Abstract
Aims
We sought to determine whether NAFLD is associated with poorer β-cell function and if any β-cell dysfunction is associated with abnormal markers of iron or inflammation.
Methods
This was a cross-sectional study of 15 non-diabetic adults with NAFLD and 15 non-diabetic age and BMI-matched controls. Insulin sensitivity was measured by isotope-labeled hyperinsulinemic-euglycemic clamps and β-cell function by both oral (OGTT) and intravenous glucose tolerance tests. Liver and abdominal fat composition was evaluated by CT scan. Fasting serum levels of ferritin, transferrin-iron saturation, IL-6, TNFα and hsCRP were measured.
Results
Compared to controls, subjects with NAFLD had lower hepatic and systemic insulin sensitivity and β-cell function was decreased as measured by the oral disposition index. Fasting serum ferritin and transferrin-iron saturation were higher in NAFLD and were positively associated with liver fat. Serum ferritin was negatively associated with β-cell function measured by both oral and intravenous tests, but was not associated with insulin sensitivity. IL-6, TNFα and hsCRP did not differ between groups and did not correlate with serum ferritin, liver fat or measures of β-cell function.
Conclusions
These findings support a potential pathophysiological link between iron metabolism, liver fat and diabetes risk.
Keywords: insulin sensitivity, ferritin, insulin secretion in vivo, fatty liver
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD), defined as fat accumulation in the liver in the absence of excessive alcohol intake, is strongly associated with insulin resistance, obesity and type 2 diabetes (1). NAFLD has also been shown to be a risk factor for the development of type 2 diabetes (2; 3). Factors underlying this increased risk to develop type 2 diabetes have not been fully elucidated. Obesity and insulin resistance are certainly factors that may contribute, but β-cell dysfunction is a key feature that contributes to the development of type 2 diabetes (4). However, studies to date have not shown an association between liver fat and β-cell function (5–7). These studies included subjects with normal or impaired glucose tolerance and diabetes using oral glucose tolerance tests to assess β-cell function.
Factors that have been associated with increased diabetes risk in this population include markers of iron metabolism, specifically ferritin which has been found to be increased in NAFLD (8–10). Iron overload conditions are well known to be associated with β-cell dysfunction and can lead to diabetes (11–13). Serum ferritin levels are higher in patients with diabetes (14) and the metabolic syndrome (15; 16), suggesting that body iron stores may play a detrimental role in glucose metabolism even in the absence of overt iron overload (16). Further, higher ferritin levels have been shown to predict the development of type 2 diabetes (17). The ability of iron depletion to improve insulin sensitivity and β-cell function in healthy individuals (18) and those with type 2 diabetes (19) provides additional support for a role of iron in glucose metabolism. Thus, hyperferritinemia in NAFLD may be a necessary cofactor in the NAFLD/diabetes connection by contributing to insulin resistance and/or β-cell dysfunction.
Elevated ferritin levels observed in NAFLD may also reflect the inflammatory milieu within the steatotic liver. The evidence suggests that inflammatory cytokines themselves may play an important role in β-cell dysfunction and β-cell apoptosis, both key features in the pathogenesis of type 2 diabetes. Inflammatory markers such as C-reactive protein, TNFα (20; 21) and IL-6 (21) have been shown to be elevated in NAFLD, with higher levels in those with steatohepatitis and fibrosis (22).
We hypothesized that subjects with NAFLD would have lower insulin sensitivity and poorer β-cell function and that higher ferritin levels and inflammatory cytokines in NAFLD may contribute to diabetes risk by being associated with lower insulin sensitivity and/or poorer β-cell function. To examine this hypothesis we studied non-diabetic subjects diagnosed with NAFLD and compared them to age- and BMI-matched control subjects without liver disease. First, we carefully characterized study subjects by performing isotope labeled, hyperinsulinemic-euglycemic clamps to directly measure both hepatic and peripheral insulin sensitivity, oral and intravenous (IV) glucose tolerance tests to measure β-cell function, and fasting iron and inflammatory markers. We then determined associations between liver fat, serum ferritin and transferrin-iron saturation, markers of inflammation and measures of insulin sensitivity and β-cell function.
RESEARCH DESIGN AND METHODS
Subjects
This cross-sectional study compared subjects with NAFLD to age- and BMI-matched control subjects. All subjects gave written informed consent to participate and the study was approved by the Human Subjects Review Committees at the VA Puget Sound Health Care System and the University of Washington.
Subjects underwent an initial screening visit which included a history, physical exam and fasting blood tests. Case subjects were recruited from local area gastroenterologists and were defined as having NAFLD based on either a liver biopsy within the past 3 years meeting criteria for >5% fatty infiltration or the presence of elevated liver enzymes in conjunction with imaging suggestive of fatty liver. Biopsy samples were available for review from 12/15 subjects. These were reviewed by a single pathologist and scored using the NASH Clinical Research Network criteria (23). Exclusion criteria for case subjects included cirrhosis on liver biopsy, significant weight loss (>5%) since the liver biopsy, other known causes of elevated liver enzymes or a serum alanine aminotransferase (ALT) >5 times the upper limit of normal (lab normal range: 0–39 U/L). Control subjects were recruited by advertisement and fliers from the Seattle area. They were required to have normal liver enzymes and no history of liver disease. Additional exclusion criteria for all subjects included: self-reported alcohol intake >20 grams per day, positive hepatitis C antibody or hepatitis B surface antigen, transferrin-iron saturation >55%, serum creatinine >1.4 mg/dl in men and >1.3 mg/dl in women, hematocrit <33%, pregnancy or lactation, any serious medical condition, or use of any of the following medications: corticosteroids, estrogens at doses higher than standard replacement therapy, tamoxifen, amiodarone, accutane, sertraline, atypical antipsychotics, anti-retroviral medications, niacin, gemfibrozil, fenofibrate, glucose-lowering agents, ursodeoxycholic acid, betaine or milk thistle. HFE gene mutation analysis was not routinely performed.
A total of 34 subjects were studied, and data on 30 were eligible for analysis. Three subjects (two cases and one control) were excluded based on oral glucose tolerance test (OGTT) results in the diabetic range. One subject was determined to have <5% fat on his liver biopsy upon review by the study pathologist and was therefore excluded.
Study Procedures
All study procedures were performed after an overnight fast of 10–12 hours on separate days within two weeks. Plasma samples were placed immediately on ice and processed in a refrigerated centrifuge at 4°C and aliquots frozen at −70°C until assayed.
Oral glucose tolerance test (OGTT)
Seventy-five grams of glucose was consumed within 5 minutes and blood samples drawn at -10, -5, -1, 10, 20, 30, 60, 90 and 120 minutes. The three basal samples were averaged for the 0 time point. Glucose tolerance status was determined according to American Diabetes Association guidelines (24).
Intravenous glucose tolerance test (IVGTT)
Two peripheral intravenous lines were established. The sampling arm was wrapped in a heating pad to “arterialize” the blood samples. Glucose (11.4 g/m2) was injected over 60 seconds and blood samples for glucose, insulin and C-peptide were drawn at -10, -5, -1, 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 19, 22, 25 and 30 minutes relative to the start of the glucose injection.
Hyperinsulinemic-euglycemic clamp
Subjects were admitted the night before and fed a standardized dinner from 7–8 pm consisting of 7 kcal/kg (50% calories from carbohydrate, 30% fat and 20% protein). An intravenous catheter was placed in each arm. The sampling arm was wrapped in a heating pad to “arterialize” the blood. At 5 a.m. a primed (200 mg/m2 × glucose/100 given over 5 minutes), continuous (2 mg/m2/minute) infusion of 6,6 2d glucose was started and continued throughout the clamp procedure. The two-step hyperinsulinemic-euglycemic clamp procedure started at 8 am and included a low dose insulin infusion (20 mU/m2/min) for 3 hours followed by a primed high dose insulin infusion (160 mU/m2/min × 5 minutes then 80 mU/m2/min) for two hours. Blood glucose was measured every 5 minutes using an iStat machine and a variable rate infusion of 20% dextrose enriched with 2% 6,6 2d glucose was titrated to maintain the blood glucose concentration at 5 mmol/L (90 mg/dl). Samples were drawn for glucose and insulin every 30 minutes throughout the clamp. Samples for glucose, insulin and 6,6 2d glucose were drawn every 15 minutes during the final half hour of the basal, low dose and high dose insulin periods. Samples for free fatty acids (FFAs) were drawn into tubes containing the lipolysis inhibitor orlistat at -30, -15, -1, 10, 20, 30 and 60 minutes relative to the start of the low dose insulin infusion and placed immediately on ice. FFA samples were processed within 30 minutes and the plasma flash frozen.
Body composition analyses
Body fat mass (FM) and lean mass (LM) were determined using dual-energy x-ray absorptiometry (Lunar, GE Medical Systems). Unenhanced CT scan images were obtained on a General Electric Discovery HD750 CT scanner. Intra-abdominal (IAF) and abdominal subcutaneous fat (SQF) areas were measured at the top of the iliac crest and quantified using the Tomovision program (SliceOMatic V4.3) by one trained technologist with an intra-observer CV of <7% for IAF and <3% for SQF.
Liver fat was estimated by CT scan by measuring the density ratio between the liver and spleen in Hounsfield units (liver/spleen ratio), which has been previously correlated with liver fat quantification by magnetic resonance spectroscopy (25). A liver/spleen ratio <1 is consistent with fatty liver. Ten separate measurements equally distributed throughout the liver and spleen were obtained and the Hounsfield units averaged. In subjects with more than one slice through the liver and spleen, the values for all slices were averaged.
Analyses of Samples
Plasma glucose was determined by the glucose oxidase method. Plasma insulin and serum ferritin were measured by an automated electrochemiluminescence immunoassay (Cobas e 601, Indianapolis, IN). C-peptide was measured by radioimmunoassay. Iron and unsaturated iron binding capacity were measured by a colorimetric assay (Cobas e 501, Indianapolis, IN), highly sensitive C-reactive protein (hsCRP) by nephelometry (Siemens, Tarrytown, NY) and adiponectin by radioimmunoassay (Millipore, Billerica, MA). Fasting plasma levels of TNFα and IL-6 were measured in duplicate using ELISA (eBioscience, San Diego, CA) and had intra-assay CVs of 2.09 and1.62% respectively. The lowest limit of detection for hsCRP was 0.8 mg/L, TNFα was 4 pg/ml and for IL-6 was 2 pg/ml. Values below the lowest detectable limit were set to 0.8, 4 and 2 respectively. The β quantification procedure was used to measure serum cholesterol, triglycerides, LDL and HDL (26) and plasma free fatty acid concentrations were determined by an enzymatic method (Wako, Richmond, VA). Levels of 6,6 2d glucose were measured by mass spectrometry as previously described (27; 28).
Calculations
OGTT
Area under the curves (AUC) above basal were calculated using the trapezoidal method. The early insulin response during the OGTT (insulinogenic index) was calculated as the change in insulin divided by the change in glucose from 0–30 minutes (ΔI/ΔG). The oral disposition index (ΔI/ΔG × 1/fasting insulin) was calculated as a measure of β-cell function (29). Model derived measures of β-cell responsivity indices (Φstatic, Φdynamic, Φtotal) were estimated using the C-peptide minimal model (30).
IVGTT
The acute insulin (AIRg) and C-peptide (ACRg) responses to IV glucose were calculated as the AUC above basal from 0–10 minutes. The glucose disappearance constant (Kg), a measure of intravenous glucose tolerance, was calculated as the slope of the natural log of glucose from 10 to 30 minutes during the IVGTT. The intravenous (iv) disposition index was calculated as AIRg × insulin sensitivity as measured by the clamp.
Clamp data
Isotopic steady state levels were achieved during the final 30 minutes of the basal, low and high dose insulin periods of the clamp. Rate of glucose appearance (Ra) was calculated using Steele’s steady state equations (31). Whole body insulin sensitivity was calculated as the glucose infusion rate/lean body mass (M) and adjusted for steady state insulin (M/I). Hepatic insulin sensitivity was determined as: 1) the hepatic insulin resistance index (HIR index: basal EGP × fasting plasma insulin) (32) and 2) percent suppression of EGP at the end of the low dose insulin clamp.
Statistical Methods
Data were loge transformed as needed to achieve a normal distribution. Variables were compared between cases and controls using independent student’s t-test. Multiple linear regression analysis was used to determine independent predictors of metabolic outcomes. All multiple linear regression models were adjusted for age and sex. A p value of <0.05 was considered significant. Analyses were performed using SPSS V16.0 (IBM).
RESULTS
General Subject Characteristics
Characteristics of case (10M/5F) and control (8M/7F) subjects are provided in Table 1. Only two women were pre-menopausal (both controls). The racial/ethnic distribution was similar (Controls: 12 Caucasian, 1 African American, 2 Native American and NAFLD: 12 Caucasian, 2 Hispanic, 1 Japanese/Caucasian). The two groups were well matched for age, BMI, reported alcohol intake, body adiposity and abdominal fat distribution. Liver fat was increased in the NAFLD subjects as estimated by a decreased liver/spleen ratio. There were no significant differences between groups in lipid levels, TNFα, IL-6, hsCRP or adiponectin levels.
Table 1.
Subject characteristics
| Controls (N=15) |
NAFLD (N=15) |
P value | |
|---|---|---|---|
| Age (years) | 50.6±1.7 | 49.7±2.1 | 0.75 |
| Sex (M/F) | 8/7 | 10/5 | 0.71 |
| BMI (kg/m2) | 33.4±1.28 | 33.1±1.0 | 0.85 |
| Alcohol intake (drinks/week) | 3.5±1.1 | 2.7±1.0 | 0.61 |
| Liver/spleen ratio | 1.22±0.04 | 0.69±0.05 | <0.001 |
| Ferritin (pmol/L) | |||
| All | 206.7 (182.0) | 361.8 (532.5) | 0.001 |
| Men | 249.4 (161.8) | 496.6 (552.8) | 0.001 |
| Women | 184.3 (182.0) | 292.1 (170.8) | 0.2 |
| Transferrin-iron saturation (%) | |||
| All | 23±2 | 30±3 | 0.05 |
| Men | 23±3 | 33±3 | 0.04 |
| Women | 24±3 | 24±4 | 0.9 |
| SGOT (U/L) | 22.3±1.8 | 43.2±6.0 | 0.002 |
| SGPT (U/L) | 28.1±4.3 | 74.4±8.2 | <0.001 |
| Fat mass (%) All | |||
| Men | 40.3±2.2 | 36.7±2.2 | 0.25 |
| Women | 34.2±1.3 | 31.5±1.2 | 0.13 |
| 47.2±2.8 | 47.0±1.8 | 0.96 | |
| Body fat mass (kg) | 36.1±2.4 | 35.2±2.4 | 0.81 |
| IAF area (cm2)a | 165.5±13.6 | 197.1±22.2 | 0.22 |
| SQF area (cm2)a | 431.0±42.0 | 371.1±35.6 | 0.29 |
| Total cholesterol (mmol/L) | 5.12±0.26 | 4.94±0.23 | 0.65 |
| LDL cholesterol (mmol/L) | 3.31±0.21 | 3.00±0.16 | 0.25 |
| Triglycerides (mmol/L) | 1.29±0.15 | 1.93±0.29 | 0.06 |
| Total HDL cholesterol (mmol/L) | |||
| All | 1.21±0.09 | 1.06±0.08 | 0.23 |
| Men | 0.94±0.06 | 0.98±0.10 | 0.79 |
| Women | 1.51±0.10 | 1.21±0.13 | 0.09 |
| Fasting FFAs (mEq/L) | 0.43±0.05 | 0.53±0.04 | 0.06 |
| TNFα (pg/ml)b | 7.7 (13.3) | 4.0 (13.1) | 0.87 |
| IL-6 (pg/ml)b | 2.0 (0.4) | 2.0 (1.7) | 0.23 |
| hsCRP (mg/L) | 1.9 (6.4) | 3.1 (4.9) | 0.44 |
| Adiponectin (µg/ml) | |||
| All | 9.6±1.9 | 8.0±1.1 | 0.46 |
| Men | 6.2±1.2 | 6.7±1.3 | 0.79 |
| Women | 13.5±3.3 | 10.7±1.4 | 0.51 |
Data are presented as Mean±SEM for normally distributed data or Median (interquartile range) for non-normally distributed data.
N=13 for NAFLD.
Three outliers were excluded (1 control and 2 NAFLD). A higher liver/spleen ratio (>1) reflects lower liver fat while a lower liver/spleen ratio (<1) reflects fatty liver.
Abbreviations: IAF = intra-abdominal fat, SQF = abdominal subcutaneous fat, ALT= alanine aminotransferase, AST = aspartate aminotransferase, FFA = free fatty acids, TNFα = tumor necrosis factor α, IL-6 = interleukin-6, hsCRP = highly sensitive C-reactive protein. The normal range for ferritin in our lab is 67–899 pmol/L (30–400 ng/ml).
The median serum ferritin level was significantly higher in men with NAFLD compared to controls, but did not differ in women (Table 1). Male case subjects with NAFLD also had significantly higher transferrin-iron saturation (Table 1), although all subjects had transferrin-iron saturation values that were within the normal range (9–46%).
Of the twelve NAFLD subjects with liver biopsy specimens available for review, six met the diagnostic criteria for nonalcoholic steatohepatitis (NASH). The subjects with histological findings showing merely steatosis (n=6) and NASH (n=6) did not differ with regards to age, sex distribution, BMI, reported alcohol intake, liver enzymes, liver/spleen ratio, ferritin, transferrin-iron saturation or body composition (data not shown). There were no differences in ferritin or transferrin-iron saturation between those with fibrosis on liver biopsy and those without (n=6 per group, median[interquartile range]: ferritin 425[618] vs. 353[739] pmol/L, p=0.9).
Glucose Tolerance, Insulin Sensitivity and Β-cell Function
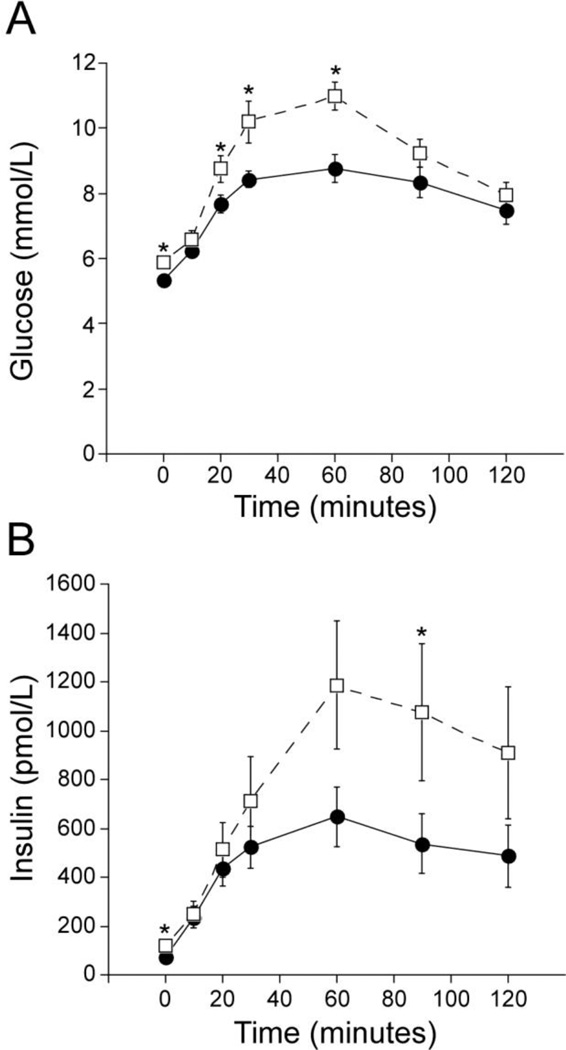
Among control subjects, five had normal glucose tolerance (NGT) and ten had either impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT). Among NAFLD subjects four had NGT and eleven had IFG and/or IGT. HbA1c values did not differ (controls 5.67±0.06 vs. NAFLD 5.61±0.05%). Those with NAFLD had higher fasting and post-challenge glucose levels at 20, 30 and 60 minutes, but did not differ at 2 hours (Figure 1A). Fasting and 90 minute insulin values were higher in the NAFLD group (Figure 1B). The incremental AUC glucose over the 120 minutes of the OGTT tended to be higher in those with NAFLD but did not reach statistical significance (controls 320±34 vs. NAFLD 392±31 mmol/L, p=0.13). The rate of glucose disappearance (Kg) during the IVGTT tended to be lower in NAFLD subjects (p=0.06) (Table 2).
Figure 1.
Glucose (A) and insulin (B) values during the oral glucose tolerance test for control (solid circle, solid line: n=15) and NAFLD (open square, dash line: n=13) subjects. Fasting, 20, 30 and 60 minutes glucose values were higher in the NAFLD subjects compared to the controls. Fasting and 90 minute insulin values were higher in the NAFLD subjects. *p<0.05 by independent t-test
Table 2.
Measures of Glucose Metabolism
| IVGTT measures of insulin secretion and glucose tolerance | |||
| Controls (N=15) | NAFLD (N=15) | P value | |
| AIRg (pmol/L) | 2426 (4704) | 4507 (4457) | 0.5 |
| Acute C-peptide response (pmol/ml) | 33 (31) | 37 (39) | 0.9 |
| IV disposition index (AIRg x M/I low; mmol/L.min(−1).kg lean mass(−1)) | 3.11 (6.06) | 2.40 (3.58) | 0.19 |
| Kg (%/minute) | 0.017±0.002 | 0.012±0.001 | 0.06 |
| OGTT measures of insulin sensitivity and β-cell function | |||
| Controls (N=15) | NAFLD (N=13) | P value | |
| Fasting insulin (pmol/L) | 54.0 (55.5) | 95.0 (93.3) | 0.01 |
| Δinsulin/Δglucose 0–30 minutes (pmol/mmol) | 117 (157) | 115 (136) | 0.6 |
| incAUC insulin/incAUC glucose from 0–120 minutes (pmol/mmol) | 133 (218) | 146 (231) | 0.8 |
| Oral disposition index (Δinsulin/Δglucose0–30 × 1/fasting insulin; mmol/L(−1)) | 1.5 (1.4) | 0.9 (0.9) | 0.003 |
| Φstatic (10−9 min(−1)) | 52.2 (37.3) | 49.8 (42.0) | 0.7 |
| Φdynamic (10−9) | 1023.5 (796.1) | 528.3 (634.2) | 0.07 |
| Φtotal (10−9 min(−1)) | 28.1 (18.1) | 28.1 (13.9) | 0.7 |
| Clamp measures of endogenous glucose production (EGP) and insulin sensitivity | |||
| Controls (N=15) | NAFLD (N=14) | P value | |
| Fasting insulin (pmol/L) | 49.8 (59.4) | 75.6 (60.5) | 0.01 |
| Basal EGP (mmol/min) | 8.9±0.8 | 8.9±0.6 | 0.9 |
| HIR index (EGP X fasting insulin; mmol.pM.min(−1)) | 30.6±4.8 | 49.9±6.8 | 0.03 |
| Suppression EGP (%) | 56.36±4.28 | 50.44±6.57 | 0.5 |
| M/I low (mmol.min(−1).kg(−1).pM(−1)) | 1.06 (1.28) | 0.55 (0.81) | 0.02 |
| M/I high (mmol.min(−1).kg(−1).pM(−1) × 10−3) | 0.23 (0.26) | 0.13 (0.18) | 0.02 |
Mean±SEM for normally distributed data. Median (interquartile range) for non-normally distributed data.
Abbreviations: IVGTT = intravenous glucose tolerance test, AIRg = acute insulin response to glucose, incAUC = incremental area under the curve, EGP = endogenous glucose production, HIR = hepatic insulin resistance, min = minutes.
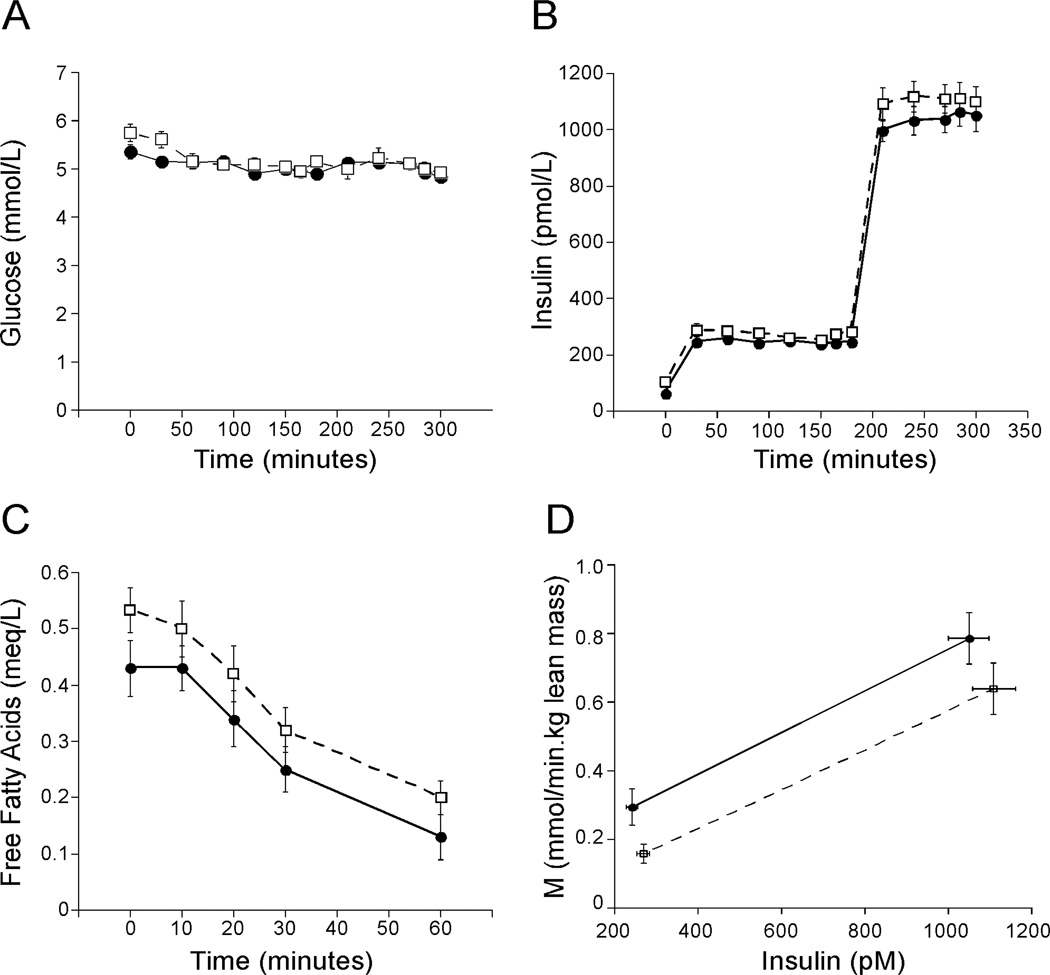
Figures 2A and 2B illustrate the glucose and insulin values throughout the clamp. Subjects with NAFLD had higher fasting insulin levels on all three study days, being 51% higher on the morning of the clamp (Table 2). Fasting free fatty acid levels tended to be higher in NAFLD (p=0.06) but the rate of fall and % suppression in response to the low dose insulin infusion were similar between controls and NAFLD (Figure 2C). EGP in the basal state and at the end of the low dose clamp and percent suppression of EGP did not differ between controls and NAFLD (Table 2). However, when adjusted for fasting insulin, the HIR index was significantly higher in NAFLD (Table 2). Whole body insulin sensitivity as measured by M/I was significantly lower in the NAFLD group at both low and high dose insulin (Table 2 and Figure 2D).
Figure 2.
Glucose (A), insulin (B), free fatty acid levels (C) and whole body insulin sensitivity (M/I) (D) during the clamp (controls: solid circle, solid line; NAFLD: open square, dashed line). Both glucose (A) and insulin (B) levels achieved steady state and did not differ during the low and high dose clamp. Fasting free fatty acid levels (C) tended to be higher in NAFLD (p=0.06 by independent t-test) but the rate of fall and % suppression during the low dose clamp was similar between controls and NAFLD. Insulin sensitivity (D) was significantly lower in the NAFLD group compared to controls during both low and high dose insulin clamps (p<0.05 for both). M = the glucose infusion rate in mmol/minute adjusted for lean body mass.
There was no difference in ΔI/ΔG, AIRg or ACRg between case and control subjects (Table 2). The oral disposition index calculated from the OGTT was lower in NAFLD compared to controls (p=0.03, Table 2). Similar results were obtained when M/I low or M/I high were used as the measure of insulin sensitivity (data not shown). Measures of β-cell function obtained by mathematical modeling of the OGTT showed that only the dynamic measure tended to be decreased in the NAFLD group (p=0.07, Table 2), while there were no differences in the static or total components. The IV disposition index did not differ between groups (p=0.19, Table 2).
Associations between Liver Fat, Ferritin, Insulin Sensitivity and Β-cell Function
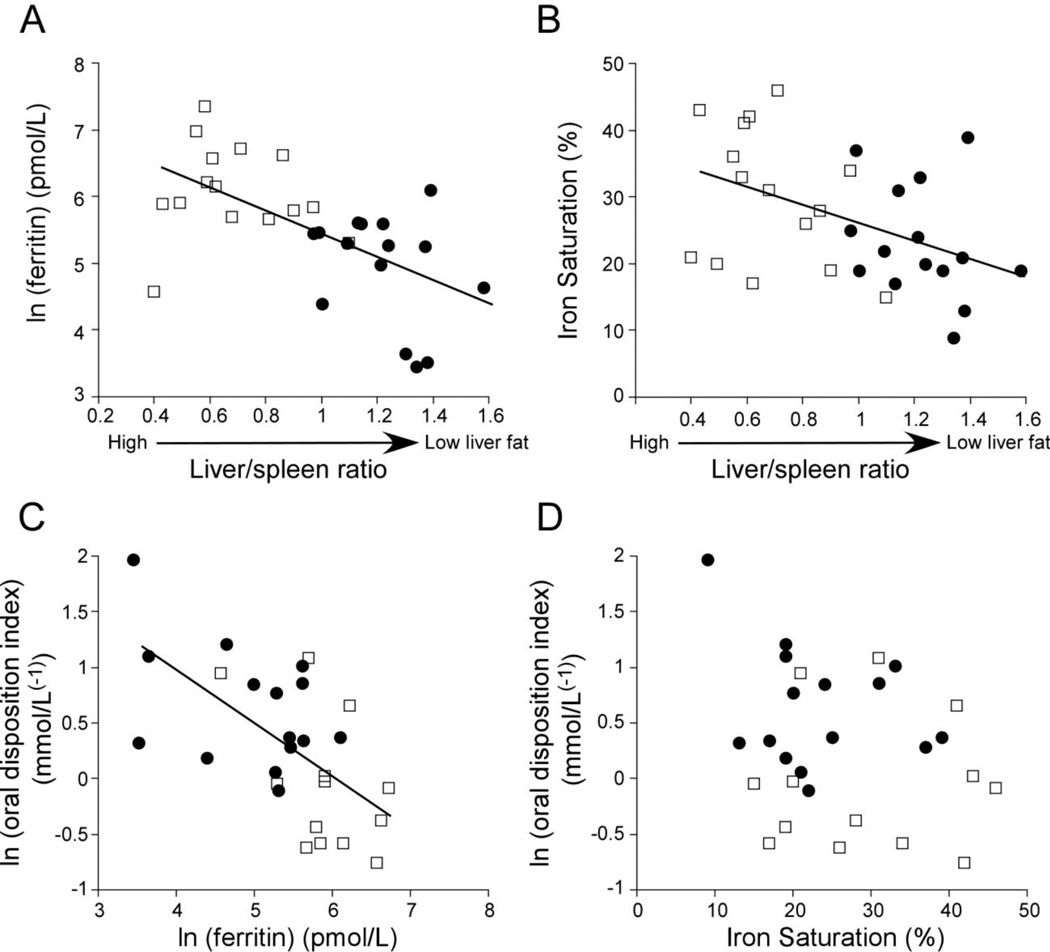
Liver fat was negatively associated with M/I low (r=−0.41, p=0.03), and M/I high (r=−0.39, p=0.03). These relationships were weakened after adjusting for age and sex (M/I low, r=−0.37, p=0.06; M/I high, r=−0.35, p=0.07). Liver fat was negatively associated with the oral disposition index (r =−0.42, p=0.03), but not modeled measures of β-cell function (Φstatic p=0.37, Φdynamic p=0.21, Φtotal p=0.49) or the IV disposition index (p=0.23). Liver fat was not associated with lnTNFα (p=0.7) or IL6 (p=0.2), but tended to be associated with higher hsCRP levels (r=0.34, p=0.07).
Both ferritin (r =0.59, p<0.001) and transferrin-iron saturation (r = 0.45, p=0.01) showed positive associations with liver fat (negative association with the liver/spleen ratio: Figure 4). These associations remained after further adjustment for age, sex and BMI, M/I low, M/I high, the basal hepatic insulin resistance index, TNFα, IL-6, or hsCRP. Ferritin was also negatively correlated with β-cell function as measured by the oral disposition index (Figure 4C; r=−0.62, p=0.001), the dynamic measure of β-cell function (Φdynamic r=−0.41, p=0.03) and the IV disposition index (r=−0.4, p=0.03). These associations remained significant after adjustment for age and sex and further addition of TNFα, IL-6, hsCRP or the liver/spleen ratio. Ferritin was not associated with any measure of insulin sensitivity (fasting insulin p=0.35, HIR index p=0.14, M/I low p=0.08, M/I high p=0.09), although due to the small sample size the power to detect a significant difference was only 60%. Transferrin-iron saturation was not associated with β-cell function (Figure 4D) or insulin sensitivity. Ferritin was not associated with inflammation on liver biopsy (p=0.9), TNFα (p=0.4), IL-6 (p=0.7) or hsCRP levels (p=0.9). Neither TNFα, IL-6, nor hsCRP was significantly associated with any of the measures of β-cell function (p all >0.05).
DISCUSSION
Our study aimed to gain a better understanding of β-cell function in NAFLD by examining the relationship between liver fat, β-cell function and markers of iron metabolism and inflammation. We found a significant association between liver fat and higher ferritin levels, but not with inflammatory markers. Further, we observed decreased β-cell function in subjects with NAFLD compared to control subjects and a negative association between liver fat and β-cell function as measured by the oral disposition index. However, these findings were not observed with other measures of β-cell function using mathematical modeling or the IV disposition index reducing the strength of this finding. Ferritin was increased in NAFLD compared to controls and demonstrated a significant positive association with liver fat and a negative association with β-cell function by all measures, independent of liver fat and inflammatory markers. These findings support a potential pathophysiological link between iron metabolism, liver fat and diabetes risk, in which diabetes risk may be mediated through adverse effects of iron on β-cell function.
Type 2 diabetes results from deficits in both insulin sensitivity and β-cell function. Our findings of a negative association between liver fat and β-cell function were only observed with the oral disposition index and were not confirmed by our other measures of β-cell function that we employed. Others have not observed a relationship between liver fat and β-cell function by oral testing (5–7). One study using model derived measures of β-cell function from an OGTT showed no difference in β-cell function between controls and those with simple steatosis, but decreased β-cell function in those with NASH compared to those with simple steatosis (33). However, the role of iron was not examined in any of these studies. One of the novel aspects and observations of our study was the association between ferritin and β-cell function which was observed with both the oral and IV disposition indices. Thus, diabetes risk in NAFLD may be driven by β-cell function and iron may be an important mediator of this relationship.
The mechanism underlying this association between ferritin and reduced β-cell function is unclear. Although none of our subjects had evidence of iron overload, high ferritin levels may reflect a state of relative iron excess which could induce oxidative stress within the β-cell. The pancreas from humans with iron overload demonstrates iron deposition in β-cells and β-cell drop-out (34). Mouse models of hemochromatosis have also demonstrated β-cell apoptosis and increased markers of oxidative stress within islets (35; 36). While it is not possible to directly measure oxidative stress in islets in humans, systemic markers of oxidative stress are known to be elevated in NAFLD (37; 38). Further, in subjects with NAFLD serum ferritin levels have been positively correlated with serum thioredoxin, a marker of oxidative stress (39).
An alternate explanation is that ferritin reflects increased inflammation, which has also been implicated as a mechanism for the development of β-cell dysfunction in type 2 diabetes (40). However, in our study plasma TNFα, IL-6 and hsCRP did not differ between NAFLD and controls. This is not surprising as TNFα and IL-6 are associated with obesity and cases and controls were well matched for obesity. Both expression and secretion of IL-6 and TNFα from adipose tissue is increased in obesity (41; 42) and circulating levels of IL-6 are strongly associated with obesity and abdominal adiposity (43). These inflammatory markers also were not correlated with serum ferritin levels and were not associated with measures of β-cell function. These findings suggest that ferritin is not simply a marker of inflammation but may have a more direct influence on the β-cell.
Using labeled hyperinsulinemic-euglycemic clamps we found that ferritin was positively associated with liver fat independent of insulin sensitivity. This contrasts with the findings by Zelber-Sagi et al, who observed a significant interaction between NAFLD and hyperinsulinemia in determining ferritin levels and concluded that the association between ferritin and the metabolic syndrome was mediated by NAFLD (9). We did not observe a significant association between ferritin and insulin sensitivity, but this negative finding was limited by our small sample size. Gastaldelli et al found a significant inverse association between ferritin and insulin sensitivity measured by an OGTT in a study of 159 morbidly obese subjects prior to and one year following gastric banding surgery. In that study, ferritin was two-fold higher in those with high liver enzymes compared to those with normal liver enzymes. Liver fat was not quantified. Interestingly, one year after surgery, ferritin was unchanged in the subjects with high liver enzymes despite decreased body weight and improved glucose tolerance and insulin sensitivity (44). A few small studies have found that iron depletion in subjects with NAFLD results in an improvement in insulin sensitivity measured by HOMA (45; 46). However, this surrogate measure is based solely on fasting measures and does not discriminate between hepatic and peripheral insulin sensitivity. Further studies are needed to determine the mechanism for improvement in insulin sensitivity with phlebotomy.
We found serum ferritin levels to be significantly higher in subjects with NAFLD compared to well-matched controls, consistent with the findings of others (8–10). We specifically excluded subjects with iron saturation levels >55% to decrease the potential for enrolling subjects homozygous for hereditary hemochromatosis gene mutations and iron overload. However, as we did not perform genotyping we cannot rule out the possibility that some subjects may have had HFE gene mutations, the most common genetic cause for hereditary hemochromatosis (47). In large studies, serum ferritin is associated with the presence of NASH and advanced fibrosis in NAFLD (48). Hepatic expression of the iron-export protein ferroportin-1 was decreased and inversely correlated with tumor necrosis factor α (TNFα) levels, suggesting iron retention within the liver due to inflammation (49). Additional support for this concept is the observation that TNFα decreased ferroportin-1 mRNA levels in the hepatoma cell line HepG2 cells (49). While we did not observe any correlation between ferritin levels and inflammatory markers to support this hypothesis, the number of subjects in our study was small and controls did not undergo liver biopsy.
The strengths of our study include the use of “gold standard” techniques to measure insulin sensitivity and the use of both oral and intravenous testing to assess β-cell function. One limitation is that the sample size was quite small which limits our statistical power and thus the ability to fully interpret the lack of an association between ferritin and insulin sensitivity and between ferritin and fibrosis. Others have shown inverse associations between ferritin and insulin sensitivity using larger sample sizes (9). Despite the small sample size, the relationship between ferritin and β-cell function in our study remained robust.
In summary, both serum ferritin and transferrin-iron saturation were positively associated with the degree of hepatic steatosis independent of insulin sensitivity and ferritin was negatively associated with β-cell function. This is an important observation as NAFLD is a risk factor for the development of type 2 diabetes (2; 3). Whether higher ferritin levels in NAFLD reflect abnormal iron metabolism or inflammation and whether the higher ferritin is a cause or consequence of liver fat accumulation is unclear. Further research is needed to better understand the mechanisms underlying the relationship between ferritin levels and β-cell function and to determine if lowering iron levels can decrease the future risk for developing type 2 diabetes associated with NAFLD.
Figure 3.
Correlations between iron and liver fat measured by the liver/spleen ratio (A and B) and between iron and β-cell function as measured by the oral disposition index (C and D): controls (solid circle) and NAFLD (open square). There was a significant correlation between liver fat and (A) ln(ferritin) (r=0.59, p<0.001) and (B) iron saturation (r=0.45, p=0.01). There was a significant correlation between the ln(oral disposition index) and (C) ln(ferritin) (r=0.62, p=0.001), but not (D) iron saturation.
ACKNOWLEDGEMENTS
We are grateful to the study participants for their contribution and time. We also thank the nursing staff on the General Clinical Research Center, Tiffany Speron, Sherree Miller, George Ioannou and Jeff Maki for assistance with the study.
FUNDING
This work was supported by the United States Department of Veterans Affairs (Office of Research and Development Medical Research Service) and grant numbers UL1RR025014, P30DK017047, P30DK035816 and T32DK007247.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have nothing to disclose. There is no conflict of interest.
AUTHOR CONTRIBUTIONS
KMU designed the study, performed the study procedures, analyzed the data and wrote the manuscript, AL and AB assisted with the OGTT modeling, JEN assisted with recruitment and study procedures and contributed to writing the manuscript, SM contributed to the sample analysis, MMY read the liver biopsy specimens, KVK and SEK assisted in the design of the study and contributed to analysis of the data and writing of the manuscript.
REFERENCES
- 1.Utzschneider KM, Kahn SE. Review: The role of insulin resistance in nonalcoholic fatty liver disease. The Journal of Clinical Endocrinology and Metabolism. 2006;91:4753–4761. doi: 10.1210/jc.2006-0587. [DOI] [PubMed] [Google Scholar]
- 2.Chon CW, Kim BS, Cho YK, Sung KC, Bae JC, Kim TW, Won HS, Joo KJ. Effect of nonalcoholic Fatty liver disease on the development of type 2 diabetes in nonobese, nondiabetic korean men. Gut Liver. 2012;6:368–373. doi: 10.5009/gnl.2012.6.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sung KC, Jeong WS, Wild SH, Byrne CD. Combined influence of insulin resistance, overweight/obesity, and fatty liver as risk factors for type 2 diabetes. Diabetes Care. 2012;35:717–722. doi: 10.2337/dc11-1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahn SE, Zraika S, Utzschneider KM, Hull RL. The beta cell lesion in type 2 diabetes: there has to be a primary functional abnormality. Diabetologia. 2009;52:1003–1012. doi: 10.1007/s00125-009-1321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rijkelijkhuizen JM, Doesburg T, Girman CJ, Mari A, Rhodes T, Gastaldelli A, Nijpels G, Dekker JM. Hepatic fat is not associated with beta-cell function or postprandial free fatty acid response. Metabolism. 2009;58:196–203. doi: 10.1016/j.metabol.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Tushuizen M, Bunck M, Pouwels P, Bontemps S, Mari A, Diamant M. Lack of Association of Liver Fat with Model Parameters of Beta-Cell Function in Men with Impaired Glucose Tolerance and Type 2 Diabetes. Eur J Endocrinol. 2008 doi: 10.1530/EJE-08-0424. [DOI] [PubMed] [Google Scholar]
- 7.Bedogni G, Gastaldelli A, Manco M, De Col A, Agosti F, Tiribelli C, Sartorio A. Relationship between fatty liver and glucose metabolism: a cross-sectional study in 571 obese children. Nutr Metab Cardiovasc Dis. 2012;22:120–126. doi: 10.1016/j.numecd.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Hsiao TJ, Chen JC, Wang JD. Insulin resistance and ferritin as major determinants of nonalcoholic fatty liver disease in apparently healthy obese patients. Int J Obes Relat Metab Disord. 2004;28:167–172. doi: 10.1038/sj.ijo.0802519. [DOI] [PubMed] [Google Scholar]
- 9.Zelber-Sagi S, Nitzan-Kaluski D, Halpern Z, Oren R. NAFLD and hyperinsulinemia are major determinants of serum ferritin levels. J Hepatol. 2007;46:700–707. doi: 10.1016/j.jhep.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Licata A, Nebbia ME, Cabibbo G, Iacono GL, Barbaria F, Brucato V, Alessi N, Porrovecchio S, Di Marco V, Craxi A, Camma C. Hyperferritinemia is a risk factor for steatosis in chronic liver disease. World Journal of Gastroenterology: WJG. 2009;15:2132–2138. doi: 10.3748/wjg.15.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fargion S, Mandelli C, Piperno A, Cesana B, Fracanzani AL, Fraquelli M, Bianchi PA, Fiorelli G, Conte D. Survival and prognostic factors in 212 Italian patients with genetic hemochromatosis. Hepatology. 1992;15:655–659. doi: 10.1002/hep.1840150417. [DOI] [PubMed] [Google Scholar]
- 12.McClain DA, Abraham D, Rogers J, Brady R, Gault P, Ajioka R, Kushner JP. High prevalence of abnormal glucose homeostasis secondary to decreased insulin secretion in individuals with hereditary haemochromatosis. Diabetologia. 2006;49:1661–1669. doi: 10.1007/s00125-006-0200-0. [DOI] [PubMed] [Google Scholar]
- 13.Dmochowski K, Finegood DT, Francombe W, Tyler B, Zinman B. Factors determining glucose tolerance in patients with thalassemia major. The Journal of Clinical Endocrinology and Metabolism. 1993;77:478–483. doi: 10.1210/jcem.77.2.8345055. [DOI] [PubMed] [Google Scholar]
- 14.Ford ES, Cogswell ME. Diabetes and serum ferritin concentration among U.S. adults. Diabetes Care. 1999;22:1978–1983. doi: 10.2337/diacare.22.12.1978. [DOI] [PubMed] [Google Scholar]
- 15.Vari IS, Balkau B, Kettaneh A, Andre P, Tichet J, Fumeron F, Caces E, Marre M, Grandchamp B, Ducimetiere P. Ferritin and transferrin are associated with metabolic syndrome abnormalities and their change over time in a general population: Data from an Epidemiological Study on the Insulin Resistance Syndrome (DESIR) Diabetes Care. 2007;30:1795–1801. doi: 10.2337/dc06-2312. [DOI] [PubMed] [Google Scholar]
- 16.Jehn M, Clark JM, Guallar E. Serum ferritin and risk of the metabolic syndrome in U.S. adults. Diabetes Care. 2004;27:2422–2428. doi: 10.2337/diacare.27.10.2422. [DOI] [PubMed] [Google Scholar]
- 17.Forouhi NG, Harding AH, Allison M, Sandhu MS, Welch A, Luben R, Bingham S, Khaw KT, Wareham NJ. Elevated serum ferritin levels predict new-onset type 2 diabetes: results from the EPIC-Norfolk prospective study. Diabetologia. 2007;50:949–956. doi: 10.1007/s00125-007-0604-5. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez-Real JM, Lopez-Bermejo A, Ricart W. Iron stores, blood donation, and insulin sensitivity and secretion. Clin Chem. 2005;51:1201–1205. doi: 10.1373/clinchem.2004.046847. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Real JM, Penarroja G, Castro A, Garcia-Bragado F, Hernandez-Aguado I, Ricart W. Blood letting in high-ferritin type 2 diabetes: effects on insulin sensitivity and beta-cell function. Diabetes. 2002;51:1000–1004. doi: 10.2337/diabetes.51.4.1000. [DOI] [PubMed] [Google Scholar]
- 20.Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology. 2004;40:46–54. doi: 10.1002/hep.20280. [DOI] [PubMed] [Google Scholar]
- 21.Kugelmas M, Hill DB, Vivian B, Marsano L, McClain CJ. Cytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin E. Hepatology. 2003;38:413–419. doi: 10.1053/jhep.2003.50316. [DOI] [PubMed] [Google Scholar]
- 22.Yoneda M, Mawatari H, Fujita K, Iida H, Yonemitsu K, Kato S, Takahashi H, Kirikoshi H, Inamori M, Nozaki Y, Abe Y, Kubota K, Saito S, Iwasaki T, Terauchi Y, Togo S, Maeyama S, Nakajima A. High-sensitivity C-reactive protein is an independent clinical feature of nonalcoholic steatohepatitis (NASH) and also of the severity of fibrosis in NASH. Journal of Gastroenterology. 2007;42:573–582. doi: 10.1007/s00535-007-2060-x. [DOI] [PubMed] [Google Scholar]
- 23.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 24.Diagnosis and classification of diabetes mellitus. Diabetes care. 2011;34(Suppl 1):S62–S69. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Longo R, Ricci C, Masutti F, Vidimari R, Croce LS, Bercich L, Tiribelli C, Dalla Palma L. Fatty infiltration of the liver. Quantification by 1H localized magnetic resonance spectroscopy and comparison with computed tomography. Invest Radiol. 1993;28:297–302. [PubMed] [Google Scholar]
- 26.Warnick GR. Enzymatic methods for quantification of lipoprotein lipids. Methods Enzymol. 1986;129:101–123. doi: 10.1016/0076-6879(86)29064-3. [DOI] [PubMed] [Google Scholar]
- 27.Kelley DE, McKolanis TM, Hegazi RA, Kuller LH, Kalhan SC. Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance. American Journal of Physiology Endocrinology and Metabolism. 2003;285:E906–E916. doi: 10.1152/ajpendo.00117.2003. [DOI] [PubMed] [Google Scholar]
- 28.Yarasheski KE, Cade WT, Overton ET, Mondy KE, Hubert S, Laciny E, Bopp C, Lassa-Claxton S, Reeds DN. Exercise training augments the peripheral insulin-sensitizing effects of pioglitazone in HIV-infected adults with insulin resistance and central adiposity. American Journal of Physiology Endocrinology and Metabolism. 2011;300:E243–E251. doi: 10.1152/ajpendo.00468.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Utzschneider KM, Prigeon RL, Faulenbach M, Tong J, Carr DB, Boyko EJ, Leonetti DL, McNeely MJ, Fujimoto WY, Kahn SE. Justification for Use of an Oral Disposition Index to Assess β-cell Function Across Glucose Tolerance Categories. Diabetes. 2008;57:A46. [Google Scholar]
- 30.Dalla Man C, Campioni M, Polonsky KS, Basu R, Rizza RA, Toffolo G, Cobelli C. Two-hour seven-sample oral glucose tolerance test and meal protocol: minimal model assessment of beta-cell responsivity and insulin sensitivity in nondiabetic individuals. Diabetes. 2005;54:3265–3273. doi: 10.2337/diabetes.54.11.3265. [DOI] [PubMed] [Google Scholar]
- 31.Wolfe R. Radioactive and Stable Isotope Tracers in Biomedicine. New York: Wiley-Liss; 1992. [Google Scholar]
- 32.Groop LC, Bonadonna RC, DelPrato S, Ratheiser K, Zyck K, Ferrannini E, DeFronzo RA. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. The Journal of Clinical Investigation. 1989;84:205–213. doi: 10.1172/JCI114142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Musso G, Cassader M, De Michieli F, Rosina F, Orlandi F, Gambino R. Nonalcoholic steatohepatitis versus steatosis: Adipose tissue insulin resistance and dysfunctional response to fat ingestion predict liver injury and altered glucose and lipoprotein metabolism. Hepatology. 2012 doi: 10.1002/hep.25739. [DOI] [PubMed] [Google Scholar]
- 34.Rahier J, Loozen S, Goebbels RM, Abrahem M. The haemochromatotic human pancreas: a quantitative immunohistochemical and ultrastructural study. Diabetologia. 1987;30:5–12. doi: 10.1007/BF01788899. [DOI] [PubMed] [Google Scholar]
- 35.Jouihan HA, Cobine PA, Cooksey RC, Hoagland EA, Boudina S, Abel ED, Winge DR, McClain DA. Iron-mediated inhibition of mitochondrial manganese uptake mediates mitochondrial dysfunction in a mouse model of hemochromatosis. Mol Med. 2008;14:98–108. doi: 10.2119/2007-00114.Jouihan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooksey RC, Jouihan HA, Ajioka RS, Hazel MW, Jones DL, Kushner JP, McClain DA. Oxidative stress, beta-cell apoptosis, and decreased insulin secretory capacity in mouse models of hemochromatosis. Endocrinology. 2004;145:5305–5312. doi: 10.1210/en.2004-0392. [DOI] [PubMed] [Google Scholar]
- 37.Madan K, Bhardwaj P, Thareja S, Gupta SD, Saraya A. Oxidant stress and antioxidant status among patients with nonalcoholic fatty liver disease (NAFLD) Journal of Clinical Gastroenterology. 2006;40:930–935. doi: 10.1097/01.mcg.0000212608.59090.08. [DOI] [PubMed] [Google Scholar]
- 38.Yesilova Z, Yaman H, Oktenli C, Ozcan A, Uygun A, Cakir E, Sanisoglu SY, Erdil A, Ates Y, Aslan M, Musabak U, Erbil MK, Karaeren N, Dagalp K. Systemic markers of lipid peroxidation and antioxidants in patients with nonalcoholic Fatty liver disease. The American Journal of Gastroenterology. 2005;100:850–855. doi: 10.1111/j.1572-0241.2005.41500.x. [DOI] [PubMed] [Google Scholar]
- 39.Nakashima T, Sumida Y, Yoh T, Kakisaka Y, Nakajima Y, Ishikawa H, Mitsuyoshi H, Kashima K, Nakamura H, Yodoi J. Thioredoxin levels in the sera of untreated viral hepatitis patients and those treated with glycyrrhizin or ursodeoxycholic acid. Antioxid Redox Signal. 2000;2:687–694. doi: 10.1089/ars.2000.2.4-687. [DOI] [PubMed] [Google Scholar]
- 40.Donath MY, Schumann DM, Faulenbach M, Ellingsgaard H, Perren A, Ehses JA. Islet inflammation in type 2 diabetes: from metabolic stress to therapy. Diabetes Care. 2008;31(Suppl 2):S161–S164. doi: 10.2337/dc08-s243. [DOI] [PubMed] [Google Scholar]
- 41.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. The Journal of Clinical Investigation. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. American Journal of Physiology Endocrinology and Metabolism. 2001;280:E745–E751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 43.Rexrode KM, Pradhan A, Manson JE, Buring JE, Ridker PM. Relationship of total and abdominal adiposity with CRP and IL-6 in women. Annals of Epidemiology. 2003;13:674–682. doi: 10.1016/s1047-2797(03)00053-x. [DOI] [PubMed] [Google Scholar]
- 44.Gastaldelli A, Perego L, Paganelli M, Sesti G, Hribal M, Chavez AO, Defronzo RA, Pontiroli A, Folli F. Elevated concentrations of liver enzymes and ferritin identify a new phenotype of insulin resistance: effect of weight loss after gastric banding. Obesity Surgery. 2009;19:80–86. doi: 10.1007/s11695-008-9690-9. [DOI] [PubMed] [Google Scholar]
- 45.Facchini FS, Hua NW, Stoohs RA. Effect of iron depletion in carbohydrate-intolerant patients with clinical evidence of nonalcoholic fatty liver disease. Gastroenterology. 2002;122:931–939. doi: 10.1053/gast.2002.32403. [DOI] [PubMed] [Google Scholar]
- 46.Valenti L, Fracanzani AL, Dongiovanni P, Bugianesi E, Marchesini G, Manzini P, Vanni E, Fargion S. Iron depletion by phlebotomy improves insulin resistance in patients with nonalcoholic fatty liver disease and hyperferritinemia: evidence from a case-control study. The American Journal of Gastroenterology. 2007;102:1251–1258. doi: 10.1111/j.1572-0241.2007.01192.x. [DOI] [PubMed] [Google Scholar]
- 47.Utzschneider KM, Kowdley KV. Hereditary hemochromatosis and diabetes mellitus: implications for clinical practice. Nat Rev Endocrinol. 2010;6:26–33. doi: 10.1038/nrendo.2009.241. [DOI] [PubMed] [Google Scholar]
- 48.Kowdley KV, Belt P, Wilson LA, Yeh MM, Neuschwander-Tetri BA, Chalasani N, Sanyal AJ, Nelson JE. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2012;55:77–85. doi: 10.1002/hep.24706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aigner E, Theurl I, Theurl M, Lederer D, Haufe H, Dietze O, Strasser M, Datz C, Weiss G. Pathways underlying iron accumulation in human nonalcoholic fatty liver disease. The American Journal of Clinical Nutrition. 2008;87:1374–1383. doi: 10.1093/ajcn/87.5.1374. [DOI] [PubMed] [Google Scholar]