Abstract
Ceramide, the backbone structure of all sphingolipids, as well as a minor component of cellular membranes, has a unique role in the skin, by forming the epidermal permeability barrier at the extracellular domains of the outermost layer of skin, the stratum corneum, which is required for terrestrial mammalian survival. In contrast to the role of ceramide in forming the permeability barrier, the signaling roles of ceramide and its metabolites have not yet been recognized. Ceramide and/or its metabolites regulate proliferation, differentiation, and apoptosis in epidermal keratinocytes. Recent studies have further demonstrated that a ceramide metabolite, sphingosine-1-phosphate, modulates innate immune function. Ceramide already has been applied to therapeutic approaches for treatment of eczema associated with attenuated epidermal permeability barrier function. Pharmacological modulation of ceramide and its metabolites signaling can also be applied to cutaneous disease prevention and therapy. The author here describes the signaling roles of ceramide and its metabolites in mammalian cells and tissues, including epidermis.
Keywords: Sphingolipid, Ceramide, Signaling, Keratinocyte, Epidermis
1. Introduction
Ceramide constitutes the backbone structure of all sphingolipids, as well as being a minor component of cellular membranes. In addition, ceramide has a unique role in the skin, forming the epidermal permeability barrier at the extracellular domains of the outermost layer of skin, the stratum corneum, which is required for terrestrial mammalian survival (Fig. 1) [1,2] (also see J.A. Bouwstra, K. Sandhoff, Y. Uchida et al. chapters elsewhere in this volume). In addition to ceramide’s structural roles in cells and tissues, over the past two decades, ceramide and its metabolites have been recognized as signaling lipids that modulate cellular function (Fig. 1) [3,4]. Concurrent with elucidating the signaling roles of ceramide and its metabolites in cells, pharmacological modulation of cellular ceramide levels has been applied as a therapeutic approach for the treatment and prevention of diseases, such as cancers, cardiovascular disease, immune dysfunction [4–6], and eczema associated with attenuated epidermal permeability barrier function [7]. The author describes here the signaling roles of ceramide and its metabolites in mammalian cells and tissues, including epidermis.
Fig. 1.
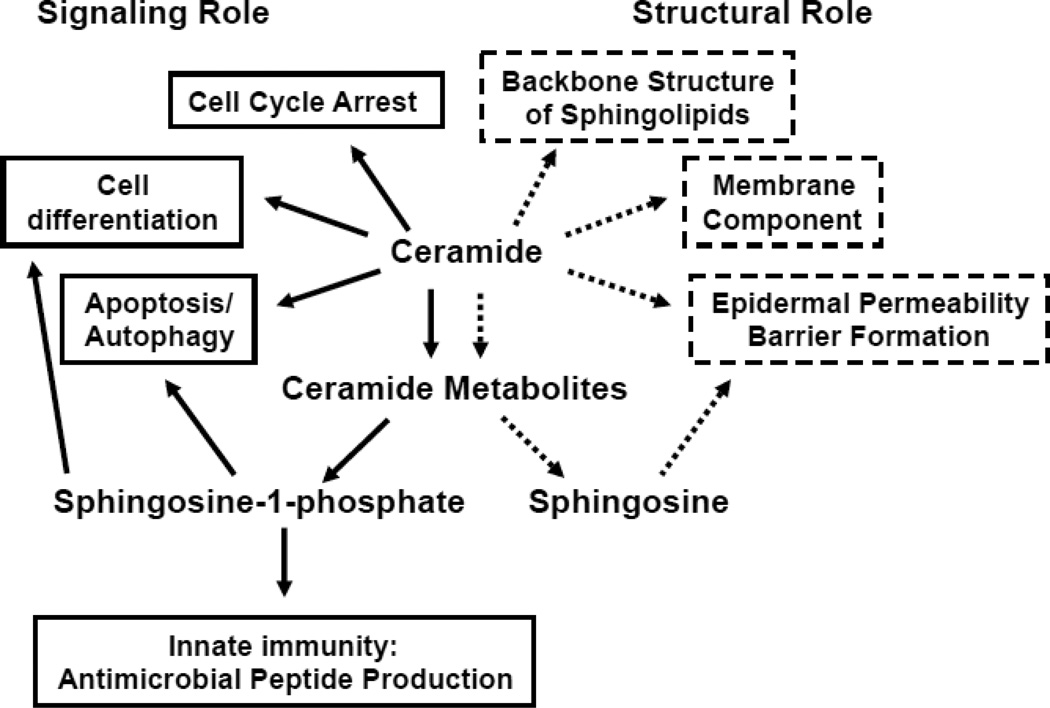
Roles of Ceramide and Its Metabolites in Epidermis
Glycosphingolipid lipids, ceramide metabolites, also are signaling lipid (not included in this figure, see 7 and 9.3, below)
2. Ceramide Structures in Epidermis
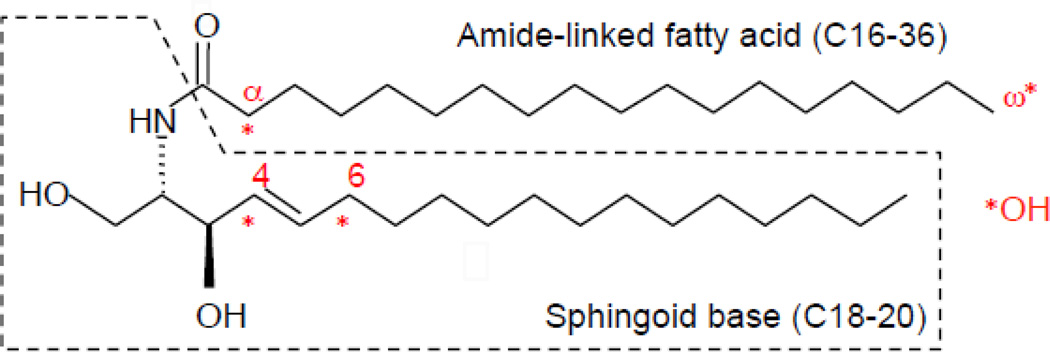
Ceramides consist of long-chain amino alcohol, sphingoid bases, and amide-linked fatty acid (Fig. 2). Sphingosine (carbon chain lengths 18–20) and non-hydroxy fatty acids (carbon chain length 16–24) are major ceramide constituents in mammalian cells. In addition to these ceramide species, dihydrosphingosine (sphinganine) and 1,3,4-trihydroxydihydrosphingosine (phytosphingosine), which contain ceramide species, are present. Ceramide species containing 6-hydroxysphingosine are contained in epidermis. 2-hydroxy fatty acids also make up ceramide (as amide-linked fatty acids), and are relatively abundant in brain, kidney [8], and differentiated layers of epidermis [9]. Moreover, ultra-long chain non-hydroxy, omega-hydroxy and omega-O-acyl fatty acids (carbon chain lengths 36), essential to forming the epidermal permeability barrier, are contained within ceramide species in differentiated layers of the epidermis [1]. In contrast to glycerolipids, which contain saturated, mono, and polyunsaturated O-esterified fatty acids, saturated or monosaturated fatty acids dominate in amide-linked (N-acylated) fatty acids of ceramides. Recent studies have revealed that different structures of ceramide play distinct signaling roles in cells (see below, 3. Ceramides signal to modulate cellular function).
Fig. 2.
Ceramide (N-octadecanoyl sphingosine) Structure
Ceramides in the stratum corneum show structural variation, including hydroxylation (α, ω, 4, and 6 position). Sphingosine (carbon chain lengths 18–20) and non-hydroxy fatty acids (carbon chain length 16–24) are major ceramide constituents in mammalian cells and these major ceramdie species ubiquitously serve as signaling lipid in mammalian cells, including epidermis.
3. Signaling Ceramide Generation
Three sources of ceramide contribute to the initiation of signals; i.e., 1) Increases in sphingomyelin hydrolysis by activation of sphingomyelinases, as described above. Six isoforms of sphingomyelinase have been identified to date in mammals; i.e., acid sphingomyelinase, four types of neutral sphingomyelinase, and alkaline sphingomyelinase [10]. But the sphingomyelinase(s) that produces ceramide signals are localized in the plasma membrane and endoplasmic reticulum (ER). Alkaline sphingomyelinase catalyzes the hydrolysis of sphingomyelin to lysophosphatidylcholine and platelet activating factor (PAF) to suppress inflammatory responses, in addition to sphingomyelin to ceramide conversion (signaling role of alkaline sphingomyelinase is unknown); 2) increased de novo synthesis of ceramide due to activation of serine palmitoyl transferase and/or ceramide synthase [11,12]. Six isoforms of ceramide synthase have been characterized which showed different substrates as well as tissue specificity; e.g., chain length of fatty acyl-CoA (also see R. Sandhoff chapter elsewhere in this issue). Different chain lengths of the amide-linked fatty acid chain of ceramide, generated by specific isoforms of ceramide synthase, demonstrate different biological activities; i.e., acyl carbon chain length 16 ceramide, synthesized by ceramide synthase 6, protects squamous carcinoma cells from ER stress and apoptosis, while carbon chain length 18 ceramide, synthesized by ceramide synthase 1, inhibits cell growth [13]; and 3) increased sphingosine-1-phosphate hydrolysis by sphingosine-1-phosphatase to produce sphingosine followed by ceramide synthesis by ceramide synthase. In addition to these three pathways, hydrolysis of glucosylceramide, galactosylceramide, and ceramide-1-phosphate by β-glucocerebrosidase, β-galactocerebrosidase and ceramide-1-phosphate phosphatase, respectively, conversely increase cellular ceramide levels and decrease ceramidase activity, albeit these pathways have not yet been defined to generate a subsequent signaling mechanism.
4. Ceramide signaling modulates cellular function
The first report of ceramides modulating cellular function was in 1974 [14]. During studies involving erythropoietic activity of lipid soluble extracts of leukocytes, ceramides were identified as stimulating rabbit erythroblast maturation [14]. This study further characterized the most potent ceramide species that enhanced erythroblast maturation; i.e., ceramides containing amide-linked C24:0 or C24:1 fatty acid and sphingosine, dihydrosphingosine (sphinganine) or 1,3,4, trihydroxy dihydrosphinganine (phytosphingosine); shorter amide-linked fatty acid (<C20) ceramide species were less potent [14]. The signaling roles of glycosphingolipids and their downstream signaling mechanisms began to be explored in the 1980s in several mammalian cell types. Ten years after the first report of ceramide signaling, the signaling roles of ceramide in mammalian cells were rediscovered. Increased cellular ceramide from sphingomyelin hydrolysis by activation of sphingomyelinase, in response to either vitamin D or phorbol ester treatment, induced cell cycle arrest and stimulated differentiation of leukemia cells [15–17]. Moreover, increased cellular ceramide occurred following various stimuli such as oxidative stress [12,18], pathogenic bacterial invasion [19,20], and initiation of an inflammatory cytokine cascade [21]. Shortly thereafter, it was also demonstrated that ceramides induce both apoptosis [22] and autophagy [23,24].
Mimicking activation of endogenous sphingomyelinase, both exogenous bacterial sphingomyelinase and cell-permeable, synthetic short chain amide-linked fatty acid (C2–8) ceramide demonstrated ceramide-dependent regulation of functions in many types of cells, including keratinocytes [25,26]. Because natural ceramides consisting of amide-linked longer chain fatty acid (>C12) have a low solubility in aqueous solutions, short chain ceramides and bacterial sphingomyelinase have been used instead to advance research as ceramide signaling. Yet, since short chain ceramide is absent or only at residual levels in cells, concerns were raised that the effects of short chain ceramides do not represent natural cellular phenomena in response to exogenous stimuli. However, incorporated short chain ceramide cells are hydrolyzed to sphingosine and fatty acid producing sphingosine as a substrate for ceramide synthesis with the increase of endogenous, longer chain length fatty acids (natural ceramide) [27,28]. Therefore, utilization of short chain ceramide can be justified in most studies (although monitoring changes in ceramide levels is important). Alternatively, natural ceramides dissolved in dodecane-ethanol solution become cell permeable [29], but optimization of dodecane-ethanol concentration is necessary to minimize non-specific effects, including cellular toxicity.
It is noted that ceramide (or its metabolites) signaling roles and their mechanisms in carcinoma cells or immortalized cells will not always be identical to those in normal cells. Yet, for example, increased metabolic conversions of ceramide to its metabolites account for a drug-resistant mechanism in some cancer cells. These metabolic conversions serve as a rescue mechanism from ceramide-induced cell death in normal keratinocytes (and possibly in other normal cells), suggesting that the insights gained from carcinoma cells could dictate some signaling roles of ceramide and its metabolites in normal cells. In addition, modulating cellular functions in response to signals by ceramide and its metabolites is dependent upon tissues and cells.
5. Ceramide signaling mechanism
Several downstream signals, initiated by elevation in ceramide, have now been characterized, including activation of ceramide-activating serine/threonine phosphatases (CAPS), i.e., protein phosphatase 1A (PP1A) and protein phosphatase 2A (PP2A) [30], protein kinase C (PKC) ζ [31], catepsin D [32], and kinase suppressor RAS (KSR) [33]. CAPS, PP1A and PP2A inactivate PKCα [34], and AKT (or protein kinase B [PFB]) [35]. These downstream signaling activities mediate diverse responses in a variety of cell responses, which are dependent upon cell and tissue types, and changes in the levels of ceramide (for signal intensities, period, see below, 7.2 Metabolic pathways that rescue keratinocytes from ceramide-induced apoptosis). In addition to these specific targets, ceramide permeabilizes mitochondrial outer membranes releasing molecules <60,000-dalton, leading to mitochondrial-mediated apoptosis [36]. This non-protein-mediated signal of ceramide could occur ubiquitously in mammalian cells.
6. Ceramide-mediated cell death pathways
The mechanisms accounting for ceramide-induced apoptosis have been extensively investigated. Ceramide-mediated cell death, including apoptosis and autophagy, is a mechanism of chemotherapy for cancer [37,38].
A) TNF receptor pathways
The activation of the TNF receptor super family, which includes TNFR1 and the TNF-related apoptosis-inducing ligand (TRAIL) receptors, such as TRAILR1 (DR4), TRAILR2 (DR5) and CD95, increases ceramide production by activation of sphingomyelinase. Both acidic and neutral sphingomyelinases account for these pathways [39–41]. Formation of a platform on plasma membranes and modulating membrane protein localization and/or clustering by increased ceramide have been postulated to initiate specific apoptosis signaling pathways [19,42–47]. Acidic sphingomyelinase localized in the lumen of lysosomes is transferred to plasma membranes in response to TRAIL2 and CD95 activation. In addition to these sphingomyelinase activation mechanisms, internalization of TNF-receptors following ligand binding activates lysosomal acidic sphingomyelinase, followed by increasing ceramide to activating cathepsin D [32,48,49]. Activated cathepsin D then translocates into the cytosol, where it stimulates apoptotic signals [32,48,49].
B) Non-TNF receptor mediated mechanism
Oxidative stress occurs within cells in response to a myriad of stimuli, including irradiation (e.g., ultraviolet, infrared, γ-irradiation), inflammation, bacterial infection, and metal ions. These forms of stress stimulate ceramide production by sphingomyelinase activation, as well as by stimulating de novo ceramide production. Increased ceramide then activates specific proteins (as above in #3. Ceramide signaling mechanism), initiating mitochondrial-mediated-caspase-dependent apoptosis, via cytochrome release [50], activations of SMAC (Second Mitochondria-derived Activator of Caspase) [51], DIABRO (Direct IAP-Binding protein with low PI) [52], and AIF (apoptosis inducing factor) [53]. AIF, HtrA serine protease 2, and endonuclease activation account for caspase-independent apoptosis. In addition to activation of specific apoptosis-inducing proteins, as above, ceramide-mediated mitochondrial outer membrane permeabilization contributes to mitochondrial-dependent apoptosis [36].
DNA breakage occurs in response to γ-irradiation for tumor therapy. Insights from acid sphingomyelinase-deficient mouse studies reveal that increased ceramide by activation of acid sphingomyeliase induces apoptosis in both cancer cells and epithelial cells, where acidic sphingomyelinase is highly expressed; e.g., in the gastrointestinal tract, [54–56]. These studies are not only elucidating roles of acid ceramidase in radiation therapy, but they also could point to more potent therapy for cancer with minimal adverse effects of γ-irradiation to normal cells.
7. Signaling roles of ceramide in epidermis
Signaling roles for ceramide in proliferation, differentiation, and apoptosis also have been demonstrated in epidermis and cultured keratinocytes (Table 1). Exogenous short chain ceramides suppressed cell proliferation of the human squamous cell carcinoma cell line, DJM-1 [25]. DNA synthesis is suppressed for a few hours following exogenous bacterial sphingomyelinase, but then is restored in parallel with increased ceramide to glucosylceramide conversion by glucosylceramide synthase activation in spontaneously immortalized, nontransformed HaCat human keratinocytes [57]. Ceramide also regulates the interferon-gamma-induced intercellular adhesion molecule (ICAM)-1 and human leukocyte antigen (HLA)-DR expression in normal human keratinocytes [26]. Exogenous short chain ceramides activate apoptosis signal-regulating kinase (ASK1), and then p38 MAP kinase, resulting in enhanced differentiation in normal human keratinocytes [58]. Exogenous short chain ceramides also stimulate the production of caspase-14 (a key enzyme involved in epidermal terminal differentiation) [59]. Moreover, ceramides stimulate the transmembrane lipid transporter, ATP binding cassette transporter, family 12 (ABCA12) expression [60]. Ceramide-induced ABCA12 expression is attenuated by silencing proliferator-activated receptor (PPAR) δ expression, suggesting that ceramide activates PPAR δ, leading to stimulation of ABCA12 production [60]. Since PPAR δ stimulates keratinocyte differentiation [61], ceramide-induced keratinocyte differentiation could be via this PPAR δ mechanism. ABCA12 is critical in delivering glucosylceramide into lamellar bodies, a prerequisite for epidermal permeability barrier formation [62]. ABCA12 mutations underlie the pathogenesis of the most severe ichthyosis, Harlequin ichthyosis, as well as a subgroup of autosomal recessive ichthyoses, with lamellar ichthyosis phenotypes [62] (also see M. Akiyama). In keratinocytes, ABCA12 transports glucosylceramides, which are immediate precursors of all ceramide species in the stratum corneum, into epidermal lamellar bodies [62]. Recent studies demonstrate that neonatal demise occurs in glucosylceramide-deficient mice associated with abnormal epidermal lamellar body formation [63,64]. Therefore, both glucosylceramide and ABCA12 are required for epidermal permeability barrier formation [63].
Table 1.
Ceramide and its metabolites signal to modulate keratinocyte function
| Treatment | Cell | Effect | Mechanism |
|---|---|---|---|
| Ceramide | |||
| Exogenous | |||
| short chain ceramide sphingosine |
DJM | ↓proliferation, ↑differentiation ↑(modest) proliferation, ↔differentiation |
not determined [25] |
| sphingomyelinase | HaCaT | ↓proliferation | not determined [57] |
| inhibitors of ceramide synthesis (fumonisin B1, cycloserine) |
NHK | ↓Interferon gamma-induced ICAM1 and HLA-DR expression |
not determined [26] |
| short chain ceramide | NHK | ↑differentiation | ASK1 and p38MAP kinase activation [58] |
| short chain ceramide | NHK | ↑caspase-14 expression | not determined [59] |
| short chain ceramide and/or inhibition of ceramide conversion to its metabolites |
NHK | ↑ABCA12 | PPARδ activation [60] |
| 1α,25-dihydroxyvitamin D3 | HaCaT | ↑differentiation (1 α,25-dihydroxy- vitamin D3→↑TNFa→↑ceramide) |
not determined [65] |
| ultravioret B irradiation | NHK | irradiation→↑ceramide→↑apoptosis | caspase independent? [12] |
| ultravioret B irradiation | irradiation→↑ceramide→↑apoptosis | not determined [66] | |
| ultravioret A irradiation | NHK | irradiation→↑ceramide →↑ICAM1 (enzyme independent sphingimyelin→↑ceramide and later ↑Cer→↑serine palmitoyltransferase) |
AP2 activation [67,68] |
| Glucosylceramide | |||
| Exogenous | |||
| glucosyl ceramides, including epidermal unique glucosyl |
FRSK | ↑differentiation | ↑intracellular Ca2+ |
| omega-O-acylceramide | and PKC activation [73] | ||
| (chemicallysynthesized) glucosyl omega-O-acylceramide |
NHK | ↑differentiation | not determined [74] |
| GM3 ganglioside | A431 and KB | ↓proliferation | ↓tyrosine phosphorylation of EGF [149] |
| GM3 ganglioside | NHK | ↓proliferation | ↓tyrosine phosphorylation of EGF [150] |
| GT1b ganglioside | NHK | ↑differentiation | protein kinase C independent [151] |
| NHK | ↓cell adhesion | not determined [152] | |
| Sphingosine-1-phosphate | |||
| Exogenous | |||
| sphingosine-1-phosphate | NHK | ↑chemotaxis and induce differentiation | sphingosine-1-phosphate receptor 2/3→↑Ca2+ [153] |
| sphingosine-1-phosphate | NHK | ↑differentiation | ↑sphingosine-1-phosphate receptor dependent / independent/[121] |
| sphingosine-1-phosphate | NHK | protect against TNFα-induced apoptosis | ↑ sphingosine-1-phosphate receptor 3→↑eNOS [154] |
| sphingosine-1-phosphate | NHK | ↑laminin 5 synthesis→?↑wound healing | not determined [117] |
| sphingosine-1-phosphate | NHK | ↑migration, ↓cell proliferation | Smad3 activation [113] |
| sphingosine-1-phosphate | NHK | protect against 1α, 25-dihydroxyvitamin D3-induced apoptosis |
not determined [120] |
| sphingosine-1-phosphate | NHK | restrains insulin-induced cell proliferation | ↑sphingosine-1-phosphate receptor 2→↑PKCδ ↓AKT [128] |
| ER stress→↑ceramide→ | NHK | ER stress→↑→↑ceramide→ sphingosine-1-phosphate →↑cathelicidin anti microbial peptide |
↑NF-κB→↑c/EBPα [28] |
DJM, human squamous cell carcinoma cell; HaCaT, immortalized, nontransformed human keratinocyte; FRSK, fetal rat skin keratinocyte cell; NHK, primary normal human keratinocyte; epidermal cartinoma cell, A431 (human) and KB (mouse) cells
Ceramide signals are responsible in part for TNFα and 1α,25-dihydroxyvitamin D3-mediated keratinocyte differentiation; i.e., 1α,25-dihydroxyvitamin D3 increases TNFα production and TNFα increases ceramide production from sphingomyelin by activating sphingomyelinase [65]. Increased cellular ceramide induces apoptosis in keratinocytes in response to ultraviolet (UV) B irradiation [12,66]. UVA increases ceramide production, without activating ceramide metabolic enzymes, but leads to increased ICAM-1 expression by activation of transcription factor AP2 [67], while increased ceramide following irradiation in turn activates serine palmitoyl transferase (by autocrine pathway), which is a first step and a rate-limiting enzyme of ceramide synthesis (Fig. 3) [68]. Because UV irradiation alters cellular metabolism, including increased hydrolysis of esterified lipids to free fatty acids, an elevated pool of the precursor to ceramide synthesis, free fatty acid, can increase ceramide production without activating enzymes.
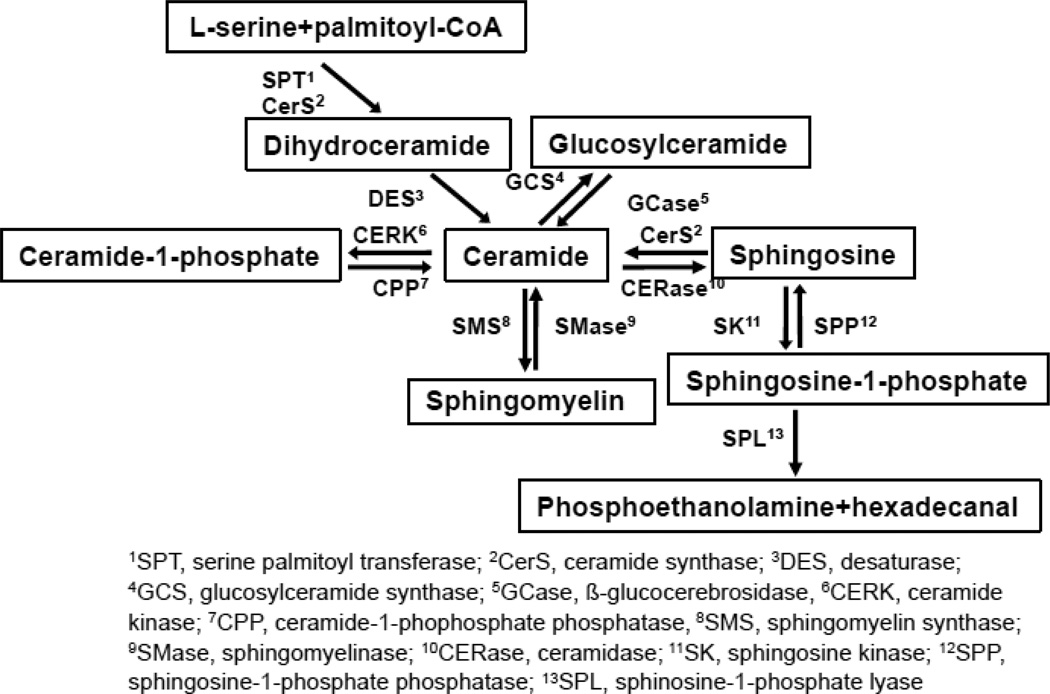
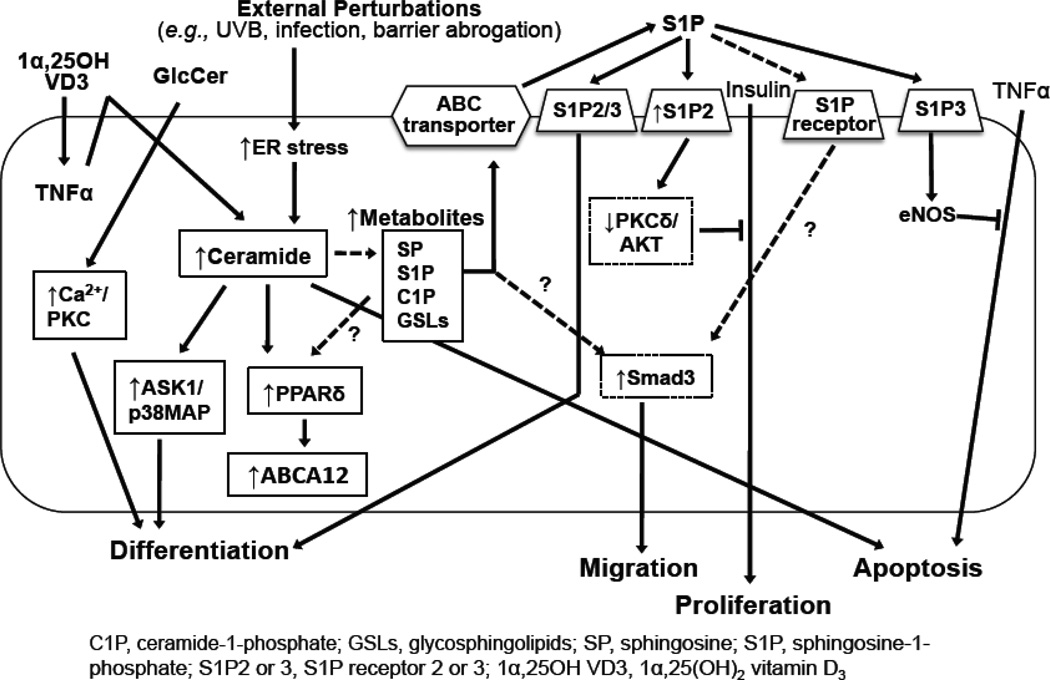
Fig. 3.
Ceramide Metabolic Pathway in Epidermis
However, increased sphingomyelin to ceramide conversion occurs in protein-free liposomes following either UVA irradiation or singlet oxygen exposure [67]. Yet, no other studies are available that show non-enzymatic conversion of sphingomyelin to ceramide by oxygen radicals. Finally, ceramides also alter cellular function in other epidermal cells, such as melanocytes. Exogenous, short chain ceramides inhibit melanocyte proliferation via Akt inactivation and induction of melanin production in the spontaneously immortalized mouse melanocyte cell line, Mel-Ab [69]. As noted above, ceramide molecular heterogeneity is unique to epidermis. These heterogeneous ceramides are distributed in the extracellular spaces of the stratum corneum, where they incorporate into stable lamellar membrane structures [2,70]. Therefore, it is unlikely that ceramide in the stratum corneum-derived signals alter cellular function in the nucleated layer of epidermis. Most of the immediate precursors of these barrier ceramides, glucosylceramides, which are not used for cellular membrane constitution, are sequestrated into lamellar bodies [71,72]. These sequestrated lipids are unable to generate signals because of being located in cellular compartments to modulate cellular function; e.g., plasma membranes, mitochondria. Yet, prior studies demonstrated that exogenous glucosyl omega-O-acyl-ultralong chain ceramide induces keratinocyte differentiation [73,74]. If a non-sequestrated pool of epidermal unique glucosylceramide and/or its metabolites is available and/or lamellar bodies diffuse to apical surface of plasma membrane during transition of stratum granulosum to stratum corneum, these lipid species could generate regulatory signals.
Note: Alterations of proliferation, differentiation, cell death, cell-cell attachment, and other cellular functions in epidermis of transgenic mice affect epidermal permeability barrier function. Diminished barrier function also affects cellular proliferation and differentiation in epidermis and results in secondarily changing other epidermal functions. Therefore, it is difficult to distinguish between primary and secondary effects of a targeted enzyme. Hence, utilization of transgenic mice to elucidate a signaling role of ceramide in epidermis should be limited. Pharmacological inhibition or gene silencing of a specific ceramide metabolic enzyme in cultured epidermal keratinocytes should be more precise in representing roles of ceramide and its metabolites in epidermis than use of transgenic animals.
8. Protective mechanisms used against ceramide-induced apoptosis
Ceramide-induced apoptosis plays a beneficial role in cancer treatment, as well as in the elimination of abnormal cells that carry potentially detrimental mutated genes. Yet, increased apoptosis in normal cells allows expansion of cancer cell population. Epidermis is situated uniquely at the environmental interface, so this external organ is continuously at risk from exposure to oxidative stressors such as UV irradiation and xenotoxic compounds. Moreover, during differentiation, keratinocytes produce abundant amounts of ceramides to form the epidermal permeability barrier [71,75,76]. Therefore, normal cells, in particular keratinocytes, need to deploy protective mechanisms against ceramide-induced apoptosis.
8.1 Metabolic conversion of ceramide to non-apoptotic metabolites
The metabolic conversion of ceramides to non-apoptotic metabolites (Figs. 3 and 4) increases in some cancer cells, as well as in drug-resistant cancer cells. For example acidic ceramidase expression increases in head and neck squamous carcinoma cells [77], and in some melanoma [78], prostate [78], and colon cancer [78] cell lines. Sphingosine kinase 1 is overexpressed in head and neck squamous carcinoma cells [79] and prostate cancer [80], suggesting ceramide conversion beyond sphingosine to sphingosine-1-phosphate. Ceramide kinase, which generates ceramide-1-phosphate, is overexpressed in breast cancer cells [81]. Glucosylceramide synthase expression increases in some cell lines (including multidrug resistance) of breast cancer [82], prostate cancer [82], colon cancer [83,84], and leukemia [84,85]. Thus, inhibition of one or more of these ceramide metabolic enzymes could represent a strategy to enhance anti-cancer treatments. Conversely, the metabolic conversions of ceramides to its non-apoptotic metabolites also serve to protect normal cells from ceramide-induced apoptosis.
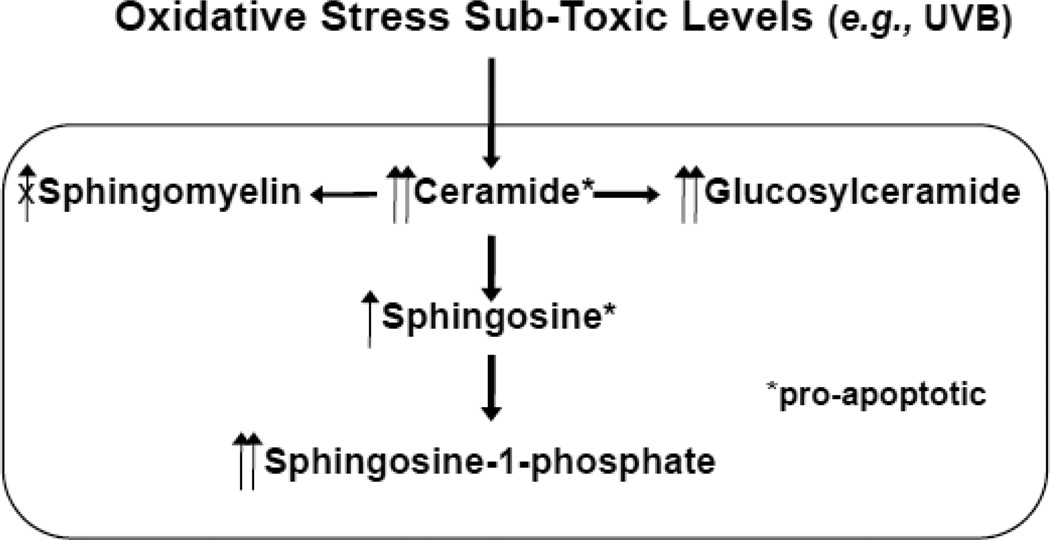
Fig. 4.
Protective Mechanism Against Ceramide-Induced Apoptosis in Response to Oxidative Stress in Epidermis
Toxic levels of stress overwhelms these protective mechanism and results in increased apoptosis [93]. Since CERT (ceramide-transfer protein) forms stable homotrimer that diminishes ceramide transfer function under oxidative stress, sphingomyelin synthesis does not increase [104].
8.1.1 Conversion of ceramide to sphingosine to sphingosine-1-phosphate
Ceramide is hydrolyzed to sphingoid base and fatty acid by ceramidase (Fig. 3). Five ceramidase isoforms, which show different pH optima and different subcellular distributions, have been characterized in mammals; i.e., 1) acid ceramidase (lysosomal distribution); 2) neutral ceramidase (plasma membrane and mitochondrial membrane distribution) [86,87]; 3) alkaline ceramidase 1 (a differentiated keratinocyte specific isoform expressed in Golgi apparatus and endoplasmic reticulum [ER]) [88]; 4) alkaline ceramidase 2 (Golgi apparatus and ER) [89]; and 5) alkaline ceramidase 3 (or phytoalkaline ceramidase), which hydrolyzes ceramide species containing dihydrosphingosine (sphinganine), 1,3,4-trihydroxydihydrosphingosine (phytosphingosine), and amide-linked unsaturated fatty acids (Golgi apparatus and ER) [90]. Epidermal keratinocytes express all five isoforms of ceramidase, with different expression profiles of each isomer across the different cell layers of epidermis [88]. The sphingosine kinase converts sphingosine to sphingosine-1-phosphate (Fig. 3). Two isoforms of sphingosine kinase, sphingosine kinase 1 [91] and sphingosine kinase 2 have been identified in mammals [92]. The sphingosine kinase isoform, sphingosine kinase 1, is localized in cytosol [91], while sphingosine kinase 2 localizes in ER and nucleus [92]. Both isoforms are expressed in keratinocytes [93]. Sphingosine-1-phosphate can be further metabolized to phosphoethanolamine and hexadecanal by spingosine-1-phosphate lyase [94,95].
8.1.2 Conversion of ceramide to ceramide-1-phosphate
Ceramide kinase phosphorylates ceramide, generating synthesize ceramide-1-phosphate in the trans Golgi network (Fig. 3). Two isoforms of ceramide kinase, ceramide kinase 1 and 2, have been characterized in mammalian tissues [96], including epidermis . Ceramide delivered from ER by ceramide transfer protein (CERT) is utilized for the generation of ceramide-1-phosphate generation [97].
8.1.3 Conversion of ceramide to glucosylceramide
Ceramide is transferred from ER to cis Golgi by vesicle transport and then glucosylated to glucosylceramide at cis Golgi by glucosylceramide synthase (Fig. 3) [98,99]. Glucosylceramide is a dominant glycosphingolipid species (>95%) in epidermis [100]. Other glycosphingolipids’ (such as gangliosides) syntheses also occur in Golgi. Gangliosides also regulate epidermal functions (see Section 9.4).
8.1.4 Conversion of ceramide to sphingomyelin
Differing from ceramide transfer for glucosylceramide synthesis from ER to Golgi, ceramide transferred from ER to trans Golgi by ceramide transfer protein (CERT) [101] precedes ceramide to sphingomyelin conversion, which is synthesized by sphingomyelin synthase 1 (Fig. 3) [102,103]. Sphingomyelin synthesis also occurs at the plasma membrane by sphingomyelin synthase 2 [102,103].
8.2 Metabolic pathways that rescue keratinocytes from ceramide-induced apoptosis
Ceramide-induced apoptosis occurs in keratinocytes following high doses of ultraviolet B (UVB) irradiation [12], while low doses of UVB irradiation only inhibit cell proliferation [93]. Interestingly, both low and high doses of UVB irradiation increase ceramide production to the comparable levels at early time points, but ceramide levels return toward normal ranges following low (but not high) doses of UVB irradiation by metabolic conversion of ceramide to sphingosine, followed by further conversion to sphingosine-1-phosphate in human keratinocytes [93]. This ceramide metabolic pathway does not operate efficiently in cells after high levels of ultraviolet insults [93]. Because the ceramide transfer function, CERT, declines after formation of a stable production of homotrimer following oxidative stress [104], ceramide to sphingomyelin (and likely ceramide to ceramide-1-phosphate) conversion does not upregulate in response to oxidative stress [104].
9. Ceramide metabolites and their downstream signals
9.1 Sphingoid base
Sphingosine inhibits protein kinase C activated by diacylglycerol, Ca2+, and phorbol ester [105,106], while sphingosine activates sphingosine dependent kinase, which is produced by proteolysis of protein kinase δ [107,108]. Sphingosine-dependent kinase accounts for apoptosis in astrocytes [107]. Sphingosine induces apoptosis through caspase-dependent pathways [109–111]. In addition, since sphingosine has detergent properties, increased sphingosine could alter membrane fluidity (membrane curvature) and alter cellular functions.
9.2 Sphingosine-1-phosphate
Sphingosine-1-phosphate is generated by sphingosine kinase 1 and has been shown to activate anti-apoptotic activity to protect cells from ceramide-induced apoptosis, to stimulate cell proliferation, to increase cell motility [112,113], and to stimulate wound healing [114–118], while sphingosine kinase 2 localized in nucleus generates sphingosine-1-phosphate, which induces cell cycle arrest [119]. Sphingosine-1-phosphate produced in endoplasmic reticulum by sphingosine kinase 2 is dephosphorylated by sphingosine-1-phosphate phosphatase and is converted to ceramide by ceramidase synthase, resulting in apoptosis [120]. In keratinocytes, sphingosine-1-phosphate induces differentiation, but does not stimulate proliferation [121]. Lipid transporters, ATP binding cassette, i.e., ABCA1, ABCC1 [122], and Spinster 2 [123,124] are involved in sphingosine-1-phosphate efflux from cells. Sphingosine-1-phosphate regulates cellular functions through the activation of plasma membrane localized G-protein coupled sphingosine-1-phosphate receptor. Five isoforms of sphingosine-1-phosphate receptors have been characterized in mammals, and all five receptors are expressed in keratinocytes. Activation of sphingosine-1-phosphate receptors modulates cellular function through a number of downstream signaling pathways, including activations of phospholipase C followed by increased intracellular Ca2+ [125,126], and PI3 kinase followed by Akt/Rac activation [127], PKCδ activation accompanied with Akt inactivation [128], and Smad 3 activation [113]. In addition to sphingosine-1-phosphate receptor-dependent signal, sphingosine-1 -phosphate modulates cellular functions through sphingosine-1-phosphate receptor independent pathways; i.e., sphingosine-1-phosphate can directly modulate histone acetylation [129] and is a cofactor in the classical RelA pathway leading to polyubiquitination of receptor interacting protein 1 (RIP1) and NF- κB activation [130].
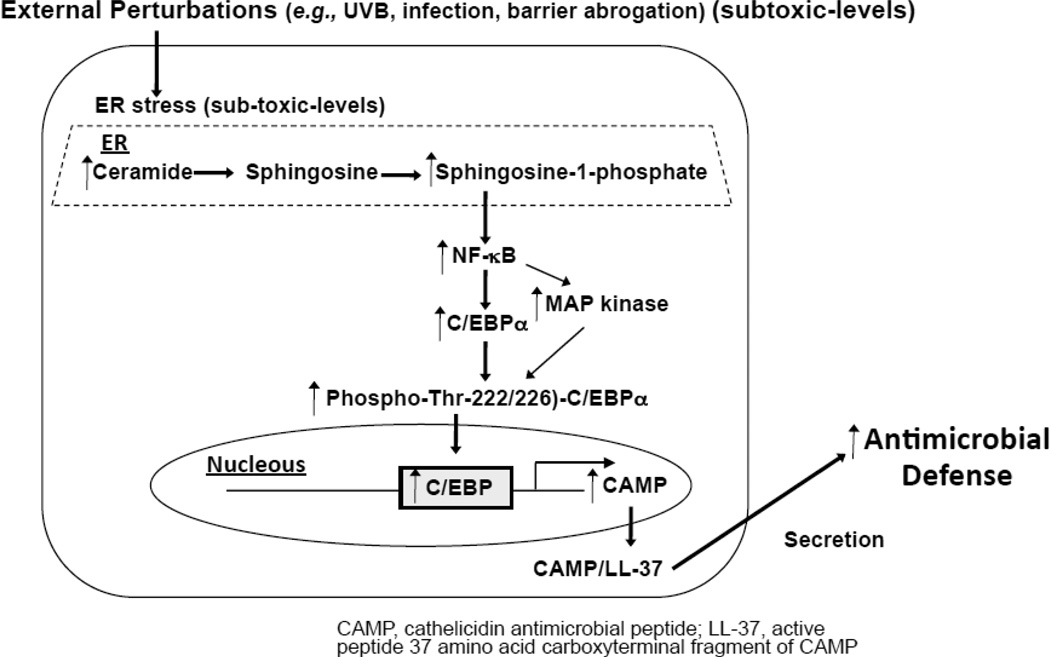
Moreover, recent studies demonstrated that sphingosine-1-phosphate produced in epithelial cells, including keratinocytes, in response to subtoxic levels of ER stress (which can be initiated by subtoxic levels of external stress, such as UVB irradiation, or epidermal permeability barrier perturbation) stimulate a major innate immune element, cathelicidin antimicrobial peptide via increased ceramide production followed by increasing sphingosine-1-phosphate that activates NF-κB and then c/EBPα (Fig. 5) [28]. In addition, the mechanism of sphingosine-1-phosphate-induced NF-κB activation has been shown through a receptor-independent pathway [28].
Fig. 5.
Ceramide metabolite, sphingosine-1-phosphate signals to stimulate antimicrobial defense.
As above, acid ceramidase expression increases in prostate cancer, suggesting the involvement of tumor growth [131]. It is likely that pro-mitogenic sphingosine-1-phosphate rather than ceramide or sphingosine is responsible for tumor growth. Keratinocytes, acid ceramidase and alkaline ceramidase 1 enhance Ca2+-induced cell cycle arrest and differentiation [116]. Overexpression of alkaline ceramidase 2 increases β1 integrine maturation and cell adhesion [132]. Alkaline ceramidase 2 and 3 have been shown to coordinately regulate keratinocyte proliferation and apoptosis [133]; i.e., silencing alkaline ceramidase 3 increases alkaline ceramidase 2 expression and results in increased ceramide, which contains unsaturated fatty acids, that inhibit cell proliferation through upregulation of increased cycline-dependent kinase inhibitor p21C1P/WAF1 expression and suppressed apoptosis [133].
9.3 Ceramide-1-phosphate
Ceramide-1-phosphate stimulates cell proliferation [134] and is implicated in neutrophil phagocytosis [135]. Ceramide-1-phosphate receptor has not been identified. Instead, ceramide-1-phosphate directly interacts with and activates cytosolic phospholipases, and releases arachidonate to increase prostanoid production. Ceramide-1-phosphate-mediated increases in prostaglandin E2 production to stimulate inflammatory responses in cells [136–138]. However, ceramide-1-phosphate inhibits TNFα converting enzyme to suppress TNFα-induced-NF-κB activation [139] and also Toll-like receptor 4 (TLR4)-induced NF-κB activation [140], suggesting ceramide-1-phosphate has an anti-inflammatory effect. Ceramide-1-phosphate stimulates cell proliferation [141,142] and cell migration [143,144], inhibits apoptosis [141,145,146] and increases glucose uptake [147].
9.4 Glycosphingolipids
Prior studies demonstrated the signaling roles of glycosphingolipid in epidermal cellular function, c.f. review articles [148]. In epidermis, glucosylceramide is a dominant glycosphingolipid (>98 %) species, while di- or polyglycosylated sphingolipid species, such as GM3 gangliosides and other gangliosides, are also present as minor components [100]. An earlier study demonstrated that GM3 ganglioside modulates tyrosine phosphorylation of the epidermal growth factor receptor and suppresses cell growth of cell lines of epidermal carcinoma cell, A431 (human) and KB (mouse) cells [149]. In normal human keratinocytes, GM3, GD3, 9-O-acetyl GD3, and GD1b ganglioside also inhibit proliferation, but do not induce differentiation [150], while GT1b ganglioside likely increases keratinocyte differentiation [151] and inhibits cell adhesion [152]. In addition, as above, epidermal unique, glucosyl omega-O-acyl-ultralong chain ceramide induce keratinocyte differentiation [73,74].
10. Conclusion
The role of ceramide in epidermal permeability barrier structure in the stratum corneum is widely-recognized. Ceramide also serves as a signaling lipid to modulate epidermal function. As discussed above, ceramide, including epidermal unique omega-O-acyl-ultralong chain ceramide, which constitute the epidermal barrier in the stratum corneum, becomes a signaling agent. Most of the immediate precursors of these barrier ceramides (glucosylceramides), which are not used for cellular membrane constitution, are sequestrated into lamellar bodies, and are unable to generate signals because of not being located in the cellular compartment (unlike plasma membranes and mitochondria). Yet, it is possible that a non-sequestrated epidermal unique glucosylceramide and/or diffusing lamellar bodies into plasma membrane, these lipid species serve as a signaling lipid. Finally, sphingosine-1-phosphate, a distal metabolite of ceramide, stimulates innate immunity. Modulation of ceramide and its metabolite signals could regulate self defense systems and also modulate inflammatory responses in skin.
Fig. 6.
Signalings of ceramide and its metabolites to alter cellular functions in normal keratinocytes
Highlights.
Ceramide has a unique role in the skin to form the epidermal permeability barrier.
This barrier role of ceramide is not the focus of this review.
Ceramide and its metabolites have a signaling role in regulating cellular function.
Cells deploy protective mechanism against ceramide-induced cell death.
Acknowledgments
The author gratefully acknowledges Drs. Peter M. Elias#, Kenneth R. Feingold#* and Walter M. Holleran# (#Department of Dermatology, *Depart of Medicine, University of California, San Francisco and Department of Veterans Affairs Medical Center San Francisco, CA) for numerous critical discussions. The author acknowledges the superb editorial assistance of Ms. Joan Wakefield. Finally, the author sincerely acknowledges the late Dr. Norman S. Radin (1921–2013) (Emeritus Professor, Molecular and Behavioral Neuroscience Institute, University of Michigan, Ann Arbor, MI), his pioneering work in the exploration of sphingolipid biology and for critical comments and discussion of the author’s projects. This study was supported by National Institute of Health grants AR051077 and AR062025 (the National Institute of Arthritis and Musculoskeletal and Skin Diseases) and a National Rosacea Society Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Uchida Y, Holleran WM. Omega-O-acylceramide, a lipid essential for mammalian survival. J Dermatol Sci. 2008;51:77–87. doi: 10.1016/j.jdermsci.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Elias PM, Menon GK. Structural and lipid biochemical correlates of the epidermal permeability barrier. Advances in Lipid Research. 1991;24:1–26. doi: 10.1016/b978-0-12-024924-4.50005-5. [DOI] [PubMed] [Google Scholar]
- 3.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 4.Morad SA, Cabot MC. Ceramide-orchestrated signalling in cancer cells. Nat Rev Cancer. 2013;13:51–65. doi: 10.1038/nrc3398. [DOI] [PubMed] [Google Scholar]
- 5.Mullen TD, Spassieva S, Jenkins RW, Kitatani K, Bielawski J, Hannun YA, Obeid LM. Selective knockdown of ceramide synthases reveals complex interregulation of sphingolipid metabolism. J Lipid Res. 2011;52:68–77. doi: 10.1194/jlr.M009142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hla T, Dannenberg AJ. Sphingolipid signaling in metabolic disorders. Cell Metab. 2012;16:420–434. doi: 10.1016/j.cmet.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uchida Y, Hamanaka S. Stratum corneum ceramides: function, origins, and therapeutic applications., in Skin Barrier. Taylor & Francis; New Yorkp: 2006. pp. 43–65. [Google Scholar]
- 8.Iwamori M, Costello C, Moser HW. Analysis and quantitation of free ceramide containing nonhydroxy and 2-hydroxy fatty acids, and phytosphingosine by high-performance liquid chromatography. J Lipid Res. 1979;20:86–96. [PubMed] [Google Scholar]
- 9.Uchida Y, Hama H, Alderson NL, Douangpanya S, Wang Y, Crumrine DA, Elias PM, Holleran WM. Fatty acid 2-hydroxylase, encoded by FA2H, accounts for differentiation-associated increase in 2-OH ceramides during keratinocyte differentiation. J Biol Chem. 2007;282:13211–13219. doi: 10.1074/jbc.M611562200. [DOI] [PubMed] [Google Scholar]
- 10.Wu J, Cheng Y, Jonsson BA, Nilsson A, Duan RD. Acid sphingomyelinase is induced by butyrate but does not initiate the anticancer effect of butyrate in HT29 and HepG2 cells. J Lipid Res. 2005;46:1944–1952. doi: 10.1194/jlr.M500118-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Perry DK, Carton J, Shah AK, Meredith F, Uhlinger DJ, Hannun YA. Serine palmitoyltransferase regulates de novo ceramide generation during etoposide-induced apoptosis. J Biol Chem. 2000;275:9078–9084. doi: 10.1074/jbc.275.12.9078. [DOI] [PubMed] [Google Scholar]
- 12.Uchida Y, Nardo AD, Collins V, Elias PM, Holleran WM. De novo ceramide synthesis participates in the ultraviolet B irradiation-induced apoptosis in undifferentiated cultured human keratinocytes. J Invest Dermatol. 2003;120:662–669. doi: 10.1046/j.1523-1747.2003.12098.x. [DOI] [PubMed] [Google Scholar]
- 13.Senkal CE, Ponnusamy S, Bielawski J, Hannun YA, Ogretmen B. Antiapoptotic roles of ceramide-synthase-6-generated C16-ceramide via selective regulation of the ATF6/CHOP arm of ER-stress-response pathways. Faseb J. 2009 doi: 10.1096/fj.09-135087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clayton RB, Cooper JM, Curstedt T, Sjovall J, Borsook H, Chin J, Schwarz A. Stimulation of erythroblast maturation in vitro by sphingolipids. J Lipid Res. 1974;15:557–562. [PubMed] [Google Scholar]
- 15.Okazaki T, Bielawska A, Bell RM, Hannun YA. Role of ceramide as a lipid mediator of 1 alpha,25-dihydroxyvitamin D3-induced HL-60 cell differentiation. J Biol Chem. 1990;265:15823–15831. [PubMed] [Google Scholar]
- 16.Okazaki T, Bell RM, Hannun YA. Sphingomyelin turnover induced by vitamin D3 in HL-60 cells. Role in cell differentiation. J Biol Chem. 1989;264:19076–19080. [PubMed] [Google Scholar]
- 17.Kolesnick RN. Sphingomyelinase action inhibits phorbol ester-induced differentiation of human promyelocytic leukemic (HL-60) cells. J Biol Chem. 1989;264:7617–7623. [PubMed] [Google Scholar]
- 18.Huang C, Ma W, Ding M, Bowden GT, Dong Z. Direct evidence for an important role of sphingomyelinase in ultraviolet-induced activation of c-Jun N-terminal kinase. J Biol Chem. 1997;272:27753–27757. doi: 10.1074/jbc.272.44.27753. [DOI] [PubMed] [Google Scholar]
- 19.Grassme H, Jendrossek V, Riehle A, von Kurthy G, Berger J, Schwarz H, Weller M, Kolesnick R, Gulbins E. Host defense against Pseudomonas aeruginosa requires ceramide-rich membrane rafts. Nat Med. 2003;9:322–330. doi: 10.1038/nm823. [DOI] [PubMed] [Google Scholar]
- 20.Delogu G, Famularo G, Amati F, Signore L, Antonucci A, Trinchieri V, Di Marzio L, Cifone MG. Ceramide concentrations in septic patients: a possible marker of multiple organ dysfunction syndrome. Crit Care Med. 1999;27:2413–2417. doi: 10.1097/00003246-199911000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Mathias S, Younes A, Kan CC, Orlow I, Joseph C, Kolesnick RN. Activation of the sphingomyelin signaling pathway in intact EL4 cells and in a cell-free system by IL-1 beta. Science. 1993;259:519–522. doi: 10.1126/science.8424175. [DOI] [PubMed] [Google Scholar]
- 22.Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science. 1993;259:1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- 23.Daido S, Kanzawa T, Yamamoto A, Takeuchi H, Kondo Y, Kondo S. Pivotal role of the cell death factor BNIP3 in ceramide-induced autophagic cell death in malignant glioma cells. Cancer Res. 2004;64:4286–4293. doi: 10.1158/0008-5472.CAN-03-3084. [DOI] [PubMed] [Google Scholar]
- 24.Scarlatti F, Bauvy C, Ventruti A, Sala G, Cluzeaud F, Vandewalle A, Ghidoni R, Codogno P. Ceramide-mediated macroautophagy involves inhibition of protein kinase B and up-regulation of beclin 1. J Biol Chem. 2004;279:18384–18391. doi: 10.1074/jbc.M313561200. [DOI] [PubMed] [Google Scholar]
- 25.Wakita H, Tokura Y, Yagi H, Nishimura K, Furukawa F, Takigawa M. Keratinocyte differentiation is induced by cell-permeant ceramides and its proliferation is promoted by sphingosine. Archives for Dermatological Research. Archiv fur Dermatologische Forschung. 1994;286:350–354. doi: 10.1007/BF00402228. [DOI] [PubMed] [Google Scholar]
- 26.Wakita H, Nishimura K, Tokura Y, Furukawa F, Takigawa M. Inhibitors of sphingolipid synthesis modulate interferon (IFN)-gamma-induced intercellular adhesion molecule (ICAM)-1 and human leukocyte antigen (HLA)-DR expression on cultured normal human keratinocytes: possible involvement of ceramide in biologic action of IFN-gamma. J Invest Dermatol. 1996;107:336–342. doi: 10.1111/1523-1747.ep12363279. [DOI] [PubMed] [Google Scholar]
- 27.Sultan I, Senkal CE, Ponnusamy S, Bielawski J, Szulc Z, Bielawska A, Hannun YA, Ogretmen B. Regulation of the sphingosine-recycling pathway for ceramide generation by oxidative stress, and its role in controlling c-Myc/Max function. Biochem J. 2006;393:513–521. doi: 10.1042/BJ20051083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park K, Elias PM, Shin KO, Lee YM, Hupe M, Borkowski AW, Gallo RL, Saba J, Holleran WM, Uchida Y. A novel role of a lipid species, sphingosine-1-phosphate, in epithelial innate immunity. Mol Cell Biol. 2013;33:752–762. doi: 10.1128/MCB.01103-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji L, Zhang G, Uematsu S, Akahori Y, Hirabayashi Y. Induction of apoptotic DNA fragmentation and cell death by natural ceramide. Febs Letters. 1995;358:211–214. doi: 10.1016/0014-5793(94)01428-4. [DOI] [PubMed] [Google Scholar]
- 30.Chalfant CE, Szulc Z, Roddy P, Bielawska A, Hannun YA. The structural requirements for ceramide activation of serine-threonine protein phosphatases. J Lipid Res. 2004;45:496–506. doi: 10.1194/jlr.M300347-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Lozano J, Berra E, Municio MM, Diaz-Meco MT, Dominguez I, Sanz L, Moscat J. Protein kinase C zeta isoform is critical for kappa B-dependent promoter activation by sphingomyelinase. J Biol Chem. 1994;269:19200–19202. [PubMed] [Google Scholar]
- 32.Heinrich M, Wickel M, Schneider-Brachert W, Sandberg C, Gahr J, Schwandner R, Weber T, Saftig P, Peters C, Brunner J, Kronke M, Schutze S. Cathepsin D targeted by acid sphingomyelinase-derived ceramide. EMBO J. 1999;18:5252–5263. doi: 10.1093/emboj/18.19.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Yao B, Delikat S, Bayoumy S, Lin XH, Basu S, McGinley M, Chan-Hui PY, Lichenstein H, Kolesnick S. Kinase suppressor of Ras is ceramide-activated protein kinase. Cell. 1997;89:63–72. doi: 10.1016/s0092-8674(00)80183-x. [DOI] [PubMed] [Google Scholar]
- 34.Lee JY, Hannun YA, Obeid LM. Ceramide inactivates cellular protein kinase Calpha. J Biol Chem. 1996;271:13169–13174. doi: 10.1074/jbc.271.22.13169. [DOI] [PubMed] [Google Scholar]
- 35.Zhou H, Summers SA, Birnbaum MJ, Pittman RN. Inhibition of Akt kinase by cell-permeable ceramide and its implications for ceramide-induced apoptosis. J Biol Chem. 1998;273:16568–16575. doi: 10.1074/jbc.273.26.16568. [DOI] [PubMed] [Google Scholar]
- 36.Siskind LJ, Kolesnick RN, Colombini M. Ceramide channels increase the permeability of the mitochondrial outer membrane to small proteins. J Biol Chem. 2002;277:26796–26803. doi: 10.1074/jbc.M200754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lacour S, Hammann A, Grazide S, Lagadic-Gossmann D, Athias A, Sergent O, Laurent G, Gambert P, Solary E, Dimanche-Boitrel MT. Cisplatin-induced CD95 redistribution into membrane lipid rafts of HT29 human colon cancer cells. Cancer Res. 2004;64:3593–3598. doi: 10.1158/0008-5472.CAN-03-2787. [DOI] [PubMed] [Google Scholar]
- 38.Modrak DE, Cardillo TM, Newsome GA, Goldenberg DM, Gold DV. Synergistic interaction between sphingomyelin and gemcitabine potentiates ceramide-mediated apoptosis in pancreatic cancer. Cancer Res. 2004;64:8405–8410. doi: 10.1158/0008-5472.CAN-04-2988. [DOI] [PubMed] [Google Scholar]
- 39.Scheel-Toellner D, Wang K, Craddock R, Webb PR, McGettrick HM, Assi LK, Parkes N, Clough LE, Gulbins E, Salmon M, Lord JM. Reactive oxygen species limit neutrophil life span by activating death receptor signaling. Blood. 2004;104:2557–2564. doi: 10.1182/blood-2004-01-0191. [DOI] [PubMed] [Google Scholar]
- 40.Grassme H, Cremesti A, Kolesnick R, Gulbins E. Ceramide-mediated clustering is required for CD95-DISC formation. Oncogene. 2003;22:5457–5470. doi: 10.1038/sj.onc.1206540. [DOI] [PubMed] [Google Scholar]
- 41.Grassme H, Jekle A, Riehle A, Schwarz H, Berger J, Sandhoff K, Kolesnick R, Gulbins E. CD95 signaling via ceramide-rich membrane rafts. J Biol Chem. 2001;276:20589–20596. doi: 10.1074/jbc.M101207200. [DOI] [PubMed] [Google Scholar]
- 42.Gulbins E, Kolesnick R. Raft ceramide in molecular medicine. Oncogene. 2003;22:7070–7077. doi: 10.1038/sj.onc.1207146. [DOI] [PubMed] [Google Scholar]
- 43.Haimovitz-Friedman A, Kan CC, Ehleiter D, Persaud RS, McLoughlin M, Fuks Z, Kolesnick RN. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J Exp Med. 1994;180:525–535. doi: 10.1084/jem.180.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riboni L, Prinetti A, Bassi R, Caminiti A, Tettamanti G. A mediator role of ceramide in the regulation of neuroblastoma Neuro2a cell differentiation. J Biol Chem. 1995;270:26868–26875. doi: 10.1074/jbc.270.45.26868. [DOI] [PubMed] [Google Scholar]
- 45.Gulbins E, Coggeshall KM, Brenner B, Schlottmann K, Linderkamp O, Lang F. Fas-induced apoptosis is mediated by activation of a Ras and Rac protein-regulated signaling pathway. J Biol Chem. 1996;271:26389–26394. doi: 10.1074/jbc.271.42.26389. [DOI] [PubMed] [Google Scholar]
- 46.Jarvis WD, Kolesnick RN, Fornari FA, Traylor RS, Gewirtz DA, Grant S. Induction of apoptotic DNA damage and cell death by activation of the sphingomyelin pathway. Proc Natl Acad Sci. 1994;91:73–77. doi: 10.1073/pnas.91.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schütze S, Machleidt T, Krönke M. The role of diacylglycerol and ceramide in tumor necrosis factor and interleukin-1 signal transduction. J Leuk Biol. 1994;56:533–541. doi: 10.1002/jlb.56.5.533. [DOI] [PubMed] [Google Scholar]
- 48.Edelmann B, Bertsch U, Tchikov V, Winoto-Morbach S, Perrotta C, Jakob M, Adam-Klages S, Kabelitz D, Schutze S. Caspase-8 and caspase-7 sequentially mediate proteolytic activation of acid sphingomyelinase in TNF-R1 receptosomes. EMBO J. 2011;30:379–394. doi: 10.1038/emboj.2010.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heinrich M, Neumeyer J, Jakob M, Hallas C, Tchikov V, Winoto-Morbach S, Wickel M, Schneider-Brachert W, Trauzold A, Hethke A, Schutze S. Cathepsin D links TNF-induced acid sphingomyelinase to Bid-mediated caspase-9 and −3 activation. Cell Death Differ. 2004;11:550–563. doi: 10.1038/sj.cdd.4401382. [DOI] [PubMed] [Google Scholar]
- 50.Boesen-de Cock JG, Tepper AD, Vries Ede, van Blitterswijk WJ, Borst J. Common regulation of apoptosis signaling induced by CD95 and the DNA-damaging stimuli etoposide and gamma-radiation downstream from caspase-8 activation. J Biol Chem. 1999;274:14255–14261. doi: 10.1074/jbc.274.20.14255. [DOI] [PubMed] [Google Scholar]
- 51.Kashkar H, Wiegmann K, Yazdanpanah B, Haubert D, Kronke M. Acid sphingomyelinase is indispensable for UV light-induced Bax conformational change at the mitochondrial membrane. J Biol Chem. 2005;280:20804–20813. doi: 10.1074/jbc.M410869200. [DOI] [PubMed] [Google Scholar]
- 52.Stoica BA, Movsesyan VA, Knoblach SM, Faden AI. Ceramide induces neuronal apoptosis through mitogen-activated protein kinases and causes release of multiple mitochondrial proteins. Mol Cell Neurosci. 2005;29:355–371. doi: 10.1016/j.mcn.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 53.Susin SA, Zamzami N, Castedo M, Daugas E, Wang HG, Geley S, Fassy F, Reed JC, Kroemer G. The central executioner of apoptosis: multiple connections between protease activation and mitochondria in Fas/APO-1/CD95- and ceramide-induced apoptosis. J Exp Med. 1997;186:25–37. doi: 10.1084/jem.186.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, Haimovitz-Friedman A, Cordon-Cardo C, Kolesnick R. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 55.Lozano J, Menendez S, Morales A, Ehleiter D, Liao WC, Wagman R, Haimovitz-Friedman A, Fuks Z, Kolesnick R. Cell autonomous apoptosis defects in acid sphingomyelinase knockout fibroblasts. J Biol Chem. 2001;276:442–448. doi: 10.1074/jbc.M006353200. [DOI] [PubMed] [Google Scholar]
- 56.Rotolo JA, Maj JG, Feldman R, Ren D, Haimovitz-Friedman A, Cordon-Cardo C, Cheng EH, Kolesnick R, Fuks Z. Bax and Bak do not exhibit functional redundancy in mediating radiation-induced endothelial apoptosis in the intestinal mucosa. Int J Radiat Oncol Biol Phys. 2008;70:804–815. doi: 10.1016/j.ijrobp.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 57.Uchida Y, Murata S, Schmuth M, Behne MJ, Lee JD, Ichikawa S, Elias PM, Hirabayashi Y, Holleran WM. Glucosylceramide synthesis and synthase expression protect against ceramide-induced stress. J Lipid Res. 2002;43:1293–1302. [PubMed] [Google Scholar]
- 58.Sayama K, Hanakawa Y, Shirakata Y, Yamasaki K, Sawada Y, Sun L, Yamanishi K, Ichijo H, Hashimoto K. Apoptosis signal-regulating kinase 1 (ASK1) is an intracellular inducer of keratinocyte differentiation. J Biol Chem. 2001;276:999–1004. doi: 10.1074/jbc.M003425200. [DOI] [PubMed] [Google Scholar]
- 59.Jiang YJ, Kim P, Uchida Y, Elias PM, Bikle DD, Grunfeld C, Feingold KR. Ceramides stimulate caspase-14 expression in human keratinocytes. Exp Dermatol. 2013;22:113–118. doi: 10.1111/exd.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang YJ, Uchida Y, Lu B, Kim P, Mao C, Akiyama M, Elias PM, Holleran WM, Grunfeld C, Feingold KR. Ceramide stimulates ABCA12 expression via peroxisome proliferator-activated receptor {delta} in human keratinocytes. J Biol Chem. 2009;284:18942–18952. doi: 10.1074/jbc.M109.006973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmuth M, Haqq CM, Cairns WJ, Holder JC, Dorsam S, Chang S, Lau P, Fowler AJ, Chuang G, Moser AH, Brown BE, Mao-Qiang M, Uchida Y, Schoonjans K, Auwerx J, Chambon P, Willson TM, Elias PM, Feingold KR. Peroxisome proliferator-activated receptor (PPAR)-beta/delta stimulates differentiation and lipid accumulation in keratinocytes. J Invest Dermatol. 2004;122:971–983. doi: 10.1111/j.0022-202X.2004.22412.x. [DOI] [PubMed] [Google Scholar]
- 62.Akiyama M, Sugiyama-Nakagiri Y, Sakai K, McMillan JR, Goto M, Arita K, Tsuji-Abe Y, Tabata N, Matsuoka K, Sasaki R, Sawamura D, Shimizu H. Mutations in lipid transporter ABCA12 in harlequin ichthyosis and functional recovery by corrective gene transfer. J Clin Invest. 2005;115:1777–1784. doi: 10.1172/JCI24834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jennemann R, Sandhoff R, Langbein L, Kaden S, Rothermel U, Gallala H, Sandhoff K, Wiegandt H, Grone HJ. Integrity and barrier function of the epidermis critically depend on glucosylceramide synthesis. J Biol Chem. 2007;282:3083–3094. doi: 10.1074/jbc.M610304200. [DOI] [PubMed] [Google Scholar]
- 64.Amen N, Mathow D, Rabionet M, Sandhoff R, Langbein L, Gretz N, Jackel C, Grone HJ, Jennemann R. Differentiation of epidermal keratinocytes is dependent on glucosylceramide:ceramide processing. Hum Mol Genet. 2013 doi: 10.1093/hmg/ddt264. [DOI] [PubMed] [Google Scholar]
- 65.Geilen CC, Bektas M, Wieder T, Kodelja V, Goerdt S, Orfanos CE. 1alpha,25-dihydroxyvitamin D3 induces sphingomyelin hydrolysis in HaCaT cells via tumor necrosis factor alpha. J Biol Chem. 1997;272:8997–9001. doi: 10.1074/jbc.272.14.8997. [DOI] [PubMed] [Google Scholar]
- 66.Magnoni C, Euclidi E, Benassi L, Bertazzoni G, Cossarizza A, Seidenari S, Giannetti A. Ultraviolet B radiation induces activation of neutral and acidic sphingomyelinases and ceramide generation in cultured normal human keratinocytes. Toxicol In Vitro. 2002;16:349–355. doi: 10.1016/s0887-2333(02)00024-3. [DOI] [PubMed] [Google Scholar]
- 67.Grether-Beck S, Bonizzi G, Schmitt-Brenden H, Felsner I, Timmer A, Sies H, Johnson JP, Piette J, Krutmann J. Non-enzymatic triggering of the ceramide signalling cascade by solar UVA radiation. EMBO J. 2000;19:5793–5800. doi: 10.1093/emboj/19.21.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grether-Beck S, Timmer A, Felsner I, Brenden H, Brammertz D, Krutmann J. Ultraviolet A-induced signaling involves a ceramide-mediated autocrine loop leading to ceramide de novo synthesis. J Invest Dermatol. 2005;125:545–553. doi: 10.1111/j.0022-202X.2005.23782.x. [DOI] [PubMed] [Google Scholar]
- 69.Kim DS, Kim SY, Moon SJ, Chung JH, Kim KH, Cho KH, Park KC. Ceramide inhibits cell proliferation through Akt/PKB inactivation and decreases melanin synthesis in Mel-Ab cells. Pigment Cell Res. 2001;14:110–105. doi: 10.1034/j.1600-0749.2001.140206.x. [DOI] [PubMed] [Google Scholar]
- 70.Bouwstra JA, Ponec M. The skin barrier in healthy and diseased state. Biochim Biophys Acta. 2006;1758:2080–2095. doi: 10.1016/j.bbamem.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 71.Grayson S, Johnson-Winegar AG, Wintroub BU, Isseroff RR, Epstein EH, Jr., Elias PM. Lamellar body-enriched fractions from neonatal mice: preparative techniques and partial characterization. J Invest Dermatol. 1985;85:289–294. doi: 10.1111/1523-1747.ep12276826. [DOI] [PubMed] [Google Scholar]
- 72.Hamanaka S, Nakazawa S, Yamanaka M, Uchida Y, Otsuka F. Glucosylceramide accumulates preferentially in lamellar bodies in differentiated keratinocytes. Br J Dermatol. 2005;152:426–434. doi: 10.1111/j.1365-2133.2004.06333.x. [DOI] [PubMed] [Google Scholar]
- 73.Uchida Y, Ogawa T, Iwamori M, Nagai Y. Enhancement of keratin synthesis induced by lipokeratinogenoside, N-(O-linoleoyl)-omega-hydroxy fatty acyl sphingosyl glucose, in association with alteration of the intracellular Ca(2+)-content and protein kinase in cultured keratinocytes (FRSK) J Biochem (Tokyo) 1991;109:462–465. doi: 10.1093/oxfordjournals.jbchem.a123404. [DOI] [PubMed] [Google Scholar]
- 74.Uchida Y, Iwamori M, Nagai Y. Activation of keratinization of keratinocytes from fetal rat skin with N-(O-linoleoyl) omega-hydroxy fatty acyl sphingosyl glucose (lipokeratinogenoside) as a marker of epidermis. Biochemical and Biophysical Research Communications. 1990;170:162–168. doi: 10.1016/0006-291x(90)91254-p. [DOI] [PubMed] [Google Scholar]
- 75.Gray GM, White RJ, Williams RH, Yardley HJ. Lipid composition of the superficial stratum corneum cells of pig epidermis. Br J Dermatol. 1982;106:59–63. doi: 10.1111/j.1365-2133.1982.tb00902.x. [DOI] [PubMed] [Google Scholar]
- 76.Gray GM, Yardley HJ. Lipid compositions of cells isolated from pig, human, and rat epidermis. J Lipid Res. 1975;16:434–440. [PubMed] [Google Scholar]
- 77.Elojeimy S, Liu X, McKillop JC, El-Zawahry AM, Holman DH, Cheng JY, Meacham WD, Mahdy AE, Saad AF, Turner LS, Cheng J, T AD, Dong JY, Bielawska A, Hannun YA, Norris JS. Role of acid ceramidase in resistance to FasL: therapeutic approaches based on acid ceramidase inhibitors and FasL gene therapy. Mol Ther. 2007;15:1259–1263. doi: 10.1038/sj.mt.6300167. [DOI] [PubMed] [Google Scholar]
- 78.Musumarra G, Barresi V, Condorelli DF, Scire S. A bioinformatic approach to the identification of candidate genes for the development of new cancer diagnostics. Biol Chem. 2003;384:321–327. doi: 10.1515/BC.2003.037. [DOI] [PubMed] [Google Scholar]
- 79.Shirai K, Kaneshiro T, Wada M, Furuya H, Bielawski J, Hannun YA, Obeid LM, Ogretmen B, Kawamori T. A role of sphingosine kinase 1 in head and neck carcinogenesis. Cancer Prev Res (Phila) 2011;4:454–462. doi: 10.1158/1940-6207.CAPR-10-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Malavaud B, Pchejetski D, Mazerolles C, Paiva GRde, Calvet C, Doumerc N, Pitson S, Rischmann P, Cuvillier O. Sphingosine kinase-1 activity and expression in human prostate cancer resection specimens. Eur J Cancer. 2010;46:3417–3424. doi: 10.1016/j.ejca.2010.07.053. [DOI] [PubMed] [Google Scholar]
- 81.Ruckhaberle E, Karn T, Rody A, Hanker L, Gatje R, Metzler D, Holtrich U, Kaufmann M. Gene expression of ceramide kinase, galactosyl ceramide synthase and ganglioside GD3 synthase is associated with prognosis in breast cancer. J Cancer Res Clin Oncol. 2009;135:1005–1013. doi: 10.1007/s00432-008-0536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu YY, Patwardhan GA, Xie P, Gu X, Giuliano AE, Cabot MC. Glucosylceramide synthase, a factor in modulating drug resistance, is overexpressed in metastatic breast carcinoma. Int J Oncol. 2011;39:425–431. doi: 10.3892/ijo.2011.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Klappe K, Hinrichs JW, Kroesen BJ, Sietsma H, Kok JW. MRP1 and glucosylceramide are coordinately over expressed and enriched in rafts during multidrug resistance acquisition in colon cancer cells. Int J Cancer. 2004;110:511–522. doi: 10.1002/ijc.20140. [DOI] [PubMed] [Google Scholar]
- 84.Gouaze V, Yu JY, Bleicher RJ, Han TY, Liu YY, Wang H, Gottesman MM, Bitterman A, Giuliano AE, Cabot MC. Overexpression of glucosylceramide synthase and P-glycoprotein in cancer cells selected for resistance to natural product chemotherapy. Mol Cancer Ther. 2004;3:633–639. [PubMed] [Google Scholar]
- 85.Uchida Y, Itoh M, Taguchi Y, Yamaoka S, Umehara H, Ichikawa S, Hirabayashi Y, Holleran WM, Okazaki T. Ceramide Reduction and Transcriptional Up-Regulation of Glucosylceramide Synthase through Doxorubicin-Activated Sp1 in Drug-Resistant HL-60/ADR Cells. Cancer Res. 2004;64:6271–6279. doi: 10.1158/0008-5472.CAN-03-1476. [DOI] [PubMed] [Google Scholar]
- 86.Mitsutake S, Tani M, Okino N, Mori K, Ichinose S, Omori A, Iida H, Nakamura T, Ito M. Ito, Purification, characterization, molecular cloning, and subcellular distribution of neutral ceramidase of rat kidney. J Biol Chem. 2001;276:26249–26259. doi: 10.1074/jbc.M102233200. [DOI] [PubMed] [Google Scholar]
- 87.el Bawab S, Mao C, Obeid LM, Hannun YA. Ceramidases in the regulation of ceramide levels and function. Subcell Biochem. 2002;36:187–205. doi: 10.1007/0-306-47931-1_10. [DOI] [PubMed] [Google Scholar]
- 88.Houben E, Holleran WM, Yaginuma T, Mao C, Obeid LM, Rogiers V, Takagi Y, Elias PM, Uchida Y. Differentiation-associated expression of ceramidase isoforms in cultured keratinocytes and epidermis. J Lipid Res. 2006;47:1063–1070. doi: 10.1194/jlr.M600001-JLR200. [DOI] [PubMed] [Google Scholar]
- 89.Xu R, Jin J, Hu W, Sun W, Bielawski J, Szulc Z, Taha T, Obeid LM, Mao C. Golgi alkaline ceramidase regulates cell proliferation and survival by controlling levels of sphingosine and S1P. Faseb J. 2006;20:1813–1825. doi: 10.1096/fj.05-5689com. [DOI] [PubMed] [Google Scholar]
- 90.Mao C, Xu R, Szulc ZM, Bielawska A, Galadari SH, Obeid LM. Cloning and characterization of a novel human alkaline ceramidase. A mammalian enzyme that hydrolyzes phytoceramide. J Biol Chem. 2001;276:26577–26588. doi: 10.1074/jbc.M102818200. [DOI] [PubMed] [Google Scholar]
- 91.Kohama T, Olivera A, Edsall L, Nagiec MM, Dickson R, Spiegel S. Molecular cloning and functional characterization of murine sphingosine kinase. J Biol Chem. 1998;273:23722–23728. doi: 10.1074/jbc.273.37.23722. [DOI] [PubMed] [Google Scholar]
- 92.Liu B, Hannun YA. Purification of rat brain membrane neutral sphingomyelinase. Methods in Enzymology. 2000;311:156–164. doi: 10.1016/s0076-6879(00)11076-6. [DOI] [PubMed] [Google Scholar]
- 93.Uchida Y, Houben E, Park K, Douangpanya S, Lee YM, Wu BX, Hannun YA, Radin NS, Elias PM, Holleran WM. Hydrolytic pathway protects against ceramide-induced apoptosis in keratinocytes exposed to UVB. J Invest Dermatol. 2010;130:2472–2480. doi: 10.1038/jid.2010.153. [DOI] [PubMed] [Google Scholar]
- 94.Saba JD, Nara F, Bielawska A, Garrett S, Hannun YA. The BST1 gene of Saccharomyces cerevisiae is the sphingosine-1-phosphate lyase. J Biol Chem. 1997;272:26087–26090. doi: 10.1074/jbc.272.42.26087. [DOI] [PubMed] [Google Scholar]
- 95.Zhou J, Saba JD. Identification of the first mammalian sphingosine phosphate lyase gene and its functional expression in yeast. Biochem Biophys Res Commun. 1998;242:502–507. doi: 10.1006/bbrc.1997.7993. [DOI] [PubMed] [Google Scholar]
- 96.Sugiura M, Kono K, Liu H, Shimizugawa T, Minekura H, Spiegel S, Kohama T. Ceramide kinase, a novel lipid kinase. Molecular cloning and functional characterization. J Biol Chem. 2002;277:23294–23300. doi: 10.1074/jbc.M201535200. [DOI] [PubMed] [Google Scholar]
- 97.Lamour NF, Stahelin RV, Wijesinghe DS, Maceyka M, Wang E, Allegood JC, Merrill AH, Jr., Cho W, Chalfant CE. Ceramide kinase uses ceramide provided by ceramide transport protein: localization to organelles of eicosanoid synthesis. J Lipid Res. 2007;48:1293–1304. doi: 10.1194/jlr.M700083-JLR200. [DOI] [PubMed] [Google Scholar]
- 98.Ichikawa S, Ozawa K, Hirabayashi Y. Molecular cloning and expression of mouse ceramide glucosyltransferase. Biochem Mol Biol Int. 1998;44:1193–1202. doi: 10.1080/15216549800202282. [DOI] [PubMed] [Google Scholar]
- 99.Ichikawa S, Ozawa K, Hirabayashi Y. Molecular cloning and characterization of the mouse ceramide glucosyltransferase gene. Biochem Biophys Res Commun. 1998;253:707–711. doi: 10.1006/bbrc.1998.9855. [DOI] [PubMed] [Google Scholar]
- 100.Uchida Y, Iwamori M, Nagai Y. Distinct differences in lipid composition between epidermis and dermis from footpad and dorsal skin of guinea pigs. J J Exp Med. 1988;58:153–161. [PubMed] [Google Scholar]
- 101.Hanada K, Kumagai K, Yasuda S, Miura Y, Kawano M, Fukasawa M, Nishijima M. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 2003;426:803–809. doi: 10.1038/nature02188. [DOI] [PubMed] [Google Scholar]
- 102.Yamaoka S, Miyaji M, Kitano T, Umehara H, Okazaki T. Expression cloning of a human cDNA restoring sphingomyelin synthesis and cell growth in sphingomyelin synthase-defective lymphoid cells. J Biol Chem. 2004;279:18688–18693. doi: 10.1074/jbc.M401205200. [DOI] [PubMed] [Google Scholar]
- 103.Huitema K, van den Dikkenberg J, Brouwers JF, Holthuis JC. Identification of a family of animal sphingomyelin synthases. Embo J. 2004;23:33–44. doi: 10.1038/sj.emboj.7600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Charruyer A, Bell SM, Kawano M, Douangpanya S, Yen TY, Macher BA, Kumagai K, Hanada K, Holleran WM, Uchida Y. Decreased ceramide transport protein, cert,function alters sphingomyelin production following UVB irradiation. J Biol Chem. 2008;283:16682–16692. doi: 10.1074/jbc.M800799200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hannun YA, Loomis CR, Merrill AH, Jr., Bell RM. Sphingosine inhibition of protein kinase C activity and of phorbol dibutyrate binding in vitro and in human platelets. J Biol Chem. 1986;261:12604–12609. [PubMed] [Google Scholar]
- 106.el Touny S, Khan W, Hannun Y. Regulation of platelet protein kinase C by oleic acid. Kinetic analysis of allosteric regulation and effects on autophosphorylation, phorbol ester binding, and susceptibility to inhibition. J Biol Chem. 1990;265:16437–16443. [PubMed] [Google Scholar]
- 107.Kanno T, Nishizaki T. Sphingosine induces apoptosis in hippocampal neurons and astrocytes by activating caspase-3/-9 via a mitochondrial pathway linked to SDK/14-3-3 protein/Bax/cytochrome c. J Cell Physiol. 2011;226:2329–2337. doi: 10.1002/jcp.22571. [DOI] [PubMed] [Google Scholar]
- 108.Suzuki E, Handa K, Toledo MS, Hakomori S. Sphingosine-dependent apoptosis: a unified concept based on multiple mechanisms operating in concert. Proc Natl Acad Sci U S A. 2004;101:14788–14793. doi: 10.1073/pnas.0406536101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cuvillier O. Sphingosine in apoptosis signaling. Biochim Biophys Acta. 2002;1585:153–162. doi: 10.1016/s1388-1981(02)00336-0. [DOI] [PubMed] [Google Scholar]
- 110.Thayyullathil F, Pallichankandy S, Rahman A, Kizhakkayil J, Chathoth S, Patel M, Galadari S. Caspase-3 mediated release of SAC domain containing fragment from Par-4 is necessary for the sphingosine-induced apoptosis in Jurkat cells. J Mol Signal. 2013;8:2. doi: 10.1186/1750-2187-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Okuwa H, Kanno T, Fujita Y, Gotoh A, Tabata C, Fukuoka K, Nakano T, Nishizaki T. Sphingosine suppresses mesothelioma cell proliferation by inhibiting PKC-delta and inducing cell cycle arrest at the G(0)/G(1) phase. Cell Physiol Biochem. 2012;30:995–1004. doi: 10.1159/000341476. [DOI] [PubMed] [Google Scholar]
- 112.Yatomi Y, Ruan F, Hakomori S, Igarashi Y. Sphingosine-1-phosphate: a platelet-activating sphingolipid released from agonist-stimulated human platelets. Blood. 1995;86:193–202. [PubMed] [Google Scholar]
- 113.Sauer B, Vogler R, Wenckstern Hvon, Fujii M, Anzano MB, Glick AB, Schafer-Korting M, Roberts AB, Kleuser B. Involvement of Smad signaling in sphingosine 1-phosphate-mediated biological responses of keratinocytes. J Biol Chem. 2004;279:38471–38479. doi: 10.1074/jbc.M313557200. [DOI] [PubMed] [Google Scholar]
- 114.Sun L, Xu L, Henry FA, Spiegel S, Nielsen TB. A new wound healing agent--sphingosylphosphorylcholine. J Invest Dermatol. 1996;106:232–237. doi: 10.1111/1523-1747.ep12340570. [DOI] [PubMed] [Google Scholar]
- 115.Lee H, Goetzl EJ, An S. Lysophosphatidic acid and sphingosine 1-phosphate stimulate endothelial cell wound healing. Am J Physiol Cell Physiol. 2000;278:C612–C618. doi: 10.1152/ajpcell.2000.278.3.C612. [DOI] [PubMed] [Google Scholar]
- 116.Vogler R, Sauer B, Kim DS, Schafer-Korting M, Kleuser B. Sphingosine-1-phosphate and its potentially paradoxical effects on critical parameters of cutaneous wound healing. J Invest Dermatol. 2003;120:693–700. doi: 10.1046/j.1523-1747.2003.12096.x. [DOI] [PubMed] [Google Scholar]
- 117.Kawanabe T, Kawakami T, Yatomi Y, Shimada S, Soma Y. Sphingosine 1-phosphate accelerates wound healing in diabetic mice. J Dermatol Sci. 2007;48:53–60. doi: 10.1016/j.jdermsci.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 118.Amano S, Akutsu N, Ogura Y, Nishiyama T. Increase of laminin 5 synthesis in human keratinocytes by acute wound fluid, inflammatory cytokines and growth factors, and lysophospholipids. Br J Dermatol. 2004;151:961–970. doi: 10.1111/j.1365-2133.2004.06175.x. [DOI] [PubMed] [Google Scholar]
- 119.Nakamura T, Abe A, Balazovich KJ, Wu D, Suchard SJ, Boxer LA, Shayman JA. Ceramide regulates oxidant release in adherent human neutrophils. J Biol Chem. 1994;269:18384–18389. [PubMed] [Google Scholar]
- 120.Maceyka M, Sankala H, Hait NC, Stunff HLe, Liu H, Toman R, Collier C, Zhang M, Satin LS, Merrill AH, Jr., Milstien S, Spiegel S. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J Biol Chem. 2005;280:37118–37129. doi: 10.1074/jbc.M502207200. [DOI] [PubMed] [Google Scholar]
- 121.Manggau M, Kim DS, Ruwisch L, Vogler R, Korting HC, Schafer-Korting M, Kleuser B. 1Alpha, 25-dihydroxyvitamin D3 protects human keratinocytes from apoptosis by the formation of sphingosine-1-phosphate. J Invest Dermatol. 2001;117:1241–1249. doi: 10.1046/j.0022-202x.2001.01496.x. [DOI] [PubMed] [Google Scholar]
- 122.Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci U S A. 2006;103:16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fukuhara S, Simmons S, Kawamura S, Inoue A, Orba Y, Tokudome T, Sunden Y, Arai Y, Moriwaki K, Ishida J, Uemura A, Kiyonari H, Abe T, Fukamizu A, Hirashima M, Sawa H, Aoki J, Ishii M, Mochizuki N. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J Clin Invest. 2012;122:1416–1426. doi: 10.1172/JCI60746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nagahashi M, Kim EY, Yamada A, Ramachandran S, Allegood JC, Hait NC, Maceyka M, Milstien S, Takabe K, Spiegel S. Spns2, a transporter of phosphorylated sphingoid bases, regulates their blood and lymph levels, and the lymphatic network. FASEB J. 2013;27:1001–1011. doi: 10.1096/fj.12-219618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Meyer zu Heringdorf D, Lass H, Alemany R, Laser KT, Neumann E, Zhang C, Schmidt M, Rauen U, Jakobs KH, Koppen CJvan. Sphingosine kinase-mediated Ca2+ signalling by G-protein-coupled receptors. EMBO J. 1998;17:2830–2837. doi: 10.1093/emboj/17.10.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Okamoto H, Takuwa N, Gonda K, Okazaki H, Chang K, Yatomi Y, Shigematsu H, Takuwa Y. EDG1 is a functional sphingosine-1-phosphate receptor that is linked via a Gi/o to multiple signaling pathways, including phospholipase C activation, Ca2+ mobilization, Ras-mitogen-activated protein kinase activation, and adenylate cyclase inhibition. J Biol Chem. 1998;273:27104–27110. doi: 10.1074/jbc.273.42.27104. [DOI] [PubMed] [Google Scholar]
- 127.Ambesi A, McKeown-Longo PJ. Anastellin, the angiostatic fibronectin peptide, is a selective inhibitor of lysophospholipid signaling. Mol Cancer Res. 2009;7:255–265. doi: 10.1158/1541-7786.MCR-08-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schuppel M, Kurschner U, Kleuser U, Schafer-Korting M, Kleuser B. Sphingosine 1-phosphate restrains insulin-mediated keratinocyte proliferation via inhibition of Akt through the S1P2 receptor subtype. J Invest Dermatol. 2008;128:1747–1756. doi: 10.1038/sj.jid.5701259. [DOI] [PubMed] [Google Scholar]
- 129.Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, Luo C, Marmorstein R, Kordula T, Milstien S, Spiegel S. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–1247. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, Maceyka M, Jiang H, Luo C, Kordula T, Milstien S, Spiegel S. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465:1084–1088. doi: 10.1038/nature09128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Saad AF, Meacham WD, Bai A, Anelli V, Elojeimy S, Mahdy AE, Turner LS, Cheng J, Bielawska A, Bielawski J, Keane TE, Obeid LM, Hannun YA, Norris JS, Liu X. The functional effects of acid ceramidase overexpression in prostate cancer progression and resistance to chemotherapy. Cancer Biol Ther. 2007;6:1455–1460. doi: 10.4161/cbt.6.9.4623. [DOI] [PubMed] [Google Scholar]
- 132.Sun W, Jin J, Xu R, Hu W, Szulc ZM, Bielawski J, Obeid LM, Mao C. Substrate specificity, membrane topology, and activity regulation of human alkaline ceramidase 2 (ACER2) J Biol Chem. 2010;285:8995–9007. doi: 10.1074/jbc.M109.069203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hu W, Xu R, Sun W, Szulc ZM, Bielawski J, Obeid LM, Mao C. Alkaline ceramidase 3 (ACER3) hydrolyzes unsaturated long-chain ceramides, and its down-regulation inhibits both cell proliferation and apoptosis. J Biol Chem. 2010;285:7964–7976. doi: 10.1074/jbc.M109.063586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gomez-Munoz A, Duffy PA, Martin A, O’Brien L, Byun HS, Bittman R, Brindley DN. Short-chain ceramide-1-phosphates are novel stimulators of DNA synthesis and cell division: antagonism by cell-permeable ceramides. Mol Pharmacol. 1995;47:833–839. [PubMed] [Google Scholar]
- 135.Hinkovska-Galcheva VT, Boxer LA, Mansfield PJ, Harsh D, Blackwood A, Shayman JA. The formation of ceramide-1-phosphate during neutrophil phagocytosis and its role in liposome fusion. J Biol Chem. 1998;273:33203–33209. doi: 10.1074/jbc.273.50.33203. [DOI] [PubMed] [Google Scholar]
- 136.Pettus BJ, Bielawska A, Spiegel S, Roddy P, Hannun YA, Chalfant CE. Ceramide kinase mediates cytokine- and calcium ionophore-induced arachidonic acid release. J Biol Chem. 2003;278:38206–38213. doi: 10.1074/jbc.M304816200. [DOI] [PubMed] [Google Scholar]
- 137.Pettus BJ, Bielawska A, Subramanian P, Wijesinghe DS, Maceyka M, Leslie CC, Evans JH, Freiberg J, Roddy P, Hannun YA, Chalfant CE. Ceramide 1-phosphate is a direct activator of cytosolic phospholipase A2. J Biol Chem. 2004;279:11320–11326. doi: 10.1074/jbc.M309262200. [DOI] [PubMed] [Google Scholar]
- 138.Pettus BJ, Kitatani K, Chalfant CE, Taha TA, Kawamori T, Bielawski J, Obeid LM, Hannun YA. The coordination of prostaglandin E2 production by sphingosine-1-phosphate and ceramide-1-phosphate. Mol Pharmacol. 2005;68:330–335. doi: 10.1124/mol.104.008722. [DOI] [PubMed] [Google Scholar]
- 139.Lamour NF, Wijesinghe DS, Mietla JA, Ward KE, Stahelin RV, Chalfant CE. Ceramide kinase regulates the production of tumor necrosis factor alpha (TNFalpha) via inhibition of TNFalpha-converting enzyme. J Biol Chem. 2011;286:42808–42817. doi: 10.1074/jbc.M111.310169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hankins JL, Fox TE, Barth BM, Unrath KA, Kester M. Exogenous ceramide-1-phosphate reduces lipopolysaccharide (LPS)-mediated cytokine expression. J Biol Chem. 2011;286:44357–44366. doi: 10.1074/jbc.M111.264010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gangoiti P, Granado MH, Arana L, Ouro A, Gomez-Munoz A. Activation of protein kinase C-alpha is essential for stimulation of cell proliferation by ceramide 1-phosphate. FEBS Lett. 2010;584:517–524. doi: 10.1016/j.febslet.2009.11.086. [DOI] [PubMed] [Google Scholar]
- 142.Kim TJ, Kang YJ, Lim Y, Lee HW, Bae K, Lee YS, Yoo JM, Yoo HS, Yun YP. Ceramide 1-phosphate induces neointimal formation via cell proliferation and cell cycle progression upstream of ERK1/2 in vascular smooth muscle cells. Exp Cell Res. 2011;317:2041–2051. doi: 10.1016/j.yexcr.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 143.Kim C, Schneider G, Abdel-Latif A, Mierzejewska K, Sunkara M, Borkowska S, Ratajczak J, Morris AJ, Kucia M, Ratajczak MZ. Ceramide-1-phosphate regulates migration of multipotent stromal cells and endothelial progenitor cells--implications for tissue regeneration. Stem Cells. 2013;31:500–510. doi: 10.1002/stem.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hankins JL, Ward KE, Linton SS, Barth BM, Stahelin RV, Fox TE, Kester M. Ceramide-1-phosphate mediates endothelial cell invasion via the annexin a2/p11 heterotetrameric protein complex. J Biol Chem. 2013 doi: 10.1074/jbc.M113.481622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gomez-Munoz A, Kong JY, Parhar K, Wang SW, Gangoiti P, Gonzalez M, Eivemark S, Salh B, Duronio V, Steinbrecher UP. Ceramide-1-phosphate promotes cell survival through activation of the phosphatidylinositol 3-kinase/protein kinase B pathway. FEBS Lett. 2005;579:3744–3750. doi: 10.1016/j.febslet.2005.05.067. [DOI] [PubMed] [Google Scholar]
- 146.Gomez-Munoz A, Kong JY, Salh B, Steinbrecher UP. Ceramide-1-phosphate blocks apoptosis through inhibition of acid sphingomyelinase in macrophages. J Lipid Res. 2004;45:99–105. doi: 10.1194/jlr.M300158-JLR200. [DOI] [PubMed] [Google Scholar]
- 147.Ouro A, Arana L, Gangoiti P, Rivera IG, Ordonez M, Trueba M, Lankalapalli RS, Bittman R, Gomez-Munoz A. Ceramide 1-phosphate stimulates glucose uptake in macrophages. Cell Signal. 2013;25:786–795. doi: 10.1016/j.cellsig.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lingwood CA. Glycosphingolipid functions. Cold Spring Harb Perspect Biol. doi: 10.1101/cshperspect.a004788. 32011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bremer EG, Schlessinger J, Hakomori S. Ganglioside-mediated modulation of cell growth. Specific effects of GM3 on tyrosine phosphorylation of the epidermal growth factor receptor. J Biol Chem. 1986;261:2434–2440. [PubMed] [Google Scholar]
- 150.Paller AS, Arnsmeier SL, Alvarez-Franco M, Bremer EG. Ganglioside GM3 inhibits the proliferation of cultured keratinocytes. J Invest Dermatol. 1993;100:841–845. doi: 10.1111/1523-1747.ep12476755. [DOI] [PubMed] [Google Scholar]
- 151.Paller AS, Arnsmeier SL, Fisher GJ, Yu QC. Ganglioside GT1b induces keratinocyte differentiation without activating protein kinase C. Experimental Cell Research. 1995;217:118–124. doi: 10.1006/excr.1995.1070. [DOI] [PubMed] [Google Scholar]
- 152.Paller AS, Arnsmeier SL, Chen JD, Woodley DT. Ganglioside GT1b inhibits keratinocyte adhesion and migration on a fibronectin matrix. J Invest Dermatol. 1995;105:237–242. doi: 10.1111/1523-1747.ep12317572. [DOI] [PubMed] [Google Scholar]
- 153.Lichte K, Rossi R, Danneberg K, ter Braak M, Kurschner U, Jakobs KH, Kleuser B, Meyer zu Heringdorf D. Lysophospholipid receptor-mediated calcium signaling in human keratinocytes. J Invest Dermatol. 2008;128:1487–1498. doi: 10.1038/sj.jid.5701207. [DOI] [PubMed] [Google Scholar]
- 154.Schmitz EI, Potteck H, Schuppel M, Manggau M, Wahydin E, Kleuser B. Sphingosine 1-phosphate protects primary human keratinocytes from apoptosis via nitric oxide formation through the receptor subtype S1P(3) Mol Cell Biochem. 2012;371:165–176. doi: 10.1007/s11010-012-1433-5. [DOI] [PubMed] [Google Scholar]