Abstract
Objective
To investigate the role of leptin on reproductive hormones and ovulation.
Study Design
The BioCycle Study (2005–2007) followed 259, healthy premenopausal women not using hormonal contraceptives for ≤2 menstrual cycles (N=509 cycles). Serum leptin, estradiol, progesterone, luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone were measured ≤8 times per cycle. The association of time-varying leptin and reproductive hormones over the cycle was estimated using linear mixed models adjusted for percent body fat and age with inverse probability weighting for time-varying physical activity, caloric intake and other reproductive hormones. The odds ratio (OR) for sporadic anovulation (n=42 cycles) was estimated using generalized linear models, adjusted for percent body fat and age.
Results
Geometric mean serum leptin increased from menses to the late luteal phase (from 16.7 to 20.4 ng/mL; p <0.01), with a mid-cycle peak (21.7 ng/mL) at the time of the LH surge (p <0.01). A 10% higher leptin level across the menstrual cycle was associated with higher estradiol (2.2%, 95% confidence interval [CI]: 1.5 to 3.0), luteal progesterone (2.1%, CI: 0.5 to 3.7), ovulatory LH (1.2%, CI: 0.0 to 2.3) and testosterone (0.6%, CI: 0.3 to 0.9), and lower FSH (−0.7%, CI:−1.1 to −0.4). Leptin at the time of the expected LH surge was moderately inversely associated with sporadic anovulation (per log increase in leptin, adjusted OR=0.58, CI: 0.28 to 1.22).
Conclusions
The association observed between leptin and reproductive function points to a possible relationship between serum leptin level and enhanced fertility.
Keywords: anovulation, leptin, menstrual cycle, reproductive hormones
BACKGROUND AND OBJECTIVE
Leptin, a product of the LEP gene, is widely known to regulate appetite and energy expenditure.1 Its involvement in the reproductive system was first suspected in 1949 when leptin homozygous recessive female mice were observed to be not only obese but sterile.2 Future research demonstrating that the administration of recombinant leptin to these mice restored fertility led researchers to theorize that leptin served as a signal of adequate fat deposition, allowing for the energy-intensive reproduction system to function appropriately.3,4 Recent studies on the administration of recombinant leptin to women with lipodystrophy (i.e. leptin deficiency) have also demonstrated restored menstrual cycle regularity and fertility.5,6 Despite the clear involvement of leptin in the female reproductive system, its relationship to reproductive hormone production, menstrual cycle characteristics, and ovarian function remains unclear.
The role of leptin on menstrual cycle regulation was first suggested more than a decade ago by researchers who found that leptin levels varied across the menstrual cycle while remaining stable for men and postmenopausal women over a 28-day period.7 Subsequently, a number of studies have either found serum leptin to increase from the follicular to the luteal phase (in a cyclic fashion) or show no trend across the menstrual cycle.7–22 Limitations of previous work include the small number of women studied, the limited number of serum samples collected over the cycle, and unverified menstrual cycle phase determination. Furthermore, associations between leptin and reproductive hormones have been primarily identified by statistical correlations, without further consideration for factors such as diet, physical activity, and other hormone levels, which may have resulted in bias. In addition, because adipose tissue is a source of both leptin and estradiol production,23 adjustment for adiposity is critical for understanding leptin’s effect on reproductive hormones outside of the influence of body fat and could help inform future clinical interventions.
The primary objective of our study was to describe leptin levels across the menstrual cycle among a cohort of premenopausal women. Our secondary objectives were to examine the associations between leptin and reproductive hormones (including estradiol, progesterone, luteinizing hormone [LH], follicle-stimulating hormone [FSH], and testosterone), menstrual cycle characteristics and the odds of sporadic anovulation. The results of our study are important for understanding the role of leptin on reproduction and fertility.
METHODS
Study population
The BioCycle Study (2005–2007) was a prospective cohort study of 259 regularly menstruating, healthy premenopausal women from Western New York who were followed over 1 (n=9) or 2 (n=250) menstrual cycles. Women were not eligible for the study if they were using oral contraceptives or medications for a chronic medical condition; had been pregnant or breastfeeding within the past 6 months; had been diagnosed with a menstrual or ovulatory disorder; or self-reported their body mass index (BMI) as less than 18 or greater than 35 kg/m2 at screening. Additional information about the study population is described in more detail elsewhere.24 The University at Buffalo Health Sciences Institutional Review Board (IRB) approved the study and served as the IRB designated by the National Institutes of Health for this study under a reliance agreement. All participants provided written informed consent.
Measures
Leptin and reproductive hormones
Women provided morning fasting blood samples up to 8 times per cycle. Fertility monitors (Clearblue Easy Fertility Monitor; Inverness Medical, Waltham, Massachusetts) were used to time mid-cycle visits, with the remaining visits scheduled according to an algorithm that considered each woman’s typical cycle length.25 Consequently, blood samples were collected during the following expected phases of the menstrual cycle: menses; the middle and late follicular phase; LH surge; ovulation; and the early, mid and late luteal phase. Most women adhered to the study protocol with 94% providing blood samples for at least 7 visits per cycle. Blood samples were processed according to standard protocols and frozen at −80°C within 90 minutes of phlebotomy.24 Frozen sera were later shipped on dry ice to analytical laboratories. Samples from each participant were measured within a single run to limit analytical variability.
Leptin concentration was measured in multiple batches by immunoassay using the Mercodia Leptin ELISA (Mercodia AB, Uppsala, Sweden) at the Advanced Research and Diagnostics Laboratory, University of Minnesota, Minneapolis, MN. No values were below the lower limit of detection (LOD) for this assay (0.05 ng/mL). Select batches of measurements were recalibrated post-assay by a calibration curve estimated from all the calibration data.26 The maximum interassay coefficient of variation (CV) was 10.2% after recalibration.
Estradiol, progesterone, LH, and FSH concentrations were measured by solid-phase competitive chemiluminescent enzymatic immunoassays on the Immulite 2000 analyzer (Siemens Medical Solutions Diagnostics, Deerfield, Illinois) at Kaleida Laboratories in Buffalo, NY. Total testosterone was measured by liquid chromatography/tandem mass spectrometry using the Shimadzu Prominence Liquid Chromatogram with an ABSceix 5500 tandem mass spectrometer at the Advanced Research and Diagnostics Laboratory (see above). Increased sensitivity was achieved by using 100% acetronitrile mobile phase B as the solvent gradient elution and adding a low standard of 4 ng/dL. The interassay CVs were: < 10% for estradiol; < 14% for progesterone; < 4% for LH and FSH; and < 7% for testosterone. Values falling below the LOD for each assay were rare (< 3%) and were replaced with values equal to the LOD divided by the square root of 2.27 All hormone measurements, including leptin, were logarithmically transformed for the analysis.
Sporadic anovulation and menstrual cycle characteristics
Sporadic anovulatory cycles were defined as cycles with a peak progesterone concentration ≤ 5 ng/mL and no observed serum LH peak among samples collected during the later cycle visits (n=42 cycles).28 In a sensitivity analysis, a subgroup of anovulatory cycles with peak progesterone concentrations ≤ 3 ng/mL (n=28 cycles) were examined as an alternative definition of anovulation. Menstrual cycle characteristics (menstrual cycle length, menses length, and total blood loss during menses) were determined from daily diaries which documented bleeding days and blood loss using validated pictograms.29 Follicular and luteal phase lengths were determined based on the expected date of ovulation using information from the fertility monitors and serum hormone levels.30
Hormone realignment
To correct for any residual errors in blood collection timing, hormone measurements were realigned within ovulatory cycles according to an algorithm based on the day of the serum LH peak.31 This realignment affected 70% of ovulatory cycles, with 42% of realignments due to an LH peak detected at the visits adjacent to the expected LH surge visit. All hormones measurements were realigned together for a given cycle. Realignment procedures sometimes resulted in missing hormone levels; therefore, longitudinal multiple imputation methods were applied to these missing data. Anovulatory cycles were excluded from the realignment and imputation procedures as no ovulation was presumed to have occurred.
Covariates
Self-administered questionnaires were used to assess demographics, smoking behavior, physical activity and perceived stress (measured by the Cohen Perceived Stress Scale)32 during a baseline study visit scheduled 1–2 weeks prior to the participant’s next expected start of menses. Physical activity was assessed by measuring past-week MET-hours per week (MET-h/week) using the long-form International Physical Activity Questionnaire (IPAQ).33 BMI was calculated using weight and height as measured by trained personnel at the baseline visit. At the end of the follow-up period, body composition was determined using duel energy X-ray absorptiometry (DXA) scans (Hologic Discovery Elite, software version 12.4.1, Waltham, MA) and total percent body fat was derived.24 Past-week MET-h/week and caloric intake were assessed 4 times during each cycle using the short-form IPAQ and the 24-hour dietary recall, respectively.33,34 These within cycle measures were realigned and imputed along with the hormone measurements.
Statistical analysis
Participant characteristics were compared by tertile of average leptin concentration across the study period with Fisher’s exact and ANOVA tests used to examine differences. Unadjusted linear mixed models were used to estimate geometric mean leptin by menstrual cycle phase and assess differences in leptin between phases. To evaluate the effect of our realignment and imputations procedures, leptin concentrations across the cycle were also examined using the original data before realignment. Unadjusted linear mixed models were also used to estimate mean reproductive hormones and menstrual cycle characteristics by tertile of leptin within each cycle. Unadjusted generalized linear models were used to assess differences in the proportion of anovulatory cycles by tertile of leptin within each cycle.
Linear mixed models with random intercepts were used to evaluate the association between time-varying leptin and reproductive hormones (estradiol, luteal progesterone, ovulatory LH, FSH, and testosterone). In these models hormone levels varied over the cycle within women, allowing for the estimation of the average association between leptin and reproductive hormones rather than the association within women categorized by average leptin level. Models were adjusted for 1) age and 2) age and percent body fat. For the 11 women missing DXA scans, percent body fat was estimated using the following internally derived linear regression formula: {percent body fat=1.7+ BMI x 1.2}. Results of the models were presented as average percent change in nontransformed reproductive hormone values per 10% increase in nontransformed leptin value using the following formula: {[(1.1^β)−1] x 100%}. Because leaner women may be more sensitized to the effects of leptin,12 we conducted a secondary analysis by limiting our regression models to records from women with a percent body fat <30% (50th percentile).
In order to account for the possibility that time-varying confounders could be both causes and consequences of leptin levels, we used marginal structural models with inverse probability weights to adjust for these confounders.35 These models were estimated using weighted linear mixed models and stabilized inverse probability weights were constructed using concurrent MET-h/week and caloric intake and prior reproductive hormone measurements (estradiol, progesterone, FSH, LH). Confounders included in the weight models were based on a directed acyclic graph (DAG) approach. Model weights were the cumulative product of all previous time-points’ weights for each woman and had a mean value of 1.0 (range 0.01 to 16.9).
Sporadic anovulation
Linear mixed models were used to estimate leptin levels across the menstrual cycle in ovulatory and anovulatory cycles, adjusting for age and percent body fat. The odds of sporadic anovulation was estimated using average leptin levels only from the first half of the menstrual cycle to preserve the temporal ordering of the exposure-outcome relationship. Leptin on the day of the expected LH surge was also examined in relation to sporadic anovulation. We used generalized linear models to estimate the odds ratio (OR), adjusting for age and percent body fat. In addition, we explored the relationship between leptin and sporadic anovulation non-parametrically with restricted cubic splines.36,37
All analyses accounted for multiple imputations and were performed using SAS v9.3 (SAS Institute, Cary, NC, USA).
RESULTS
Study population
Study participants had a mean age of 27.3 years (standard deviation [SD], 8.2), BMI of 24.1 (SD, 3.9) and percent body fat of 29.5 (SD, 6.0) (Table 1). BMI and percent body fat increased with leptin tertile (p<0.01, respectively) and lower educational attainment was associated with higher leptin (p=0.01), but no other significant differences were observed.
Table 1.
Participant characteristics by tertile of average leptin measured across ≤ 2 menstrual cycles (N=259 women)
| Tertile of average leptin across ≤ 2 menstrual cycles: Mean± SD (min, max)n(column %) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Participant Characteristics |
All (N=259b) |
1st Tertile < 13.7 ng/mL (n=85) |
2nd Tertile 13.7 to 28.2 ng/mL (n=85) |
3rd Tertile ≥ 28.2 ng/mL (n=89) |
P-valuea | ||||
| Age, years | 27.3 ± 8.2 | (18 , 44) | 27.8 ± 8 | (18 , 43) | 26.9 ± 8.8 | (18 , 44) | 27.2 ± 8 | (18 , 44) | 0.78 |
| Body mass index (kg/m2) | 24.1 ± 3.9 | (16.1 , 35.0) | 21.2 ± 2 | (16.1 , 27.3) | 23.6 ± 2.6 | (18.5 , 30.2) | 27.3 ± 3.9 | (18.4 , 35.0) | <0.0001 |
| Percent body fatc | 29.5 ± 6.0 | (15.1 , 45.2) | 23.7 ± 3.4 | (15.8 , 33.7) | 29.6 ± 3.6 | (15.1 , 37.6) | 35.1 ± 4.1 | (24.8 , 45.2) | <0.0001 |
| Race | 0.13 | ||||||||
| White | 154 | ( 59.5 ) | 56 | ( 65.9 ) | 54 | ( 63.5 ) | 44 | ( 49.4 ) | |
| African American | 51 | ( 19.7 ) | 13 | ( 15.3 ) | 13 | ( 15.3 ) | 25 | ( 28.1 ) | |
| Other race | 54 | ( 20.8 ) | 16 | ( 18.8 ) | 18 | ( 21.2 ) | 20 | ( 22.5 ) | |
|
Education (highest grade completed) |
0.01 | ||||||||
| Post-secondary | 226 | ( 87.3 ) | 79 | ( 92.9 ) | 77 | ( 90.6 ) | 70 | ( 78.7 ) | |
| ≤ High school | 33 | ( 12.7 ) | 6 | ( 7.1 ) | 8 | ( 9.4 ) | 19 | ( 21.3 ) | |
| Marital status | 0.85 | ||||||||
| Single/divorced | 193 | ( 74.5 ) | 62 | ( 72.9 ) | 65 | ( 76.5 ) | 66 | ( 74.2 ) | |
| Married/living as married | 66 | ( 25.5 ) | 23 | ( 27.1 ) | 20 | ( 23.5 ) | 23 | ( 25.8 ) | |
| Nulliparous | 187 | ( 74 ) | 57 | ( 67.1 ) | 65 | ( 76.5 ) | 65 | ( 73.0 ) | 0.51 |
|
Physical activity at baseline |
1.0 | ||||||||
| Low | 25 | ( 9.7 ) | 8 | ( 9.4 ) | 8 | ( 9.4 ) | 9 | ( 10.1 ) | |
| Moderate | 92 | ( 35.5 ) | 30 | ( 35.3 ) | 31 | ( 36.5 ) | 31 | ( 34.8 ) | |
| High | 142 | ( 54.8 ) | 47 | ( 55.3 ) | 46 | ( 54.1 ) | 49 | ( 55.1 ) | |
| Smoking | 0.44 | ||||||||
| Nonsmoker | 249 | ( 96.1 ) | 80 | ( 94.1 ) | 82 | ( 96.5 ) | 87 | ( 97.8 ) | |
| Current smoker | 10 | ( 3.9 ) | 5 | ( 5.9 ) | 3 | ( 3.5 ) | 2 | ( 2.2 ) | |
| ≥1 Anovulatory cycled | 35 | ( 13.5 ) | 15 | ( 17.6 ) | 8 | ( 9.4 ) | 12 | ( 13.5 ) | 0.30 |
| Perceived stress scoree | 20.2 ± 6.8 | (3 , 44) | 19.8 ± 6.2 | (6 , 34) | 19.7 ± 6.2 | (7 , 38) | 21.0 ± 7.9 | (3 , 44) | 0.34 |
| Dietary intakef | |||||||||
| Total caloric intake, kcal | 1607± 354 | (793, 2686) | 1626 ± 364 | (793 , 2622) | 1611 ± 359 | (858 , 2686) | 1587 ± 344 | (821 , 2667) | 0.76 |
| Total fat, g | 62.1 ± 18.5 | (18.5 , 133.9) | 62.9 ± 16.4 | (28.6 , 113.4) | 62.1 ± 19.8 | (26.1 , 124.1) | 61.5 ± 19.4 | (18.5 , 133.9) | 0.88 |
| Total carbohydrate, g | 201.1 ± 49.0 | (75.7 , 330.3) | 202.9 ± 52.9 | (92.5 , 325.3) | 200.6 ± 47.0 | (94.2 , 330.3) | 199.9 ± 47.4 | (75.7 , 312.4) | 0.91 |
| Total protein, g | 62.2 ± 15.6 | (26.3 , 132.1) | 63.2 ± 17.0 | (28.5 , 132.1) | 63.0 ± 14.7 | (37.6 , 108.8) | 60.4 ± 15.1 | (26.3 , 100.1) | 0.41 |
Abbreviations: SD, standard deviation; kcal, kilocalorie
Two-sided P-values for continuous variables calculated using ANOVA and categorical variables using Fisher’s Exact test.
250 women were followed for 2 cycles, 9 women were followed for 1 cycle, for a total of 509 cycles.
248 participants were assessed for percent body fat at end of study.
Twenty-eight women in the study had only one anovulatory cycle, and seven women in the study had two anovulatory cycles.
Perceived stress score measured at baseline.
Averaged over up to 8 24-hour dietary recalls conducted during the study.
Leptin
The geometric mean of average leptin levels within-women over the study period was 19.7 ng/mL (95% confidence interval [CI]:18.1 to 21.4) and varied across the menstrual cycle (p <0.01). Leptin concentrations increased over the menstrual cycle (from 16.7 [CI: 15.3 to 18.2] during menses to 20.4 [CI: 18.7 to 22.3] ng/mL during the late luteal phase, p <0.01), with a mid-cycle peak at the time of the LH surge (21.7 [CI: 19.9 to 23.7] ng/mL; p <0.01 for comparisons with late follicular and ovulation, respectively). In the original data before realignment, the mid-peak (20.5 [SD, 2.0] ng/mL) was discernible, but only statistically different from the late follicular phase (p< 0.03 and 0.65 for comparisons with late follicular and ovulation, respectively).
Menstrual cycle characteristics
Menses length differed by tertile of average leptin within each cycle (p=0.01), with the shortest menses length observed for cycles within the highest leptin tertile (Table 2). This relationship was attenuated after post-hoc adjustment for percent body fat (p=0.09, data not shown). No other differences in menstrual cycle characteristics were notable, including the proportion of cycles that were anovulatory (overall 42/509, 8.3%).
Table 2.
Reproductive hormones, menstrual cycle characteristics and the proportion of anovulatory cycles by tertile of average leptin within each cycle (N=509 cycles)
| Tertile of aver age leptin (ng/mL) w ithin each cycle | |||||
|---|---|---|---|---|---|
| Cycle characteristics | Total Number of Cycles (N=509b) |
1st Tertile < 13.6 ng/mL (n=167) |
2nd Tertile 13.6 to 28.6 ng/mL (n=168) |
3rd Tertile ≥ 28.6 ng/mL (n=174) |
P-valuea |
|
Reproductive hormonesc mean (95% CI) |
|||||
| Estradiol (pg/mL) | 87.3 ( 83.9, 90.9) | 83.5 ( 78.2, 89.2) | 89.3 ( 84.0, 94.9) | 89.3 ( 83.8, 95.2) | 0.25 |
| Progesterone (ng/mL) | 1.4 ( 1.4, 1.5) | 1.4 ( 1.3, 1.5) | 1.5 ( 1.4, 1.6) | 1.4 ( 1.3, 1.5) | 0.33 |
| FSH (mIU/mL) | 5.2 ( 5.0, 5.4) | 5.7 ( 5.4, 6) | 5.1 ( 4.8, 5.3) | 4.9 ( 4.6, 5.2) | <0.01 |
| LH (ng/mL) | 6.4 ( 6.2, 6.7) | 6.4 ( 6.0, 6.9) | 6.5 ( 6.1, 6.9) | 6.3 ( 5.9, 6.7) | 0.63 |
| Testosterone (ng/dL) | 27.8 ( 26.7, 29.0) | 26.8 ( 25.3, 28.3) | 28.1 ( 26.8, 29.5) | 28.6 ( 27.1, 30.2) | 0.15 |
|
Menstrual cycle characteristics, mean (95% CI) |
|||||
| Menstrual cycle length, daysd | 28.8 ( 28.4, 29.3) | 28.4 ( 27.6, 29.1) | 28.9 ( 28.2, 29.6) | 29.3 ( 28.6, 30.0) | 0.24 |
| Follicular phase length, dayse | 15.3 ( 14.9, 15.8) | 15.1 ( 14.4, 15.9) | 15.4 ( 14.6, 16.1) | 15.4 ( 14.7, 16.1) | 0.87 |
| Luteal phase length, dayse | 13.9 ( 13.7, 14.1) | 13.6 ( 13.1, 14) | 13.9 ( 13.5, 14.3) | 14.3 ( 13.9, 14.7) | 0.09 |
| Menses length, daysf | 5.4 ( 5.2, 5.5) | 5.5 ( 5.3, 5.8) | 5.6 ( 5.3, 5.8) | 5.0 ( 4.8, 5.3) | 0.01 |
| Total blood loss, gg | 43.8 ( 38.9, 49.3) | 46.1 ( 38.2, 55.7) | 46.9 ( 39.4, 55.8) | 38.8 ( 32.3, 46.7) | 0.24 |
|
Anovulatory cyclesh, n (column %) |
42 (8.3%) | 18 (10.8%) | 10 (6.0%) | 14 (8.1%) | 0.34 |
Abbreviations: CI, confidence interval; FSH, follicle-stimulating hormone; LH, luteinizing hormone
P-value for continuous measures assessed by Type III effects from unadjusted linear mixed models. Anovulatory cycle p-value assessed by Type III effects from unadjusted generalized linear regression.
250 women were followed for 2 cycles, 9 women were followed for 1 cycle.
Geometric mean calculated within-women using up to 8 measurements over the cycle. Testing performed on log hormone mean, then transformed by exponentiation for table display.
Menstrual cycle length available for 476 cycles.
Among ovulatory cycles, follicular phase length available for 467 women; luteal phase length available for 443 women.
Menses length available for 497 cycles.
Total blood loss available for 467 cycles. Geometric mean calculated within-women using up to 2 measurements over the study. Testing performed on log total blood loss mean, then transformed by exponentiation for table display.
Anovulatory cycles defined as progesterone concentration < 5 ng/mL and no luteinizing hormone peak detected in serum during the later cycle visits.
Reproductive hormones
FSH concentrations differed by tertile of average leptin within each cycle (p<0.01), but the other reproductive hormones were similar among tertiles (Table 2). In the time-varying analysis, a 10% increase in leptin across the menstrual cycle was associated with increased estradiol (2.2%, CI: 1.5 to 3.0), luteal progesterone (2.1%, CI: 0.5 to 3.7), ovulatory LH (1.2%, CI: 0.0 to 2.3), and testosterone (0.6%, CI: 0.3 to 0.9), and decreased FSH (−0.7%, CI: −1.1 to −0.4) (Table 3). Adjustment for time-varying physical activity, caloric intake and other hormone concentrations had little impact on the effect estimates, indicating that confounding by these factors was minimal. Estimates of the association between leptin and reproductive hormones in women with <30% body fat were similar (±20%) to those found overall, with the exception of ovulatory LH (which increased by 22% from 1.4% [CI: 0.5 to 2.3] to 1.7% [CI: 0.4 to 3.0]) and FSH (which decreased by 26% from −0.7% [CI: −1.1 to −0.3] to −0.5% [CI: −1.0 to 0.0]).
Table 3.
Associations between serum leptin levels and concentrations of reproductive hormones across the menstrual cycle
| Hormone | Modela | Per 10% increase in leptin (ng/mL) |
||
|---|---|---|---|---|
| % change | 95% CI | |||
| Estradiol (pg/mL) | Model 1 | 1.5** | 1.1 | 1.9 |
| Model 2 | 1.5** | 1.1 | 1.9 | |
| Model 3 | 2.1** | 1.6 | 2.7 | |
| Model 4 | 2.2** | 1.5 | 3.0 | |
| Luteal Progesteroneb (ng/mL) | Model 1 | 0.9 | 0.0 | 1.7 |
| Model 2 | 1.0* | 0.1 | 1.9 | |
| Model 3 | 2.0* | 0.6 | 3.3 | |
| Model 4 | 2.1* | 0.5 | 3.7 | |
| FSH (mIU/mL) | Model 1 | −0.7** | −1.0 | −0.4 |
| Model 2 | −0.7** | −1.0 | −0.4 | |
| Model 3 | −0.7* | −1.1 | −0.3 | |
| Model 4 | −0.7* | −1.1 | −0.4 | |
| Ovulatory LHc (ng/mL) | Model 1 | 0.7* | 0.1 | 1.2 |
| Model 2 | 0.7* | 0.1 | 1.3 | |
| Model 3 | 1.4* | 0.5 | 2.3 | |
| Model 4 | 1.2 | 0.0 | 2.3 | |
| Testosterone (ng/dL) | Model 1 | 0.6** | 0.3 | 0.8 |
| Model 2 | 0.5** | 0.3 | 0.7 | |
| Model 3 | 0.6** | 0.4 | 0.8 | |
| Model 4 | 0.6* | 0.3 | 0.9 | |
Abbreviations: CI, confidence interval; FSH, follicle stimulating hormone; LH, luteinizing hormone
P-value < 0.05,
P-value < 0.0001
Percent change, 95% confidence interval and p-value were assessed using linear mixed models. Model 1: Unadjusted; Model 2: Adjusted for age; Model 3: Adjusted for age and percent body fat; Model 4: Adjusted for age, percent body fat, and, through the use of inverse probability weights, other reproductive hormones, caloric intake, and physical activity.
Included progesterone measured in serum collected during the early, mid and late luteal phases of the menstrual cycle.
Included LH measured in serum collected during the late follicular, LH surge, and ovulation phases of the menstrual cycle.
Sporadic anovulation
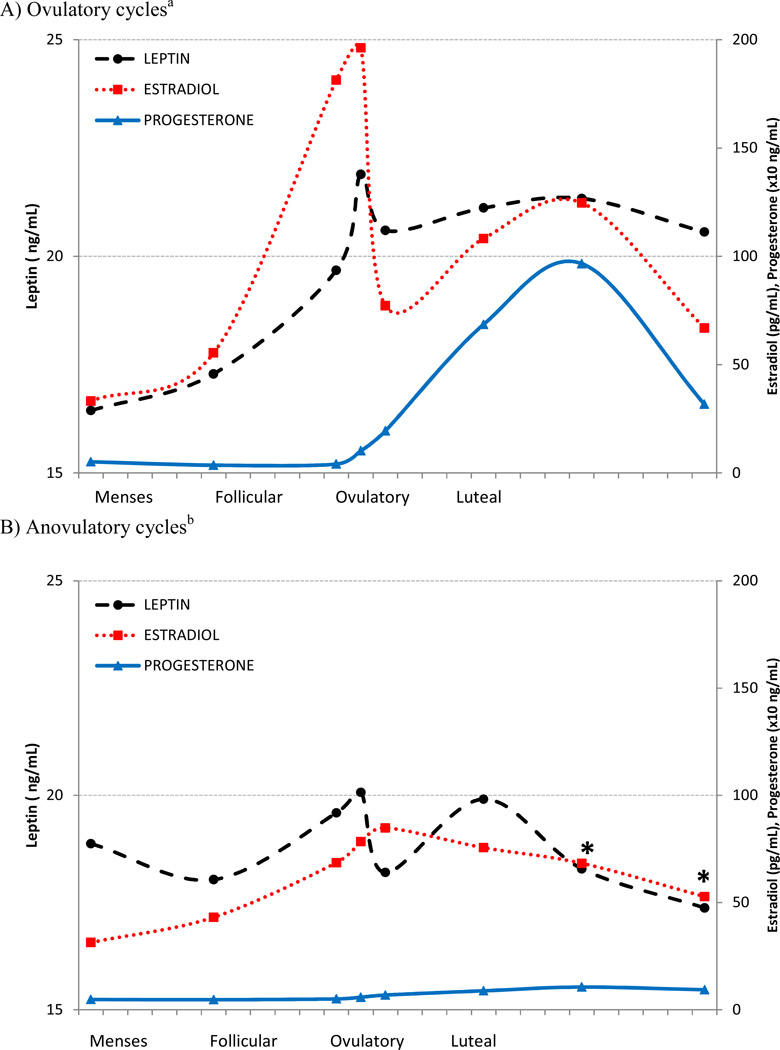
Average leptin levels within ovulatory cycles were similar to those within anovulatory cycles overall (p=0.60), however leptin was lower in anovulatory cycles compared with ovulatory cycles during the latter half of the cycle (p<0.05) (Figure 1).
Figure 1.
Average estradiol (dotted line), progesterone (solid line) and leptin (dashed line) concentrations across a standardized menstrual cycle for 467 ovulatory (A) and 42 anovulatory cycles (B), after adjustment for differences in percent body fat and age.
*Leptin was significantly lower in anovulatory cycles compared with ovulatory cycles (p<0.05). Geometric means were estimated and p-value was assessed using linear mixed models adjusted for percent body fat and age.
a Leptin during menses, middle follicular and late follicular phases, respectively, were lower compared to all subsequent phases and leptin at the time of the LH surge was higher than all subsequent phases except the mid luteal phase (p <0.05). P-values were assessed using linear mixed models.
b Expected menstrual cycle phases for anovulatory cycles were determined based on fertility monitor readings and each participant’s self-reported typical menstrual cycle length. Mean cycle day of blood collection was as follows: menses (2.6), middle follicular (7.8), late follicular (13.8), LH surge (15.9), ovulation (18.1), early luteal (20.6), mid-luteal (23.4), and late luteal (26.1). Leptin at the time of the expected LH surge was higher than at the time of expected ovulation and the late luteal phase, and leptin during the expected early luteal phase was higher than at the expected late luteal phase (p<0.05). P-values were assessed using linear mixed models.
Leptin levels averaged across the first half of the menstrual cycle were not associated with the odds of sporadic anovulation (unadjusted and adjusted p>0.31). Leptin levels on the expected day of the LH surge did show an inverse relationship with sporadic anovulation (per log increase in leptin: unadjusted OR= 0.66, CI: 0.44 to 1.0, p=0.05; adjusted OR= 0.58, CI: 0.28 to 1.22, p=0.15). Using a more restrictive definition of anovulation resulted in similar findings: no association for leptin averaged across the first half of the cycle (p>0.42), and a moderate inverse association for leptin on the expected day of the LH surge (per log increase in leptin: unadjusted OR= 0.74, CI: 0.47 to 1.17, p=0.20; adjusted OR= 0.63, CI: 0.31 to 1.25, p=0.19). Restricted cubic spline regression failed to show a non-linear relationship was likely for either of these leptin exposure definitions.
COMMENTS
This study observed increasing leptin levels across the menstrual cycle, with a mid-cycle peak at the time of the LH surge, among a cohort of healthy, regularly menstruating, premenopausal women. We also observed modest associations between time-varying leptin levels and reproductive hormones. Specifically, 10% higher leptin levels throughout the menstrual cycle were associated with 1–2% higher levels of estradiol, luteal progesterone, ovulatory LH, and testosterone and 1% lower levels of FSH. Leptin levels averaged across the first half of the cycle were not associated with the odds of sporadic anovulation; however, leptin at the time of the LH surge was moderately inversely associated.
Previous studies of leptin across the menstrual cycle have found a similar pattern of higher leptin levels in the luteal phase compared with the first half of the cycle in regularly menstruating women.7–19 The source of luteal phase leptin may be the corpus luteum, which has been shown to produce leptin in humans and in a variety of animals.38–43 Supporting this idea, our study showed lower leptin during the latter half of anovulatory cycles, i.e. cycles where no luteinization was presumed to have occurred. Changes in caloric intake and physical activity did not account for the higher luteal leptin, as others have previously found.10 Higher luteal phase leptin likely plays a role in preparing the body for the energy-intensive demands of pregnancy.19,44
Consistent with our study, Cella et al and Fernández-Real et al both noted a mid-cycle peak in leptin,12,13 and several other studies show a discernible leptin peak around the time of the LH surge even though this was not noted in their findings.7,14,17 A leptin peak concurrent with the LH surge, which consequently triggers ovulation, could indicate leptin has an active role in initiating ovulation.13 Our ability to detect this mid-cycle peak was enhanced by our realignment and imputation procedures, as the peak was less clearly defined using the original data before realignment. Further evidence for leptin’s role in ovulation is the moderate inverse association we found between leptin at the time of the LH surge and the odds of sporadic anovulation.
Experimental studies support the role of leptin on ovulation through leptin’s stimulation of the pituitary to secrete LH.45–47 Leptin can also activate receptors for gonadotropin-releasing hormone in the hypothalamus, which in turn stimulate LH secretion.6 Empirically, the administration of recombinant leptin has been shown to restore menses and fertility in women with hypothalamic amenorrhea6,48 and congenital generalized lipodystrophy, most likely by increasing LH levels and pulse frequency.5
Our study expands on previous literature by examining leptin throughout the menstrual cycle with enhanced accuracy as to the timing of serum collection in a large group of women and accounting for multiple sources of confounding. Use of home fertility monitors to time serum collection at 8 key phases of the menstrual cycle and post-hoc realignment procedures based on the day of the LH surge increased our ability to detect a leptin mid-cycle peak that other studies may have missed. Leptin was measured in fasting morning serum samples, decreasing variability due to diurnal patterns and short-term response to food intake.49,50 Analytically, we were able to perform regression adjustment for percent body fat, which is highly associated with leptin levels. Our study examined leptin as a time-varying exposure, allowing for estimation of average hormone changes across the menstrual cycle while controlling for the time-varying confounding effects of caloric intake, physical activity and other reproductive hormones.
This study has several limitations. Though we set up our statistical models to examine the effect of leptin on reproductive hormones, reverse causation cannot be ruled out. For example, the association between leptin and estradiol that we observed could be due to estrogen’s effect on leptin production.13,51,52 More likely, these hormonal relationships are not unidirectional but part of a feedback loop, which was partly addressed in our analysis by use of marginal structural modeling. In addition, we limited our ability to detect the risk of chronic anovulation by excluding women with known ovulatory disorders and irregular menstrual cycle lengths. We also restricted our study to women with a self-reported BMI at screening between 18 and 35. While this likely enhanced our ability to control for adiposity by design, it limits the generalizability of our findings given the leptin insensitivity that develops in obese individuals.53 Indeed we observed small differences in the relationship between leptin and estradiol and FSH in women with <30% body fat compared to those overall. Future research on leptin’s effect on menstrual cycle function in obese women is needed, particularly comparisons between ovulatory and anovulatory cycles in these women.
In our cohort of healthy, regularly menstruating premenopausal women, leptin increased over the menstrual cycle, with a mid-cycle peak concurrent with the LH surge. Leptin was associated with modest changes in reproductive hormones and may be associated with triggering ovulation. The physiological significance of higher leptin levels during mid-cycle and in the luteal phase are not well understood, but taking into consideration prior research, are likely related to enhanced fertility and preparing the body for the increased energy demands of pregnancy.
Acknowledgments
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (Contracts #HHSN275200403394C, HHSN275201100002I Task 1 HHSN27500001).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflict of interest.
A preliminary version of this analysis was presented at the 26th annual meeting of the Society for Pediatric and Perinatal Epidemiologic Research (SPER): June 17–19, 2013, Boston, MA.
References
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Ingalls A, Dickie M, Snell G. Obese, a new mutation in the house mouse. J Hered. 1950;41(12):317–318. doi: 10.1093/oxfordjournals.jhered.a106073. [DOI] [PubMed] [Google Scholar]
- 3.Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet. 1996;12(3):318–320. doi: 10.1038/ng0396-318. [DOI] [PubMed] [Google Scholar]
- 4.Barash IA, Cheung CC, Weigle DS, et al. Leptin is a metabolic signal to the reproductive system. Endocrinology. 1996;137(7):3144–3147. doi: 10.1210/endo.137.7.8770941. [DOI] [PubMed] [Google Scholar]
- 5.Maguire M, Lungu A, Gorden P, Cochran E, Stratton P. Pregnancy in a woman with congenital generalized lipodystrophy: leptin's vital role in reproduction. Obstet Gynecol. 2012;119(2 Pt 2):452–455. doi: 10.1097/AOG.0b013e31822cecf7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou SH, Chamberland JP, Liu X, et al. Leptin is an effective treatment for hypothalamic amenorrhea. Proc Natl Acad Sci U S A. 2011;108(16):6585–6590. doi: 10.1073/pnas.1015674108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riad-Gabriel MG, Jinagouda SD, Sharma A, Boyadjian R, Saad MF. Changes in plasma leptin during the menstrual cycle. Eur J Endocrinol. 1998;139(5):528–531. doi: 10.1530/eje.0.1390528. [DOI] [PubMed] [Google Scholar]
- 8.Hardie L, Trayhurn P, Abramovich D, Fowler P. Circulating leptin in women: a longitudinal study in the menstrual cycle and during pregnancy. Clin Endocrinol (Oxf) 1997;47(1):101–106. doi: 10.1046/j.1365-2265.1997.2441017.x. [DOI] [PubMed] [Google Scholar]
- 9.Messinis IE, Milingos S, Zikopoulos K, Kollios G, Seferiadis K, Lolis D. Leptin concentrations in the follicular phase of spontaneous cycles and cycles superovulated with follicle stimulating hormone. Hum Reprod. 1998;13(5):1152–1156. doi: 10.1093/humrep/13.5.1152. [DOI] [PubMed] [Google Scholar]
- 10.Paolisso G, Rizzo MR, Mazziotti G, et al. Lack of association between changes in plasma leptin concentration and in food intake during the menstrual cycle. Eur J Clin Invest. 1999;29(6):490–495. doi: 10.1046/j.1365-2362.1999.00488.x. [DOI] [PubMed] [Google Scholar]
- 11.Quinton ND, Laird SM, Okon MA, et al. Serum leptin levels during the menstrual cycle of healthy fertile women. Br J Biomed Sci. 1999;56(1):16–19. [PubMed] [Google Scholar]
- 12.Fernández-Real JM, Gutierrez C, Vendrell J, Casamitjana R, Ricart W. Plasma soluble tumor necrosis factor-alpha receptors circulate in proportion to leptin levels during the menstrual cycle in lean but not in obese women. Eur J Endocrinol. 2000;143(2):235–241. doi: 10.1530/eje.0.1430235. [DOI] [PubMed] [Google Scholar]
- 13.Cella F, Giordano G, Cordera R. Serum leptin concentrations during the menstrual cycle in normalweight women: effects of an oral triphasic estrogen-progestin medication. Eur J Endocrinol. 2000;142(2):174–178. doi: 10.1530/eje.0.1420174. [DOI] [PubMed] [Google Scholar]
- 14.Ludwig M, Klein HH, Diedrich K, Ortmann O. Serum leptin concentrations throughout the menstrual cycle. Arch Gynecol Obstet. 2000;263(3):99–101. doi: 10.1007/s004040050004. [DOI] [PubMed] [Google Scholar]
- 15.Geisthövel F, Jochmann N, Widjaja A, Horn R, Brabant G. Serum pattern of circulating free leptin, bound leptin, and soluble leptin receptor in the physiological menstrual cycle. Fertil Steril. 2004;81(2):398–402. doi: 10.1016/j.fertnstert.2003.06.020. [DOI] [PubMed] [Google Scholar]
- 16.Wunder DM, Yared M, Bersinger NA, Widmer D, Kretschmer R, Birkhäuser MH. Serum leptin and Creactive protein levels in the physiological spontaneous menstrual cycle in reproductive age women. Eur J Endocrinol. 2006;155(1):137–142. doi: 10.1530/eje.1.02178. [DOI] [PubMed] [Google Scholar]
- 17.Asimakopoulos B, Milousis A, Gioka T, et al. Serum pattern of circulating adipokines throughout the physiological menstrual cycle. Endocr J. 2009;56(3):425–433. doi: 10.1507/endocrj.k08e-222. [DOI] [PubMed] [Google Scholar]
- 18.Einollahi N, Dashti N, Nabatchian F. Serum leptin concentrations during the menstrual cycle in Iranian healthy women. Acta Med Iran. 2010;48(5):300–303. [PubMed] [Google Scholar]
- 19.Ajala M, Ogunro P, Elusanmi G, Ogunyemi E, Bolarinde A. Changes in serum leptin during phases of the menstrual cycle of fertile women: relationship to age groups and fertility. International Journal of Endocrinology and Metabolism. 2013;11(1):27–33. doi: 10.5812/ijem.6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teirmaa T, Luukkaa V, Rouru J, Koulu M, Huupponen R. Correlation between circulating leptin and luteinizing hormone during the menstrual cycle in normal-weight women. Eur J Endocrinol. 1998;139(2):190–194. doi: 10.1530/eje.0.1390190. [DOI] [PubMed] [Google Scholar]
- 21.Stock SM, Sande EM, Bremme KA. Leptin levels vary significantly during the menstrual cycle, pregnancy, and in vitro fertilization treatment: possible relation to estradiol. Fertil Steril. 1999;72(4):657–662. doi: 10.1016/s0015-0282(99)00321-0. [DOI] [PubMed] [Google Scholar]
- 22.Lin KC. Changes of circulating leptin levels during normal menstrual cycle: relationship to estradiol and progesterone. Kaohsiung J Med Sci. 1999;15(10):597–602. [PubMed] [Google Scholar]
- 23.Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab. 2000;11(8):327–332. doi: 10.1016/s1043-2760(00)00301-5. [DOI] [PubMed] [Google Scholar]
- 24.Wactawski-Wende J, Schisterman EF, Hovey KM, et al. BioCycle study: design of the longitudinal study of the oxidative stress and hormone variation during the menstrual cycle. Paediatric and Perinatal Epidemiology. 2009;23(2):171–184. doi: 10.1111/j.1365-3016.2008.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howards PP, Schisterman EF, Wactawski-Wende J, Reschke JE, Frazer AA, Hovey KM. Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: the BioCycle Study. American Journal of Epidemiology. 2009;169(1):105–112. doi: 10.1093/aje/kwn287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitcomb BW, Perkins NJ, Albert PS, Schisterman EF. Treatment of batch in the detection, calibration, and quantification of immunoassays in large-scale epidemiologic studies. Epidemiology. 2010;21(Suppl 4):S44–S50. doi: 10.1097/EDE.0b013e3181dceac2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hurnong R, Reed L. Estimation of average concentration in the presence of non-detectable values. Applied Occupational and Environmental Hygiene. 1990;5:46–51. [Google Scholar]
- 28.Gaskins AJ, Mumford SL, Zhang C, et al. Effect of daily fiber intake on reproductive function: the BioCycle Study. American Journal of Clinical Nutrition. 2009;90(4):1061–1069. doi: 10.3945/ajcn.2009.27990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dasharathy SS, Mumford SL, Pollack AZ, et al. Menstrual bleeding patterns among regularly menstruating women. American Journal of Epidemiology. 2012;175(6):536–545. doi: 10.1093/aje/kwr356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mumford SL, Steiner AZ, Pollack AZ, et al. The utility of menstrual cycle length as an indicator of cumulative hormonal exposure. Journal of Clinical Endocrinology and Metabolism. 2012;97(10):E1871–E1879. doi: 10.1210/jc.2012-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mumford SL, Schisterman EF, Gaskins AJ, et al. Realignment and multiple imputation of longitudinal data: an application to menstrual cycle data. Paediatr Perinat Epidemiol. 2011;25(5):448–459. doi: 10.1111/j.1365-3016.2011.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social behavior. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 33.Committee IR. [Accessed December 27, 2005];Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ) 2005 Nov; www.ipaq.ki.se.
- 34.Institute NC. [Accessed February 2013, 2013];ASA Automated Self-Administerd 24-hour Recall. 2009 http://riskfactor.cancer.gov/tools/instruments/asa24/
- 35.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 37.Hertzmark E, Li R, Hong B, Spiegelman D. The SAS GLMCURV9 Macro. 2012 [Google Scholar]
- 38.Löffler S, Aust G, Köhler U, Spanel-Borowski K. Evidence of leptin expression in normal and polycystic human ovaries. Mol Hum Reprod. 2001;7(12):1143–1149. doi: 10.1093/molehr/7.12.1143. [DOI] [PubMed] [Google Scholar]
- 39.Ryan NK, Van der Hoek KH, Robertson SA, Norman RJ. Leptin and leptin receptor expression in the rat ovary. Endocrinology. 2003;144(11):5006–5013. doi: 10.1210/en.2003-0584. [DOI] [PubMed] [Google Scholar]
- 40.Balogh O, Kowalewski MP, Reichler IM. Leptin and leptin receptor gene expression in the canine corpus luteum during diestrus, pregnancy and after aglepristone-induced luteolysis. Reprod Domest Anim. 2012;47(Suppl 6):40–42. doi: 10.1111/rda.12005. [DOI] [PubMed] [Google Scholar]
- 41.Archanco M, Muruzábal FJ, Llopiz D, et al. Leptin expression in the rat ovary depends on estrous cycle. J Histochem Cytochem. 2003;51(10):1269–1277. doi: 10.1177/002215540305101003. [DOI] [PubMed] [Google Scholar]
- 42.Kumar L, Panda RP, Hyder I, et al. Expression of leptin and its receptor in corpus luteum during estrous cycle in buffalo (Bubalus bubalis) Anim Reprod Sci. 2012;135(1–4):8–17. doi: 10.1016/j.anireprosci.2012.08.030. [DOI] [PubMed] [Google Scholar]
- 43.Sarkar M, Schilffarth S, Schams D, Meyer HH, Berisha B. The expression of leptin and its receptor during different physiological stages in the bovine ovary. Mol Reprod Dev. 2010;77(2):174–181. doi: 10.1002/mrd.21129. [DOI] [PubMed] [Google Scholar]
- 44.Tessier DR, Ferraro ZM, Gruslin A. Role of leptin in pregnancy: Consequences of maternal obesity. Placenta. 2013 doi: 10.1016/j.placenta.2012.11.035. [DOI] [PubMed] [Google Scholar]
- 45.Fenichel RM, Dominguez JE, Mayer L, Walsh BT, Boozer C, Warren MP. Leptin levels and luteinizing hormone pulsatility in normal cycling women and their relationship to daily changes in metabolic rate. Fertil Steril. 2008;90(4):1161–1168. doi: 10.1016/j.fertnstert.2007.07.1350. [DOI] [PubMed] [Google Scholar]
- 46.De Biasi SN, Apfelbaum LI, Apfelbaum ME. In vitro effect of leptin on LH release by anterior pituitary glands from female rats at the time of spontaneous and steroid-induced LH surge. Eur J Endocrinol. 2001;145(5):659–665. doi: 10.1530/eje.0.1450659. [DOI] [PubMed] [Google Scholar]
- 47.Sir-Petermann T, Maliqueo M, Palomino A, Vantman D, Recabarren SE, Wildt L. Episodic leptin release is independent of luteinizing hormone secretion. Hum Reprod. 1999;14(11):2695–2699. doi: 10.1093/humrep/14.11.2695. [DOI] [PubMed] [Google Scholar]
- 48.Welt CK, Chan JL, Bullen J, et al. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004;351(10):987–997. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- 49.Sinha MK, Ohannesian JP, Heiman ML, et al. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J Clin Invest. 1996;97(5):1344–1347. doi: 10.1172/JCI118551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borer KT, Wuorinen E, Ku K, Burant C. Appetite responds to changes in meal content, whereas ghrelin, leptin, and insulin track changes in energy availability. J Clin Endocrinol Metab. 2009;94(7):2290–2298. doi: 10.1210/jc.2008-2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Geber S, Brandão AH, Sampaio M. Effects of estradiol and FSH on leptin levels in women with suppressed pituitary. Reprod Biol Endocrinol. 2012;10:45. doi: 10.1186/1477-7827-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brann DW, De Sevilla L, Zamorano PL, Mahesh VB. Regulation of leptin gene expression and secretion by steroid hormones. Steroids. 1999;64(9):659–663. doi: 10.1016/s0039-128x(99)00049-5. [DOI] [PubMed] [Google Scholar]
- 53.Mantzoros CS, Magkos F, Brinkoetter M, et al. Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab. 2011;301(4):E567–E584. doi: 10.1152/ajpendo.00315.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]