Abstract
Aims
To determine if type 2 diabetes mellitus (T2D) differentiates endothelial function and plasma nitrite response (a marker of nitric oxide bioavailability) during exercise in peripheral arterial disease (PAD) subjects prior to and following 3 months supervised exercise training (SET).
Methods
In subjects with T2D + PAD (n = 13) and PAD-only (n = 14), endothelial function was measured using brachial artery flow-mediated dilation. On a separate day, venous blood draws were performed at rest and 10 min following a symptom-limited graded treadmill test (SL-GXT). Plasma samples were snap-frozen for analysis of nitrite by reductive chemiluminescence. All testing was repeated following 3 months of SET.
Results
Prior to training both groups demonstrated endothelial dysfunction, which was correlated with a net decrease in plasma nitrite following a SL-GXT (p ≤ 0.05). Following SET, the PAD-only group demonstrated an improvement in endothelial function (p ≤ 0.05) and COT (p ≤ 0.05), which was related to a net increase in plasma nitrite following the SL-GXT (both p ≤ 0.05). The T2D + PAD group had none of these increases.
Conclusions
T2D in the presence of PAD attenuated improvements in endothelial function, net plasma nitrite, and COT following SET. This suggests that T2D maybe associated with an inability to endogenously increase vascular NO bioavailability to SET.
Keywords: Nitric Oxide, Nitrite, Peripheral Arterial Disease, Diabetes Mellitus, Exercise
1. Introduction
The incidence of peripheral arterial disease (PAD) in subjects with diabetes mellitus is so greatly increased,(Dormandy, Heeck, & Vig, 1999; Jude et al., 2001) that the American Diabetes Association recommends screening for PAD even in asymptomatic subjects with diabetes(Marso & Hiatt, 2006; Association, 2001). Intermittent claudication, where arterial occlusive disease results in reduced blood flow during exercise, is the major clinical manifestation of PAD (Hiatt, 2001; Hiatt, Hoag, & Hamman, 1995; Hirsch et al., 2001). In the presence of diabetes, PAD patients are at increased risk for disease progression to critical leg ischemia, lower extremity amputation, and cardiovascular events than their non-diabetic counterparts.
Dysfunction of the vascular endothelium is an early event in the development of cardiovascular disease and PAD. Endothelial dysfunction is characterized by abnormal arterial vasoreactivity, mediated in part, by reduced production of nitric oxide (NO). NO plays an important role in inflammation, thrombosis, vascular tone, blood flow, tissue perfusion and exercise capacity. Reduced bioavailable vascular NO may be a particularly pertinent issue in diabetes given that endothelial derived NO production has been shown to be decreased by hyperglycemia(Tesfamariam, Brown, & Cohen, 1991; Williams et al., 1998), and NO is inactivated by advanced glycation end products (Tan et al., 2002) and reactive oxygen species(Ting et al., 1996).
Largely due to the difficulties involved in measuring the levels of NO in vivo (Lauer et al., 2001), including its short-half life and rapid metabolism, plasma nitrite has evolved as a key marker of vascular NO bioavailability(Allen, Cobb, & Gow, 2005). In fact, it has been estimated that approximately 70% of resting plasma nitrite is derived from endothelial nitric oxide synthase (eNOS) activity in humans and other mammalian species(Kleinbongard et al., 2003). More recently in-vivo (Pinder et al., 2009) experiments support the theory of a “push–pull” model for plasma nitrite, whereby eNOS produces NO during normoxia and increases plasma nitrite (via NO oxidation) and conversely during hypoxia (when eNOS is dysfunctional) nitrite can be reduced back to NO to cause vasodilation(Allen, Giordano, & Kevil, 2012). This complementary system enables NO to be available to vessels across the oxygen gradient. It also suggests that changes in net plasma nitrite during physical stress could be a dynamic marker of NO bioavailability and vascular health. PAD subjects with atherosclerotic occlusions in the lower limbs which limit blood supply to working tissues and produce physical manifestations of ischemic pain (claudication) during exercise stress may be an ideal model to examine these changes.
We have previously shown that subjects with PAD have significantly blunted responses in NO-dependent endothelial function (brachial artery flow-mediated dilation), the ability to increase plasma nitrite to exercise stress (net plasma nitrite), and reduced exercise tolerance in comparison to those with subclinical disease(Allen et al., 2009). Following supervised exercise training these subjects had significant improvements in exercise tolerance, endothelial function and net plasma nitrite(Allen et al., 2010). We then subsequently examined the effects of acute oral high inorganic nitrate supplementation on exercise performance in PAD subjects(Kenjale et al., 2011). We found no changes in endothelial function, but a six-fold increase in plasma nitrite 3 h following beverage consumption and an 18% and 17% increase in COT and TTE, which was correlated with the change in plasma nitrite. These studies suggest that plasma nitrite levels, either endogenous or exogenous, appear to be important for exercise tolerance in subjects with PAD without concomitant T2DM.
Exercise is recognized as a cornerstone of diabetes management. Along with diet and medication(Sigal et al., 2006), exercise has been shown to reduce risk factors for vascular disease(Metkus, Baughman, & Thompson, 2010). Studies have demonstrated the beneficial effects of exercise training on endothelial function and exercise tolerance in both subjects with diabetes (Fuchsjager-Mayrl et al., 2002; Hamdy et al., 2003; Maiorana et al., 2001; Okada et al., 2010) and one in those with PAD (Brendle et al., 2001). To our knowledge, there are no reports to date of the effects of exercise training on endothelial function and changes in plasma nitrite to exercise stress in subjects with PAD + T2D. The purpose of this study was to understand the potential role of T2D on endothelial function and net changes in plasma nitrite in subjects with PAD who complete 3 months of supervised aerobic exercise training.
2. Methods
2.1. Participants
All subjects for this paper were recruited at Duke University Medical Center. The PAD-only subjects were part of a larger Angiogenesis and Mechanisms of Exercise Training in Peripheral Arterial Disease (AMNESTI) study(Mitchell et al., 2007), which was conducted in conjunction with The University of Colorado School of Medicine at Denver. T2D + PAD subjects were recruited separately at Duke. For this paper we included only PAD patients that completed the 3 months of supervised training. This investigation conforms to the principles outlined in the Declaration of Helsinki and prior to participation all subjects signed an informed consent document approved by Duke University Institutional Review Board.
All subjects had a history of stable intermittent claudication for 3 or more months and an ankle-brachial pressure index <0.9 at rest (ankle-brachial index is the ratio of blood pressure in the lower legs to the blood pressure in the arms, a ratio of <0.9 is considered a diagnosis of peripheral arterial disease). The subjects with T2D + PAD had an established diagnosis of type 2 diabetes mellitus by their physician. All subjects were receiving anti-platelet and lipid lowering therapy unless medically contraindicated by their physician. Details of other medications are shown in Table 1. Exclusions were based on a past medical history of gangrene, impending limb loss or osteomyelitis, lower extremity vascular surgery, angioplasty or lumbar sympathectomy within 3 months of enrollment, severe peripheral neuropathy, any condition other than PAD that limits walking, unstable angina, history of significant coronary artery disease) or recent myocardial infarction (≤6 weeks), chest pain during treadmill exercise which appears before the onset of claudication, or >3 mm ST depression during exercise. Subjects were not placed on a specialized diet during this study.
Table 1.
Subject baseline characteristics.
| PAD (10 Male, 4 Female) |
PAD + T2D (6 Male, 7 Female) |
|
|---|---|---|
| Age (year) | 69 ± 3 | 66 ± 3 |
| Ht (cm) | 173 ± 3 | 168 ± 2 |
| Wt (kg) | 75.0 ± 3.5 | 78.5 ± 4.3 |
| BMI | 25 ± 1.3 | 27 ± 1.6 |
| SBP (mmHg) | 153 ± 6 | 146 ± 6 |
| DBP (mmHg) | 79 ± 3 | 74 ± 2 |
| ABI | 0.60 ± 0.06 | 0.77 ± 0.08 |
| VO2peak (ml/kg/min) | 15.1 ± 0.7 | 15.2 ± 1.0 |
| COT (s) | 205 ± 57 | 285 ± 76 |
| PWT (s) | 467 ± 50 | 531 ± 102 |
| Resting Brachial Diameter (mm) | 3.5 ± 0.1 | 3.6 ± 0.2 |
| Resting Plasma NO2- (nM) | 87.8 ± 14.6 | 103.58 ± 16.2 |
| Resting Plasma NO3- (μM) | 24 ± 5 | 28 ± 5 |
| Smoking % | ||
| Never | 14 | 39 |
| Former | 50 | 46 |
| Current | 36 | 15 |
| Medications % | ||
| Ace Inhibitor | 41 | 67 |
| Beta Blocker | 41 | 50 |
| Statin | 71 | 38 |
| Aspirin | 69 | 78 |
| Metformin | N/A | 44.4 |
| Insulin | N/A | 38.9 |
| Thiazolidinediones | N/A | 11.1 |
| Sulfonylurea | N/A | 11.1 |
PAD = Peripheral Arterial Disease Subjects, T2D + PAD = Peripheral Arterial Disease Subjects with Type 2 Diabetes Mellitus, ABI = Ankle-Brachial Index, COT = Claudication Onset Time, PWT = Peak Walk Time, CAD = Coronary Artery Disease, CVA = Cerebrovascular Disease, CHF = Congestive Heart Failure.
Significantly different within groups at the p ≤ 0.05 level.
Subjects free from symptomatic coronary artery disease, an ankle-brachial index (ABI) of > 1.0, no complicating illnesses, with resting systolic blood pressure <170 and resting diastolic <100, and not actively enrolled in an exercise program were used as baseline controls in the AMNESTI study and have been included here (where appropriate) as a reference group.
2.2. Study design
Subjects were tested at baseline and following the completion of 3 months of supervised aerobic exercise training intervention. The exercise protocol was based on published optimal programs for improving claudication pain distances in patients with PAD (Gardner & Poehlman, 1995; Regensteiner, Steiner, & Hiatt, 1996). Exercise training was performed 3 times per week for 3 months. No subject exceeded 16 weeks to complete the 36 sessions. All sessions were supervised by a trained exercise physiologist. Subjects walked until claudication pain became moderately severe, at which time they stepped off the treadmill and rested until claudication pain subsided. Exercise and rest periods were repeated during each training session until a total of 30 to 40 min of walk time was achieved. The initial training intensity was set to the workload that brought on claudication pain during the maximal treadmill test performed at baseline. In subsequent visits, the speed and/or elevation of the treadmill was increased once the subject could walk for greater than 8–10 min or longer without reaching moderate pain.
2.3. Endothelial function measures
Prior to imaging (on a separate day from cardiopulmonary exercise testing), subjects were instructed to hold medications, fast and refrain from exercise for 12 h, alcohol and smoking for 48 h. All vascular imaging and ankle-brachial index measures were performed between 8 am and 11 am with the subject in a supine position. Brachial artery assessments were obtained on the left arm, with the forearm extended and slightly supinated using high resolution ultrasound and a 7.5 MHz linear array transducer (Accuson, Sequoia 512). Measures were taken at baseline (following 10 min of supine rest), during five minutes of forearm occlusion, and continuously on an r-wave trigger for 2 min following cuff release (hyperemia) (for further details and reproducibility data see(Welsch, Allen, & Geaghan, 2002)). The percent change in brachial artery diameter was calculated by the equation:
2.4. Plasma nitrite measures
Prior to initiation of the cardiopulmonary exercise test, a 20 gauge I.V. catheter was placed in the cephalic vein. Approximately, 5 ml of blood was taken prior to (rest), and 10 min following the exercise test termination. Samples were divided into 1 ml Eppendorf tubes containing 5 μL heparin (1 to 1000 U/ml) and centrifuged at 5000 g for 1 min. Plasma samples were then removed into separate tubes, snap-frozen in liquid nitrogen and stored at −70 °C until analysis.
All nitric oxide metabolite concentrations were measured (within 30 min of defrosting) by chemiluminescence using an Ionics/Sievers nitric oxide analyzer (NOA 280i), as per manufacturer’s instructions (GE Analytical Instruments, Boulder, CO). The reductant used for nitrite analysis was potassium iodide in acetic acid, which has the reduction potential to convert nitrite to nitric oxide but is insufficient to reduce any higher oxides of nitrogen such as nitrate and thus is relatively specific for nitrite. To obtain concentrations of total plasma nitrogen oxides we used the same apparatus with a stronger reductant, vanadium chloride in hydrochloric acid at 94 °C. This stronger reductant reduces the sum of all nitrogen oxides with an oxidation state of +2 or higher which is predominantly nitrate [μM] but also includes both nitrite [nM] and nitrosothiols [nM].
2.5. Exercise performance measures
All subjects performed maximal cardiopulmonary exercise testing on a treadmill with gas exchange analysis and a 12-lead electrocardiograph. The Gardner treadmill protocol, which maintains 2 mph with a 2% grade increase every 2 min was used as it is specifically designed for a claudication-limited peripheral arterial disease population(Gardner et al., 1991).
Expired gases were analyzed continuously using a ParvoMedics unit (Sandy UT, USA) and averaged in 15-s intervals. Subjects were encouraged to walk until they could no longer continue (Peak Walking Time-PWT). During the test subjects were asked to indicate the point at which they initially felt claudication related leg pain. This was recorded as claudication onset time (COT). Blood pressure measurements and ratings of perceived exertion were also obtained at the end of each minute throughout the test.
2.6. Statistical analysis
All statistical analyses were performed using SPSS for Windows (version 15.0). Baseline differences between groups were initially examined using between groups independent t-tests. In order to determine if the PAD or T2D + PAD groups changed significantly over time, paired t-tests were used within each group to compare the levels of the following variables at baseline and 3 months: nitric oxide metabolites (“net plasma nitrite” and plasma nitrate markers), endothelial function (brachial artery flow-mediated dilation), ankle-brachial index, and functional performance (claudication onset pain, peak walking time and VO2peak). An analysis of variance (ANOVA) was used to determine differences in BAFMD peak dilation from the comparison control group.
Repeated measures analyses of variance were then used to examine group differences over time in the outcome variables of VO2peak, PWT, COT, BAFMD and Plasma Nitrite Flux. In these repeated measures ANOVAs, we examined the main effect of group, the main effect of time, and the group*time interaction. The significance of the interaction term reflects a difference of does one group improve more over time than the other group on the outcomes of interest? An alpha level of p ≤ 0.05 was required for statistical significance. For all outcome variables, we report unadjusted mean levels by group and by time.
Pearson product moment correlations were used to examine univariate relations between variable change scores.
3. Results
3.1. Participants
Twenty seven PAD subjects (13 subjects had T2D) aged 48 to 83 years completed vascular and exercise testing measures. Rest and recovery blood samples were obtained at both treadmill tests (pre and post 3 M training) on 23 subjects (10 had T2D). Details of group values for subject characteristics are shown in Table 1. The diabetic subjects had, as expected, elevated HbA1c of 7.35% at enrollment and were taking diabetic medications (collected from medical records).
3.2. Endothelial function measures
There were no significant differences between the two groups for blood pressure, ABIs, or resting brachial artery diameters before (Table 1) or following 3 months of supervised exercise training (data not shown). All groups significantly increased brachial diameters (mm) in response to flow stimulus, as shown by within group paired t-test analysis (both p ≤ 0.01).
Before training there were no differences in the peak percent dilation response to hyperemia (brachial artery flow-mediated dilation) between the groups (PAD = 2.1% ± 0.74% vs. T2D + PAD = 1.9% ± 0.9%, Fig. 1). There were no significant differences in blood flow velocities or calculated volumes between the groups at any stage in the protocol indicating a similar vasodilatory stimulus for all groups.
Fig. 1.
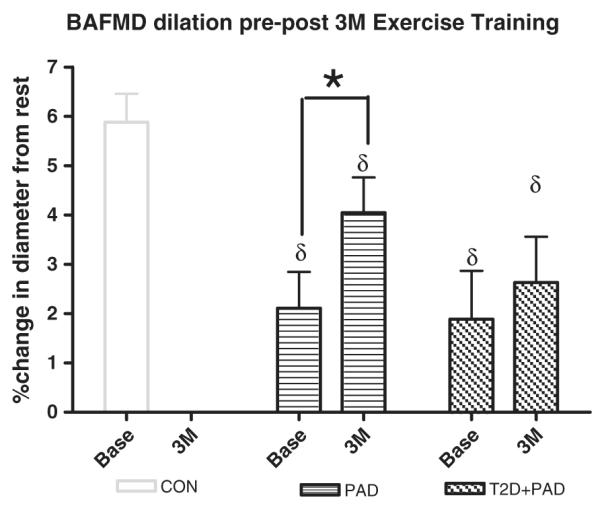
Brachial Artery Flow-Mediated Dilation at Baseline (Base) and following 3 months (3 M) of supervised exercise training. Values are mean ± SE. * = p ≤ 0.05 within groups, δ = significantly lower than control subjects by ANOVA at the p ≤ 0.05. CON = non-PAD controls, PAD = Peripheral Arterial Disease Subjects, T2D + PAD = Peripheral Arterial Disease Subjects with Type 2 Diabetes Mellitus.
Following the completion of 3 months of supervised exercise, the PAD group increased brachial artery flow-mediated dilation by +1.9% (absolute) versus pre-intervention (p ≤ 0.05 paired t-test) whereas the T2D + PAD changed by +0.74% (p = 0.8, Fig. 1). For comparison purposes a non-PAD control group (CON) is shown with a peak dilation of 5.88% ± 0.6%. These subjects had greater than 2 risk factors for, but no clinically diagnosed CVD. They did not undergo exercise training.
3.3. Plasma nitrite measures
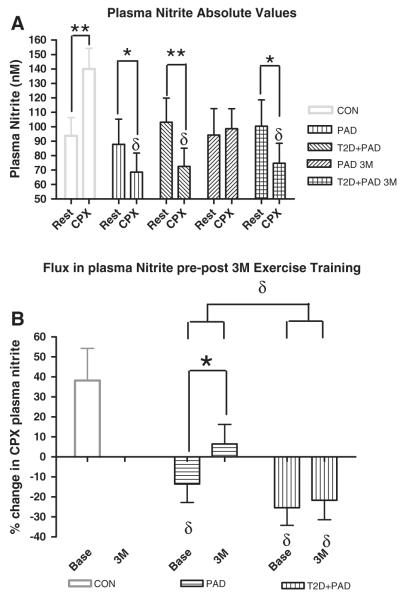
There were no significant differences between the two groups for resting plasma nitrite values at pre training (Table 1) or following the completion of 3 months of supervised exercise (Fig. 2A) by ANOVA analysis. The absolute (nM) and relative (%) changes in plasma nitrite following the acute cardiopulmonary exercise test (net plasma nitrite) can be seen in Fig. 2A and B respectively. For comparison purposes a non-PAD control group had an increase (+49%) in net plasma nitrite at baseline testing(Allen et al., 2010) whereas there were decreases in both the PAD (−22%) and T2D + PAD (−28%) groups. Following the 3 months of supervised exercise training the PAD group showed a non-significant increase in absolute (within visit) net plasma nitrite (+4.6) whereas the T2D + PAD group remained negative (−26%) (Fig. 2A). The relative change in plasma nitrite flux from baseline to 3 months (between visits) showed a significant increase for the PAD group (Fig. 2B). Additionally, a repeated measures ANOVA revealed a significant main effect for group*time; i.e., the PAD group increased significantly more than the T2D + PAD group for plasma nitrite flux over the 3 month training period (p ≤ 0.05, Fig. 2B). The T2D + PAD group showed no changes in plasma nitrite flux between visits.
Fig. 2.
A–B. Plasma Nitrite prior to and following a maximal Cardiopulmonary Exercise Testing (CPX). Changes in (A) Circulating plasma nitrite concentration (nM) and (B) plasma nitrite flux (% change in plasma nitrite concentration for pre to post CPX) at baseline (Base) and following 3 month supervised exercise training (3 M). (A) Samples were collected prior to (Rest), and 10 min following maximal CPX (CPX). * = significantly different within groups at the p ≤ 0.05 level. δ = significantly lower than control subjects CPX values by ANOVA at the p ≤ 0.05 level. ∞ = p ≤ 0.05 for group*time interaction using a repeated measures analyses of variance. PAD = peripheral arterial disease subjects, T2D + PAD = peripheral arterial disease subjects with type 2 diabetes mellitus, CON = Controls.
3.4. Exercise performance measures
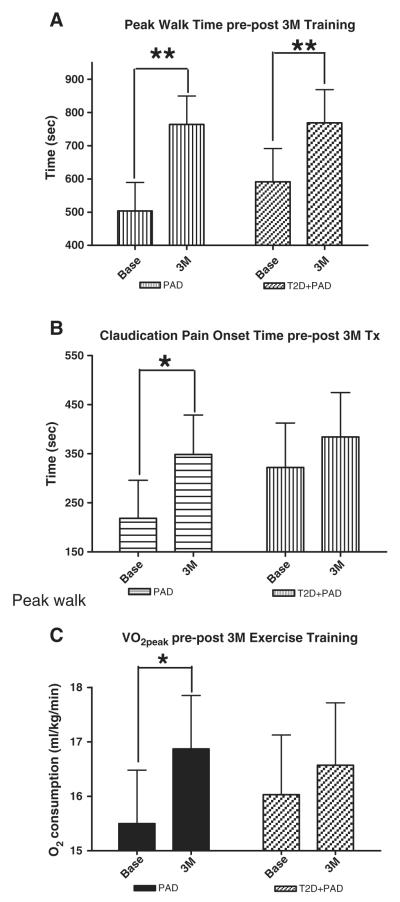
Prior to the start of exercise training there were no significant differences between the PAD and T2D + PAD groups for claudication pain onset time (218 ± 78 v 322 ± 78 s), peak walk time (503 ± 85 v 591 ± 100 s) or VO2peak, (15.5 ± 1.0 v 16.03 ± 1.1 ml/kg/min) (Fig. 3A–C). According to ACSM classification all subjects are regarded as having very poor fitness(ACSM, 2013).
Fig. 3.
Exercise performance measures at baseline (Base) and after 3 months (3 M) of supervised exercise training. Changes in (A) Peak Walk Time (s), (B) Claudication Pain Onset Time (s), and (C) VO2peak (ml/kg/min). Values are means ± standard error. *p ≤ 0.05, **p ≤ 0.01. PAD = peripheral arterial disease subjects, T2D + PAD = peripheral arterial disease subjects with type 2 diabetes mellitus.
Following the completion of 3 months of supervised exercise training, the PAD group increased peak walk time by 52% and the T2D + PAD group by 29% (both p ≤ 0.01 vs. baseline) showing both groups had adaptations to the training (Fig. 3A). Interestingly the PAD group increased pain free walking time prior to claudication onset by 59% (p ≤ 0.05) whereas there was no statistical difference for the T2D + PAD group (19%) (Fig. 3B), possibly suggesting a greater supply or utilization of oxygen by the PAD-only subjects. Peak oxygen utilization (VO2peak) responses to training were similar with an 8% (p ≤ 0.05) and 3% (NS) increase for PAD and T2D + PAD subjects respectively (Fig. 3C). This study was not powered for and indeed we did not detect any group*time interaction effect by repeated measures ANOVA for any of the performance measures.
3.5. Relationships between plasma nitrite, endothelial function and exercise performance
At baseline we have previously reported univariate relationships for net plasma nitrite during cardiopulmonary exercise testing (ΔnM) with exercise capacity (VO2peak) (r2 = 0.12, p ≤ 0.05) and localized endothelial function measured by brachial artery flow-mediated dilation (% change) (r2 = 0.18, p ≤ =0.01), and for brachial artery flow-mediated dilation and VO2peak (r2 = 0.14, p ≤ 0.05) (Allen et al., 2009).
Following the supervised exercise training intervention there was a significant positive correlation between the Δclaudication onset pain time and the Δplasma nitrite flux (from baseline to the completion of 3 months) for the PAD subjects (r2 = 0.47, p ≤ 0.05) (Fig. 4). In the subjects with T2D + PAD this relationship was not evident (r2 = −0.00, p = 0.90), although the range of Δnet plasma nitrite values was also much smaller.
Fig. 4.
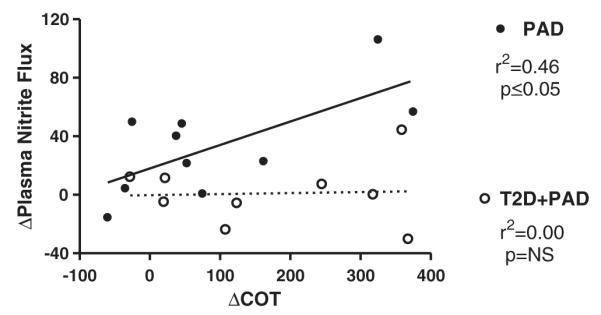
Relationship between the Change in Time to Claudication Onset Pain and Change in Plasma Nitrite Flux following 3 months of study intervention. PAD = peripheral arterial disease subjects, T2D + PAD = peripheral arterial disease subjects with type 2 diabetes mellitus.
No relationships were seen in the T2D + PAD subjects between level of diabetic control at baseline, as measured by HbA1c, and any of the above measures.
4. Discussion
The findings of this study demonstrate that the presence of T2D attenuates improvements in endothelial function (Fig. 1), net plasma nitrite response (Fig. 2A & B), and pain free walking time (Fig. 3A) in subjects with PAD following 3 months of supervised exercise training. Additionally, in PAD-only subjects the change in net plasma nitrite to exercise is related to the improvement in claudication pain onset time; a potential marker of hypoxia in the calf muscle (Fig. 4). In the subjects with T2D + PAD there were no significant increases in endothelial function, net plasma nitrite or COT following training and, thus, this relationship is absent. T2D + PAD subjects did increase overall peak walking time following training.
These findings suggest that in PAD subjects without concomitant T2D, exercise training may increase nitrite/NO bioavailability, in so much as endogenous endothelial generation outstrips the demand of hypoxic working skeletal muscle. Additionally, the acute change in plasma nitrite flux may be a mediator of peripheral tissue perfusion and pain free exercise performance in PAD. This hypothesis is based upon the tenet that plasma nitrite can function as a protected NO-species which can be carried in the circulation and converted back to NO under hypoxic conditions (see below).
Plasma nitrite is formed as a primary oxidation product of NO. NO itself is formed endogenously by endothelial nitric oxide synthase, via metabolism of L-arginine to l-citrulline in the presence of oxygen. NO is a free radical and as such is highly reactive and therefore limited both temporally and spatially to the proximity of its site of production. However, studies of inhaled and intravenously applied NO demonstrate effects on vasodilation(Cosby et al., 2003), reduction in blood pressure(Larsen et al., 2006), inhibition of platelet function(Webb et al., 2008) and protection from ischemia-reperfusion injury(Webb et al., 2004) suggesting that there may be other mechanisms for transportation of NO to be used at critical areas of the circulation.
Enzyme independent formation of NO from nitrite in biological tissues was first observed in rat hearts following 30 min of ischemia by spin-trapping and electron paramagnetic resonance spectrometry(Zweier et al., 1995). This led to observations of arterial to venous nitrite consumption in the human forearm during handgrip exercise (Gladwin et al., 2000) and a recognition that nitrite can function as a protected NO-species that can be carried in the circulation and converted back to NO under hypoxic conditions. Although the exact mechanism of reduction of nitrite to NO in hypoxia is not yet clear, several pathways have been described including, chemical acidification (Zweier et al., 1995), xanthine oxidase (Webb et al., 2004), deoxyhemoglobin (Cosby et al., 2003), deoxymyoglobin (Shiva et al., 2007) and mitochondrial enzymes(Nohl et al., 2000). Alternatively, studies provide evidence of increases in RBC-nitrite along with S-nitrosothiols in both the plasma (SNO-albumin and SNO-glutathione) and the red cells (SNO-Hb) following supplementation of plasma nitrite.
Patients with PAD limited by intermittent claudication pain during exercise are an ideal population in which to investigate the role of plasma nitrite and its contribution to physical function. These subjects can reliably approximate the point of ischemia via verbal reporting of claudication pain onset time during testing(Cachovan et al., 1999) and we can therefore document the amount of work performed when lower limb hypoxia impacts the muscle tissues and track changes over time. Prior to exercise training, there appears to be a net loss of plasma nitrite stores in both PAD and T2D + PAD groups, compared to healthier individuals, suggesting a decrease in the bioavailable NO pool during periods of exercise induced ischemia, consistent with attempts to normalize blood flow and oxygen delivery. Following 3 months of supervised training however, those with both T2D + PAD showed no increase in bioavailable NO/nitrite during acute exercise stress (compared to PAD only) and failed to significantly increase COT. Given that both groups were treated in exactly the same manner it seems logical that the diabetic condition contributed to this differential response.
Both the PAD-only and T2D + PAD subjects significantly increased peak walk time by 261 s and 171 s respectively (both p ≤ 0.05), suggesting an adaption to training for both groups. Although we did not measure any other potential mechanisms for this change it is expected that changes in muscle sarcomere number along with aerobic and anaerobic enzymes and increases in capillary density did occur in both the PAD-only and the T2D + PAD groups. In support of this idea, it is noteworthy that in the PAD-only subjects approximately 50% (128 s) of this peak walk time was due to increased pain free walk time, whereas in the T2D + PAD group only 34% (61 s) was additional pain free walking.
Traditional models of diabetes support the idea that hyperglycemia decreases endothelium-derived NO production via eNOS (Tesfamariam et al., 1991; Williams et al., 1998), which is reflected in our inhibited BAFMD response, and also increases inactivation of NO by advanced glycation end products (Tan et al., 2002) and reactive oxygen species (Beckman et al., 2001). This may make the ability or inability to deliver NO from other distant sites via protected species particularly pertinent in the development of cardiovascular disease and micro-vascular complications in the diabetic model.
The clinical significance of this finding lies in the therapeutic potential to provide an endogenous source of nitrite to augment the plasma reserves. This therapy would be specifically targeted at areas of ischemia to acutely improve oxygenation and may avoid systemic cytotoxicity effects. In support of this, we have previously shown in PAD-only subjects that acute increases in plasma nitrite concentration (via oral plasma nitrate beverage) increase exercise COT by 18% when compared to placebo(Kenjale et al., 2011). The performance increases were accompanied by a reduction in fractional O2 extraction at the working tissues, but there were no changes in ABI or BAFMD suggesting that stenosis and endogenous NO-production were not changed. This novel targeted delivery of NO to the tissues that are under-perfused due to occlusive vascular disease and/or rarefaction may be a more beneficial therapy in subjects with T2D + PAD who are unable to increase eNOS function in response to exercise training.
5. Limitations
This paper used data from two 2 parallel training studies. We only included subjects who completed the exercise regimen and therefore the study is not designed to assess effectiveness of the regimen in an intent-to-treat approach. Our findings do suggest a need to incorporate or analyze studies that focus on the mechanisms of exercise training and nitric oxide bioavailability based on the presence or absence of T2D.
Subjects in this trial were diagnosed via ABI testing and the presence of intermittent claudication, rather than angiographic assessment of PAD.
The two subject groups were not evenly matched for gender. The PAD-only group contained 4 females and the T2D + PAD group contained 7 females. Future confirmatory studies should contain more subjects per group and an equal gender distribution.
We did not measure the oxygen saturation of the working tissues in our subjects during the cardiopulmonary exercise testing. Although we used claudication onset time as a marker of tissue ischemia, it would be interesting, in future studies, to monitor the relationship between lower limb hemoglobin saturation and plasma nitrite flux during cardiopulmonary exercise testing in subjects with PAD.
We did not measure the concentration of reactive oxygen species in any of the blood samples. It is likely that individuals with peripheral arterial disease generate greater reactive oxygen species during the cardiopulmonary exercise testing than non-PAD subjects. For those with T2D + PAD this may play an even greater role in the consumption of nitric oxide especially in ischemic tissues. This may partly contribute to the lack of vascular responses to training in subjects with T2D + PAD. We also did not measure nitric oxide-species on the red blood cell. It is well documented that nitric oxide is carried on the red blood cell and can be released during hypoxia (Gladwin, 2008; McMahon et al., 2002). It has also been shown that plasma nitrite may be converted by deoxyhemoglobin to nitric oxide and iron-nitrosylated hemoglobin (Cosby et al., 2003).
This study was underpowered to see repeated measures ANOVA group*time interaction effects for most of the measured variables. From our current results, power calculations indicate for 80% power at the p = 0.05 level we would require approximately 64 subjects to observe differences in changes for VO2peak and PWT, and greater than 200 subjects for changes in COT and BAFMD.
6. Conclusion
These findings suggest PAD-only subjects are able to up-regulate endothelial function, and change plasma nitrite signaling following exercise training and that these changes mediate a more pronounced functional response in COT. In those subjects with T2D + PAD there were no changes in plasma nitrite signaling following training. These differences did not appear to be due to the extent of PAD or response to training and additional studies are needed to understand the impact of these finding on the overall clinical course of PAD.
Acknowledgments
Assistance for treadmill testing and exercise training in this study was provided by staff at the Duke University Medical Center, Center for Living Campus, Durham, NC 27710.
Abbreviations
- PAD
Peripheral Arterial Disease
- T2D
Type II Diabetes Mellitus
- IC
Intermittent Claudication
- NO
Nitric Oxide
- eNOS
endothelial nitric oxide synthase
- ABI
ankle-brachial index
- PWT
Peak Walking Time
- COT
Claudication Onset Time
- BAFMD
Brachial Artery Flow-Mediated Dilation
- NO2
Nitrite
- NO3
Nitrate
Footnotes
Declaration of Conflicting Interests: There are no conflicts of interest for any of the authors.
Funding: This work was supported in part by R01 HL755752 from the National Heart, Lung, and Blood Institute as well as the Division of Women’s Health, Office of the Director, National Institutes of Health, and by grant 1-08-CR-03 from the American Diabetes Association both to BHA.
References
- ACSM . ACSM’s guidelines for exercise testing and prescription. Ninth edition. 6th ed. Williams and Wilkins; Baltimore: 2013. [DOI] [PubMed] [Google Scholar]
- Allen JD, Cobb FR, Gow AJ. Regional and whole-body markers of nitric oxide production following hyperemic stimuli. Free Radical Biology & Medicine. 2005;38(9):1164–1169. doi: 10.1016/j.freeradbiomed.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Allen JD, Giordano T, Kevil CG. Nitrite and nitric oxide metabolism in peripheral artery disease. Nitric Oxide. 2012;26(4):217–222. doi: 10.1016/j.niox.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J, et al. Plasma nitrite response and arterial reactivity differentiate cardiovascular health status and performance. Nitric Oxide Biology and Chemistry. 2009;20:231–237. doi: 10.1016/j.niox.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JD, et al. Plasma nitrite flux predicts exercise performance in peripheral arterial disease following 3 months of exercise training. Free Radical Biology & Medicine. 2010;49:1138–1144. doi: 10.1016/j.freeradbiomed.2010.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association AD. Standards of medical care for patients with diabetes mellitus. Diabetes Care. 2001;24(Suppl. 1):S33–S43. doi: 10.2337/diacare.26.2007.s33. [DOI] [PubMed] [Google Scholar]
- Beckman JA, et al. Ascorbate restores endothelium-dependent vasodilation impaired by acute hyperglycemia in humans. Circulation. 2001;103(12):1618–1623. doi: 10.1161/01.cir.103.12.1618. [DOI] [PubMed] [Google Scholar]
- Brendle DC, et al. Effects of exercise rehabilitation on endothelial reactivity in older patients with peripheral arterial disease. The American Journal of Cardiology. 2001;87(3):324–329. doi: 10.1016/s0002-9149(00)01367-9. [DOI] [PubMed] [Google Scholar]
- Cachovan M, et al. Randomized reliability study evaluating constant-load and graded-exercise treadmill test for intermittent claudication. Angiology. 1999;50(3):193–200. doi: 10.1177/000331979905000303. [DOI] [PubMed] [Google Scholar]
- Cosby K, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nature Medicine. 2003;9(12):1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- Dormandy J, Heeck L, Vig S. Predicting which patients will develop chronic critical leg ischemia. Seminars in Vascular Surgery. 1999;12(2):138–141. [PubMed] [Google Scholar]
- Fuchsjager-Mayrl G, et al. Exercise training improves vascular endothelial function in patients with type 1 diabetes. Diabetes Care. 2002;25(10):1795–1801. doi: 10.2337/diacare.25.10.1795. [DOI] [PubMed] [Google Scholar]
- Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain. A meta-analysis. Journal of the American Medical Association. 1995;274(12):975–980. [PubMed] [Google Scholar]
- Gardner A, et al. Progressive vs single-stage treadmill tests for evaluation of claudication. Medicine and Science in Sports and Exercise. 1991;23:402–408. [PubMed] [Google Scholar]
- Gladwin MT. Evidence mounts that nitrite contributes to hypoxic vasodilation in the human circulation. Circulation. 2008;117(5):594–597. doi: 10.1161/CIRCULATIONAHA.107.753897. [DOI] [PubMed] [Google Scholar]
- Gladwin MT, et al. Role of circulating nitrite and S-nitrosohemoglobin in the regulation of regional blood flow in humans. Proceedings of the National Academy of Sciences. 2000;97(21):11482–11487. doi: 10.1073/pnas.97.21.11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdy O, et al. Lifestyle modification improves endothelial function in obese subjects with the insulin resistance syndrome. Diabetes Care. 2003;26(7):2119–2125. doi: 10.2337/diacare.26.7.2119. [DOI] [PubMed] [Google Scholar]
- Hiatt WR. Medical treatment of peripheral arterial disease and claudication. The New England Journal of Medicine. 2001;344(21):1608–1621. doi: 10.1056/NEJM200105243442108. [DOI] [PubMed] [Google Scholar]
- Hiatt WR, Hoag S, Hamman RF. Effect of diagnostic criteria on the prevalence of peripheral arterial disease: The San Luis Valley Diabetes Study. Circulation. 1995;91(5):1472–1479. doi: 10.1161/01.cir.91.5.1472. [DOI] [PubMed] [Google Scholar]
- Hirsch AT, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. Journal of the American Medical Association. 2001;286(11):1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- Jude EB, et al. Peripheral arterial disease in diabetic and nondiabetic patients: A comparison of severity and outcome. Diabetes Care. 2001;24(8):1433–1437. doi: 10.2337/diacare.24.8.1433. [DOI] [PubMed] [Google Scholar]
- Kenjale AA, et al. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. Journal of Applied Physiology. 2011;110(6):1582–1591. doi: 10.1152/japplphysiol.00071.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinbongard P, et al. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radical Biology & Medicine. 2003;35(7):790–796. doi: 10.1016/s0891-5849(03)00406-4. [DOI] [PubMed] [Google Scholar]
- Larsen FJ, et al. Effects of dietary nitrate on blood pressure in healthy volunteers. The New England Journal of Medicine. 2006;355(26):2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- Lauer T, et al. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. PNAS. 2001;98(22):12814–12819. doi: 10.1073/pnas.221381098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiorana A, et al. The effect of combined aerobic and resistance exercise training on vascular function in type 2 diabetes. Journal of the American College of Cardiology. 2001;38(3):860–866. doi: 10.1016/s0735-1097(01)01439-5. [DOI] [PubMed] [Google Scholar]
- Marso SP, Hiatt WR. Peripheral arterial disease in patients with diabetes. Journal of the American College of Cardiology. 2006;47(5):921–929. doi: 10.1016/j.jacc.2005.09.065. [DOI] [PubMed] [Google Scholar]
- McMahon TJ, et al. Nitric oxide in the human respiratory cycle. Nature Medicine. 2002;8(7):711–717. doi: 10.1038/nm718. [DOI] [PubMed] [Google Scholar]
- Metkus TS, Jr., Baughman KL, Thompson PD. Exercise prescription and primary prevention of cardiovascular disease. Circulation. 2010;121(23):2601–2604. doi: 10.1161/CIRCULATIONAHA.109.903377. [DOI] [PubMed] [Google Scholar]
- Mitchell RG, et al. Increased levels of apoptosis in gastrocnemius skeletal muscle in patients with peripheral arterial disease. Vascular Medicine. 2007;12(4):285–290. doi: 10.1177/1358863X07084858. [DOI] [PubMed] [Google Scholar]
- Nohl H, et al. Mitochondria recycle nitrite back to the bioregulator nitric monoxide. Acta Biochimica Polonica. 2000;47(4):913–921. [PubMed] [Google Scholar]
- Okada S, et al. Effect of exercise intervention on endothelial function and incidence of cardiovascular disease in patients with type 2 diabetes. Journal of Atherosclerosis and Thrombosis. 2010 doi: 10.5551/jat.3798. [DOI] [PubMed] [Google Scholar]
- Pinder A, et al. Nitrite directly vasodilates hypoxic vasculature via nitric oxide dependent and independent pathways. British Journal of Pharmacology. 2009;158(7):1523–1530. doi: 10.1111/j.1476-5381.2009.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regensteiner J, Steiner J, Hiatt W. Exercise training improves functional status in patients with peripheral arterial disease. Journal of Vascular Surgery. 1996;23(1):104–115. doi: 10.1016/s0741-5214(05)80040-0. [DOI] [PubMed] [Google Scholar]
- Shiva S, et al. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circulation Research. 2007;100(5):654–661. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- Sigal RJ, et al. Physical activity/exercise and type 2 diabetes: A consensus statement from the American Diabetes Association. Diabetes Care. 2006;29(6):1433–1438. doi: 10.2337/dc06-9910. [DOI] [PubMed] [Google Scholar]
- Tan KCB, et al. Advanced glycation end products and endothelial dysfunction in type 2 diabetes. Diabetes Care. 2002;25(6):1055–1059. doi: 10.2337/diacare.25.6.1055. [DOI] [PubMed] [Google Scholar]
- Tesfamariam B, Brown ML, Cohen RA. Elevated glucose impairs endothelium-dependent relaxation by activating protein kinase C. Journal of Clinical Investigation. 1991;87:1643–1648. doi: 10.1172/JCI115179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting H, et al. Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. Journal of Clinical Investigation. 1996;97(1):22–28. doi: 10.1172/JCI118394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb A, et al. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia–reperfusion damage. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(37):13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AJ, et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51(3):784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch MA, Allen JD, Geaghan JP. Stability and reproducibility of brachial artery flow-mediated dilation. Medicine and Science in Sports and Exercise. 2002;34(6):960–965. doi: 10.1097/00005768-200206000-00009. [DOI] [PubMed] [Google Scholar]
- Williams SB, et al. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation. 1998;97(17):1695–1701. doi: 10.1161/01.cir.97.17.1695. [DOI] [PubMed] [Google Scholar]
- Zweier J, et al. Enzyme-independent formation of nitric oxide in biological tissues. Nature Medicine. 1995;8:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]