicities in NPC [1-3]. Distant metastasis is the main reason for treatment failure [4]. Identifying the high-risk group for distant metastasis and increasing chemotherapy intensity are the most important approaches to increasing the overall survival (OS) rate in NPC patients.
The main prognostic factors of NPC widely used in clinical work include the staging system, tumor size, and plasma Epstein-Barr virus (EBV) DNA level [5-12]. Currently, the 7th edition of the American Joint Committee on Cancer (AJCC) staging system is used to predict prognoses and determine treatment strategies worldwide [5]. Using the 7th edition system instead of the 6th edition system, Chen et al. observed better segregation of survival curves in NPC patients [6]. Previous research has demonstrated that tumor sizes, including primary tumor (PT) volume and maximum PT diameter, could serve as important prognostic factors in NPC [7-9]. Chong et al. indicated that it might be possible to incorporate tumor volume as an additional prognostic factor within the existing TNM staging system [10]. The plasma EBV DNA level is useful for predicting prognosis and evaluating treatment failure in NPC [11]. In patients with Stage I–II NPC, pretherapy plasma EBV DNA levels identified a high-risk group with a probability of distant failure similar to that of patients with advanced-stage disease [12].
All of these pretreatment factors, which reflect severity of disease and predict prognosis, form the basis of determining treatment strategies. The Response Evaluation Criteria in Solid Tumors (RECIST) is used to evaluate the response of tumor regression. Overall responses are divided into four levels: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) [13]. The relationship between recent tumor regression and long-term survival in NPC patients remains unknown. If poor tumor regression can predict potential treatment failure, such patients may require timely strengthening treatment.
Therefore, we performed a retrospective study to observe PT regression speed after RT and to evaluate its prognostic significance in NPC patients. The purpose of this study was to investigate whether patients with poor tumor regression require strengthening treatment, thereby increasing the effectiveness of treatment. Our data may improve understanding of the biological nature of NPC.
Methods
Study population
This retrospective study was approved by the ethics committee of the First People’s Hospital of Foshan, Foshan, China. The inclusion criteria were as follows: patients with newly diagnosed, nonmetastatic, and histologically proven NPC. Patients were treated with RT alone or concurrent chemoradiotherapy (CCRT), but did not receive neoadjuvant chemotherapy or adjuvant chemotherapy. In addition, all patients underwent nasopharyngeal fiberscope and magnetic resonance imaging (MRI) scan of the nasopharynx before RT, during RT when the accumulated dose was 46–50 Gy, at the end of RT, and 3–4 months after RT. Between June 2004 and November 2009, 188 eligible patients were included in the study, including 138 male and 50 female patients (male:female, 2.8:1). The median age was 50 years (range, 14–85 years). Histologically, 98.9% of patients had nonkeratinizing NPC, 0.5% had keratinizing NPC, and the remainder (0.5%) had other types. All patients underwent pretreatment evaluation that included complete history, physical and neurological examinations, hematology and biochemistry profiles, nasopharyngeal fiberscopy, nasopharynx and neck MRI, chest radiography, and abdominal sonography. Medical and imaging records were retrospectively reviewed and all patients were restaged according to the 7th edition of the AJCC staging system [5]. The TNM stage distribution of all patients was T1 in 19.7%, T2 in 10.1%, T3 in 37.8%, and T4 in 32.4%; N0 in 6.9%, N1 in 50.0%, N2 in 42.6%, and N3 in 0.5%; Stage I in 2.7%, Stage II in 14.9%, Stage III in 49.5%, and Stage IVA-B in 33.0%.
Imaging
All patients underwent MRI using a 1.0 Tesla scanner (Siemens Magnetom Impact, Siemens Healthcare, Erlangen, Germany). The area from the suprasellar cistern to the inferior margin of the sternal end of the clavicle was examined using a head and neck segregate coil. T1-weighted fast spin-echo images in the axial, coronal, and sagittal planes (repetition time, 500 ms; echo time, 12 ms) and T2-weighted fast spin-echo MR images in the axial plane (repetition time, 3,304 ms; echo time, 96 ms) were obtained before the injection of contrast material. After intravenous injection of gadopentetate dimeglumine (Gd-DTPA; 0.1 mmol/kg body weight; Magnevist, Schering, Berlin, Germany), spin-echo T1-weighted axial and sagittal sequences and spin-echo T1-weighted fat-suppressed coronal sequences were performed sequentially using similar parameters to those used prior to Gd-DTPA injection. We used a section thickness of 5 mm and a 256 × 256 matrix size.
Image assessment
Two radiologists with clinical focus on head and neck cancer and certifications for professional diagnostic imaging in China, who have been on staff for 10 years, evaluated the MR images separately. Any discord in evaluation was resolved by consensus every two weeks. Tumor soft tissue had low signal intensity on T1-weighted images; it had intermediate signal intensity superior to the muscle signal on T2-weighted images; tumor soft tissues had moderate enhancement on contrast-enhanced T1-weighted images, replacing the normal anatomy of the structure. Tumor regression was evaluated by the change in the maximum primary tumor diameter (MPTD) on the same plane after RT. The method applied to measure MPTD was as follows [9]: firstly, MPTD was measured on post-Gd-DTPA T1-weighted images. Secondly, tumor signal was not interrupted, but continuous on the maximum diameter. Finally, the maximum diameters in the axial, coronal and sagittal planes were measured separately; the largest value was recorded as the MPTD. Tumor regression was divided into four levels: CR, PR, SD, and PD, according to RECIST [13].
Treatment
All patients were treated with definitive-intent RT. One hundred and five patients (55.9%) were treated with conventional 2-dimensional RT (2-DRT) and 83 (44.1%) were treated with 3-dimensional conformal RT (3-DCRT). The accumulated doses were 68–70 Gy to the gross tumor, 60–62 Gy to involved areas of the neck, and 50 Gy to uninvolved areas. Boost irradiation to the parapharyngeal space, skull-base, and primary or nodal sites were administered if indicated, and did not exceed 16 Gy. Ninety patients (57.7%) were treated with boost irradiation.
Platinum-based concomitant chemotherapy was administered to 130 patients with Stage III or Stage IVA-B disease (classified as T3-T4 or N2-N3). The remaining 25 patients with advanced-stage disease did not receive chemotherapy due to advanced age, heart disease, hepatitis, severe diabetes, inadequate renal function, patient refusal, or economic problems. When possible, salvage treatment (including afterloading, surgery, and chemotherapy) was provided in the event of documented relapse or if the disease persisted despite therapy.
Statistical analysis
Patients were assessed every two months during the first year, every three months for the next two years, and every six months thereafter until death. All events were measured from the date of commencement of treatment. The following end points (time to the first defining event) were assessed: OS, local relapse-free survival (LRFS), distant metastasis-free survival (DMFS), and failure-free survival (FFS). Local recurrence was established by fiberoptic endoscopy and biopsy and/or MRI. Distant metastases were diagnosed based on clinical symptoms, physical examination, and imaging methods, including chest radiography, bone scan, computed tomography, and abdominal sonography.
All statistical analyses were performed using Statistical Package for the Social Sciences version 12.0 (SPSS, Chicago, IL, USA). Actuarial rates were calculated using the Kaplan-Meier method and differences were compared using the log-rank test. Multivariate analyses with the Cox proportional hazards model were used to test for independent significance by backward elimination of insignificant explanatory variables. Demographic characteristics (age, sex) were introduced into the models as covariates for all statistical tests. No correction for multiple testing was performed. The criterion for statistical significance was set at α = 0.05; P-values were based on two-sided tests.
Results
PT regression speed after RT and survival
Among 188 patients, 21.8% had CRPT, 39.9% had PRPT, and 38.3% had SDPT during RT. At the end of RT, 40.4% of patients had CRPT, 44.7% had PRPT, and 14.9% had SDPT. After 3–4 months of RT, 97.4% of patients had CRPT and 2.6% had persistent PT (Figure 1).
Figure 1.
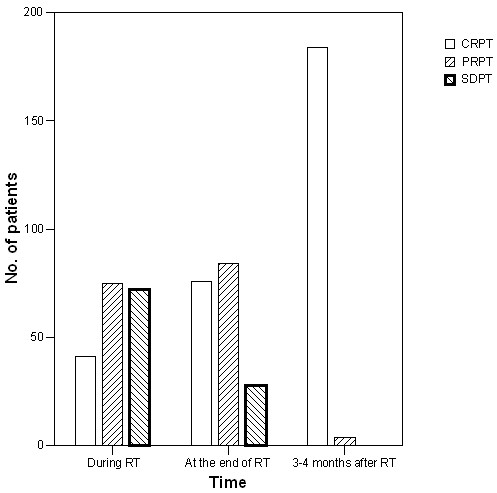
Tumor regression of primary tumor after radiotherapy in 188 nasopharyngeal carcinoma patients. CRPT, Complete response of primary tumor; PRPT, partial response of primary tumor; SDPT, stable disease of primary tumor.
The median follow-up period for the whole group was 57 months (range, 2–110 months). Altogether, 41 patients (21.8%) had locoregional failure or distant metastases; 53 patients (28.2%) died. For all patients, the 5-year OS, FFS, DMFS, and LRFS rates were 72.1%, 76.8%, 85.1%, and 89.8%, respectively.
Prognostic value of PT regression speed at the end of RT
Based on PT regression speed at the end of RT, the 188 patients were divided into three groups: CRPT, PRPT and SDPT. The 5-year OS rates for patients with CRPT, PRPT and SDPT were 84.0%, 70.7%, and 44.3%, respectively. The differences among these rates were highly significant (hazard ratio [HR] = 2.177, 95% confidence interval [CI] = 1.480-3.202; P < 0.001; Figure 2A). The 5-year FFS rates also differed significantly (87.8% vs. 74.3% vs. 52.7%, P = 0.001, HR = 2.148, 95% CI, 1.384-3.333, Figure 2B). Similarly, the 5-year DMFS rates were 91.7%, 84.7%, and 66.1%, respectively (HR = 2.252, 95% CI = 1.296-3.912; P = 0.004; Figure 2C). The 5-year LRFS rates were not significantly different (95.8% vs. 86.0% vs. 81.8%, P = 0.137, HR = 1.975, 95% CI, 0.976-3.995, Figure 2D).
Figure 2.

Survival rates of 188 nasopharyngeal carcinoma patients. (A) Overall survival, (B) failure-free survival, (C) distant metastasis-free survival, and (D) local relapse–free survival rates of patients with complete response of primary tumor (CRPT), partial response of primary tumor (PRPT), and stable disease of primary tumor (SDPT) at the end of radiotherapy. Hazard ratios (HR) were calculated with the unadjusted Cox proportional hazards model; P-values were calculated with the unadjusted log-rank test. 95% CI, 95% confidence interval.
To adjust for prognostic factors, the following parameters were introduced into the Cox regression model: age (≤ 50 vs. > 50 years), sex, chemotherapy (yes vs. no), radiation technique (2-DRT vs. 3-DCRT), boosting (yes vs. no), T stage (T1-2 vs. T3-4), N stage (N0-1 vs. N2-3), PT volume (< 19 cm3 vs. ≥ 19 cm3) and PT regression speed at the end of RT (CRPT vs. PRPT vs. SDPT). PT regression speed at the end of RT was the only independent prognostic factor of OS, FFS, and DMFS (P < 0.001, P = 0.001 and P = 0.004; Table 1).
Table 1.
Multivariate analyses of prognostic factors in 188 NPC patients
| Endpoint | Variable | Estimate | HR † | 95% CI* | P value ‡ |
|---|---|---|---|---|---|
| OS |
Regression speed |
0.778 |
2.177 |
1.480-3.203 |
< 0.001 |
| FFS |
Regression speed |
0.764 |
2.148 |
1.384-3.333 |
0.001 |
| DMFS |
Regression speed |
0.812 |
2.252 |
1.296-3.912 |
0.004 |
| LRFS | Regression speed | 0.681 | 1.975 | 0.976-3.995 | 0.058 |
†HR, Hazard ratio from Cox proportional hazards model; *CI, confidence interval; ‡ P-values were calculated using an adjusted Cox proportional hazards model. OS, Overall survival; FFS, Failure-free survival; DMFS, Distant metastasis-free survival; LRFS, Local relapse-free survival. The following parameters were included in the model as covariates for each analysis: age (≤ 50 vs. > 50 years), sex, chemotherapy (yes vs. no), radiation technique (2-DRT vs. 3-DCRT), boosting (yes vs. no), T stage (T1-2 vs. T3-4), N stage (N0-1 vs. N2-3), primary tumor volume (< 19 cm3 vs. ≥ 19 cm3) and primary tumor regression speed at the end of RT (CRPT vs. PRPT vs. SDPT). Table contains only the results for primary tumor regression speed and other statistically significant variables.
Prognosis comparison of patients with CRPT during RT and patients with CRPT only at the end of RT
Seventy-six patients with CRPT at the end of RT were divided into two groups: Group A (with CRPT during RT), 41 patients; Group B (with CRPT only at the end of RT), 35 patients. The 5-year OS, FFS, DMFS, and LRFS rates of Group A and B were 79.9% vs. 88.6% (P = 0.383), 80.2% vs. 97.1% (P = 0.033), 87.1% vs. 97.1% (P = 0.146), and 92.4% vs. 100% (P = 0.113), respectively, the details for which are listed in Table 2.
Table 2.
Survival outcomes in patients with CRPT during RT and only at the end of RT
| Variable | CRPT during RT (N = 41) | CRPT at the end of RT (N = 35) | HR † (95% CI*) | P ‡ |
|---|---|---|---|---|
| 5-yr OS rate |
79.9% |
88.6% |
0.590 (0.177-1.962) |
0.383 |
| 5-yr FFS rate |
80.2% |
97.1% |
0.144 (0.018-1.151) |
0.033 |
| 5-yr DMFS rate |
87.1% |
97.1% |
0.233 (0.027-1.992) |
0.146 |
| 5-yr LRFS rate | 92.4% | 100% | 0.018 (0.000-206.004) | 0.113 |
†HR, Hazard ratios calculated using the unadjusted Cox proportional hazards model; *CI, confidence interval; ‡P-values were calculated by the unadjusted log-rank test. RT, Radiotherapy; OS, Overall survival; FFS, Failure-free survival; DMFS, Distant metastasis-free survival; LRFS, Local relapse-free survival; CRPT, Complete response of primary tumor.
Value of boost irradiation in patients with persistent PT at the end of RT
One hundred and twelve patients had persistent PT at the end of RT; 27 were not treated with boost irradiation (Group C) and 85 were treated with boost irradiation (Group D). The 5-year OS, FFS, DMFS, and LRFS rates of Group C and D were 65.5% vs. 63.9% (P = 0.477), 73.1% vs. 67.7% (P = 0.579), 84.4% vs. 79.2% (P = 0.589), and 87.1% vs. 84.6% (P = 0.812), respectively; the details are listed in Table 3.
Table 3.
Prognosis of patients with persistent PT at RT end with and without boost irradiation
| Variable | Without boost irradiation (N = 27) | With boost irradiation (N = 85) | HR † (95% CI*) | P ‡ |
|---|---|---|---|---|
| 5-yr OS rate |
65.5% |
63.9% |
1.308 (0.622-2.748) |
0.477 |
| 5-yr FFS rate |
73.1% |
67.7% |
1.267 (0.547-2.934) |
0.579 |
| 5-yr DMFS rate |
84.4% |
79.2% |
1.350 (0.451-4.039) |
0.589 |
| 5-yr LRFS rate | 87.1% | 84.6% | 1.170 (0.322-4.252) | 0.812 |
†HR, Hazard ratios calculated using the unadjusted Cox proportional hazards model; *CI, confidence interval; ‡P-values were calculated by the unadjusted log-rank test. PT, Primary tumor; RT, Radiotherapy; OS, Overall survival; FFS, Failure-free survival; DMFS, Distant metastasis-free survival; LRFS, Local relapse-free survival.
Discussion
In NPC patients, the determination of a treatment regimen is mainly based on tumor stage. Clinical response is usually evaluated 3–4 months after RT. The lack of monitoring for tumor response during RT results in patients with poor tumor regression not receiving immediate strengthening treatment.
PT regression speed after RT
There are two treatment response evaluation criteria for solid tumors: that of the World Health Organization (WHO) and RECIST [13]. The WHO criteria adopt bidimensional measurement; RECIST adopts unidimensional measurement. Compared to the WHO criteria, that of RECIST underscores reevaluation and follow-up. Therefore, we adopted the RECIST criteria. In this study, 97.4% of patients had CRPT after 3–4 months of RT, similar to the 96.5%–100% reported in previous studies [14-16]. Further, we considered it important to observe the speed of CRPT. Although only 21.8% of patients had CRPT during RT and 40.4% of patients had CRPT at the end of RT, 97.4% of patients had CRPT after 3–4 months of RT. In the study of Kwong et al., 803 patients underwent serial post-RT nasopharyngeal biopsies. A high proportion of early positive histology remitted spontaneously, and 93.1% of patients had negative histology at week 12 after RT [17]. The phenomenon is well known as delayed regression.
Prognostic value of PT regression speed at the end of RT
In this study, PT regression speed at the end of RT was an independent prognostic factor of OS, FFS, and DMFS. In addition, CRPT at the end of RT suggested the best prognosis, followed by PRPT, and SDPT suggested the worst prognosis. Mäntylä et al. reported that, in head and neck cancers, prognosis was significantly more favorable when an advanced tumor had disappeared at the end of treatment [18]. Strengthening treatment may be administered immediately to patients with poor tumor regression. Although CCRT is the standard treatment for locoregionally advanced NPC, the role of adjuvant chemotherapy is debated [19-26]. The Phase III multicentre randomized controlled trial by Chen et al. helped resolve this issue, demonstrating that adjuvant chemotherapy did not contribute any benefit to CCRT. However, the median follow-up was only 37.8 months, and longer follow-up is needed to fully assess survival and late toxic effects [27]. Adjuvant chemotherapy, an important chemotherapy regimen, should not be abandoned in NPC patients; instead, it may be used to assess whether it confers survival benefit to patients with obvious persistent tumor at the end of RT in clinical trials.
The 5-year LRFS rates among the CRPT, PRPT, and SDPT groups were not significantly different (95.8% vs. 86.0% vs. 81.8%, P = 0.137). The study by Jaulerry et al. demonstrated that tumor regression during external RT was an independent predictive factor of local control in head and neck carcinomas [28]. Bataini et al. also confirmed that complete tumor clearance following RT is a reliable indicator of permanent local control for squamous cell carcinoma of the oropharynx and pharyngolarynx [29]. The differing results might be due to the following reasons: first, NPC possesses obviously unique pathological types as compared to other head and neck cancers, where > 95% of patients in endemic areas have nonkeratinizing NPC [30]. The treatment method and prognosis for this histologic subtype is very different from that of other subtypes. Second, distant metastasis, which is the main reason for treatment failure in NPC patients, usually occurs in the first two years after treatment [31]. Such patients might die due to distant metastasis before possible local relapse. Third, 5-year LRFS rates are as high as 86.8-94.9% in NPC patients [1,4]. This justifies the call for enrollment of a large number of patients for observing significant statistical differences in LRFS rates among groups. The 5-year LRFS rates for patients with CRPT, PRPT, and SDPT gradually declined (95.8% vs. 86.0% vs. 81.8%, P = 0.137), but with no statistical difference. However, the number of patients in the three groups was small, which might have led to false-negative results.
Prognostic comparison of rapid and slow regression
We compared the prognosis of patients with CRPT when the radiation dose was 46–50 Gy and those with CRPT only at the end of RT. The OS, DMFS, and LRFS rates of patients in the latter group were higher than that of patients in the former group, but the P-values were not significantly different; however, the FFS rate was significantly different (80.2% vs. 97.1%, P = 0.033). Wang et al. confirmed that the tumor regression rate during radical RT had significant predictive value for 5-year OS, FFS, DMFS and locoregional FFS. In that study, the prognosis of slow response was significantly better than that of rapid regression [32]. The exact radiobiological mechanism through which tumor regression speed influences prognosis in NPC patients remains unknown. Patients with rapid tumor regression might have higher incidence rates of distant metastasis. Li et al. divided NPC into four RT-related types: radiosensitive and non–metastasis-prone, radioresistant and non–metastasis-prone, radiosensitive and metastasis-prone, and radioresistant and metastasis-prone [33]. Local control was better when a tumor was radiosensitive and poorer when a tumor was radioresistant. As distant metastasis could also result in treatment failure, a curative effect might not be achieved in radiosensitive tumors for which excellent local control is obtained [33].
Value of boost irradiation in patients with persistent PT at the end of RT
In 1999, Tate et al. reported that stereotactic radiosurgical boost following fractionated external beam RT (EBRT) provided excellent local control in advanced-stage NPC [34]. Subsequent studies also supported the premise that stereotactic RT boost after EBRT results in excellent local control for patients with NPC [35,36]. Leung et al. and Yeo et al. both reported that brachytherapy boost supplementing radical EBRT improved local control in NPC patients with early T1-2B disease [37,38]. However, Schinagl et al. reported that while EBRT with endocavitary brachytherapy produced excellent rates of local control for T1-2 tumors, the high incidence of late toxicity suggested overtreatment [39]. In this study, boost irradiation did not confer any survival benefit to patients with persistent PT at the end of RT. It has been recommended that boost radiation to residual PTs be withheld unless positive biopsy samples persist at ≥10 weeks after RT [40].
Limitations
Due to limited medical resources, 2-DRT and 3-DCRT were used instead of IMRT. Although excellent local control can be achieved in NPC by IMRT, distant metastasis remains the major cause of treatment failure [1,2,4]. The number of patients in the two-subgroup analysis of prognostic comparison of patients with CRPT during RT to patients with CRPT only at the end of RT and the value of boost irradiation in patients with persistent PT at the end of RT was too small, which might have led to false-negative results. Moreover, this was a retrospective study, and the conclusions need to be confirmed by future prospective studies.
Conclusions
PT regression speed at the end of RT was an independent prognostic factor of OS, FFS, and DMFS in NPC patients. Immediate strengthening treatment may be administered to patients with poor tumor regression at the end of RT. Patients with fast tumor regression could obtain excellent local control but a curative effect might still not be achieved due to distant metastasis. There is a need for awareness of the requirement of boost irradiation after radical RT because of the high incidence of late toxicity. However, this was a retrospective study with a limited number of patients, and future studies focusing on tumor regression speed and its prognostic impact are warranted.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
NZ and S-BL participated in literature research, study design, data collection, data analysis, interpretation of findings and the draft of the manuscript. Y-MD, H-YC, Z-QL, SQL and LY carried out the data collection. R-LL and HZ reviewed MR images. D-SL performed the statistical analysis. YC contributed with study design, data collection, interpretation of findings and critical edit of the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Ning Zhang, Email: zning@fsyyy.com.
Shao-Bo Liang, Email: drshaoboliang@hotmail.com.
Yan-Ming Deng, Email: fsdyming@163.com.
Rui-Liang Lu, Email: luruiliang126@126.com.
Hai-Yang Chen, Email: chenhaiyangjlu@163.com.
Hai Zhao, Email: woaixiaoduoduo@yahoo.cn.
Zhi-Qian Lv, Email: lzhiqian@fsyyy.com.
Shao-Qiang Liang, Email: lsqiang@fsyyy.com.
Lin Yang, Email: yanglin90412@163.com.
Dong-Sheng Liu, Email: ldsheng@fsyyy.com.
Yong Chen, Email: chenyong@sysucc.org.cn.
Acknowledgements
The study was supported by grants from the Science Foundation from the Sci-Tech Office of Guangdong Province (No. 2010B080701014), the Science Foundation from the Sci-Tech Office of Foshan City (No. 200908048), and the Science Foundation from the Health Bureau of Foshan City (No. 2013064).
References
- Lai SZ, Li WF, Chen L, Luo W, Chen YY, Liu LZ, Sun Y, Lin AH, Liu MZ, Ma J. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. 2011;80:661–668. doi: 10.1016/j.ijrobp.2010.03.024. [DOI] [PubMed] [Google Scholar]
- Peng G, Wang T, Yang KY, Zhang S, Zhang T, Li Q, Han J, Wu G. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol. 2012;104:286–293. doi: 10.1016/j.radonc.2012.08.013. [DOI] [PubMed] [Google Scholar]
- Fang FM, Chien CY, Tsai WL, Chen HC, Hsu HC, Lui CC, Huang TL, Huang HY. Quality of life and survival outcome for patients with nasopharyngeal carcinoma receiving three-dimensional conformal radiotherapy vs. intensity-modulated radiotherapy-a longitudinal study. Int J Radiat Oncol Biol Phys. 2008;72:356–364. doi: 10.1016/j.ijrobp.2007.12.054. [DOI] [PubMed] [Google Scholar]
- Xiao WW, Huang SM, Han F, Wu SX, Lu LX, Lin CG, Deng XW, Lu TX, Cui NJ, Zhao C. Local control, survival, and late toxicities of locally advanced nasopharyngeal carcinoma treated by simultaneous modulated accelerated radiotherapy combined with cisplatin concurrent chemotherapy: long-term results of a phase 2 study. Cancer. 2011;117:1874–1883. doi: 10.1002/cncr.25754. [DOI] [PubMed] [Google Scholar]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- Chen L, Mao YP, Xie FY, Liu LZ, Sun Y, Tian L, Tang LL, Lin AH, Li L, Ma J. The seventh edition of the UICC/AJCC staging system for nasopharyngeal carcinoma is prognostically useful for patients treated with intensity-modulated radiotherapy from an endemic area in China. Radiother Oncol. 2012;104:331–337. doi: 10.1016/j.radonc.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Chua DT, Sham JS, Kwong DL, Tai KS, Wu PM, Lo M, Yung A, Choy D, Leong L. Volumetric analysis of tumor extent in nasopharyngeal carcinoma and correlation with treatment outcome. Int J Radiat Oncol Biol Phys. 1997;39:711–719. doi: 10.1016/S0360-3016(97)00374-X. [DOI] [PubMed] [Google Scholar]
- Guo R, Sun Y, Yu XL, Yin WJ, Li WF, Chen YY, Mao YP, Liu LZ, Li L, Lin AH, Ma J. Is primary tumor volume still a prognostic factor in intensity modulated radiation therapy for nasopharyngeal carcinoma? Radiother Oncol. 2012;104:294–299. doi: 10.1016/j.radonc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Liang SB, Deng YM, Zhang N, Lu RL, Zhao H, Chen HY, Li SE, Liu DS, Chen Y. Prognostic significance of maximum primary tumor diameter in nasopharyngeal carcinoma. BMC Cancer. 2013;13:260. doi: 10.1186/1471-2407-13-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong VF, Zhou JY, Khoo JB, Chan KL, Huang J. Correlation between MR imaging-derived nasopharyngeal carcinoma tumor volume and TNM system. Int J Radiat Oncol Biol Phys. 2006;64:72–76. doi: 10.1016/j.ijrobp.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Chai SJ, Pua KC, Saleh A, Yap YY, Lim PV, Subramaniam SK, Lum CL, Krishnan G, Mahiyuddin WR, Teo SH, Khoo AS, Yap LF. Malaysian NPC Study Group. Clinical significance of plasma Epstein-Barr Virus DNA loads in a large cohort of Malaysian patients with nasopharyngeal carcinoma. J Clin Virol. 2012;55:34–39. doi: 10.1016/j.jcv.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Leung SF, Chan AT, Zee B, Ma B, Chan LY, Johnson PJ, Lo YM. Pretherapy quantitative measurement of circulating Epstein-Barr virus DNA is predictive of posttherapy distant failure in patients with early-stage nasopharyngeal carcinoma of undifferentiated type. Cancer. 2003;98:288–291. doi: 10.1002/cncr.11496. [DOI] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the United States, national cancer institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- Chen QY, Wen YF, Guo L, Liu H, Huang PY, Mo HY, Li NW, Xiang YQ, Luo DH, Qiu F, Sun R, Deng MQ, Chen MY, Hua YJ, Guo X, Cao KJ, Hong MH, Qian CN, Mai HQ. Concurrent chemoradiotherapy vs radiotherapy alone in stage II nasopharyngeal carcinoma: phase III randomized trial. J Natl Cancer Inst. 2011;103:1761–1770. doi: 10.1093/jnci/djr432. [DOI] [PubMed] [Google Scholar]
- Lim AM, Corry J, Collins M, Peters L, Hicks RJ, D’Costa I, Coleman A, Chua M, Solomon B, Rischin D. A phase II study of induction carboplatin and gemcitabine followed by chemoradiotherapy for the treatment of locally advanced nasopharyngeal carcinoma. Oral Oncol. 2013;49:468–474. doi: 10.1016/j.oraloncology.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Chen Y, Liu MZ, Liang SB, Zong JF, Mao YP, Tang LL, Guo Y, Lin AH, Zeng XF, Ma J. Preliminary results of a prospective randomized trial comparing concurrent chemoradiotherapy plus adjuvant chemotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma in endemic regions of china. Int J Radiat Oncol Biol Phys. 2008;71:1356–1364. doi: 10.1016/j.ijrobp.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Kwong DL, Nicholls J, Wei WI, Chua DT, Sham JS, Yuen PW, Cheng AC, Wan KY, Kwong PW, Choy DT. The time course of histologic remission after treatment of patients with nasopharyngeal carcinoma. Cancer. 1999;85:1446–1453. doi: 10.1002/(SICI)1097-0142(19990401)85:7<1446::AID-CNCR4>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Mäntylä M, Kortekangas AE, Valavaara RA, Nordman EM. Tumor regression during radiation treatment as a guide to prognosis. Br J Radiol. 1979;52:972–977. doi: 10.1259/0007-1285-52-624-972. [DOI] [PubMed] [Google Scholar]
- Chan AT, Teo PM, Ngan RK, Leung TW, Lau WH, Zee B, Leung SF, Cheung FY, Yeo W, Yiu HH, Yu KH, Chiu KW, Chan DT, Mok T, Yuen KT, Mo F, Lai M, Kwan WH, Choi P, Johnson PJ. Concurrent chemotherapy-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: progression-free survival analysis of a phase III randomized trial. J Clin Oncol. 2002;20:2038–2044. doi: 10.1200/JCO.2002.08.149. [DOI] [PubMed] [Google Scholar]
- Chan AT, Leung SF, Ngan RK, Teo PM, Lau WH, Kwan WH, Hui EP, Yiu HY, Yeo W, Cheung FY, Yu KH, Chiu KW, Chan DT, Mok TS, Yau S, Yuen KT, Mo FK, Lai MM, Ma BB, Kam MK, Leung TW, Johnson PJ, Choi PH, Zee BC. Overall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst. 2005;97:536–539. doi: 10.1093/jnci/dji084. [DOI] [PubMed] [Google Scholar]
- Lin JC, Jan JS, Hsu CY, Liang WM, Jiang RS, Wang WY. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol. 2003;21:631–637. doi: 10.1200/JCO.2003.06.158. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhao C, Peng PJ, Lu LX, Huang PY, Han F, Wu SX. Phase III study comparing standard radiotherapy with or without weekly oxaliplatin in treatment of locoregionally advanced nasopharyngeal carcinoma: preliminary results. J Clin Oncol. 2005;23:8461–8468. doi: 10.1200/JCO.2004.00.3863. [DOI] [PubMed] [Google Scholar]
- Baujat B, Audry H, Bourhis J, Chan AT, Onat H, Chua DT, Kwong DL, Al-Sarraf M, Chi KH, Hareyama M, Leung SF, Thephamongkhol K, Pignon JP. MAC-NPC Collaborative Group. Chemotherapy in locally advanced nasopharyngeal carcinoma: an individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys. 2006;64:47–56. doi: 10.1016/j.ijrobp.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Langendijk JA, Leemans CR, Buter J, Berkhof J, Slotman BJ. The additional value of chemotherapy to radiotherapy in locally advanced nasopharyngeal carcinoma: a meta-analysis of the published literature. J Clin Oncol. 2004;22:4604–4612. doi: 10.1200/JCO.2004.10.074. [DOI] [PubMed] [Google Scholar]
- Rossi A, Molinari R, Boracchi P, del Vecchio M, Marubini E, Nava M, Morandi L, Zucali R, Pilotti S, Grandi C. Adjuvant chemotherapy with vincristine, cyclophosphamide and doxorubicin after radiotherapy in local-regional nasopharyngeal cancer: results of a 4 year multi-center randomized study. J Clin Oncol. 1988;6:1401–1410. doi: 10.1200/JCO.1988.6.9.1401. [DOI] [PubMed] [Google Scholar]
- Chi KH, Chang YC, Guo WY, Leung MJ, Shiau CY, Chen SY, Wang LW, Lai YL, Hsu MM, Lian SL, Chang CH, Liu TW, Chin YH, Yen SH, Perng CH, Chen KY. A phase III study of adjuvant chemotherapy in advanced nasopharyngeal carcinoma patients. Int J Radiat Oncol Biol Phys. 2002;52:1238–1244. doi: 10.1016/S0360-3016(01)02781-X. [DOI] [PubMed] [Google Scholar]
- Chen L, Hu CS, Chen XZ, Hu GQ, Cheng ZB, Sun Y, Li WX, Chen YY, Xie FY, Liang SB, Chen Y, Xu TT, Li B, Long GX, Wang SY, Zheng BM, Guo Y, Sun Y, Mao YP, Tang LL, Chen YM, Liu MZ, Ma J. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2012;13:163–171. doi: 10.1016/S1470-2045(11)70320-5. [DOI] [PubMed] [Google Scholar]
- Jaulerry C, Dubray B, Brunin F, Rodriguez J, Point D, Blaszka B, Asselain B, Mosseri V, Brugere J, Cosset JM. Prognostic value of tumor regression during radiotherapy for head and neck cancer: a prospective study. Int J Radiat Oncol Biol Phys. 1995;33:271–279. doi: 10.1016/0360-3016(95)00157-T. [DOI] [PubMed] [Google Scholar]
- Bataini JP, Jaulerry C, Brunin F, Ponvert D, Ghossein NA. Significance and therapeutic implications of tumor regression following radiotherapy in patients treated for squamous cell carcinoma of the oropharynx and pharyngolarynx. Head Neck. 1990;12:41–49. doi: 10.1002/hed.2880120106. [DOI] [PubMed] [Google Scholar]
- Sun Y, Tang LL, Chen L, Li WF, Mao YP, Liu LZ, Lin AH, Li L, Ma J. Promising treatment outcomes of intensity-modulated radiation therapy for nasopharyngeal carcinoma patients with N0 disease according to the seventh edition of the AJCC staging system. BMC Cancer. 2012;12:68. doi: 10.1186/1471-2407-12-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AW, Poon YF, Foo W, Law SC, Cheung FK, Chan DK, Tung SY, Thaw M, Ho JH. Retrospective analysis of 5037 patients with nasopharyngeal carcinoma treated during 1976–1985: overall survival and patterns of failure. Int J Radiat Oncol Biol Phys. 1992;23:261–270. doi: 10.1016/0360-3016(92)90740-9. [DOI] [PubMed] [Google Scholar]
- Wang XS, Hu CS, Wu YR, Feng Y. Influence of speed in tumor regression on prognosis of nasopharyngeal carcinoma. Chin J of Radiat Oncol. 2005;14:6–9. [Google Scholar]
- Li ZQ, Xia YF, Liu Q, Yi W, Liu XF, Han F, Luo W, Lu TX. Radiotherapy-related typing in 842 patients in canton with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2006;66:1011–1016. doi: 10.1016/j.ijrobp.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Tate DJ, Adler JR Jr, Chang SD, Marquez S, Eulau SM, Fee WE, Pinto H, Goffinet DR. Stereotactic radiosurgical boost following radiotherapy in primary nasopharyngeal carcinoma: impact on local control. Int J Radiat Oncol Biol Phys. 1999;45:915–921. doi: 10.1016/S0360-3016(99)00296-5. [DOI] [PubMed] [Google Scholar]
- Hara W, Loo BW Jr, Goffinet DR, Chang SD, Adler JR, Pinto HA, Fee WE, Kaplan MJ, Fischbein NJ, Le QT. Excellent local control with stereotactic radiotherapy boost after external beam radiotherapy in patients with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2008;71:393–400. doi: 10.1016/j.ijrobp.2007.10.027. [DOI] [PubMed] [Google Scholar]
- Yau TK, Sze WM, Lee WM, Yeung MW, Leung KC, Hung WM, Chan WI. Effectiveness of brachytherapy and fractionated stereotactic radiotherapy boost for persistent nasopharyngeal carcinoma. Head Neck. 2004;26:1024–1030. doi: 10.1002/hed.20093. [DOI] [PubMed] [Google Scholar]
- Leung TW, Wong VY, Sze WK, Lui CM, Tung SY. High-dose-rate intracavitary brachytherapy boost for early T stage nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2008;70:361–367. doi: 10.1016/j.ijrobp.2007.06.078. [DOI] [PubMed] [Google Scholar]
- Yeo R, Fong KW, Hee SW, Chua ET, Tan T, Wee J. Brachytherapy boost for T1/T2 nasopharyngeal carcinoma. Head Neck. 2009;31:1610–1618. doi: 10.1002/hed.21130. [DOI] [PubMed] [Google Scholar]
- Schinagl DA, Marres HA, Kappelle AC, Merkx MA, Pop LA, Verstappen SM, Kaanders JH. External beam radiotherapy with endocavitary boost for nasopharyngeal cancer: treatment results and late toxicity after extended follow-up. Int J Radiat Oncol Biol Phys. 2010;78:689–695. doi: 10.1016/j.ijrobp.2009.08.072. [DOI] [PubMed] [Google Scholar]
- Sham JS, Wei WI, Kwan WH, Chan CW, Kwong WK, Choy D. Nasopharyngeal carcinoma. Pattern of tumor regression after radiotherapy. Cancer. 1990;65:216–220. doi: 10.1002/1097-0142(19900115)65:2<216::AID-CNCR2820650206>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]


