Abstract
Prior studies have revealed the key roles played by Th1/Th2 cell dysregulation, IgE production, mast cell hyperactivity, and dendritic cell signaling in the evolution of the chronic, pruritic, inflammatory dermatosis that characterizes atopic dermatitis (AD). We review here increasing evidence that the inflammation in AD results primarily from inherited abnormalities in epidermal structural and enzymatic proteins that impact permeability barrier function. We also will show that the barrier defect can be attributed to a paracellular abnormality due to a variety of abnormalities in lipid composition, transport and extracellular organization. Accordingly, we also review the therapeutic implications of this emerging pathogenic paradigm, including several current and potentially novel, lipid-based approaches to corrective therapy.
Keywords: antimicrobial peptides, atopic dermatitis, barrier function, ceramides, cytokines, filaggrin, kallikreins, lamellar bodies, lipid composition, pH, serine protease inhibitors, Th2 cells
Introduction
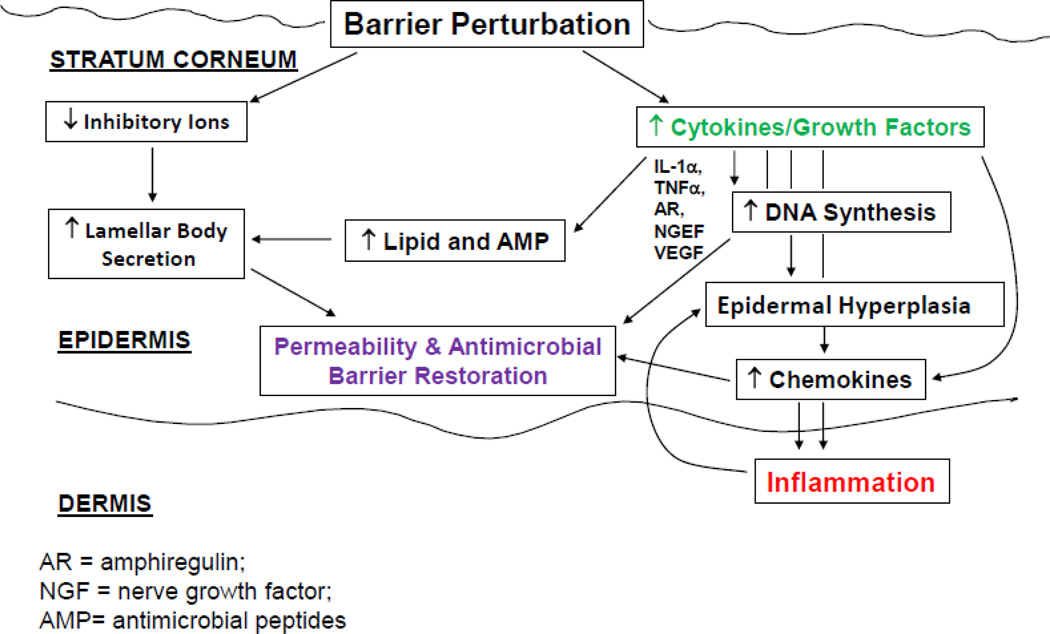
Because both a defective epidermal permeability barrier [1–4], as well as a propensity to develop secondary infections [5] are well-recognized features of AD, we and others proposed several years ago that the barrier abnormalities in AD are not merely epiphenomena, but rather the ‘driver’ of disease activity (i.e., an ‘outside-to-inside’ view of disease pathogenesis) [6–8] (Fig. 1), because: 1) the extent of the permeability barrier abnormality parallels severity of disease phenotype in AD [1, 2, 4]; 2) both clinically-uninvolved skin sites, as well as skin cleared of inflammation for several years, can continue to display significant barrier abnormalities [2]; and 3) emollient therapy comprises effective ancillary therapy for AD [9]. Much more is now known about inherited and acquired abnormalities in AD, which have fortified this ‘outside-to-inside’ view of disease pathogenesis, with broad implications for what should comprise rational therapy.
Fig. 1.
‘OUTSIDE-INSIDE’ HOMEOSTATIC RESPONSES CAN ALSO PROVOKE A CYTOKINE CASCADE LEADING TO INFLAMMATION
Basis for the Permeability Barrier in Normal Skin
The epidermis generates a set of protective/defensive functions, mediated by its unique differentiation end-product, the stratum corneum [10] (see also article by Feingold & Elias in this volume). These functions include the permeability barrier, which retards transcutaneous evaporative water loss, allowing survival in a potentially desiccating external environment, while simultaneously impeding the ingress of noxious substances, including toxins, allergens, and pathogenic microbes. Yet, the permeability barrier shares many features with the antimicrobial barrier, which impedes the growth of pathogenic organisms, while simultaneously encouraging colonization by non-pathogenic ‘normal’ flora (see article by Wertz, et al., in this issue). This antimicrobial system comprises a key distal component of the cutaneous innate immune system [11].
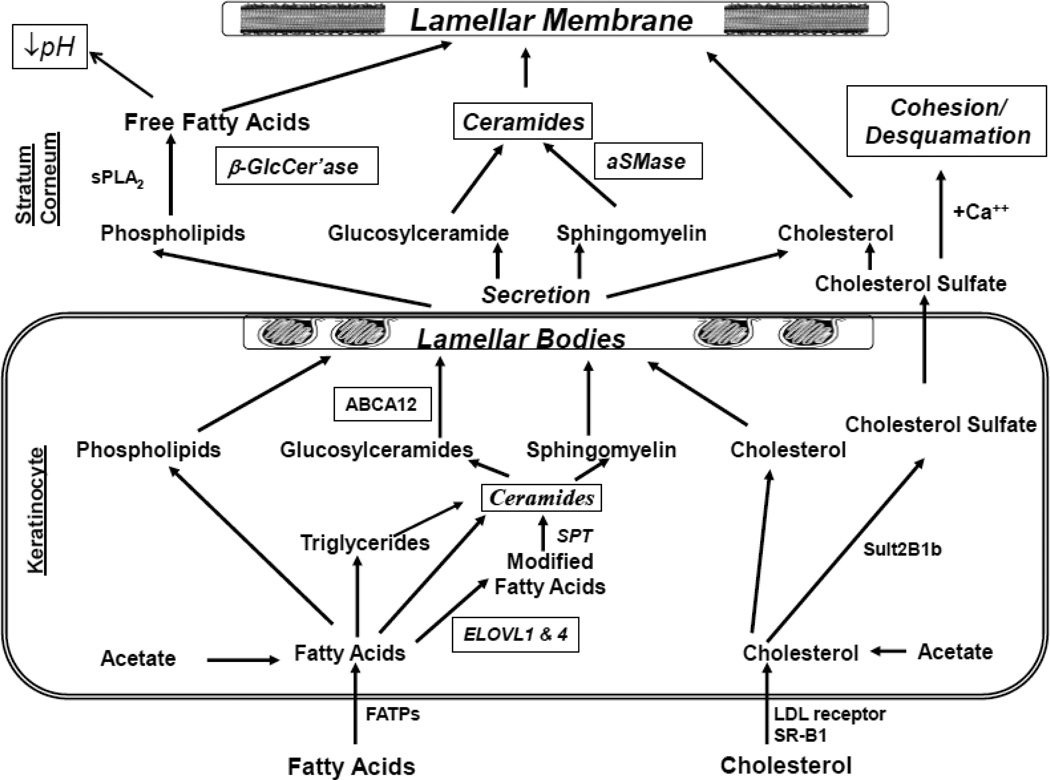
The stratum corneum (SC) comprises a multilayered tissue composed of flattened, geometrical, anucleate corneocytes, surrounded by multiple stacks of board, planar lamellae, enriched in ceramides, cholesterol, and free fatty acids (FFA) [12]. It is the localization of these highly-hydrophobic lipids within the extracellular domains of the SC that inhibits both the outward movement of water, and the access of noxious substances and pathogenic microbes from the environment (Ibid.). These lipids are delivered to the SC as their precursors through secretion of a unique organelle, the epidermal lamellar body [13]. As the SC forms, this organelle delivers lipid precursors (e.g., glucosylceramides and phospholipids, as well as a set of hydrolytic, lipid-processing enzymes, such as β-glucocerebrosidase, acidic sphingomylinase, secretory phospholipase A2 and steroid sulfatase, required to generate ceramides (Cer), free fatty acids (FFA), and much of the cholesterol that is required for the organization of these non-polar lipids into mature lamellar membrane structures [13] (see also article by Feingold & Elias, and K. Sandhoff in this issue). In parallel, lamellar body-derived proteases and their inhibitors orchestrate the orderly digestion of corneodesmosomes, transient intercellular rivets that are progressively degraded, initiating the invisible shedding of corneocytes from the skin surface [14–16]. Finally, at least two antimicrobial peptides, human β-defensin 2 and the carboxyterminal cathelicidin peptide, LL-37, are delivered to the SC intercellular domains through secretion of lamellar body contents [17–19]. Thus, the epidermal lamellar body is a multi-functional organelle, whose contents influence not only permeability barrier status, but also at least two other key functions, SC cohesion/desquamation and cutaneous antimicrobial defense.
Inherited Causes of a Barrier Abnormality in Atopic Dermatitis
1) Deficiency of Filaggrin and other S100 Proteins
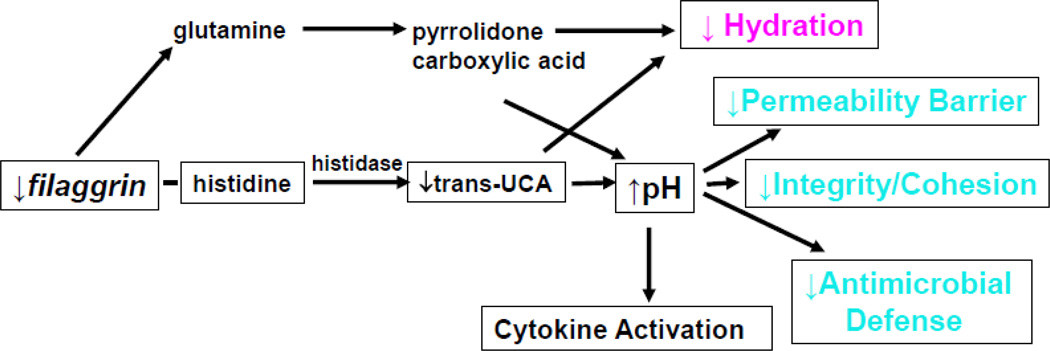
The strongest evidence that a primary structural abnormality underlies the pathogenesis of AD derives from the recent work that links loss-of-function mutations in the gene encoding, filament aggregating protein (filaggrin, FLG) in humans with AD [20, 21]. Up to 50% of northern European kindreds with AD reveal either single or double allele mutations in the gene encoding for FLG, which is located in the differentiation complex on chromosome 1q21. The initial product of FLG translation is pro-FLG, a large, histidine-rich, highly cationic phosphoprotein, consisting of ten to twelve FLG repeats, connected by peptide segments enriched in hydrophobic amino acids [22–24]. Pro-FLG contains an aminoterminal sequence, including a calcium-binding A domain as well as a B domain of uncertain function, with a putative S100-like, calcium binding domain. In contrast to the cytoplasmatic location of the C-terminal FLG monomers, the N-terminal portion of pro-FLG tethers to the nucleus, consistent with its nuclear localization sequence. During cornification in normal, non-atopic humans, pro-FLG is dephosphorylated and proteolytically processed to FLG monomers. Immunolocalization studies suggest that processed FLG peptides associate with, and induce aggregation of keratins within the corneocyte cytosol, while also attaching to the cornified envelope, a unique structure that forms under the plasma membrane as granular cells transform into corneocytes [25, 26]. The CE provides an inflexible, mechanically-resistant physical barrier. However, as the water content of the SC drops in the mid-to-outer stratum corneum of humans, FLG detaches from the cornified envelope, with the C-terminal portion of FLG proteolyzed by caspase 14 into its constituent amino acids. These amino acids subsequently are further deiminated into polycarboxylic acids that comprise the ‘natural moisturizing factor’ of the SC (Fig. 2) [27, 28].
Fig. 2.
How Filaggrin Deficiency Predisposes to Atopic Dermatitis Trans-urocanic acid (t-UCA) is the most potent endogenous UV-B filter in lightlypigmented skin. Loss of t-UCA could account for the higher incidence of nonmelanoma skin cancers in AD. (Elias, P. & Williams, M. J Invest Dermatol. 133(6): 1,676–1,677, 2013.)
FLG deficiency in AD has been ascribed to both nonsense and frameshift mutations that result in partial or complete loss of FLG expression, as well as the reduction-to-loss of keratohyalin granules in the epidermis. Although more than 40 different mutations are now reported [29], 4 mutations predominate in northern and central Europeans [30, 31]. These mutations exhibit an allele-dose effect, wherein heterozygous patients show diminished FLG expression with a mild IV phenotype, as well as minor abnormalities in surface pH, hydration, and barrier function [32]. But IV patients with homozygous and compound heterozygous FLG mutations, who lack FLG expression, exhibit more severe scaling, more pronounced abnormalities in stratum corneum structure and function [32], and a further propensity to develop AD [29]. Yet importantly, a substantial proportion of these double-allele IV patients still do not exhibit inflammation (AD), emphasizing the role of exogenous (acquired) factors in AD pathogenesis.
FLG is the main component of keratohyalin granules located in the outer nucleated layers of the epidermis, that account for the designation of this cell layer as the stratum granulosum. Accordingly, decreased FLG expression results in a paucity of keratohyalin granules, a hallmark of ichthyosis vulgaris (IV) [33, 34], the forme fruste of AD, and often accompanied by allergic rhinitis and/or asthma. But an acquired reduction in epidermal FLG expression also occurs in AD [3, 35–37], in part due to Th2-induced down-regulation of a broad range of proteins associated with epidermal differentiation [38, 39].
Yet, there is increasing evidence that inherited abnormalities not only in FLG, but also in other proteins that are important for barrier maintenance, also can lead to AD. It is important to note that inherited abnormalities in FLG occur primarily in populations of northern European descent [29]. AD in other populations will likely prove to be associated with other inherited abnormalities. Very recent studies have shown an association of AD with other S100 proteins, including hornerin [40] and FLG-2 [41]. But a still broader view might be that any inherited abnormality that leads to a chronic barrier abnormality could predispose to AD. Note the association of AD with loss-of-function mutations in the fatty acid transporter, FATP4 [42]. It is also likely that any mutations that occur in the lamellar body secretory system should predispose to AD, as suggested by the association of the trans-membrane, trans-Golgi-associated protein, Tmem, with an ichthyosiform phenotype in mice [43], an association now also reported in some humans with AD (Irvine, A. & Fallon P, J Allergy Clin Immunol, In Press 2013).
2) Protease-Anti-Protease Expression
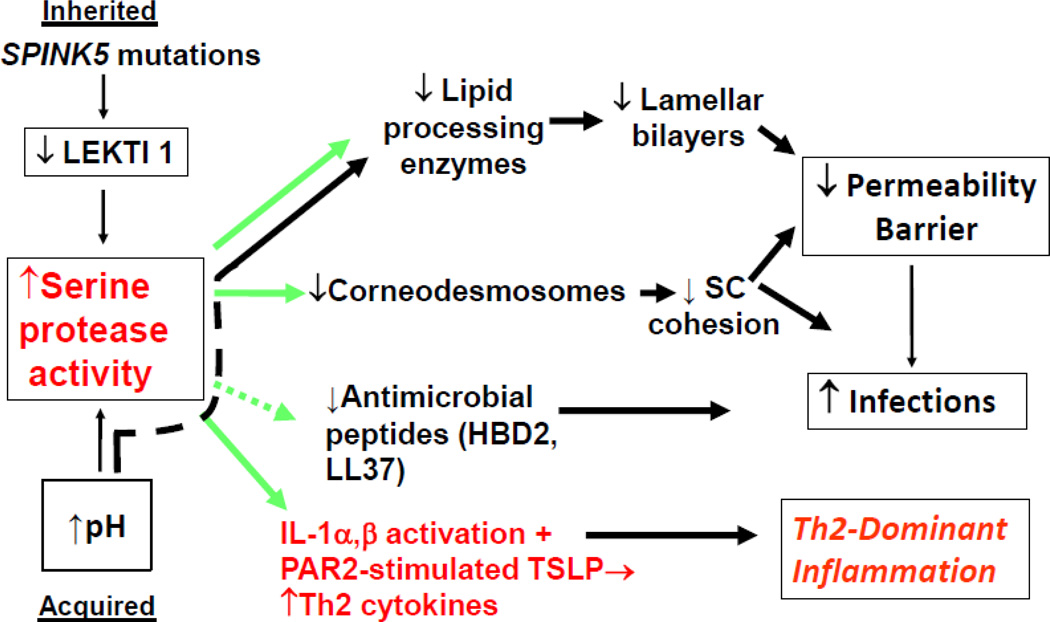
In addition to loss-of-function mutations in FLG and other structural proteins, inherited abnormalities in either serine protease (SP) or anti-protease expression lead to defects in the structure and function of the SC, and predispose to AD (Fig. 1) [44]. The most compelling case for the role of excess SP activity in the pathogenesis of AD comes from Netherton syndrome (NS), an autosomal recessive disorder due to loss-of-function mutations in SPINK5, the gene encoding the SP inhibitor, lymphoepithelial Kazal-type trypsin inhibitor type 1 (LEKTI 1) [45]. NS is characterized by severe AD, mucosal atopy, and anaphylactic reactions to food antigens. Residual LEKTI expression in humans with NS correlates inversely with excess SP activity within the outer epidermis [46], resulting in a severe permeability barrier defect and dramatic thinning of SC due to unrestrained, SP-dependent degradation of lipid-processing enzymes and corneodesmosome-constituent proteins, respectively [46]. Pertinently, several European, American, and Japanese case-control studies of humans, with AD or mucosal atopy, have found an increased frequency of single nucleotide polymorphisms (Glu420Lys) in SPINK5 [45]. Conversely, a British case-control study described putative, gain-of-function polymorphisms (AACCAACC vs. AACC) in the 3’ region of KLK7, which encodes the serine protease SC chymotryptic enzyme or KLK7 [47]. Moreover, transgenic mice forced to express human KLK7 display a severe AD-like dermatosis. Yet, the incidence of both of these polymorphisms is also quite high in unaffected normals [48, 49]. Nonetheless, in experimental animals, a net increase in SP activity, achieved by a variety of unrelated means, compromises barrier function through accelerated degradation of both corneodesmosomes (accounting for flawed SC cohesion) and degradation of extracellular, ceramide (Cer)-generating enzymes; i.e., β-glucocerebrosidase and acidic sphingomyelinase [50] (Fig. 3). As shown most dramatically in lesional skin of humans with NS [46], SP-mediated degradation of these enzymes contributes to the depletion of Cer, a characteristic lipid abnormality in AD [51, 52].
Fig. 3.
Lessons from Netherton Syndrome: Central Role of KLKs
Basis for Global Lipid Abnormalities in Atopic Dermatitis (Fig. 4)
Fig. 4.
Lipid Metabolic Events Leading to Barrier Formation (Italics indicate abnormalities in atopic dermatitis)
Abbreviations: β-Glccer’ase, β-glucocerebrosidase; aSMase, acidic sphingomyelinase; FATPs, fatty acid transport proteins; SSase, steroid sulfate; sPLA2, secretory phospholipase A2
Filaggrin-associated AD is characterized by profound abnormalities in lipid content, distribution, and lamellar membrane organization in lesional skin [53–55]. In other inherited scaling abnormalities (ichthyoses) due to abnormal structural proteins, as occur not only in IV, but also in epidermolytic ichthyosis, transglutaminase 1-negative lamellar ichthyosis, and loricrin keratoderma, the permeability barrier defect lies in the lipid-enriched extracellular domains (rev. in [56]). But in all three of these disorders, the cellular mechanisms that account for the extracellular abnormalities differ. In humans with FLG-deficient IV, a forme fruste of AD, we observed two abnormalities: first, micro-vesicles within lamellar bodies, an indication of impaired loading of cargo into nascent organelles [57]. Second, lamellar body secretion is moderately impaired in IV, resulting in entombment of substantial quantities of lamellar bodies within corneocytes, a feature that becomes even more prominent once inflammation (AD) appears [58]. Elevated SP activity in experimental animals also provokes a secretory abnormality in AD by signaling the plasminogen activator type 2 receptor (PAR2), which in turn downregulates lamellar body (LB) secretion [59], likely providing a biochemical signal that entombs these organelles in nascent corneocytes. These animal studies suggest that increased SP activity alone induces lipid abnormalities that parallel those that occur in AD, providing a mechanistic basis that accounts in part for the global reduction in extracellular lipids and the further decline in Cer levels that occur in AD. Together, the abnormalities in lamellar body loading and secretion in FLG-deficient IV suggest that FLG deficiency produces a cytoskeletal defect sufficient to impair organelle secretion. Thus, impaired lamellar body secretion due not only to FLG deficiency, but also to TMEM79 (see above), can produce a paucity of extracellular lamellar bilayers, as accounting at least in part for the global decrease in lipid content in the SC of patients with AD [53, 54].
Further Bases for Ceramide Deficiency in Atopic Dermatitis
The most impressive hallmark of human AD, however, is a repeatedly-noted, selective reduction in Cer content [51, 52]. Several mechanisms appear to contribute to the decrease in Cer. First, the barrier-related increase in pH, and pH-induced increase in kallikrein (KLK) activity result in deactivation, and ultimately in accelerated degradation of the Cer-generating enzymes, acidic sphingomylinase and β-glucocerabrosidase (Fig. 3), demonstrated most dramatically in patients with Netherton syndrome [46]. Yet, neither sphingomyelin nor glucosylceramides accumulate in the SC of AD. Imokawa, et al. (2009) provided an alternate mechanism that could explain why sphingomyelin and glucosylceramides no not accumulate in AD [60]. AD epidermis exhibits novel N-deacylation activities that degrade both sphingomyelin and glucosylceramides, resulting in the accumulation of appropriate metabolic products (but neither sphingomyelin nor glycosylceramides) in AD scale [60]. Yet, the genes for these enzymes have not yet been identified in epidermis; hence, it is possible that these activities could be of bacterial origin. Likewise, other microbial pathogens that frequently colonize AD also elaborate acidic ceramidase activity [61, 62], which could further decrease Cer content. This scenario seems less important, since the sphingoid base content of the SC in AD is lower, not higher than in normal SC [63], arguing against an important role for microbial ceramidases in producing Cer deficiency in AD. Finally, abnormalities in the ratio of sphingoid bases, specifically sphingosine and sphinganine appears to exert important effects on lamellar membrane permeability in AD [64].
Increased production of the Th2-derived cytokines, IL-4 and IL-13, is a further important contributor to the decrease in Cer in AD [65, 66]. In experimental animals, IL-4 down-regulates not only serine palmitoyl transferase, the rate-limiting enzyme of ceramide synthesis, but it also blunts the potential beneficial effects of Th1-derived TNF-α on ceramide-generating enzymes. While Th1 cytokines instead upregulate Cer production [67], it is likely that the dominance of Th2 cytokines in AD overwhelms this Th1 response, with profound consequences for epidermal structure and function (Fig. 3).
In experimental animals, IL-4 also inhibits expression of keratinocyte differentiation-linked proteins, most notably FLG [35]. Desmoglein 3 expression is also inhibited by exogenous IL-4. Moreover, recent studies have shown that serum IgE from AD patients auto-reacts against a variety of keratinocyte antigens, suggesting yet another ‘vicious cycle’ in AD [68]. Together, these observations provide additional acquired mechanisms that could further compromise barrier function in AD [35, 69]. Thus, primary inherited barrier abnormalities in AD ultimately stimulate downstream paracrine mechanisms that could further compromise permeability barrier function, completing a potential ‘outsideinside-outside’ pathogenic loop in AD [70].
Recently, researchers in Japan and The Netherlands independently reported that the sum of sphingoid bases plus the N-acyl fatty acids (FA) in ceramides declines in lesional AD, in parallel with a decline in the chain length of free fatty acids (FFA) [71][72, 73]. These shorter chain fatty acids in turn produce abnormalities in lipid organization that likely compromise permeability barrier function in AD [71, 73, 74]. The basis for the chain length abnormalities likely will prove to be reduced expression of two fatty acid elongases, ELOVL1 and ELOVL4, that are required to generate the very long chain N-acyl FA in Cer and FFA in AD [75]. Although it is intriguing to speculate that the reduced levels of ELOVs could be an acquired abnormality due to excessive serine-protease activity, even more likely is the possibility that elevated levels of IFNγ downregulate ELOV1 and 4 [43], accounting for reduced N-acyl chain [75]. Together, these results suggest that lipid restorative measures could prevent and/or ameliorate the barrier abnormality in AD, thereby reducing the inflammatory component in AD [53, 54, 76, 77] (see below).
Basis for Inflammation in Atopic Dermatitis
One important downstream consequence of increased SP activity is generation of the primary cytokines, IL-1α and IL-1β [78], from their 33kDa pro-forms in human SC, which are stored in large quantities in the cytosol of corneocytes. This putative pH-induced increase in SP activity would generate the active, 17kDa forms of these cytokines [78], the first step in the cytokine cascade in AD, which includes production of several additional cytokines and growth factors [79–81]. However, as noted above, one of these downstream epidermal cytokines, IFNγ, downregulates ELOV1 and 4, contributing to the barrier abnormality in AD [75]. Consequently, sustained antigen ingress through a defective barrier leads to a Th2-dominant infiltrate, which then becomes a second cause of inflammation in AD (Figs. 1 & 2). Certain antigens, such as cat dander, preferentially trigger childhood AD, particularly in FLG-deficient patients [82]. But the worst offenders are mites and cockroach antigens, which themselves release and activate SP activity, resulting in further damage to the barrier [83]. Furthermore, the lipid-depleted barrier in AD may facilitate the penetration of water-soluble haptens, such as nickel. Indeed, nickel-induced, acute allergic dermatitis is more common in humans with AD than in normals [84]. Accordingly, correction of the barrier abnormality alone should ameliorate both the cytokine cascade and allergen-induced inflammation in AD.
Consequences of Failure of the Antimicrobial Barrier in Atopic Dermatitis
Like permeability barrier dysfunction, the antimicrobial barrier also is compromised in AD. Colonization by Staphylococcus aureus is a common, often disease-precipitating feature of AD. And while colonization is highest on lesional skin of AD patients, colony counts often are high on clinically normal skin of AD patients [5]. Moreover, overt secondary infections, manifesting commonly as impetiginization, widespread folliculitis, or less frequently cutaneous abscesses or cellulitis, are well-recognized complications in the management of AD. Furthermore, colonization by superantigen producing S. aureus strains is more common in steroid-resistant patients [85], and further exacerbates disease in severe AD through generalized augmentation of IgE production, as well as through development of specific IgE-directed towards staphylococcal exotoxins [rev. in [86]]. Over time, non-toxigenic strains of S. aureus that colonize AD can be replaced by enterotoxin-generating strains [87], which in turn, could aggravate AD by at least three mechanisms: 1) toxigenic strains are more likely to produce clinical infections than are non-toxigenic strains [87]; 2) some toxins stimulate pruritus [88] and production of specific IgE [5, 86]; and 3) some toxins serve as ‘superantigens’ that stimulate T and B cell proliferation, as well as immunoglobulin class-switching to allergen-specific or ‘superallergens’ that stimulate IgE production [5]. Activated T cells produce IL-31, which also induces pruritus [89]. Finally, clinical infections, particularly folliculitis, are notoriously pruritic, even in non-atopics, eliciting an ‘itch-scratch’ vicious cycle that creates additional portals of entry for pathogens. It is self-evident that excoriations create further defects in the permeability barrier, representing yet another potentially-important vicious cycle in AD pathogenesis. In addition, patients with atopic dermatitis are also susceptible to widespread cutaneous viral infections, including molluscum contagiosum, Herpes simplex (Kaposi’s varicelliform eruption), and life-threatening Vaccinia. Widespread dermatophytosis (tinea corporis) and Malassezia infections also occur in AD, and the latter, like S. aureus, can stimulate specific IgE production. Taken together, these observations point to loss of a competent antimicrobial barrier in AD. While failure of both permeability and antimicrobial function is well-recognized in AD, only recently have studies in experimental animals shown that these two functions are both co-regulated and interdependent [19]. Thus, failure of the permeability barrier in itself favors secondary infection; and conversely, pathogen colonization/infection further aggravates the permeability barrier abnormality.
Normal SC itself comprises a formidable barrier to pathogen colonization [11], but further several mechanisms can aggravate barrier function in AD. The antimicrobial barrier is intimately linked to the permeability barrier [19]; and, as with water egress, pathogen ingress occurs via the extracellular domains [90]. Moreover, an impaired permeability barrier alone predisposes to pathogen colonization, not only because of the increase in surface pH, but also because levels of FFA and the Cer metabolite, sphingosine, which exhibit potent antimicrobial activity [90, 91], decline in AD patients [11]. Surface proteins on S. aureus can down-regulate epidermal FFA production; thereby aggravating both permeability and antimicrobial function in parallel, a strategy that could also facilitate microbial invasion. In addition, members of two key families of antimicrobial peptides (AMP), the human cathelicidin (hCAP) product, LL-37, and human β-defensins (hBD) 2 and 3, are down-regulated in a TH2-dependent fashion in AD [92, 93]. Notably, both the hCAP aminoterminal fragment, cathelicidin (LL-37), and hBD3 display robust activity against S. aureus. Studies in experimental animals have shown that LL-37 is required for normal epidermal permeability barrier function [19] (notably, LL-37 is also important for the integrity of extracutaneous epithelia). Thus, it is likely that decreased LL-37 amplifies the barrier defect in AD patients.
Exogenous and Endogenous Stressors Further Aggravate Barrier Dysfunction in Atopic Dermatitis
Acquired pH-dependent increases in SP activity could also contribute to AD pathogenesis. That FLG mutations alone do not suffice is shown in ichthyosis vulgaris (IV), where the same single or double allele FLG mutations reduce FLG content, but inflammation (i.e., AD) does not always occur. Certain stressors could elicit disease by aggravating the barrier abnormality by provoking an incremental increase in pH of the SC, leading to a further amplification of SP activity (Fig. 1). Such a barrier-dependent increase in pH (and SP activity) likely accounts for the precipitation of AD following the use of neutral-to-alkaline soaps, a well-known exogenous stressor of clinical AD [77, 94].
Prolonged exposure to a reduced environmental humidity, as occurs in radiant-heated homes in temperate climates during the winter, is also a well-known risk factor for AD [95]. Under these conditions, transcutaneous water loss would accelerate across a defective SC, aggravating the underlying permeability barrier abnormality, while also amplifying cytokine signaling of inflammation. Because FLG proteolysis is regulated by changes in external humidity [96], sustained reductions in environmental relative humidities could further deplete residual FLG in single-allele FLG-deficient patients with AD. Finally, sustained psychological stress aggravates permeability barrier function in otherwise normal humans [97], and is also a well-known precipitant of AD. In the case of PS, however, the likely mechanism differs from either surfactant use or decreased environmental humidities. Increased stress in experimental animals induces an increase in endogenous glucocorticoids (GC), which in turn alter permeability barrier homeostasis, SC integrity and epidermal antimicrobial defense. In murine epidermis, the mechanism for the negative effects of psychological stress is GC-mediated inhibition of synthesis of the three key epidermal lipids that mediate barrier function; i.e., Cer, cholesterol, and FFA. Accordingly, a topical mixture of these three lipids largely normalized all of these functions, even in the face of ongoing PS or GC therapy [98]. Yet, our recent studies in experimental animals show that the increase in endogenous GC that accompanies stress improves, rather than aggravates inflammation in AD [99]. Of course, these paradoxical benefits of stress disappear as barrier function returns towards normal with topical or systemic GC therapy.
Lipid-Based Therapeutic Interventions in Atopic Dermatitis
Together, the converging pathogenic features described above create a strong rationale for the deployment of specific strategies to restore barrier function in AD [54, 77, 94, 100]. Based upon the mechanisms described above, these approaches could range from general moisturization measures, to a reduction in the pH of SC alone (hyperacidification), applications of serine protease or PAR2 inhibitors, or different forms of lipid-based therapy. Lipid-enriched moisturizers are widely used in AD [101], and when deployed with nursing supervision, they significantly reduce topical steroid usage [102]. Of the various lipid-based, topical approaches, a Cer-dominant, triple-physiologic lipid, barrier repair therapy for AD (Cer:cholesterol:free fatty acids at a 3:1:1 molar ratio), addresses the dual problem of both a global reduction in lipids, as well as the further decline in Cer in AD. Such a formulation, provided at an acidic pH, has demonstrated efficacy in humans with AD [103, 104].1 Notably, a synthetic pseudoceramide, consisting of two C16FA, linked by an amide bond, appears able to substitute for naturally-occurring Cer, with the additional advantage of not risking excessive apoptosis [105]. Several additional clinical studies support the efficacy of targeted, Cer-dominant lipid replacement therapy in AD [54, 106]. An open-label study first demonstrated dramatic improvements in clinical activity, permeability barrier function, and SC integrity, when an over-the-counter version of this technology (TriCeram®) was substituted for standard moisturizers in children with severe, recalcitrant AD [4]. More recently, a higher-strength, FDA-approved prescription formulation (EpiCeram® cream, PuraCap Pharmaceutical) demonstrated efficacy that was comparable to a mid-potency steroid (fluticasone, Cutivate® cream) in an investigator-blinded, multicenter clinical trial of pediatric patients with moderate-to-severe AD [107]. Several recent reviews summarize more recent clinical experiences with Cer-dominant, barrier repair therapy in AD [76, 104, 106, 108, 109].
Therapy with Dietary Fats
Over 30 years ago, Houtsmuller, et al. (1981) demonstrated the efficacy of dietary and topical n-6 and n-3 polyunsaturated FFA in the therapy of essential fatty acid deficiency (EFAD) in animals [110]. However, linoleic acid (C18:2w6) and α-linolenic acid (C18:3w3) represent the parent lipids of two divergent classes of very long chain FFA, the n-6 and n-3 families of FFAs, respectively. The roles of these FFA are diverse – in epidermis, linoleic acid is incorporated into ω-hydroxy-Cer, where it functions as a critical structural component of the extracellular lamellar bilayers that form the permeability barrier. In contrast, n-3 FFA such as eicosapentaenoic acid (EPA; C20:5) and docosaehexanoic acid (DHA; 22:6) modulate inflammation [111]. Certain n-6 and n-3 FFA also activate peroxisomal proliferator-activated receptors, which regulate multiple steps in epidermal differentiation and lipid metabolism, while also exerting potent anti-inflammatory activity [112, 113]. While the ideal homeostatic rates of ω-6 to ω-3 is approximately 3:1 [111], modern Western diets contain abundant ω-6-enriched fats, which are poor substrates for the Δ5 and Δ6 dextroses that generate arachidonic acid, EPA and DHA. Nonetheless, it is now widely-believed that extra-dietary supplementation with ω-3’s is desirable, if not necessary to maintain the optimal ω-6: ω-3 ratio in human tissues [114, 115].
The prominent barrier abnormality and inflammation in AD prompted early attempts to treat these patients with evening primrose oil, a rich source of both linoleic acid and α-linolenic acid [116]. Results have been mixed, although a recent meta-analysis indicates utility in AD patients who also display elevated IgE levels. Accordingly, the focus has shifted to treating pregnant mothers and infants at risk for atopy with ω-3 supplements. Briefly, the rationale for taking w-3 fats is that substitution of w-3 FFA for w-6 FFA in cell membranes will decrease the subsequent release of downstream pro-inflammatory products, derived from arachidonic acid. Indeed, several recent studies indicate the efficacy of ω-3 supplementation in delaying the emergence of AD. But the benefits of these dietary interventions apparently disappear by two years of age. Yet, atopic children may benefit from other dietary or extra-dietary interventions, including: i) reducing trans-FA intake, thereby increasing levels of arachidonic acid in infants’ serum; ii) dietary sphingolipids – such as glucosylceramides and ceramides may be helpful [117]; iii) ingestion of reducing agents, including ascorbic acid and N-acetylcysteine; iv) dietary or topical niacinamide, which stimulates Cer production in vitro; and v) dietary vitamin D, which in contrast to topical vitamin D, appears to improve AD [118].
Conclusion
Since prior studies concentrated on the key roles played by Th1/Th2 cell dysregulation in the evolution of AD, therapy has been directed largely at ameliorating Th2-mediated inflammation and pruritus. We have reviewed here emerging evidence that the inflammation in AD results from inherited and acquired insults to the barrier, and the therapeutic implications of this new paradigm. Moreover, these preliminary, recent studies suggest that pathogenesis-based therapy is effective, and it could comprise a new paradigm for the therapy of AD. Yet, an important question remains: will restoration of permeability barrier function alone simultaneously improve antimicrobial defense in AD? Since recent studies have shown that these two key functions are both regulated in parallel and interdependent [19], there is reason to be optimistic on this score, as well [119]. A final consequence of the defective epidermal barrier in AD could be that it would allow epicutaneous delivery of antigens that induce asthma and allergic rhinitis. Thus, the ‘atopic march’; i.e., the tendency for AD to precede the later development of mucosal atopy, can be explained by cutaneous penetration of aeroallergens of all types. FLG deficiency is associated with mucosal atopy, independent of AD [120], though FLG is not expressed in either bronchial or other non-keratinizing mucosal epithelia [121]. An implication of this observation is that again barrier repair therapy could block development of the ‘atopic march’.
Highlights.
Atopic dermatitis (AD) results from inherited abnormalities that impact epidermal barrier function.
The paracellular barrier defect in AD is due to abnormal lipid composition, transport and organization.
Barrier abnormality in AD also allows for pathogen and antigen access into epidermis.
Increased serine protease activity accounts for decreased lipids and further decline in ceramides in AD.
This emerging paradigm may lead to lipid-based approaches for corrective therapy in AD.
Acknowledgements
This work was supported by NIH grant AR019098, and by the Medical Research Service, Department of Veterans Affairs. These contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIAMS or NIH.
Abbreviations
- AD
atopic dermatitis
- AMP
antimicrobial peptides
- Cer
ceramides
- EFAD
essential fatty acid deficiency
- FLG
filaggrin
- FFA
free fatty acids
- GC
glucocorticoids
- hBD
human β-defensins
- hCAP
human cathelicidin
- IV
ichthyosis vulgaris
- KLK
kallikreins
- LEKTI
lymphoepithelial Kazal-type trypsin inhibitor
- NS
Netherton syndrome
- PAR2
plasminogen activator type 2 receptor
- SP
serine protease
- SC
stratum corneum
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Dr. Elias is a co-inventor of this UC patented technology. He is a consultant for PuraCap Pharmaceutical, which markets EpiCeram®in the United States.
References
- 1.Sugarman JL, Fluhr JW, Fowler AJ, Bruckner T, Diepgen TL, Williams ML. The objective severity assessment of atopic dermatitis score: an objective measure using permeability barrier function and stratum corneum hydration with computerassisted estimates for extent of disease. Arch Dermatol. 2003;139:1417–1422. doi: 10.1001/archderm.139.11.1417. [DOI] [PubMed] [Google Scholar]
- 2.Seidenari S, Giusti G. Objective assessment of the skin of children affected by atopic dermatitis: a study of pH, capacitance and TEWL in eczematous and clinically uninvolved skin. Acta Derm Venereol. 1995;75:429–433. doi: 10.2340/0001555575429433. [DOI] [PubMed] [Google Scholar]
- 3.Proksch E, Folster-Holst R, Jensen JM. Skin barrier function, epidermal proliferation and differentiation in eczema. J Dermatol Sci. 2006;43:159–169. doi: 10.1016/j.jdermsci.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Chamlin SL, Kao J, Frieden IJ, Sheu MY, Fowler AJ, Fluhr JW, Williams ML, Elias PM. Ceramide-dominant barrier repair lipids alleviate childhood atopic dermatitis: changes in barrier function provide a sensitive indicator of disease activity. J Am Acad Dermatol. 2002;47:198–208. doi: 10.1067/mjd.2002.124617. [DOI] [PubMed] [Google Scholar]
- 5.Baker BS. The role of microorganisms in atopic dermatitis. Clin Exp Immunol. 2006;144:1–9. doi: 10.1111/j.1365-2249.2005.02980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elias PM, Wood LC, Feingold KR. Epidermal pathogenesis of inflammatory dermatoses. Am J Contact Dermat. 1999;10:119–126. [PubMed] [Google Scholar]
- 7.Elias PM, Feingold KR. Does the tail wag the dog? Role of the barrier in the pathogenesis of inflammatory dermatoses and therapeutic implications. Arch Dermatol. 2001;137:1079–1081. [PubMed] [Google Scholar]
- 8.Taieb A. Hypothesis: from epidermal barrier dysfunction to atopic disorders. Contact Dermatitis. 1999;41:177–180. doi: 10.1111/j.1600-0536.1999.tb06125.x. [DOI] [PubMed] [Google Scholar]
- 9.Grimalt R, Mengeaud V, Cambazard F. The steroid-sparing effect of an emollient therapy in infants with atopic dermatitis: a randomized controlled study. Dermatology. 2007;214:61–67. doi: 10.1159/000096915. [DOI] [PubMed] [Google Scholar]
- 10.Elias PM. Stratum corneum defensive functions: an integrated view. J Invest Dermatol. 2005;125:183–200. doi: 10.1111/j.0022-202X.2005.23668.x. [DOI] [PubMed] [Google Scholar]
- 11.Elias PM. The skin barrier as an innate immune element. Sem Immunopath. 2007;29:3–14. doi: 10.1007/s00281-007-0060-9. [DOI] [PubMed] [Google Scholar]
- 12.Elias PM, Menon GK. Structural and lipid biochemical correlates of the epidermal permeability barrier. Adv Lipid Res. 1991;24:1–26. doi: 10.1016/b978-0-12-024924-4.50005-5. [DOI] [PubMed] [Google Scholar]
- 13.Feingold KR. Lamellar bodies: the key to cutaneous barrier function. J Invest Dermatol. 2012;132:1951–1953. doi: 10.1038/jid.2012.177. [DOI] [PubMed] [Google Scholar]
- 14.Caubet C, Jonca N, Brattsand M, Guerrin M, Bernard D, Schmidt R, Egelrud T, Simon M, Serre G. Degradation of corneodesmosome proteins by two serine proteases of the kallikrein family, SCTE/KLK5/hK5 and SCCE/KLK7/hK7. J Invest Dermatol. 2004;122:1235–1244. doi: 10.1111/j.0022-202X.2004.22512.x. [DOI] [PubMed] [Google Scholar]
- 15.Brattsand M, Stefansson K, Lundh C, Haasum Y, Egelrud T. A proteolytic cascade of kallikreins in the stratum corneum. J Invest Dermatol. 2005;124:198–203. doi: 10.1111/j.0022-202X.2004.23547.x. [DOI] [PubMed] [Google Scholar]
- 16.Lin TK, Crumrine D, Ackerman LD, Santiago JL, Roelandt T, Uchida Y, Hupe M, Fabrias G, Abad JL, Rice RH, Elias PM. Cellular changes that accompany shedding of human corneocytes. J Invest Dermatol. 2012;132:2430–2439. doi: 10.1038/jid.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braff MH, Di Nardo A, Gallo RL. Keratinocytes store the antimicrobial peptide cathelicidin in lamellar bodies. J Invest Dermatol. 2005;124:394–400. doi: 10.1111/j.0022-202X.2004.23443.x. [DOI] [PubMed] [Google Scholar]
- 18.Oren A, Ganz T, Liu L, Meerloo T. In human epidermis, beta-defensin 2 is packaged in lamellar bodies. Exp Mol Pathol. 2003;74:180–182. doi: 10.1016/s0014-4800(02)00023-0. [DOI] [PubMed] [Google Scholar]
- 19.Aberg KM, Man MQ, Gallo RL, Ganz T, Crumrine D, Brown BE, Choi EH, Kim DK, Schroder JM, Feingold KR, Elias PM. Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. J Invest Dermatol. 2008;128:917–925. doi: 10.1038/sj.jid.5701099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irvine AD, McLean WH. Breaking the (un)sound barrier: filaggrin is a major gene for atopic dermatitis. J Invest Dermatol. 2006;126:1200–1202. doi: 10.1038/sj.jid.5700365. [DOI] [PubMed] [Google Scholar]
- 21.Hudson TJ. Skin barrier function and allergic risk. Nat Genet. 2006;38:399–400. doi: 10.1038/ng0406-399. [DOI] [PubMed] [Google Scholar]
- 22.Lynley AM, Dale BA. The characterization of human epidermal filaggrin. A histidine-rich, keratin filament-aggregating protein. Biochim Biophys Acta. 1983;744:28–35. doi: 10.1016/0167-4838(83)90336-9. [DOI] [PubMed] [Google Scholar]
- 23.Harding CR, Scott IR. Histidine-rich proteins (filaggrins): structural and functional heterogeneity during epidermal differentiation. J Mol Biol. 1983;170:651–673. doi: 10.1016/s0022-2836(83)80126-0. [DOI] [PubMed] [Google Scholar]
- 24.Fleckman P, Dale BA, Holbrook KA. Profilaggrin, a high-molecular-weight precursor of filaggrin in human epidermis and cultured keratinocytes. J Invest Dermatol. 1985;85:507–512. doi: 10.1111/1523-1747.ep12277306. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi M, Tezuka T, Katunuma N. Filaggrin linker segment peptide and cystatin alpha are parts of a complex of the cornified envelope of epidermis. Arch Biochem Biophys. 1996;329:123–126. doi: 10.1006/abbi.1996.0199. [DOI] [PubMed] [Google Scholar]
- 26.Steinert PM, Marekov LN. The proteins elafin, filaggrin, keratin intermediate filaments, loricrin, and small proline-rich proteins 1 and 2 are isodipeptide cross-linked components of the human epidermal cornified cell envelope. J Biol Chem. 1995;270:17702–17711. doi: 10.1074/jbc.270.30.17702. [DOI] [PubMed] [Google Scholar]
- 27.Scott IR, Harding CR, Barrett JG. Histidine-rich protein of the keratohyalin granules. Source of the free amino acids, urocanic acid and pyrrolidone carboxylic acid in the stratum corneum. Biochim Biophys Acta. 1982;719:110–117. doi: 10.1016/0304-4165(82)90314-2. [DOI] [PubMed] [Google Scholar]
- 28.Rawlings AV, Scott IR, Harding CR, Bowser PA. Stratum corneum moisturization at the molecular level. J Invest Dermatol. 1994;103:731–741. doi: 10.1111/1523-1747.ep12398620. [DOI] [PubMed] [Google Scholar]
- 29.Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011;365:1315–1327. doi: 10.1056/NEJMra1011040. [DOI] [PubMed] [Google Scholar]
- 30.O'Regan GM, Sandilands A, McLean WH, Irvine AD. Filaggrin in atopic dermatitis. J Allergy Clin Immunol. 2008;122:689–693. doi: 10.1016/j.jaci.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Sandilands A, Smith FJ, Irvine AD, McLean WH. Filaggrin's fuller figure: a glimpse into the genetic architecture of atopic dermatitis. J Invest Dermatol. 2007;127:1282–1284. doi: 10.1038/sj.jid.5700876. [DOI] [PubMed] [Google Scholar]
- 32.Gruber R, Elias PM, Crumrine D, Lin TK, Brandner JM, Hachem JP, Presland RB, Fleckman P, Janecke AR, Sandilands A, McLean WH, Fritsch PO, Mildner M, Tschachler E, Schmuth M. Filaggrin genotype in ichthyosis vulgaris predicts abnormalities in epidermal structure and function. Am J Pathol. 2011;178:2252–2263. doi: 10.1016/j.ajpath.2011.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thyssen JP, Godoy-Gijon E, Elias PM. Ichthyosis vulgaris: the filaggrin mutation disease. Br J Dermatol. 2013;168:1155–1166. doi: 10.1111/bjd.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fleckman P, Brumbaugh S. Absence of the granular layer and keratohyalin define a morphologically distinct subset of individuals with ichthyosis vulgaris. Exp Dermatol. 2002;11:327–336. doi: 10.1034/j.1600-0625.2002.110406.x. [DOI] [PubMed] [Google Scholar]
- 35.Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, Debenedetto A, Schneider L, Beck LA, Barnes KC, Leung DY. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2007;120:150–155. doi: 10.1016/j.jaci.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 37.Jensen JM, Proksch E, Elias PM. In: The stratum corneum of the epidermis in atopic dermatitis. Elias PM, Feingold KR, editors. New York: Skin Barrier, Marcel Dekker; 2005. pp. 569–590. [Google Scholar]
- 38.Jungersted JM, Hellgren LI, Jemec GB, Agner T. Lipids and skin barrier function--a clinical perspective. Contact Dermatitis. 2008;58:255–262. doi: 10.1111/j.1600-0536.2008.01320.x. [DOI] [PubMed] [Google Scholar]
- 39.Howell MD. The role of human beta defensins and cathelicidins in atopic dermatitis. Curr Opin Allergy Clin Immunol. 2007;7:413–417. doi: 10.1097/ACI.0b013e3282a64343. [DOI] [PubMed] [Google Scholar]
- 40.Greisenegger EK, Zimprich F, Zimprich A, Gleiss A, Kopp T. Association of the chromosome 11q13.5 variant with atopic dermatitis in Austrian patients. Eur J Dermatol. 2013;23:142–145. doi: 10.1684/ejd.2013.1955. [DOI] [PubMed] [Google Scholar]
- 41.Pellerin L, Henry J, Hsu CY, Balica S, Jean-Decoster C, Mechin MC, Hansmann B, Rodriguez E, Weindinger S, Schmitt AM, Serre G, Paul C, Simon M. Defects of filaggrin-like proteins in both lesional and nonlesional atopic skin. J Allergy Clin Immunol. 2013;131:1094–1102. doi: 10.1016/j.jaci.2012.12.1566. [DOI] [PubMed] [Google Scholar]
- 42.Khnykin D, Ronnevig J, Johnsson M, Sitek JC, Blaas HG, Hausser I, Johansen FE, Jahnsen FL. Ichthyosis prematurity syndrome: clinical evaluation of 17 families with a rare disorder of lipid metabolism. J Am Acad Dermatol. 2012;66:606–616. doi: 10.1016/j.jaad.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 43.Sasaki T, Shiohama A, Kubo A, Kawasaki H, Ishida-Yamamoto A, Yamada T, Hachiya T, Shimizu A, Okano H, Kudoh J, Amagai M. A homozygous nonsense mutation in the gene for Tmem79, a component for the lamellar granule secretory system, produces spontaneous eczema in an experimental model of atopic dermatitis. J Allergy Clin Immunol. 2013 doi: 10.1016/j.jaci.2013.08.027. In Press. [DOI] [PubMed] [Google Scholar]
- 44.Lee SE, Jeong SK, Lee SH. Protease and protease-activated receptor-2 signaling in the pathogenesis of atopic dermatitis. Yonsei medical journal. 2010;51:808–822. doi: 10.3349/ymj.2010.51.6.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walley AJ, Chavanas S, Moffatt MF, Esnouf RM, Ubhi B, Lawrence R, Wong K, Abecasis GR, Jones EY, Harper JI, Hovnanian A, Cookson WO. Gene polymorphism in Netherton and common atopic disease. Nat Genet. 2001;29:175–178. doi: 10.1038/ng728. [DOI] [PubMed] [Google Scholar]
- 46.Hachem JP, Wagberg F, Schmuth M, Crumrine D, Lissens W, Jayakumar A, Houben E, Mauro TM, Leonardsson G, Brattsand M, Egelrud T, Roseeuw D, Clayman GL, Feingold KR, Williams ML, Elias PM. Serine protease activity and residual LEKTI expression determine phenotype in Netherton syndrome. J Invest Dermatol. 2006;126:1609–1621. doi: 10.1038/sj.jid.5700288. [DOI] [PubMed] [Google Scholar]
- 47.Vasilopoulos Y, Cork MJ, Murphy R, Williams HC, Robinson DA, Duff GW, Ward SJ, Tazi-Ahnini R. Genetic association between an AACC insertion in the 3'UTR of the stratum corneum chymotryptic enzyme gene and atopic dermatitis. J Invest Dermatol. 2004;123:62–66. doi: 10.1111/j.0022-202X.2004.22708.x. [DOI] [PubMed] [Google Scholar]
- 48.Hubiche T, Ged C, Benard A, Leaute-Labreze C, McElreavey K, de Verneuil H, Taieb A, Boralevi F. Analysis of SPINK 5, KLK 7 and FLG genotypes in a french atopic dermatitis cohort. Acta Derm Venereol. 2007;87:499–505. doi: 10.2340/00015555-0329. [DOI] [PubMed] [Google Scholar]
- 49.Folster-Holst R, Stoll M, Koch WA, Hampe J, Christophers E, Schreiber S. Lack of association of SPINK5 polymorphisms with nonsyndromic atopic dermatitis in the population of Northern Germany. Br J Dermatol. 2005;152:1365–1367. doi: 10.1111/j.1365-2133.2005.06602.x. [DOI] [PubMed] [Google Scholar]
- 50.Hachem JP, Crumrine D, Fluhr J, Brown BE, Feingold KR, Elias PM. pH directly regulates epidermal permeability barrier homeostasis, and stratum corneum integrity/cohesion. J Invest Dermatol. 2003;121:345–353. doi: 10.1046/j.1523-1747.2003.12365.x. [DOI] [PubMed] [Google Scholar]
- 51.Di Nardo A, Wertz P, Giannetti A, Seidenari S. Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. Acta Derm Venereol. 1998;78:27–30. doi: 10.1080/00015559850135788. [DOI] [PubMed] [Google Scholar]
- 52.Imokawa G, Abe A, Jin K, Higaki Y, Kawashima M, Hidano A. Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin? J Invest Dermatol. 1991;96:523–526. doi: 10.1111/1523-1747.ep12470233. [DOI] [PubMed] [Google Scholar]
- 53.Elias PM, Wakefield JS. Therapeutic implications of a barrier-based pathogenesis of atopic dermatitis. Clin Rev Allergy Immunol. 2011;41:282–295. doi: 10.1007/s12016-010-8231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elias PM, Sun R, Eder AR, Wakefield JS, Man MQ. Treating atopic dermatitis at the source: corrective barrier repair therapy based upon new pathogenic insights. Exp Rev of Dermatol. 2013;8:27–36. [Google Scholar]
- 55.Jungersted JM, Scheer H, Mempel M, Baurecht H, Cifuentes L, Hogh JK, Hellgren LI, Jemec GB, Agner T, Weidinger S. Stratum corneum lipids, skin barrier function and filaggrin mutations in patients with atopic eczema. Allergy. 2010;65:911–918. doi: 10.1111/j.1398-9995.2010.02326.x. [DOI] [PubMed] [Google Scholar]
- 56.Schmuth M, Gruber R, PM E, Williams M. Ichthyosis update: towards a functiondriven model of pathogenesis of the disorders of cornification and the role of corneocyte proteins in these disorders. Adv Dermatol. 2007;23:231–256. doi: 10.1016/j.yadr.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gruber R, Janecke A, Rabl M, Oji V, Traupe H, Sandilands A, McLean W, Utermann G, Schmuth M. R501X, 2282del4 and R2447X founder mutations in the filaggrin gene in european ichthyosis vulgaris patients, IID 2008. Kyoto, Japan: 2008. [Google Scholar]
- 58.Man MQ, Hatano Y, Lee SH, Man M, Chang S, Feingold KR, Leung DY, Holleran W, Uchida Y, Elias PM. Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. J Invest Dermatol. 2008;128:79–86. doi: 10.1038/sj.jid.5701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hachem JP, Houben E, Crumrine D, Man MQ, Schurer N, Roelandt T, Choi EH, Uchida Y, Brown BE, Feingold KR, Elias PM. Serine protease signaling of epidermal permeability barrier homeostasis. J Invest Dermatol. 2006;126:2074–2086. doi: 10.1038/sj.jid.5700351. [DOI] [PubMed] [Google Scholar]
- 60.Imokawa G. A possible mechanism underlying the ceramide deficiency in atopic dermatitis: expression of a deacylase enzyme that cleaves the N-acyl linkage of sphingomyelin and glucosylceramide. J Dermatol Sci. 2009;55:1–9. doi: 10.1016/j.jdermsci.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 61.Kita K, Sueyoshi N, Okino N, Inagaki M, Ishida H, Kiso M, Imayama S, Nakamura T, Ito M. Activation of bacterial ceramidase by anionic glycerophospholipids: possible involvement in ceramide hydrolysis on atopic skin by Pseudomonas ceramidase. Biochem J. 2002;362:619–626. doi: 10.1042/0264-6021:3620619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ohnishi Y, Okino N, Ito M, Imayama S. Ceramidase activity in bacterial skin flora as a possible cause of ceramide deficiency in atopic dermatitis. Clinical and diagnostic laboratory immunology. 1999;6:101–104. doi: 10.1128/cdli.6.1.101-104.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arikawa J, Ishibashi M, Kawashima M, Takagi Y, Ichikawa Y, Imokawa G. Decreased levels of sphingosine, a natural antimicrobial agent, may be associated with vulnerability of the stratum corneum from patients with atopic dermatitis to colonization by Staphylococcus aureus. J Invest Dermatol. 2002;119:433–439. doi: 10.1046/j.1523-1747.2002.01846.x. [DOI] [PubMed] [Google Scholar]
- 64.Loiseau N, Obata Y, Moradian S, Sano H, Yoshino S, Aburai K, Takayama K, Sakamoto J, Holleran WM, Elias PM, Uchida Y. Altered sphingoid base profiles predict compromised membrane structure and permeability in atopic dermatitis. J Dermatol Sci. 2013 doi: 10.1016/j.jdermsci.2013.08.003. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hatano Y, Adachi Y, Elias PM, Crumrine D, Sakai T, Kurahashi R, Katagiri K, Fujiwara S. The Th2 cytokine, interleukin-4, abrogates the cohesion of normal stratum corneum in mice: implications for pathogenesis of atopic dermatitis. Exp Dermatol. 2013;22:30–35. doi: 10.1111/exd.12047. [DOI] [PubMed] [Google Scholar]
- 66.Kurahashi R, Hatano Y, Katagiri K. IL-4 suppresses the recovery of cutaneous permeability barrier functions in vivo. J Invest Dermatol. 2008;128:1329–1331. doi: 10.1038/sj.jid.5701138. [DOI] [PubMed] [Google Scholar]
- 67.Sawada E, Yoshida N, Sugiura A, Imokawa G. Th1 cytokines accentuate but Th2 cytokines attenuate ceramide production in the stratum corneum of human epidermal equivalents: an implication for the disrupted barrier mechanism in atopic dermatitis. J Dermatol Sci. 2012;68:25–35. doi: 10.1016/j.jdermsci.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 68.Altrichter S, Kriehuber E, Moser J, Valenta R, Kopp T, Stingl G. Serum IgE autoantibodies target keratinocytes in patients with atopic dermatitis. J Invest Dermatol. 2008;128:2232–2239. doi: 10.1038/jid.2008.80. [DOI] [PubMed] [Google Scholar]
- 69.Proksch E, Brandner JM, Jensen JM. The skin: an indispensable barrier. Exp Dermatol. 2008;17:1063–1072. doi: 10.1111/j.1600-0625.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- 70.Elias PM, Hatano Y, Williams ML. Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol. 2008;121:1337–1343. doi: 10.1016/j.jaci.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ishikawa J, Narita H, Kondo N, Hotta M, Takagi Y, Masukawa Y, Kitahara T, Takema Y, Koyano S, Yamazaki S, Hatamochi A. Changes in the ceramide profile of atopic dermatitis patients. J Invest Dermatol. 2010;130:2511–2514. doi: 10.1038/jid.2010.161. [DOI] [PubMed] [Google Scholar]
- 72.Park YH, Jang WH, Seo JA, Park M, Lee TR, Kim DK, Lim KM. Decrease of ceramides with very long-chain fatty acids and downregulation of elongases in a murine atopic dermatitis model. J Invest Dermatol. 2012;132:476–479. doi: 10.1038/jid.2011.333. [DOI] [PubMed] [Google Scholar]
- 73.Janssens M, van Smeden J, Gooris GS, Bras W, Portale G, Caspers PJ, Vreeken RJ, Hankemeier T, Kezic S, Wolterbeek R, Lavrijsen AP, Bouwstra JA. Increase in short-chain ceramides correlates with an altered lipid organization and decreased barrier function in atopic eczema patients. J Lipid Res. 2012;53:2755–2766. doi: 10.1194/jlr.P030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Janssens M, van Smeden J, Gooris GS, Bras W, Portale G, Caspers PJ, Vreeken RJ, Kezic S, Lavrijsen AP, Bouwstra JA. Lamellar lipid organization and ceramide composition in the stratum corneum of patients with atopic eczema. J Invest Dermatol. 2011;131:2136–2138. doi: 10.1038/jid.2011.175. [DOI] [PubMed] [Google Scholar]
- 75.Tawada C, Kanoh H, Nakamura M, Mizutani Y, Fujisawa T, Banno Y, Seishima M. Interferon-gamma Decreases Ceramides with Long-Chain Fatty Acids: Possible Involvement in Atopic Dermatitis and Psoriasis. J Invest Dermatol. 2013 doi: 10.1038/jid.2013.364. [DOI] [PubMed] [Google Scholar]
- 76.Hon KL, Leung AK, Barankin B. Barrier Repair Therapy in Atopic Dermatitis: An Overview. Am J Clin Dermatol. 2013 doi: 10.1007/s40257-013-0033-9. [DOI] [PubMed] [Google Scholar]
- 77.Cork MJ, Danby SG, Vasilopoulos Y, Hadgraft J, Lane ME, Moustafa M, Guy RH, Macgowan AL, Tazi-Ahnini R, Ward SJ. Epidermal barrier dysfunction in atopic dermatitis. J Invest Dermatol. 2009;129:1892–1908. doi: 10.1038/jid.2009.133. [DOI] [PubMed] [Google Scholar]
- 78.Nylander-Lundqvist E, Back O, Egelrud T. IL-1 beta activation in human epidermis. J Immunol. 1996;157:1699–1704. [PubMed] [Google Scholar]
- 79.Wood LC, Jackson SM, Elias PM, Grunfeld C, Feingold KR. Cutaneous barrier perturbation stimulates cytokine production in the epidermis of mice. J Clin Invest. 1992;90:482–487. doi: 10.1172/JCI115884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Elias PM, Ansel JC, Woods LD, Feingold KR. Signaling networks in barrier homeostasis. The mystery widens. Arch Dermatol. 1996;132:1505–1506. [PubMed] [Google Scholar]
- 81.Elias PM, Steinhoff M. "Outside-to-inside" (and now back to "outside") pathogenic mechanisms in atopic dermatitis. J Invest Dermatol. 2008;128:1067–1070. doi: 10.1038/jid.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bisgaard H, Simpson A, Palmer CN, Bonnelykke K, McLean I, Mukhopadhyay S, Pipper CB, Halkjaer LB, Lipworth B, Hankinson J, Woodcock A, Custovic A. Gene-environment interaction in the onset of eczema in infancy: filaggrin loss-of-function mutations enhanced by neonatal cat exposure. PLoS Med. 2008;5:e131. doi: 10.1371/journal.pmed.0050131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jeong SK, Kim HJ, Youm JK, Ahn SK, Choi EH, Sohn MH, Kim KE, Hong JH, Shin DM, Lee SH. Mite and cockroach allergens activate protease-activated receptor 2 and delay epidermal permeability barrier recovery. J Invest Dermatol. 2008;128:1930–1939. doi: 10.1038/jid.2008.13. [DOI] [PubMed] [Google Scholar]
- 84.Novak N, Baurecht H, Schafer T, Rodriguez E, Wagenpfeil S, Klopp N, Heinrich J, Behrendt H, Ring J, Wichmann E, Illig T, Weidinger S. Loss-of-function mutations in the filaggrin gene and allergic contact sensitization to nickel. J Invest Dermatol. 2008;128:1430–1435. doi: 10.1038/sj.jid.5701190. [DOI] [PubMed] [Google Scholar]
- 85.Schlievert PM, Case LC, Strandberg KL, Abrams BB, Leung DY. Superantigen profile of Staphylococcus aureus isolates from patients with steroidresistant atopic dermatitis. Clin Infect Dis. 2008;46:1562–1567. doi: 10.1086/586746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leung DY, Harbeck R, Bina P, Reiser RF, Yang E, Norris DA, Hanifin JM, Sampson HA. Presence of IgE antibodies to staphylococcal exotoxins on the skin of patients with atopic dermatitis. Evidence for a new group of allergens. J Clin Invest. 1993;92:1374–1380. doi: 10.1172/JCI116711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lomholt H, Andersen KE, Kilian M. Staphylococcus aureus clonal dynamics and virulence factors in children with atopic dermatitis. J Invest Dermatol. 2005;125:977–982. doi: 10.1111/j.0022-202X.2005.23916.x. [DOI] [PubMed] [Google Scholar]
- 88.Wehner J, Neuber K. Staphylococcus aureus enterotoxins induce histamine and leukotriene release in patients with atopic eczema. Br J Dermatol. 2001;145:302–305. doi: 10.1046/j.1365-2133.2001.04352.x. [DOI] [PubMed] [Google Scholar]
- 89.Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, Alenius H, Dieu-Nosjean MC, Meller S, Rieker J, Steinhoff M, Hoffmann TK, Ruzicka T, Zlotnik A, Homey B. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol. 2006;117:411–417. doi: 10.1016/j.jaci.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 90.Miller SJ, Aly R, Shinefeld HR, Elias PM. In vitro and in vivo antistaphylococcal activity of human stratum corneum lipids. Arch Dermatol. 1988;124:209–215. [PubMed] [Google Scholar]
- 91.Bibel DJ, Aly R, Shinefield HR. Antimicrobial activity of sphingosines. J Invest Dermatol. 1992;98:269–273. doi: 10.1111/1523-1747.ep12497842. [DOI] [PubMed] [Google Scholar]
- 92.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 93.Nomura I, Goleva E, Howell MD, Hamid QA, Ong PY, Hall CF, Darst MA, Gao B, Boguniewicz M, Travers JB, Leung DY. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003;171:3262–3269. doi: 10.4049/jimmunol.171.6.3262. [DOI] [PubMed] [Google Scholar]
- 94.Cork MJ, Robinson DA, Vasilopoulos Y, Ferguson A, Moustafa M, MacGowan A, Duff GW, Ward SJ, Tazi-Ahnini R. New perspectives on epidermal barrier dysfunction in atopic dermatitis: gene-environment interactions. J Allergy Clin Immunol. 2006;118:3–21. doi: 10.1016/j.jaci.2006.04.042. quiz 22–23. [DOI] [PubMed] [Google Scholar]
- 95.Langan SM, Irvine AD. Childhood eczema and the importance of the physical environment. J Invest Dermatol. 2013;133:1706–1709. doi: 10.1038/jid.2013.128. [DOI] [PubMed] [Google Scholar]
- 96.Scott IR, Harding CR. Filaggrin breakdown to water binding compounds during development of the rat stratum corneum is controlled by the water activity of the environment. Dev Biol. 1986;115:84–92. doi: 10.1016/0012-1606(86)90230-7. [DOI] [PubMed] [Google Scholar]
- 97.Garg A, Chren MM, Sands LP, Matsui MS, Marenus KD, Feingold KR, Elias PM. Psychological stress perturbs epidermal permeability barrier homeostasis: implications for the pathogenesis of stress-associated skin disorders. Arch Dermatol. 2001;137:53–59. doi: 10.1001/archderm.137.1.53. [DOI] [PubMed] [Google Scholar]
- 98.Choi EH, Brown BE, Crumrine D, Chang S, Man MQ, Elias PM, Feingold KR. Mechanisms by which psychologic stress alters cutaneous permeability barrier homeostasis and stratum corneum integrity. J Invest Dermatol. 2005;124:587–595. doi: 10.1111/j.0022-202X.2005.23589.x. [DOI] [PubMed] [Google Scholar]
- 99.Santiago JL, Lin TK, Man MQ, Hupe M, Martin-Ezquerra G, Elias PM. Paradoxical benefits of psychological stress in mouse models of inflammatory dermatoses, in: IID 2013, vol. 133, Edinburgh. Scotland. 2013:S114. [Google Scholar]
- 100.Bieber T, Cork M, Reitamo S. Atopic dermatitis: a candidate for diseasemodifying strategy. Allergy. 2012;67:969–975. doi: 10.1111/j.1398-9995.2012.02845.x. [DOI] [PubMed] [Google Scholar]
- 101.Loden M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am J Clin Dermatol. 2003;4:771–788. doi: 10.2165/00128071-200304110-00005. [DOI] [PubMed] [Google Scholar]
- 102.Cork MJ, Britton J, Butler L, Young S, Murphy R, Keohane SG. Comparison of parent knowledge, therapy utilization and severity of atopic eczema before and after explanation and demonstration of topical therapies by a specialist dermatology nurse. Br J Dermatol. 2003;149:582–589. doi: 10.1046/j.1365-2133.2003.05595.x. [DOI] [PubMed] [Google Scholar]
- 103.Kircik LH, Del Rosso JQ, Aversa D. Evaluating Clinical Use of a Ceramidedominant, Physiologic Lipid-based Topical Emulsion for Atopic Dermatitis. The Journal of clinical and aesthetic dermatology. 2011;4:34–40. [PMC free article] [PubMed] [Google Scholar]
- 104.Bikowski J. Case studies assessing a new skin barrier repair cream for the treatment of atopic dermatitis. J Drugs Dermatol. 2009;8:1037–1041. [PubMed] [Google Scholar]
- 105.Uchida Y, Holleran WM, Elias PM. On the effects of topical synthetic pseudoceramides: Comparison of possible keratinocyte toxicities provoked by the pseudoceramides, PC104 and BIO391, and natural ceramides. J Dermatol Sci. 2008;51:37–43. doi: 10.1016/j.jdermsci.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee YB, Park HJ, Kwon MJ, Jeong SK, Cho SH. Beneficial effects of pseudoceramide-containing physiologic lipid mixture as a vehicle for topical steroids. Eur J Dermatol. 2011;21:710–716. doi: 10.1684/ejd.2011.1447. [DOI] [PubMed] [Google Scholar]
- 107.Sugarman J, Parish LJ. A topical lipid-based barrier repair formulation (EpiCeram cream) is high-effective monotherapy for moderate-to-severe pediatric atopic dermatitis, IID 2008 Kyoto. Japan. 2008 [Google Scholar]
- 108.Valdman-Grinshpoun Y, Ben-Amitai D, Zvulunov A. Barrier-restoring therapies in atopic dermatitis: current approaches and future perspectives. Dermatology research and practice. 2012;2012:923134. doi: 10.1155/2012/923134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Madaan A. Epiceram for the treatment of atopic dermatitis. Drugs Today (Barc) 2008;44:751–755. doi: 10.1358/dot.2008.44.10.1276838. [DOI] [PubMed] [Google Scholar]
- 110.Houtsmuller UM, van der Beek A. Effects of topical application of fatty acids. Prog Lipid Res. 1981;20:219–224. doi: 10.1016/0163-7827(81)90041-2. [DOI] [PubMed] [Google Scholar]
- 111.McCusker MM, Grant-Kels JM. Healing fats of the skin: the structural and immunologic roles of the omega-6 and omega-3 fatty acids. Clin Dermatol. 2010;28:440–451. doi: 10.1016/j.clindermatol.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 112.Foster RH, Hardy G, Alany RG. Borage oil in the treatment of atopic dermatitis. Nutrition. 2010;26:708–718. doi: 10.1016/j.nut.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 113.De Belilovsky C, Roo-Rodriguez E, Baudouin C, Menu F, Chadoutaud B, Msika P. Natural peroxisome proliferator-activated receptor-alpha agonist cream demonstrates similar therapeutic response to topical steroids in atopic dermatitis. J Dermatolog Treat. 2011;22:359–365. doi: 10.3109/09546634.2010.499932. [DOI] [PubMed] [Google Scholar]
- 114.Furuhjelm C, Warstedt K, Fageras M, Falth-Magnusson K, Larsson J, Fredriksson M, Duchen K. Allergic disease in infants up to 2 years of age in relation to plasma omega-3 fatty acids and maternal fish oil supplementation in pregnancy and lactation. Pediatr Allergy Immunol. 2011;22:505–514. doi: 10.1111/j.1399-3038.2010.01096.x. [DOI] [PubMed] [Google Scholar]
- 115.Kremmyda LS, Vlachava M, Noakes PS, Diaper ND, Miles EA, Calder PC. Atopy risk in infants and children in relation to early exposure to fish, oily fish, or longchain omega-3 fatty acids: a systematic review. Clin Rev Allergy Immunol. 2011;41:36–66. doi: 10.1007/s12016-009-8186-2. [DOI] [PubMed] [Google Scholar]
- 116.Senapati S, Banerjee S, Gangopadhyay DN. Evening primrose oil is effective in atopic dermatitis: a randomized placebo-controlled trial. Indian journal of dermatology, venereology and leprology. 2008;74:447–452. doi: 10.4103/0378-6323.42645. [DOI] [PubMed] [Google Scholar]
- 117.Duan J, Sugawara T, Hirose M, Aida K, Sakai S, Fujii A, Hirata T. Dietary sphingolipids improve skin barrier functions via the upregulation of ceramide synthases in the epidermis. Exp Dermatol. 2012;21:448–452. doi: 10.1111/j.1600-0625.2012.01501.x. [DOI] [PubMed] [Google Scholar]
- 118.Amestejani M, Salehi BS, Vasigh M, Sobhkhiz A, Karami M, Alinia H, Kamrava SK, Shamspour N, Ghalehbaghi B, Behzadi AH. Vitamin D supplementation in the treatment of atopic dermatitis: a clinical trial study. J Drugs Dermatol. 2012;11:327–330. [PubMed] [Google Scholar]
- 119.Park KY, Kim DH, Jeong MS, Li K, Seo SJ. Changes of antimicrobial peptides and transepidermal water loss after topical application of tacrolimus and ceramide-dominant emollient in patients with atopic dermatitis. J Korean Med Sci. 2010;25:766–771. doi: 10.3346/jkms.2010.25.5.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.De Benedetto A, Qualia CM, Baroody FM, Beck LA. Filaggrin expression in oral, nasal, and esophageal mucosa. J Invest Dermatol. 2008;128:1594–1597. doi: 10.1038/sj.jid.5701208. [DOI] [PubMed] [Google Scholar]