Abstract
Scope
In contrast to well-characterized polyunsaturated fatty acid (PUFA) levels in serum, little is known regarding their downstream metabolic products. However, many of these compounds are lipid mediators with prominent roles during pro- and anti-inflammatory processes.
Methods and Results
In this double blind crossover study on asthmatics, shifts in serum levels of ω-3 and ω-6 PUFA-derived oxidized fatty acids (e.g., eicosanoids, oxylipins) were quantified following dietary fish oil supplementation. Serum was obtained from subjects following fasting at 3 occasions; 1) Prior to supplementation, 2) Following a 3-week supplement intake of either placebo or fish oil, and 3) Following a 3-week washout period with a subsequent 3-week period of either fish oil or placebo supplement. A total of 87 oxylipins representing cyclooxygenase (COX), lipoxygenase (LOX) and cytochrome P450 (CYP) metabolic pathways were screened via liquid chromatography-tandem mass spectrometry (LC-MS/MS). The primary alterations observed were in CYP- and 15-LOX-derived EPA- and CYP-derived DHA oxylipins.
Conclusion
The results indicate that intake of an ω-3 rich diet altars not only the PUFA ratio, but also the ratio of downstream oxylipins. These data further support that dietary manipulation with ω-3 PUFAs affects not only PUFA levels, but importantly also the downstream metabolic profile.
Keywords: asthma, eicosanoid, lipidomics, omega-3, oxylipin
INTRODUCTION
The potential impacts upon health and disease of the long-chain omega-3 fatty acids (ω-3 FAs) have become an area of intense investigation [1-3]. The American Heart Association recommends that all adults eat fish at least twice per week, as well as vegetables containing plant-derived ω-3 FAs, and that patients with documented heart disease consume fish oil capsules [4, 5]. To date, the majority of studies have focused on the parent polyunsaturated fatty acid (PUFA) vs. the downstream metabolic products. However, there is a growing interest in exploring the biology associated with ω-6 and ω-3 FA-derived oxidized lipid metabolites (e.g., eicosanoids, oxylipins) [6-8]. It is well known that ω-6 FA-derived oxylipins from in particular the arachidonic acid (AA) cascade (e.g., leukotrienes, prostaglandins) have prominent roles during the inflammatory process [9-12]. In particular, eicosanoids are linked to the incidence of inflammatory events, and correlate to a number of diseases including atherosclerosis, diabetes, coronary heart disease, hypertension and obesity [13-18]. In contrast, data suggest that oxylipins derived from ω-3 FAs (e.g., resolvins and protectins) are involved in the resolution phase of inflammation, providing evidence for their beneficial health effects in the diet [19-21]. Much of the research performed to date has focused on the ω-3 FAs EPA and DHA, the so-called fish oils, although there are other sources of plant-derived ω-3 FAs in the diet (e.g., α-linolenic acid [ALA] from soybean, rapeseed and flaxseed).
The ω-6 and ω-3 FA-derived oxylipins are formed via the same 3 main enzymatic pathways: cyclooxygenase (COX), lipoxygenase (LOX) and cytochrome P450 (CYP), suggesting that lipid mediator formation can be affected by substrate abundance. It is therefore likely that the balance between ω-6 and ω-3 FAs and their downstream metabolic products are connected to disease etiology. However, to date, only a few studies have investigated how the intake of ω-3 FAs affects the overall profile of downstream oxylipins [8, 22, 23]. Accordingly, additional information is required to establish the baseline levels of ω-3 FA-derived oxylipins in clinical samples from both healthy and diseased groups as well as the effect of dietary changes.
In this double blind, randomized, placebo controlled crossover study we investigated serum levels of ω-3 and ω-6 FA-derived oxylipins in clinically mild to moderate asthmatic individuals at baseline and following placebo- and ω-3 FA-supplementation. An oxylipin metabolic profiling approach was applied in which a broad selection of compounds were quantified, representing multiple components of the relevant biosynthetic pathways (LOX, COX and CYP). Multivariate statistics were used to probe the relationship between oxylipin and PUFA levels. This report provides useful data on oxylipin serum ratios in a mild asthmatic population, and relative intra-individual shifts in ω-6/ω-3 FA-derived lipid mediators following dietary ω-3 FA supplementation. In addition, it presents one of the few oxylipin metabolic profiling studies performed to date in human serum.
MATERIALS AND METHODS
Clinical data and study design
A total of 25 clinically stable mild-moderate asthmatic patients (age 20-54 years, 11 females, BMI 19-39) were included in the study (Table 1). Inclusion criteria were non-smoking subjects with clinically diagnosed asthma and current asthma symptoms with a forced expiratory volume in one second (FEV1) >70% of predicted values. Exclusion criteria included significant gastrointestinal, hematological, cardiovascular, cerebrovascular or other system disorders. Additionally, smoking, regular fish or ω-3 FA supplement consumption, as well as intake of leukotriene receptor antagonists 4 weeks prior to study initiation were excluded. Blood sampling following 12-14 h fasting was performed at 3 occasions; 1) Prior to supplementation (baseline), 2) Following a 3 week supplement intake of A: placebo or B: ω-3 FA, and 3) Following a 3 week washout period (normal diet) with a subsequent 3 week period of A: ω-3 FA or B: placebo supplement. The 3-week washout period was chosen based upon previously reported studies for DHA and EPA [24-27] as well as the normal washout time between successive provocations in order to regain baseline airway responsiveness [28]. Case A and B were randomized. ω-3 FA (400 mg of EPA and 200 mg DHA/capsule; 40/20EE capsules; product code 4020PB1000CT; QC Lot # QC48781 [Ocean Nutrition, Nova Scotia, Canada]; equated to a daily dose of 4.0g EPA and 2.0g DHA) and placebo (50:50 mix of soybean and corn oil; product code PLACEBO1000; QC Lot # QC48748; Ocean Nutrition) supplements were administered daily in the form of 10 capsules during the supplement periods. Five capsules were taken in the morning and 5 capsules in the evening, with 12h between evening capsule consumption and blood draw. Blood was collected immediately upon patient arrival in the clinic. Samples were collected in standard non-heparinzed tubes (Vacutainer, Becton Dickinson), allowed to sit for 1 hr and spun at room temperature at 1000g for 15 min. Serum was aliquoted in eppendorf tubes via a micropipette and stored at −80°C until analysis. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Ethics Review Board of St Joseph's Healthcare, Hamilton (R.P. #06-2750) and by Health Canada (Approval No. 120532). Written informed consent was obtained from all subjects. More details of the study design and primary endpoint are available from The Clinical Trial Registration (http://www.clinicaltrials.gov; Identifier Number NCT00526357) [29].
Table 1.
Clinical data of participating study subjects
| Subject | Gender1 | Age2 | BMI3 | FEV1 (%Pred)4 |
|---|---|---|---|---|
| 1 | M | 31 | 30 | 75.6 |
| 2 | M | 38 | 23 | 88.6 |
| 3 | F | 43 | 26 | 84.7 |
| 4 | F | 26 | 30 | 80.3 |
| 5 | M | 23 | 27 | 102.6 |
| 6 | M | 30 | 37 | 76.4 |
| 7 | F | 33 | 35 | 88.1 |
| 8 | M | 24 | 28 | 98.0 |
| 9 | F | 28 | 22 | 72.1 |
| 10 | F | 21 | 37 | 71.1 |
| 11 | M | 20 | 33 | 84.7 |
| 12 | M | 28 | 34 | 89.6 |
| 13 | M | 26 | 27 | 110.0 |
| 14 | F | 33 | 25 | 81.8 |
| 15 | M | 29 | 19 | 82.5 |
| 16 | F | 22 | 22 | 99.5 |
| 17 | F | 24 | 20 | 81.1 |
| 18 | M | 54 | 27 | 76.8 |
| 19 | M | 47 | 26 | 76.2 |
| 20 | M | 19 | 39 | 70.2 |
| 21 | F | 39 | 23 | 103.2 |
| 22 | M | 26 | 30 | 79.8 |
| 23 | M | 22 | 25 | 74.5 |
| 24 | F | 42 | 32 | 87.8 |
| 25 | F | 21 | 24 | 87.8 |
Female (F) and Male (M)
Years
Body mass index: [mass(kg)]/[height(m)]2
Percent predicted Forced Expiatory Volume in one second. FEV1% predicted is defined as FEV1% of the patient divided by the average FEV1% in the population for any person of similar age, sex and body composition
Oxylipin extraction and analysis
External standards, deuterated internal standards and the technical standard N-cyclohexyl-N'-dodecanoic acid urea (CUDA) were obtained from Cayman Chemical (Ann Arbor, MI, USA), Larodan Fine Chemicals AB (Malmö, Sweden), Biomol International (Plymouth Meeting, PA, USA) or synthesized in-house [30]. Off-line SPE-extraction was performed on 220 μL serum aliquots using Waters Oasis-HBL 60 mg cartridge columns (Milford, MA, USA) as previously described [30, 31]. A detailed description of the instrument method is given elsewhere [30]. An Agilent 1200 SL separation module (Santa Clara, CA, USA) coupled to an ABI QTRAP® 4000 hybrid triple quadrupole/linear ion trap mass spectrometer (Foster City, CA, USA) was used for analyses and separation was performed via a 2.1 × 150 mm Eclipse Plus C18 column with a 1.8 μm particle size (Agilent, Santa Clara, CA, USA). Oxylipins were quantified using stable isotope internal standard methods as previously described [30].
PUFA extraction and analysis
Fatty acid compositions of total serum phospholipids were determined in the laboratory of Bruce Holub at the University of Guelph based upon previous methods [32]. Lipids were extracted from the serum samples according to the method of Folch et al. [33] and the serum phospholipids were separated from the neutral lipids by thin-layer chromatography [32]. The fatty acid methyl esters were prepared from the isolated phospholipid fraction by the method of Morrison and Smith et al. [34] and were analyzed on a Varian 3400 gas-liquid chromatograph (Palo Alto, CA) with a 60 m DB-23 capillary column (0.32 mm internal diameter).
Statistical methods
Univariate statistical analysis was performed using Student's paired t-test. Multivariate analyses by orthogonal projections to latent structures (OPLS) were performed using SIMCA v.13.0 (Umetrics, Umeå, Sweden) following log transformation, mean centering and UV scaling [35]. Model performance was reported as cumulative correlation coefficients for the model (R2), predictive performance based on cross validation calculations (Q2), and cross validated analysis of variance (CV-ANOVA).
RESULTS
Levels of EPA and DHA increased in the serum phospholipids following ω-3 FA supplementation
Levels of FAs in the serum phospholipids are provided on a percent composition basis in Table 2 for placebo and ω-3 FA supplementation (baseline values were not assessed). As expected, supplementation with EPA/DHA resulted in a concomitant increase in these species in serum indicating that all patients were compliant with capsule consumption. The percentage of EPA following supplementation was 5.7% relative to 1.0% for placebo, whereas the DHA levels were 6.2% and 3.7%, respectively (reflecting the higher dosing of EPA in the capsules). The overall percentage of ω-6 FAs was lower following ω-3 FA supplementation, driven primarily by decreases in linoleic and arachidonic acid.
Table 2.
Free fatty acid levels (%) in serum phospholipids1
| Free Fatty Acid2 | ω-3 Supplemented | Placebo | p-value | ||
|---|---|---|---|---|---|
| Average (%) | CV (%) | Average (%) | CV (%) | ||
| C14:0 | 0.39 | 29% | 0.41 | 29% | 5.22E-01 |
| C14:1 | 0.00 | 271% | 0.00 | 304% | 9.73E-01 |
| C15:0 | 0.25 | 20% | 0.27 | 18% | 3.66E-01 |
| C16:0 | 27.77 | 4% | 27.63 | 5% | 7.15E-01 |
| C16:1 | 0.33 | 41% | 0.37 | 33% | 3.53E-01 |
| C18:0 | 14.02 | 7% | 13.84 | 9% | 5.85E-01 |
| C18:1 | 10.64 | 9% | 11.43 | 9% | 5.30E-03 |
| C18:2n6 (LA) | 18.34 | 17% | 22.73 | 12% | 2.62E-06 |
| C18:3n6 | 0.02 | 140% | 0.07 | 91% | 8.31E-04 |
| C18:3n3 (ALA) | 0.21 | 46% | 0.24 | 42% | 1.64E-01 |
| C18:4n3 | 0.02 | 234% | 0.01 | 287% | 6.46E-01 |
| C20:0 | 0.14 | 71% | 0.11 | 103% | 2.45E-01 |
| C20:1 | 0.12 | 98% | 0.14 | 72% | 6.69E-01 |
| C20:2n6 | 0.19 | 84% | 0.26 | 65% | 1.43E-01 |
| C20:3n6 (DGLA) | 2.11 | 32% | 3.28 | 22% | 3.40E-07 |
| C20:4n6 (AA) | 9.94 | 14% | 11.58 | 19% | 2.93E-03 |
| C20:3n3 | 0.01 | 391% | 0.01 | 414% | 6.40E-01 |
| C20:4n3 | 0.11 | 95% | 0.09 | 82% | 4.67E-01 |
| C20:5n3 (EPA) | 5.72 | 41% | 1.01 | 40% | 3.10E-10 |
| C22:0 | 0.48 | 39% | 0.39 | 31% | 7.54E-02 |
| C22:1 | 0.00 | 500% | 0.00 | 500% | 6.38E-01 |
| C22:2n6 | 0.02 | 199% | 0.03 | 145% | 3.95E-01 |
| C22:4n6 | 0.15 | 71% | 0.34 | 28% | 2.86E-08 |
| C22:5n6 | 0.02 | 293% | 0.02 | 340% | 8.07E-01 |
| C22:5n3 | 1.50 | 21% | 0.91 | 20% | 8.44E-10 |
| C22:6n3 (DHA) | 6.24 | 19% | 3.72 | 25% | 4.66E-11 |
| C24:0 | 0.46 | 46% | 0.43 | 29% | 5.27E-01 |
| C24:1 | 0.78 | 28% | 0.66 | 26% | 3.39E-02 |
| Total | 100 | 0% | 100 | 0% | 1.00E+00 |
| Saturated | 43.51 | 2% | 43.09 | 2% | 7.76E-02 |
| Monounsaturated | 11.88 | 8% | 12.60 | 8% | 1.71E-02 |
| Polyunsaturated | 44.61 | 3% | 44.31 | 3% | 3.88E-01 |
| Total ω-3 | 13.81 | 23% | 5.98 | 19% | 1.21E-12 |
| Total ω-6 | 30.80 | 9% | 38.33 | 4% | 1.17E-13 |
| EPA + DHA | 11.96 | 25% | 4.72 | 23% | 3.08E-12 |
| ω-3/ω-6 | 0.46 | 31% | 0.16 | 21% | 5.92E-11 |
| AA/[EPA + DHA]3 | 0.83 | 66% | 2.5 | 38% | 3.13E-11 |
Levels of free fatty acids in serum are shown as percent composition of the total amount of species quantified. P-values in bold represent species that were significantly shifted at the p<0.05 level.
Free fatty acid species are shown as the number of carbon atoms in the alkyl chain, number of double bonds, and number of unsaturations (e.g., C18:2n6 is linoleic acid with an 18 carbon alkyl chain containing two double bonds and it is an ω-6 fatty acid).
Values are given as a ratio of the percentages.
Supplementation with ω-3 FAs increased serum levels of ω-3-derived oxylipin
Of the 87 oxylipins screened in serum, 41 species were detected above the method limit of quantification (mLOQ). The oxylipin levels (from all 3 sampling times combined) ranged over 3 orders of magnitude from ~50 pM to ~40 nM (Table 3). Average concentrations (nM) and coefficients of variance (CVs) are given for the 3 sampling conditions in Table 3 (i.e., Baseline [B], placebo supplement [P] and ω-3 FA supplement [ω-3]). It should be stressed that all oxylipins were quantified as the free fatty acid according to previously published methods [30]. There were no significant changes in the levels of any oxylipin species between baseline and ω-3 FA diet between individuals at time points 1 or 2.
Table 3. Oxylipin concentrations in serum (nM).
Baseline (B), Placebo supplement (P) and ω-3 supplement (ω-3)
| Class | PUFA | Oxylipin | Baseline (B) | Placebo supplement (P) | ω-3 supplement (ω-3) | p-values | Fold Change [Cω-3]/[CP]1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Av2 | CV3 | Av | CV | Av | CV | B/P | B/ω-3 | P/ω-3 | Av | CV | |||
| ω-3 | ALA | 9-HOTrE | 0.71 | 86 | 0.71 | 116 | 0.53 | 50 | 9.9E-01 | 1.7E-01 | 1.9E-01 | 1.0 | 57 |
| 13-HOTrE | 0.80 | 90 | 0.74 | 111 | 0.51 | 52 | 8.4E-01 | 7.8E-02 | 8.8E-02 | 0.9 | 47 | ||
| 15(16)-EpODE | 3.9 | 83 | 3.7 | 115 | 2.4 | 75 | 9.1E-01 | 6.8E-02 | 3.2E-02 | 0.8 | 52 | ||
| 15,16-DiHODE | 7.1 | 47 | 8.7 | 78 | 6.2 | 54 | 3.4E-01 | 2.9E-01 | 1.4E-02 | 0.8 | 32 | ||
| Sum (n=4) | 13 | 53 | 14 | 90 | 10 | 54 | 6.6E-01 | 1.2E-01 | 2.2E-02 | 0.8 | 33 | ||
| EPA | 12-HEPE | 2.4 | 291 | 0.55 | 87 | 6.9 | 188 | 2.0E-01 | 1.3E-01 | 2.2E-02 | 27 | 228 | |
| 15-HEPE | 0.07 | 129 | 0.10 | 114 | 0.56 | 88 | 3.2E-01 | 1.3E-05 | 2.4E-05 | 17 | 112 | ||
| 11,12-DiHETE | 0.04 | 83 | 0.05 | 103 | 0.22 | 59 | 2.7E-01 | 1.1E-07 | 2.6E-07 | 6.1 | 68 | ||
| 14,15-DiHETE | 0.07 | 60 | 0.09 | 87 | 0.36 | 55 | 3.7E-01 | 6.5E-08 | 2.2E-07 | 4.9 | 58 | ||
| 17,18-DiHETE | 0.47 | 48 | 0.56 | 80 | 2.2 | 59 | 3.9E-01 | 3.9E-07 | 5.1E-07 | 4.5 | 58 | ||
| Sum (n=5) | 3.1 | 236 | 1.3 | 73 | 10.2 | 141 | 2.6E-01 | 3.1E-02 | 5.1E-03 | 9.0 | 124 | ||
| DHA | 10(11)-EpDPE | 0.25 | 74 | 0.24 | 111 | 0.47 | 77 | 9.2E-01 | 4.2E-03 | 7.2E-03 | 2.5 | 58 | |
| 4,5-DiHDPE | 0.79 | 79 | 0.91 | 75 | 2.0 | 57 | 5.0E-01 | 1.5E-05 | 4.2E-05 | 2.7 | 48 | ||
| 10,11-DiHDPE | 0.19 | 71 | 0.24 | 154 | 0.38 | 55 | 5.2E-01 | 2.2E-05 | 8.1E-02 | 2.4 | 52 | ||
| 13,14-DiHDPE | 0.22 | 63 | 0.23 | 76 | 0.38 | 41 | 8.0E-01 | 9.8E-06 | 2.6E-04 | 2.0 | 41 | ||
| 16,17-DiHDPE | 0.30 | 49 | 0.33 | 76 | 0.50 | 40 | 6.0E-01 | 1.6E-05 | 4.3E-03 | 1.7 | 38 | ||
| Sum (n=5) | 1.7 | 65 | 1.9 | 85 | 3.7 | 49 | 6.1E-01 | 4.6E-06 | 1.8E-04 | 2.3 | 39 | ||
| ω-6 | LA | 9,12,13-TriHOME | 3.5 | 67 | 3.3 | 56 | 3.2 | 55 | 7.5E-01 | 6.3E-01 | 8.9E-01 | 1.2 | 52 |
| 9,10,13-TriHOME | 1.7 | 60 | 1.6 | 59 | 1.6 | 55 | 7.0E-01 | 8.0E-01 | 8.9E-01 | 1.3 | 63 | ||
| 9-HODE | 13 | 81 | 13 | 103 | 9 | 43 | 9.7E-01 | 1.2E-01 | 1.1E-01 | 0.9 | 46 | ||
| 13-HODE | 23 | 79 | 23 | 83 | 17 | 49 | 9.3E-01 | 5.0E-02 | 5.8E-02 | 0.9 | 42 | ||
| 9-KODE | 2.9 | 122 | 2.2 | 153 | 1.9 | 104 | 5.2E-01 | 2.0E-01 | 6.5E-01 | 1.5 | 87 | ||
| 9,10-DiHOME | 3.7 | 73 | 4.5 | 102 | 2.5 | 52 | 4.4E-01 | 3.3E-02 | 2.2E-02 | 0.8 | 70 | ||
| 12,13-DiHOME | 6.8 | 61 | 7.6 | 80 | 5.0 | 46 | 5.9E-01 | 5.1E-02 | 1.8E-02 | 0.8 | 53 | ||
| 9(10)-EpOME | 4.1 | 101 | 3.2 | 125 | 2.4 | 99 | 4.2E-01 | 9.1E-02 | 3.4E-01 | 1.1 | 79 | ||
| 12(13)-EpOME | 6.1 | 93 | 4.6 | 91 | 3.5 | 76 | 2.9E-01 | 5.0E-02 | 1.8E-02 | 1.0 | 67 | ||
| Sum (n=9) | 65 | 68 | 63 | 86 | 46 | 40 | 8.8E-01 | 3.6E-02 | 7.8E-02 | 0.9 | 44 | ||
| DGLA | 15-HETrE | 0.67 | 100 | 0.54 | 62 | 0.46 | 45 | 3.8E-01 | 7.5E-02 | 1.4E-01 | 0.9 | 43 | |
| Sum (n=1) | 0.67 | 100 | 0.54 | 62 | 0.46 | 45 | 3.8E-01 | 7.5E-02 | 1.4E-01 | 0.9 | 42 | ||
| AA | PGE2 | 0,34 | 251 | 0.06 | 108 | 0.16 | 156 | 1.2E-01 | 3.1E-01 | 9.0E-02 | 6.2 | 233 | |
| TXB2 | 3.1 | 139 | 1.1 | 69 | 1.4 | 127 | 3.1E-02 | 7.7E-02 | 3.8E-01 | 2.1 | 156 | ||
| 5-HETE | 1.7 | 103 | 1.2 | 78 | 1.1 | 45 | 2.6E-01 | 7.1E-02 | 6.5E-01 | 1.2 | 50 | ||
| 8-HETE | 0.55 | 235 | 0.23 | 154 | 0.31 | 87 | 2.5E-01 | 3.3E-01 | 3.3E-01 | 9.1 | 179 | ||
| 11-HETE | 0.77 | 200 | 0.29 | 100 | 0.38 | 87 | 1.4E-01 | 1.7E-01 | 2.9E-01 | 3.0 | 162 | ||
| 12-HETE | 43 | 168 | 11 | 93 | 17 | 144 | 4.1E-02 | 9.3E-02 | 2.9E-01 | 2.8 | 163 | ||
| 15-HETE | 2.0 | 129 | 1.3 | 52 | 1.3 | 45 | 2.0E-01 | 1.3E-01 | 7.4E-01 | 1.1 | 44 | ||
| 12-KETE | 4.0 | 154 | 1.7 | 81 | 3.3 | 152 | 8.7E-02 | 2.9E-01 | 1.5E-01 | 6.1 | 318 | ||
| 15-KETE | 0.39 | 230 | 0.15 | 130 | 0.25 | 102 | 2.1E-01 | 4.2E-01 | 8.7E-02 | 2.9 | 93 | ||
| 5(6)-EpETrE | 0.51 | 89 | 0.38 | 50 | 0.34 | 85 | 1.7E-01 | 8.7E-02 | 5.5E-01 | 1.0 | 82 | ||
| 8(9)-EpETrE | 0.37 | 94 | 0.27 | 90 | 0.22 | 101 | 2.2E-01 | 7.3E-02 | 4.8E-01 | 1.2 | 97 | ||
| 11(12)-EpETrE | 0.43 | 134 | 0.29 | 97 | 0.29 | 130 | 2.5E-01 | 2.6E-01 | 9.6E-01 | 1.5 | 103 | ||
| 14(15)-EpETrE | 0.48 | 140 | 0.32 | 82 | 0.38 | 105 | 2.8E-01 | 5.0E-01 | 5.8E-01 | 1.5 | 81 | ||
| 5(6)-DiHETrE | 0.29 | 65 | 0.26 | 63 | 0.19 | 40 | 5.1E-01 | 2.4E-03 | 3.3E-02 | 0.8 | 38 | ||
| 8(9)-DiHETrE | 0.31 | 74 | 0.33 | 101 | 0.22 | 45 | 8.2E-01 | 1.7E-02 | 9.3E-02 | 0.8 | 38 | ||
| 11(12)-DiHETrE | 0.75 | 62 | 0.70 | 70 | 0.51 | 30 | 7.3E-01 | 4.0E-03 | 6.3E-02 | 0.9 | 33 | ||
| 14(15)-DiHETrE | 0.91 | 67 | 0.91 | 81 | 0.64 | 26 | 9.8E-01 | 1.3E-02 | 6.6E-02 | 0.8 | 29 | ||
| Sum (n=17) | 60 | 153 | 20 | 64 | 28 | 106 | 4.7E-02 | 8.7E-02 | 2.8E-01 | 1.8 | 121 | ||
| Sum (n-15)4 | 13 | 109 | 7.8 | 63 | 7.7 | 45 | 1.1E-01 | 5.1E-02 | 9.4E-01 | 1.2 | 53 | ||
The fold change in oxylipin serum concentrations (C) comparing subjects following ω-3 supplementation (ω-3) and placebo supplementation (P), i.e., [Cω-3]/[CP]
Average
Coefficient of variance
Excluding 12-HETE and 12-KETE
Five EPA-derived and 5 DHA-derived oxylipins were detected above the mLOQ. Of these, the majority were CYP products: EPA: 11,12- 14,15- and 17,18-dihydroxyeicosatetraenoic acid (DiHETE), and DHA: 10(11)- epoxydocosapentaenoic acid (EpDPE), 4,5-, 10-11, 13,14, and 16,17-dihydroxydocosapentaenoic acid (DiHDPE). In addition, two 15-LOX-formed EPA products (12- and 15-hydroxyeicosapentaenoic acid [HEPE]) were detected. Following ω-3 FA supplementation, almost all of the quantified EPA- and DHA-derived oxylipins were significantly elevated compared to both baseline and placebo serum levels (Table 3). As shown in Figure 1 and Table 3, the relative difference between ω-3 FA and placebo ranged on average from 5-25-fold increases for EPA metabolites and 2-3-fold increases of the DHA metabolites following ω-3 FA supplementation. The largest shifts were observed in the EPA 15-LOX-produced compounds, which on average increased 20-25 fold relative to placebo.
Figure 1.
Log10 fold change in EPA-derived and DHA-derived oxylipins comparing ω-3 and placebo (P) supplementation. Values ≥0 indicate an increase following ω-3 supplement. Oxylipin nomenclature is provided in the Supporting Information Table S1.
In addition to the EPA and DHA products, metabolites originating from ω-3 FAs: α-linolenic acid (ALA, n=4) and ω-6 FAs: linoleic acid (LA, n=9), dihomo-gamma-linolenic acid (DGLA, n=1) and arachidonic acid (AA, n=17) were detected. Following ω-3 FA supplementation, 2 CYP-formed products from the ALA pathway and 4 CYP-produced compounds from the AA (n=1) and LA (n=3) pathways were significantly decreased compared to concentrations following placebo: 15(16)- epoxyoctadecadienoic acid [EpODE], 15,16-dihydroxyoctadecadienoic acid [DiHODE], 5(6)- dihydroxyeicosatrienoic acid [DiHETrE], 9,10-and 12,13-dihydroxyoctadecenoic acid [DiHOME], and 12(13)-epoxydecenoic acid [EpOME], respectively. A similar observation was observed comparing ω-3 FA supplementation with baseline concentrations that showed a significant decrease in 4 CYP-produced AA products (5[6]-DiHETrE, 8[9]-DiHETrE, 11[12]-DiHETrE and 14[15]-DiHETrE) and 2 CYP-produced LA products (9,10-DiHOME and 12(13)-EpOME).
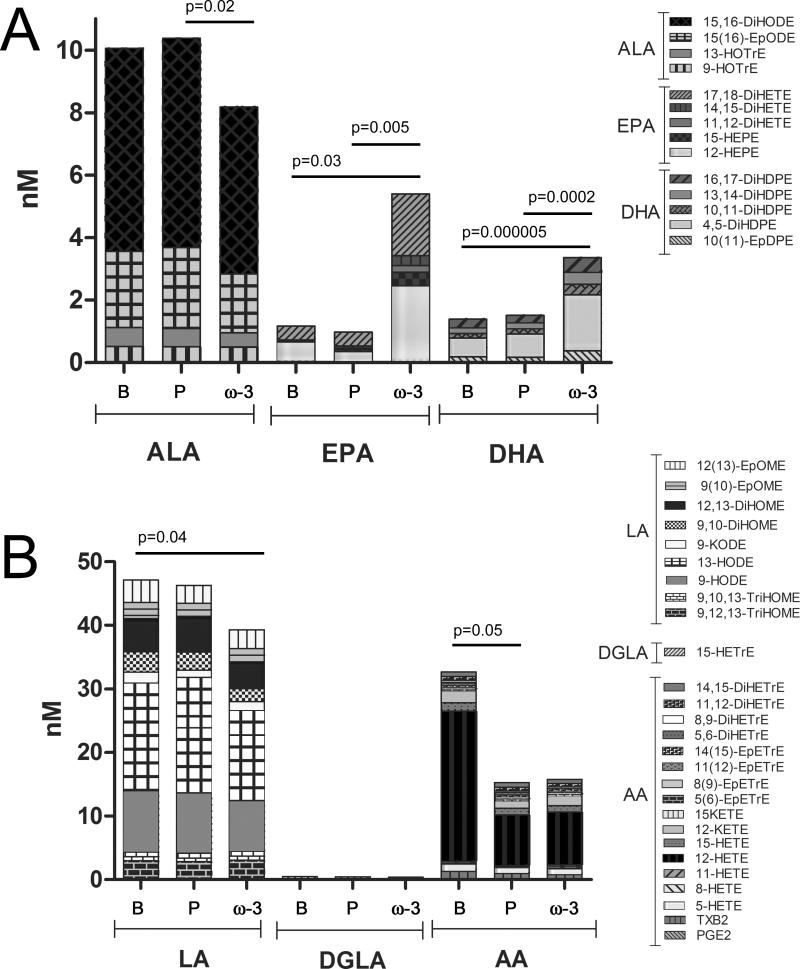
In Figure 2 the overall profile of targeted ω-3 FA-derived (Figure 2A) and ω-6 FA-derived (Figure 2B) oxylipins is presented as the sum of the median values of each fatty acid subgroup. As expected, the sum of EPA and DHA products significantly increased following ω-3 FA supplementation compared to both baseline and placebo (Table 3, Figure 2). In contrast, the sum of the ALA products significantly decreased following ω-3 FA supplementation compared to the placebo (Table 3, Figure 2). The drop in AA-derived compounds from baseline to placebo and ω-3 FA supplementation shown in Figure 2 is primarily due to the variation in the 12-HETE pathway (If 12-HETE and the 12-HETE product 12-oxoeicosatetraenoic acid [KETE] are removed, the p-value shifts from p=0.047 to p=0.10; Table 3). The sum of LA products significantly decreased following ω-3 supplementation compared to baseline (p=0.03) and the DGLA products were not significantly altered between the groups.
Figure 2.
Oxylipin levels group by substrate using the sum of the median values. (A) ω-3 derived and (B) ω-6 derived oxylipins. Significant shifts are indicated as calculated with a Student's t-test. Individual values for averages and CVs are provided in Table 3. Oxylipin nomenclature is as provided in the Supporting Information Table S2. ALA=alpha-linolenic acid; LA=linoleic acid; DGLA=dihomo-gamma-linolenic acid; AA=arachidonic acid.
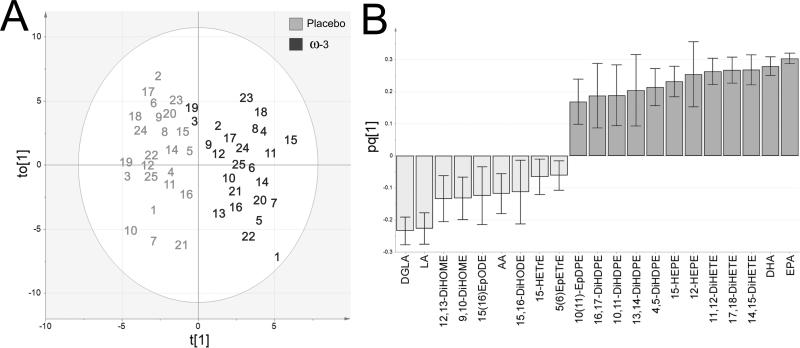
Integrated multivariate modeling demonstrated that serum PUFA levels had the largest effect on differentiating ω-3 FA vs. placebo supplementation
Multivariate statistical modeling integrating oxylipin data with relative serum PUFA levels (%) was performed in order to investigate overall trends in the data. An OPLS-DA model examining separation according to placebo versus ω-3 FA supplementation was constructed (R2[cum]=0.81, Q2[cum]=0.78, CVANOVA p-value=2.17E-14). The R2 value indicates how well the model explains the current dataset, whereas the Q2 is the correlation based on averaging the results from repeated iterations of cross-validation. As such, the Q2 represents a measure of the predictive power of the model (i.e., how well the model is expected to fit additional samples from the same groups). The model (Figure 3) was built from a single predictive and 1 orthogonal component, resulting in a robust separation between the two test conditions. One placebo subject was located outside of Hotelling's T2 in the scores plot (Subject 13). However, it was only an outlier in terms of the orthogonal vector, and further examination of the Distance to Model X (DModX) confirmed that this individual was within the 95% confidence interval. Accordingly, this individual was not excluded from the model. The loadings column plot (Figure 3B) showed that the PUFAs as well as both specific ω-3 and ω-6 derived oxylipins are primary variables contributing to subject separation. Subjects 3ω-3 and 19ω-3 (dark gray), which slightly cluster with the placebo group, are still clearly distinguished from their 3P and 19P (light gray) counterparts (the EPA- and DHA-derived oxylipins and PUFA variables were more prominent following ω-3 FA supplementation). The PUFA data are the strongest contributors to the model, with increases in EPA and DHA and decreases in DGLA and LA possessing the largest loadings. No clustering was observed according to age, gender or BMI.
Figure 3.
OPLS-DA model of placebo (light gray) versus ω-3 supplementation (dark gray). (A) Scores plot. The placebo outlier 13 is outside the shown plot range (t[1]:-4.4, to[1]:-14.5). (B) Loadings column plot. Mediators correlating with 95% confidence are shown. The model was built from a single predictive and 1 orthogonal component (R2[cum]=0.81, Q2[cum]=0.78, CVANOVA p-value=2.17E-14).
DISCUSSION
Oxylipin composition of serum and plasma evidences similar profiles
Both the human plasma [36, 37] and serum lipidomes [38] have been examined in detail, revealing a significant diversity of lipid species. However, these studies tend to focus on higher abundance structural lipids rather than signaling lipids (e.g., eicosanoids). Earlier studies have reported the baseline oxylipin profiles in plasma [39-41] and a number of studies have examined the effect of ω-3 FA supplementation [8, 22, 23]. However, it is known that the human plasma and serum metabolomes profiles differ [42, 43], which should potentially be reflected in the oxylipin levels. The current study is one of the first to measure the oxylipin component of the human serum lipidome, both at baseline and following dietary supplementation with ω-3 FAs (a recent paper examined the serum oxylipin profiles in immunoglobulin A nephropathy [IgAN] patients [44]).
A particular issue related to the measure of oxylipins in blood is the potential effects of the blood collection process as well as serum/plasma generation upon the observed oxylipin profile [40, 45, 46]. Accordingly, it is essential that blood collection, processing, and handling methods are reported in detail when providing oxylipin data. For example, higher serum values of TXB2 relative to plasma are attributed to platelet activation [47-49], and use of heparin in plasma generation is postulated to stimulate lipoprotein lipases, which results in concomitant increases in overall oxylipin levels [45]. In the current study, as expected baseline TXB2 levels were roughly 3.5-fold greater than previously published values in plasma [39]. However, overall, the serum baseline concentrations presented herein are similar to previously published values for plasma [22, 39, 50]. A comparison of serum oxylipin values with previously published plasma values evidences a few important trends (Table 4). The plasma data are from two distinct cohorts from the Human Metabolome (HM; n=3 individuals) and the Pennington Plasma (n=70 individuals) [39]. The composition of the oxylipin analytical platforms is slightly different, but there is sufficient overlap for comparison purposes. The current study observed 41 oxylipin species in serum relative to 50 in the HM plasma and 76 in the Pennington Plasma. The ratio of the serum/plasma levels for all compounds in common between both analytical platforms was 1.45 and 1.67 for the HM and Pennington Plasma, respectively. As discussed above, the notable outlier was TXB2, removal of which slightly decreased the ratios (1.38 and 1.62, respectively). The other major outlier in the current study was 12-HETE, which was significantly elevated in serum. It is unclear what is causing the elevated levels of 12-HETE and its downstream product 12-KETE; however, it is well known that platelets can produce 12-HETE [51]. Further removal of 12-HETE reduced the ratios to 1.19 and 1.32, respectively (12-KETE was not observed in the work by Psychogios et al. [39]). Accordingly, serum and plasma levels of the majority of oxylipin species appear to be relatively similar, which is in agreement with recently published data [46].
Table 4.
Oxylipin levels in serum vs. plasma (nM).
| Class | PUFA | Oxylipin | Serum (B1) | Plasma (HM2) | Plasma (PP3) | |||
|---|---|---|---|---|---|---|---|---|
| Av4 | SD5 | Av | SD | Av | SD | |||
| ω-3 | ALA | 9-HOTrE | 0.71 | 0.61 | 1.98 | 0.12 | 1.19 | 0.91 |
| 13-HOTrE | 0.80 | 0.72 | 1.9 | 0.21 | 1.11 | 0.74 | ||
| 15(16)-EpODE | 3.9 | 3.2 | 3.27 | 0.23 | 2.77 | 2.1 | ||
| 15,16-DiHODE | 7.1 | 3.3 | 14.5 | 1 | 5.93 | 2.4 | ||
| EPA | 12-HEPE | 2.4 | 7.0 | 3.19 | 0.35 | 0.195 | 0.11 | |
| 15-HEPE | 0.07 | 0.09 | 0.28 | 1.63 | 1.63 | 1.6 | ||
| 11,12-DiHETE | 0.04 | 0.03 | NR6 | -7 | NR | - | ||
| 14,15-DiHETE | 0.07 | 0.03 | ND8 | - | 0.304 | 0.1 | ||
| 17,18-DiHETE | 0.47 | 0.23 | 14.4 | 1.1 | 2.08 | 0.85 | ||
| DHA | 10(11)-EpDPE | 0.25 | 0.19 | NR | - | NR | - | |
| 4,5-DiHDPE | 0.79 | 0.62 | NR | - | NR | - | ||
| 10,11-DiHDPE | 0.19 | 0.13 | NR | - | NR | - | ||
| 13,14-DiHDPE | 0.22 | 0.14 | NR | - | NR | - | ||
| 16,17-DiHDPE | 0.30 | 0.15 | NR | - | NR | - | ||
| ω-6 | LA | 9,12,13-TriHOME | 3.5 | 2.3 | 0.827 | 21 | 4.11 | 2.2 |
| 9,10,13-TriHOME | 1.7 | 1.0 | 0.513 | 0.083 | 1.16 | 0.64 | ||
| 9-HODE | 13 | 10 | 11.7 | 0.23 | 11 | 6.1 | ||
| 13-HODE | 23 | 18 | 47.3 | 0.53 | 58.2 | 28 | ||
| 9-KODE | 2.9 | 3.5 | 2.41 | 0.29 | 5.3 | 2.7 | ||
| 9,10-DiHOME | 3.7 | 2.7 | 60.5 | 3.8 | 29.7 | 11 | ||
| 12,13-DiHOME | 6.8 | 4.1 | 7.69 | 0.59 | 5.82 | 3 | ||
| 9(10)-EpOME | 4.1 | 4.1 | 2.17 | 0.23 | 5.47 | 7.4 | ||
| 12(13)-EpOME | 6.1 | 5.7 | 4.88 | 0.34 | 7.21 | 8.8 | ||
| DGLA | 15-HETrE | 0.67 | 0.67 | 0.437 | 0.028 | 0.732 | 0.45 | |
| AA | PGE2 | 0.34 | 0.85 | 0.0967 | 0.012 | 0.172 | 0.13 | |
| TXB2 | 3.1 | 4.3 | 0.865 | 0.18 | 0.919 | 1.6 | ||
| 5-HETE | 1.7 | 1.8 | 0.901 | 0.029 | 1.02 | 0.79 | ||
| 8-HETE | 0.55 | 1.3 | 2.09 | 0.16 | 0.536 | 0.4 | ||
| 11-HETE | 0.77 | 1.5 | 0.425 | 0.0095 | 0.401 | 0.36 | ||
| 12-HETE | 43 | 72 | 6.42 | 0.74 | 3.95 | 3.3 | ||
| 15-HETE | 2.0 | 2.6 | 1.8 | 0.098 | 2.04 | 1.2 | ||
| 12-KETE | 4.0 | 6.2 | <0.1 | - | <0.1 | - | ||
| 15-KETE | 0.39 | 0.90 | 0.749 | 0.08 | 0.682 | 0.76 | ||
| 5(6)-EpETrE | 0.51 | 0.45 | NR | - | NR | - | ||
| 8(9)-EpETrE | 0.37 | 0.35 | <0.2 | - | 0.627 | 0.71 | ||
| 11(12)-EpETrE | 0.43 | 0.58 | 0.303 | 0.028 | 1.02 | 1.4 | ||
| 14(15)-EpETrE | 0.48 | 0.67 | 1.77 | 0.05 | 0.442 | 0.59 | ||
| 5,6-DiHETrE | 0.29 | 0.19 | 0.264 | 0.025 | 0.189 | 0.092 | ||
| 8,9-DiHETrE | 0.31 | 0.23 | 0.294 | 0.056 | 0.244 | 0.078 | ||
| 11,12-DiHETrE | 0.75 | 0.47 | 0.779 | 0.037 | 0.566 | 0.2 | ||
| 14,15-DiHETrE | 0.91 | 0.61 | 0.714 | 0.031 | 0.603 | 0.18 | ||
The ratio of [AA]/[EPA+DHA]-derived oxylipins from the 15-LOX and CYP pathways demonstrates inter-study homogeneity
The [AA]/[EPA+DHA] ratio of 15-LOX- and CYP-produced compounds is more consistent than the concentrations as shown in Table 5 for 3 different studies: this study (Lundström et al.) and Shearer et al.[22], in which oxylipin levels were measured in plasma, and Honstra et al.[23], in which oxylipin levels in plasma were measured following platelet activation with collagen. Interestingly, the ratios of the groups having an ω-3 FA or fish supplemented diet are similar between the studies. Additionally, the other groups (baseline pre-study, controls during study and placebo supplement) are also similar between studies, but differ from the ω-3 FA groups. Thus, results indicate that an ω-3 FA rich diet affects downstream metabolic pathways and the intra-individual ratio of ω-3 and ω-6 15-LOX-derived and CYP-derived products in a consistent manner. The shift is mainly due to changes in the concentration of EPA- and DHA-derived oxylipins, but also affects the levels in pathways derived from LA, AA and ALA (Table 3, Figure 2). Decreased levels in AA-derived oxylipins following ω-3 supplementation have previously been reported by Shearer et al [22].
Table 5.
The average [AA]/[EPA+DHA] ratios in 15-LOX- and CYP-derived oxylipins from 3 studies (Lundström et al. [this study], Honstra et al. [23] and Shearer et al. [22], respectively) comparing baseline, control and placebo groups to ω-3 or fish supplemented diet groups.1
| Pathway | Type2 | Cohort 13 | Cohort 2 | Cohort 3 | Study |
|---|---|---|---|---|---|
| 15-LOX | Baseline | 34 | - | - | Lundström et al. |
| 40 | 16 | 40 | Honstra et al. | ||
| 28 | 20 | 47 | |||
| 20 | - | - | Shearer et al. | ||
| Placebo | 26 | - | - | Lundström et al. | |
| Control | 37 | 39 | 37 | Honstra et al. | |
| ω-3 | 4 | - | - | Lundström et al. | |
| 3 | - | - | Shearer et al. | ||
| Mackerel | 4 | 4 | 6 | Honstra et al. | |
| CYP | Baseline | 1.9 | - | - | Lundström et al. |
| 3.0 | - | - | Shearer et al. | ||
| Placebo | 1.5 | - | - | Lundström et al. | |
| ω-3 | 0.5 | - | - | Lundström et al. | |
| 0.8 | - | - | Shearer et al. |
AA=arachidonic acid; 15-LOX=15-lipoxygenase; CYP=cytochrome P450.
Grouped according to sampling times. Baseline: prior study; Control: following control supplement during study; Placebo: following blind control supplement during study; ω-3: following blind EPA and DHA supplement during study; and Mackerel: following mackerel paste supplement during study.
The study by Honstra et al. [23] was divided into 3 different cohorts from Tromsø, Maastritch and Zeist.
Asthmatics evidence similar serum oxylipin profile to healthy individuals following ω-3 FA supplementation
No obvious differences were observed in the oxylipin shifts following ω-3 FA supplementation that would distinguish the healthy populations characterized by Shearer et al. [22, 23] and Honstra et al. [22, 23] to the asthmatic population investigated herein. However, we cannot exclude the possibility that there are some differences between the two populations in terms of how they handle ω-3 FAs. It is distinctly possible that differences not visible in the serum signature could be observed by pulmonary examination (e.g., bronchoalveolar lavage). It is clear that a shift to an ω-3 FA rich diet will affect not only the PUFA ratio, but also the overall downstream oxylipin profile, which can have ramifications for disease. For example, the 15-LOX-derived EPA pathway is involved in the formation of resolvins, which have anti-inflammatory or resolution-like properties [19], whereas the majority of AA-derived mediators have pro-inflammatory properties [9-11]. Accordingly, shifts in downstream ω-3 FA-derived lipid mediators such as resolvins and protectins could affect disease processes. Several studies have indicated positive effects of ω-3 FA supplementation for asthma treatment, whereas ω-6 and trans-fatty acids may adversely affect disease [52-55]. However, there are also studies arguing that a high intake of ω-3 FAs will not have protective effects [56, 57]. In addition, the observed intra-individual variability in oxylipin levels following ω-3 treatment could potentially correlate with beneficial response to dietary supplementation [8]. Accordingly, any discussion of the role of ω-3 FAs in the diet should also consider potential effects on the downstream metabolic pathways.
In conclusion, dietary supplementation of ω-3 FAs results in concomitant increases in downstream metabolic products. The oxylipin profile in serum and plasma are quite similar, with the exception of eicosanoid products due to platelet activation (e.g., TXB2). ω-3 FA supplementation appears to have similar effects upon the oxylipin profile in serum from asthmatic and healthy individuals. The major observed shifts in EPA- and DHA-derived mediators, and minor shifts in LA and AA-derived mediators, could potentially contribute to the observed beneficial effects of fish oil supplementation in a healthy population. Accordingly, studies involving ω-3 FA supplementation need to also consider the consequences of shifts in downstream lipid mediator profiles.
Supplementary Material
ACKNOWLEDGEMENTS
This work has been funded in part by the Center for Allergy Research, Åke Wibergs Stiftelse, Jeanssons Stiftelse, Fredrik and Ingrid Thurings Stiftelse, Swedish Medical Research Council (10350), CIDaT Vinnova, Torsten & Ragnar Söderberg Foundation. The work was also supported and in part by NIEHS SBRP Grant p42 ES004699. Partial support was provided by the American Asthma Association #09-0269. S.L.L. was supported by the Bernard Osher Initiative for Research on Severe Asthma. J.D.B. was supported by the Martin Hardie Travel Fellowship from the Asthma Foundation of New South Wales. B.D.H. is a George and Judy Marcus Senior Fellow of the American Asthma Foundation.
Abbreviations used
- AA
Arachidonic acid
- ALA
α-linolenic acid
- COX
cyclooxygenase
- CUDA
N-cyclohexyl-N'-dodecanoic acid urea
- CYP
cytochrome P450
- DGLA
dihomogamma linolenic acid
- DiHETrE
dihydroxyeicosatrienoic acid
- DiHDPE
dihydroxydocosapentaenoic acid
- DiHETE
dihydroxyeicosatetraenoic acid
- DiHODE
dihydroxyoctadecadienoic acid
- DModX
distance to model X
- EpETrE
epoxyeicosatrienoic acid
- EpDPE
epoxydocosapentaenoic acid
- EpODE
epoxyoctadecadienoic acid
- HETE
hydroxyeicosatetraenoic acid
- HETrE
hydroxyeicosatrienoic acid
- HEPE
hydroxy eicosapentaenoic acid
- HODE
hydroxyoctadecadienoic acid
- KODE
oxooctadecadienoic acid
- HOTE
hydroxyoctadecatrienoic acid
- LA
linoleic acid
- LT
leukotriene
- LOX
lipoxygenase
- MS/MS
tandem mass spectrometry
- mLOQ
method limit of quantification
- OPLS-DA
orthogonal projections to latent structures – discriminate analysis
- PUFA
polyunsaturated fatty acid
- TriHOME
trihydroxyoctadecenoic acid
- TX
tromboxane
Literature Cited
- 1.Baum SJ, Kris-Etherton PM, Willett WC, Lichtenstein AH, et al. Fatty acids in cardiovascular health and disease: A comprehensive update. J Clin Lipidol. 2012;6:216–234. doi: 10.1016/j.jacl.2012.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Caterina R. n-3 fatty acids in cardiovascular disease. N Engl J Med. 2011;364:2439–2450. doi: 10.1056/NEJMra1008153. [DOI] [PubMed] [Google Scholar]
- 3.Saravanan P, Davidson NC, Schmidt EB, Calder PC. Cardiovascular effects of marine omega-3 fatty acids. Lancet. 2010;376:540–550. doi: 10.1016/S0140-6736(10)60445-X. [DOI] [PubMed] [Google Scholar]
- 4.Kris-Etherton PM, Harris WS, Appel LJ, Association, A. H. A. N. C. A. H. Omega-3 fatty acids and cardiovascular disease: new recommendations from the American Heart Association. Arterioscler Thromb Vasc Biol. 2003;23:151–152. doi: 10.1161/01.atv.0000057393.97337.ae. [DOI] [PubMed] [Google Scholar]
- 5.Kris-Etherton PM, Harris WS, Appel LJ, American Heart Association. Nutrition C. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 6.Dumlao DS, Cunningham AM, Wax LE, Norris PC, et al. Dietary Fish Oil Substitution Alters the Eicosanoid Profile in Ankle Joints of Mice during Lyme Infection. J Nutr. 2012 doi: 10.3945/jn.112.157883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norris PC, Dennis EA. Omega-3 fatty acids cause dramatic changes in TLR4 and purinergic eicosanoid signaling. Proc Natl Acad Sci U S A. 2012;109:8517–8522. doi: 10.1073/pnas.1200189109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keenan AH, Pedersen TL, Fillaus K, Larson MK, et al. Basal omega-3 fatty acid status affects fatty acid and oxylipin responses to high-dose n3-HUFA in healthy volunteers. J Lipid Res. 2012;53:1662–1669. doi: 10.1194/jlr.P025577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lundstrom SL, Balgoma D, Wheelock AM, Haeggstrom JZ, et al. Lipid mediator profiling in pulmonary disease. Curr Pharm Biotechnol. 2011;12:1026–1052. doi: 10.2174/138920111795909087. [DOI] [PubMed] [Google Scholar]
- 10.Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 11.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 12.Haeggstrom JZ, Funk CD. Lipoxygenase and leukotriene pathways: biochemistry, biology, and roles in disease. Chem Rev. 2011;111:5866–5898. doi: 10.1021/cr200246d. [DOI] [PubMed] [Google Scholar]
- 13.Capra V, Back M, Barbieri SS, Camera M, et al. Eicosanoids and Their Drugs in Cardiovascular Diseases: Focus on Atherosclerosis and Stroke. Med Res Rev. 2012 doi: 10.1002/med.21251. [DOI] [PubMed] [Google Scholar]
- 14.Tremoli E, Folco G, Agradi E, Galli C. Platelet thromboxanes and serum-cholesterol. Lancet. 1979;1:107–108. doi: 10.1016/s0140-6736(79)90101-6. [DOI] [PubMed] [Google Scholar]
- 15.Capone C, Faraco G, Anrather J, Zhou P, Iadecola C. Cyclooxygenase 1-derived prostaglandin E2 and EP1 receptors are required for the cerebrovascular dysfunction induced by angiotensin II. Hypertension. 2010;55:911–917. doi: 10.1161/HYPERTENSIONAHA.109.145813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davi G, Catalano I, Averna M, Notarbartolo A, et al. Thromboxane biosynthesis and platelet function in type II diabetes mellitus. N Engl J Med. 1990;322:1769–1774. doi: 10.1056/NEJM199006213222503. [DOI] [PubMed] [Google Scholar]
- 17.Davi G, Guagnano MT, Ciabattoni G, Basili S, et al. Platelet activation in obese women: role of inflammation and oxidant stress. JAMA. 2002;288:2008–2014. doi: 10.1001/jama.288.16.2008. [DOI] [PubMed] [Google Scholar]
- 18.Di Gennaro A, Wagsater D, Mayranpaa MI, Gabrielsen A, et al. Increased expression of leukotriene C4 synthase and predominant formation of cysteinyl-leukotrienes in human abdominal aortic aneurysm. Proc Natl Acad Sci U S A. 2010;107:21093–21097. doi: 10.1073/pnas.1015166107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohli P, Levy BD. Resolvins and protectins: mediating solutions to inflammation. Br J Pharmacol. 2009;158:960–971. doi: 10.1111/j.1476-5381.2009.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. 2010;68:280–289. doi: 10.1111/j.1753-4887.2010.00287.x. [DOI] [PubMed] [Google Scholar]
- 22.Shearer GC, Harris WS, Pedersen TL, Newman JW. Detection of omega-3 oxylipins in human plasma and response to treatment with omega-3 acid ethyl esters. J Lipid Res. 2010;51:2074–2081. doi: 10.1194/jlr.M900193-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honstra G, van Houwelingen AC, Kivits GA, Fischer S, Uedelhoven W. Influence of dietary fish on eicosanoid metabolism in man. Prostaglandins. 1990;40:311–329. doi: 10.1016/0090-6980(90)90018-q. [DOI] [PubMed] [Google Scholar]
- 24.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83:1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 25.Conquer JA, Holub BJ. Effect of supplementation with different doses of DHA on the levels of circulating DHA as non-esterified fatty acid in subjects of Asian Indian background. J Lipid Res. 1998;39:286–292. [PubMed] [Google Scholar]
- 26.Marsen TA, Pollok M, Oette K, Baldamus CA. Pharmacokinetics of omega-3-fatty acids during ingestion of fish oil preparations. Prostaglandins Leukot Essent Fatty Acids. 1992;46:191–196. doi: 10.1016/0952-3278(92)90069-u. [DOI] [PubMed] [Google Scholar]
- 27.Zuijdgeest-van Leeuwen SD, Dagnelie PC, Rietveld T, van den Berg JW, Wilson JH. Incorporation and washout of orally administered n-3 fatty acid ethyl esters in different plasma lipid fractions. Br J Nutr. 1999;82:481–488. doi: 10.1017/s0007114599001737. [DOI] [PubMed] [Google Scholar]
- 28.Roquet A, Dahlen B, Kumlin M, Ihre E, et al. Combined antagonism of leukotrienes and histamine produces predominant inhibition of allergen-induced early and late phase airway obstruction in asthmatics. Am J Respir Crit Care Med. 1997;155:1856–1863. doi: 10.1164/ajrccm.155.6.9196086. [DOI] [PubMed] [Google Scholar]
- 29.Brannan JD, Larsson J, Alkhabaz A, Balgoma D, et al. Effect of omega-3 fatty acids on bronchial hyperesponsiveness, sputum eosinophilia and mast cell mediators in asthma. doi: 10.1378/chest.14-1214. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang J, Schmelzer K, Georgi K, Hammock BD. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Anal Chem. 2009;81:8085–8093. doi: 10.1021/ac901282n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lundstrom SL, Levanen B, Nording M, Klepczynska-Nystrom A, et al. Asthmatics exhibit altered oxylipin profiles compared to healthy individuals after subway air exposure. PLoS One. 2011;6:e23864. doi: 10.1371/journal.pone.0023864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stark KD, Holub BJ. Differential eicosapentaenoic acid elevations and altered cardiovascular disease risk factor responses after supplementation with docosahexaenoic acid in postmenopausal women receiving and not receiving hormone replacement therapy. Am J Clin Nutr. 2004;79:765–773. doi: 10.1093/ajcn/79.5.765. [DOI] [PubMed] [Google Scholar]
- 33.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 34.Morrison WR, Smith LM. Preparation of Fatty Acid Methyl Esters and Dimethylacetals from Lipids with Boron Fluoride--Methanol. J Lipid Res. 1964;5:600–608. [PubMed] [Google Scholar]
- 35.Trygg J, Wold S. Orthogonal Projections to Latent Structures (OPLS). J Chemometrics. 2002;16:119–128. [Google Scholar]
- 36.Quehenberger O, Dennis EA. The human plasma lipidome. N Engl J Med. 2011;365:1812–1823. doi: 10.1056/NEJMra1104901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quehenberger O, Armando AM, Brown AH, Milne SB, et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res. 2010;51:3299–3305. doi: 10.1194/jlr.M009449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szymanska E, van Dorsten FA, Troost J, Paliukhovich I, et al. A lipidomic analysis approach to evaluate the response to cholesterol-lowering food intake. Metabolomics. 2012;8:894–906. doi: 10.1007/s11306-011-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Psychogios N, Hau DD, Peng J, Guo AC, et al. The human serum metabolome. PLoS One. 2011;6:e16957. doi: 10.1371/journal.pone.0016957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strassburg K, Huijbrechts AM, Kortekaas KA, Lindeman JH, et al. Quantitative profiling of oxylipins through comprehensive LC-MS/MS analysis: application in cardiac surgery. Anal Bioanal Chem. 2012;404:1413–1426. doi: 10.1007/s00216-012-6226-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giordano RM, Newman JW, Pedersen TL, Ramos MI, Stebbins CL. Effects of dynamic exercise on plasma arachidonic acid epoxides and diols in human volunteers. Int J Sport Nutr Exerc Metab. 2011;21:471–479. doi: 10.1123/ijsnem.21.6.471. [DOI] [PubMed] [Google Scholar]
- 42.Yu Z, Kastenmuller G, He Y, Belcredi P, et al. Differences between human plasma and serum metabolite profiles. PLoS One. 2011;6:e21230. doi: 10.1371/journal.pone.0021230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Denery JR, Nunes AA, Dickerson TJ. Characterization of differences between blood sample matrices in untargeted metabolomics. Anal Chem. 2011;83:1040–1047. doi: 10.1021/ac102806p. [DOI] [PubMed] [Google Scholar]
- 44.Zivkovic AM, Yang J, Georgi K, Hegedus C, et al. Serum oxylipin profiles in IgA nephropathy patients reflect kidney functional alterations. Metabolomics. 2012;8:1102–1113. doi: 10.1007/s11306-012-0417-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goodfriend TL, Pedersen TL, Grekin RJ, Hammock BD, et al. Heparin, lipoproteins, and oxygenated fatty acids in blood: a cautionary note. Prostaglandins Leukot Essent Fatty Acids. 2007;77:363–366. doi: 10.1016/j.plefa.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mas E, Croft KD, Zahra P, Barden A, Mori TA. Resolvins D1, D2, and Other Mediators of Self-Limited Resolution of Inflammation in Human Blood following n-3 Fatty Acid Supplementation. Clin Chem. 2012;58:1476–1484. doi: 10.1373/clinchem.2012.190199. [DOI] [PubMed] [Google Scholar]
- 47.Sadilkova L, Paluch Z, Mottlova J, Bednar F, Alusik S. The effect of selected pre-analytical phase variables on plasma thromboxane A(2) measurements in humans. Int J Lab Hematol. 2012 doi: 10.1111/j.1751-553X.2012.01458.x. [DOI] [PubMed] [Google Scholar]
- 48.Warner TD, Nylander S, Whatling C. Anti-platelet therapy: cyclo-oxygenase inhibition and the use of aspirin with particular regard to dual anti-platelet therapy. Br J Clin Pharmacol. 2011;72:619–633. doi: 10.1111/j.1365-2125.2011.03943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weber C, Beetens JR, Van de Wiele R, Holler M, De Clerck F. Production of thromboxane and prostaglandins in human blood in the presence of thromboxane synthase inhibitors: a comparison of RIA and GC/MS determinations. Blood Coagul Fibrinolysis. 1991;2:7–15. doi: 10.1097/00001721-199102000-00002. [DOI] [PubMed] [Google Scholar]
- 50.Gomolka B, Siegert E, Blossey K, Schunck WH, et al. Analysis of omega-3 and omega-6 fatty acid-derived lipid metabolite formation in human and mouse blood samples. Prostaglandins Other Lipid Mediat. 2011;94:81–87. doi: 10.1016/j.prostaglandins.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 51.Hamberg M, Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc Natl Acad Sci U S A. 1974;71:3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arm JP, Horton CE, Spur BW, Mencia-Huerta JM, Lee TH. The effects of dietary supplementation with fish oil lipids on the airways response to inhaled allergen in bronchial asthma. Am Rev Respir Dis. 1989;139:1395–1400. doi: 10.1164/ajrccm/139.6.1395. [DOI] [PubMed] [Google Scholar]
- 53.McKeever TM, Britton J. Diet and asthma. Am J Respir Crit Care Med. 2004;170:725–729. doi: 10.1164/rccm.200405-611PP. [DOI] [PubMed] [Google Scholar]
- 54.Nagakura T, Matsuda S, Shichijyo K, Sugimoto H, Hata K. Dietary supplementation with fish oil rich in omega-3 polyunsaturated fatty acids in children with bronchial asthma. Eur Respir J. 2000;16:861–865. doi: 10.1183/09031936.00.16586100. [DOI] [PubMed] [Google Scholar]
- 55.Fogarty A, Britton J. The role of diet in the aetiology of asthma. Clin Exp Allergy. 2000;30:615–627. doi: 10.1046/j.1365-2222.2000.00766.x. [DOI] [PubMed] [Google Scholar]
- 56.Broadfield EC, McKeever TM, Whitehurst A, Lewis SA, et al. A case-control study of dietary and erythrocyte membrane fatty acids in asthma. Clin Exp Allergy. 2004;34:1232–1236. doi: 10.1111/j.1365-2222.2004.02032.x. [DOI] [PubMed] [Google Scholar]
- 57.McKeever TM, Lewis SA, Cassano PA, Ocke M, et al. The relation between dietary intake of individual fatty acids, FEV1 and respiratory disease in Dutch adults. Thorax. 2008;63:208–214. doi: 10.1136/thx.2007.090399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.