Abstract
Normal bone remodeling depends upon a balance between the action of bone-resorbing cells, osteoclasts, and bone-forming cells, osteoblasts. When this balance is disrupted, as is seen in inflammatory diseases such as rheumatoid arthritis (RA) and ankylosing spondylitis (AS), abnormal bone loss or bone formation occurs. In RA, proinflammatory cytokines induce osteoclast differentiation and inhibit osteoblast maturation, leading to articular bone erosions. In contrast, the inflammatory milieu in AS leads to excessive osteoblast activation and bone formation at sites of entheses. While much information exists about the effects of proinflammatory cytokines on osteoclast differentiation and function, more recent studies have begun to elucidate the impact of inflammation on the osteoblast. This review will summarize the mechanisms by which inflammation perturbs bone homeostasis, with a specific focus on the osteoblast.
Keywords: inflammation, osteoclast, osteoblast, Wnt, DKK1, sclerostin, rheumatoid arthritis, ankylosing spondylitis, bone remodeling, bone erosions, bone formation, entheses
Introduction
Bone remodeling occurs throughout adult life. This process, in which existing bone is resorbed by osteoclasts and new bone is formed by osteoblasts, is necessary to reshape and mend the skeleton [1]. Under normal physiologic conditions, the coupling of osteoclasts and osteoblasts is tightly coordinated to avoid a net loss or gain of bone. Many systemic and local factors have now been identified that regulate the interactions of these two cells [2]. Under the influence of factors that promote bone resorption, the fusion and differentiation of monocyte progenitors leads to the formation of multinucleated osteoclasts [3]. Apart from their function in bone resorption, osteoclasts have also been shown to play a role in the regulation of osteoblast differentiation and bone formation, influencing the coupling process at several levels [4]. As osteoclasts resorb bone matrix, factors from the matrix are released that can regulate osteoblast activity, including transforming growth factor beta (TGF-β) and insulin-like growth factors (IGFs) [5, 6]. Osteoclasts and osteoblasts also communicate via direct contact. For example, osteoclasts express membrane-bound ephrinB2 that binds the EphB4 receptor on osteoblast precursors. This contact both enhances osteoblast differentiation and inhibits osteoclast differentiation [7, 8]. Finally, osteoclasts secrete soluble factors that act to promote bone formation, such as cardiotrophin 1, which increases runt-related transcription factor 2 (Runx2) gene expression in the osteoblast and promotes differentiation [9].
Osteoblasts are responsible for the synthesis and mineralization of bone, as well as the modulation of osteoclast differentiation. Derived from mesenchymal stem cells, osteoblast precursor cells require upregulation of the Runx2 transcription factor for commitment to the osteoblast lineage [10, 11]. In addition to their role in bone formation, osteoblasts also regulate osteoclast differentiation through the production of several factors including, macrophage colony-stimulating factor (M-CSF), receptor activator of nuclear factor kappa-B ligand (RANKL), and osteoprotegerin (OPG). M-CSF acts to expand the pool of osteoclast precursor cells and promotes their survival [12]. RANKL, an essential factor for osteoclastogenesis, interacts with the RANK receptor on the osteoclast precursor to promote osteoclast differentiation [13]. To protect against excessive bone resorption, osteoblasts also produce OPG, a soluble decoy receptor that binds RANKL to inhibit osteoclast differentiation [14]. Other factors involved in osteoblast-osteoclast interaction include paracrine regulators, such as parathyroid hormone (PTH) and prostaglandin E2 (PGE2), which increase RANKL expression by osteoblasts [15, 16]. Additionally, bone remodeling relies upon two other key pathways that regulate osteoblast differentiation and function, the Wingless (Wnt) and bone morphogenic protein (BMP) signaling pathways.
The Wnt and BMP Signaling Pathways
Wnt signaling plays a major role in osteoblast differentiation and bone formation [17]. Three Wnt signaling pathways have been identified, including the canonical Wnt/β-catenin pathway and two non-canonical pathways, the Wnt/calcium pathway, and the planar cell polarity pathway. The canonical Wnt pathway promotes osteoblast differentiation and incorporates checks and balances, with regulatory factors in place to maintain the balance of resorption and formation. However, inappropriate expression of various antagonists and agonists can disrupt this pathway, resulting in aberrant function of osteoblasts [18]. The canonical Wnt signaling cascade is activated by Wnt ligands, such as Wnt1 and Wnt3a, which bind membrane-bound receptor complexes. These complexes are composed of a frizzled protein receptor and the low-density lipoprotein receptor-related protein (LRP)- 5 and LRP-6. Upon ligand binding to the receptor complex, the intracellular disheveled protein is activated, leading to inhibition of GSK-3β activity. This in turn stabilizes β-catenin, allowing it to translocate to the nucleus where it interacts with lymphoid enhancer factor and T-cell factor to activate the transcription of numerous genes that promote osteoblast differentiation. In the absence of Wnt ligand binding, GSK-3β actively phosphorylates β-catenin, marking it for degradation by the proteasome.
Various families of antagonists inhibit Wnt signaling and thus inhibit bone formation, including secreted frizzled-related proteins (sFRPs), the Dickkopf family members (DKKs), and sclerostin [19]. The sFRPs are a family of secreted glycoproteins that downregulate Wnt signaling by binding Wnt proteins. The overexpression of sFRP1 in osteoblasts suppresses Wnt signaling and induces apoptosis, while its overexpression in mice has been shown to decrease bone density [20, 21]. Moreover, the deletion of the sFRP1 gene in mice results in increased bone volume and increased trabecular bone mineral density [22]. Within the Dickkopf protein family, DKK1 has received the most attention as a factor that inhibits bone formation by antagonizing Wnt signaling. Specifically, DKK1 cross-links LRP5/6 with Kremen to suppress Wnt signaling in mesenchymal precursor cells [23]. Overexpression of DKK1 results in osteopenia in mice, while suppressed expression leads to high bone mass [24, 25]. An increase or decrease in the serum level of DKK1 has been associated with inflammatory diseases such as RA and AS and likely contributes to the joint pathology in these diseases, as will be discussed later in this review [26, 27].
Sclerostin is a glycoprotein secreted primarily by osteocytes that has been shown to inhibit bone formation by binding to and blocking the effects of the LRP5/6 receptor in the Wnt signaling pathway [28]. Loss-of-function mutations near or in the sclerostin-encoding gene SOST are associated with two diseases characterized by high bone mass, van Buchem's disease and sclerosteosis [29]. Van Buchem's disease is caused by a 52-kb deletion downstream of SOST, while sclerosteosis results from inactivating mutations in the SOST gene itself. These autosomal recessive disorders are characterized by bone abnormalities such as skull thickening and facial and jaw enlargement [30, 31]. The deletion of the SOST gene in mice mimics human sclerosteosis, resulting in higher rates of bone formation and high bone mass, while transgenic overexpression of SOST results in significant bone loss and decreased strength [32, 33]. In recent years, factors involved in regulating the Wnt pathway, such as sclerostin, have emerged as novel targets for the development of osteoanabolic agents [34]. Sclerostin blockade abrogates bone loss in animal models of osteoporosis and disuse-induced bone loss [35, 36]. Furthermore, in phase I clinical trials, the anti-sclerostin antibody has shown promising results, increasing bone mineral density in the lumbar spine and hips of postmenopausal women and healthy men [37].
In addition to the Wnt signaling pathway, the BMP pathway also plays a critical role in osteoblast differentiation and bone remodeling. BMPs consist of a large group of growth factors that belong to the TGF-β superfamily. The most well known BMP signaling cascade involves activation of Smad proteins. The binding of BMP ligands to their membrane-bound receptors results in phosphorylation of intracellular SMADs (SMADs 1 and 5), which associate with SMAD4 to form a complex that enters the nucleus to promote gene transcription [38]. BMPs have been implicated in playing a role in inflammatory diseases, specifically in contributing to bone formation in AS [39]. Collectively, the complex and inter-related pathways that regulate bone resorption and formation illustrate the importance of maintaining bone mass and the essential balance between osteoclast and osteoblast activity.
Inflammation Perturbs Bone Remodeling
Disruption of the bone remodeling process is seen in several rheumatic diseases. Proinflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), interleukin-6 (IL-6), and interleukin-17 (IL-17), disturb the balance between osteoclast and osteoblast activity, typically resulting in a net loss of bone. While their effects on bone are complex, these cytokines stimulate osteoclast differentiation by expansion of the osteoclast precursor pool, upregulation of RANKL expression in osteoblasts and/or synovial fibroblasts, and by synergism with RANKL itself [40-42]. Furthermore, proinflammatory cytokines can perturb the Wnt and BMP signaling pathways, leading to aberrant osteoblast activity [43]. In this review, two rheumatic diseases, RA and AS, serve as examples to elucidate the effects of inflammation on bone.
Bone Loss in Rheumatoid Arthritis
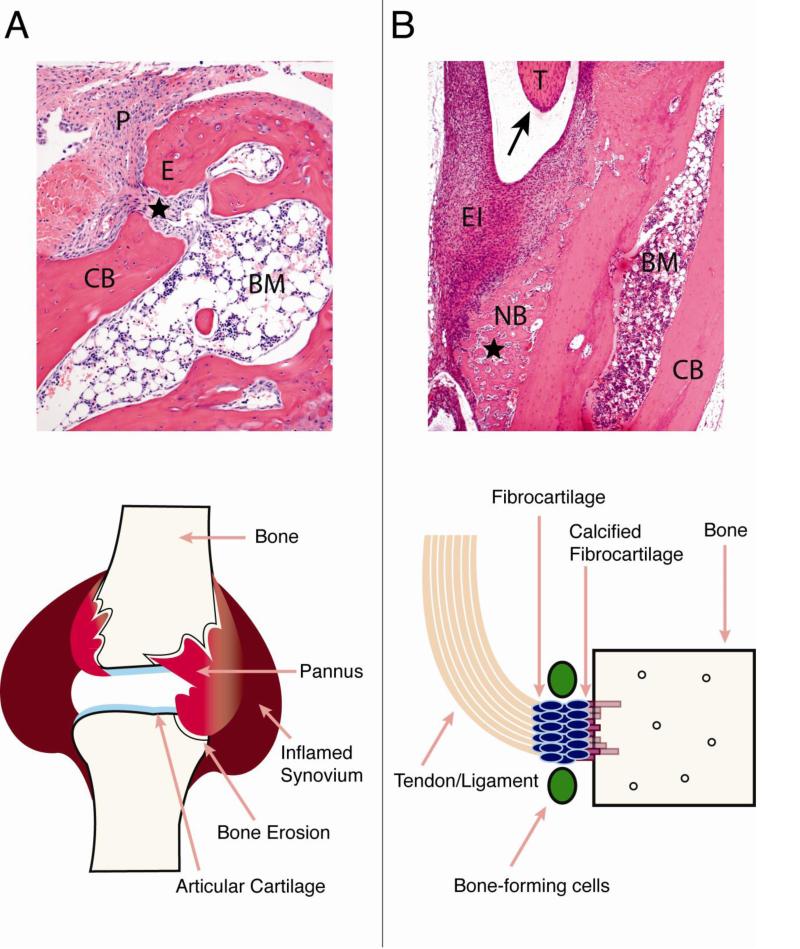
Chronic inflammation in RA leads to focal articular bone erosions within inflamed joints, as well as generalized osteoporosis in the axial and appendicular skeleton. This bone loss progresses throughout the disease, correlates with disease severity, and if left untreated, can lead to joint deformity and fractures [44]. Within the RA joint, inflamed synovially-derived “pannus” tissue invades into and erodes cortical, subchondral bone and trabecular bone (Figure 1A). Proinflammatory cytokines, secreted predominantly by macrophages, synovial fibroblasts, and lymphocytes within the inflamed synovium and pannus, mediate the erosive process by enhancing osteoclast differentiation and activity.
Figure 1.
Comparison of bone pathologies in rheumatoid arthritis (RA) and ankylosing spondylitis (AS). A) RA. Histologic section of the talus bone in the ankle joint from the K/BxN serum transfer arthritis mouse model, showing pannus eroding into bone and entering the marrow space (star). Schematic of an inflamed RA joint is shown below, demonstrating that bone erosions occur at sites of pannus invasion into bone and the bone marrow space. B) AS. Histologic section from an inflammatory mouse model showing periosteal new bone formation (NB, star), surrounded by inflammatory cells and adjacent to the insertion site of a large tendon (T, arrow). The schematic below depicts the site of new bone formation in AS, at areas of tendon/ligament insertion into bone. Code: pannus (P), erosion site (E), cortical bone (CB), bone marrow (BM), tendon (T), enthesial inflammation (EI), periosteal new bone (NB).
Impact of Inflammation on Resorption
It has been recognized since the late 1990s that the osteoclast is the cell responsible for bone resorption in RA [45]. Multinucleated TRAP-positive cells are present at the pannus-bone interface in both animal models of inflammatory arthritis and in RA [46-50]. Mice deficient in RANKL or mice that are otherwise deficient of osteoclasts are protected from bone erosions in murine models of inflammatory arthritis [51, 52]. Therapies that block the action of proinflammatory cytokines in RA not only reduce inflammation and disease symptoms, but also suppress or halt the associated bone resorption. Importantly, however, although these therapies impede bone erosions, repair of the existing erosions through new bone formation is limited [53].
In RA patients, the role of anti-TNF therapy in combination with methotrexate on bone destruction and repair has been closely examined [54]. After 12 months of treatment, magnetic resonance imaging (MRI) showed no overall progression or repair of erosions, suggesting that although bone loss was arrested, bone formation was not activated. At the level of individual patients, continued bone erosions and limited new bone formation were seen. Interestingly, this study and others have demonstrated a strong relationship between the progression of bone erosions and the presence of “bone oedema” using MRI [55, 56]. Bone oedema does not, in fact, represent fluid within bone, but rather it is the radiologic finding of osteitis, inflammation within the bone marrow space. This study also demonstrated that in the majority of patients, residual synovial inflammation persisted after 12 months of therapy. In addition, only 6% of patients showed evidence of new bone formation and repair of erosions. These findings suggest that inflammation, even at low levels, may impede the ability of osteoblasts to form new bone and mend existing erosions.
Impact of Inflammation on Formation
Indeed, recent studies have shown that proinflammatory cytokines not only induce bone resorption, but also contribute to bone loss by direct inhibition of osteoblast differentiation. In culture, TNF-α inhibits osteoblast differentiation from pluripotent progenitor cells by decreasing Runx2 expression [57]. TNF-α also induces apoptosis in osteoblasts, as well as the production of other pro-inflammatory cytokines such as IL-1β [58, 59]. Continuous exposure of osteoblasts in culture to IL-1β inhibits their differentiation, as evidenced by impaired alkaline phosphatase expression and bone nodule mineralization [60]. In addition, IL-1β has recently been shown to impair osteoblast migration toward chemotactic factors [61]. IL-6 impacts the osteoblast by binding to its sIL-6R. This interaction induces PGE2 synthesis and reduces the ratio of OPG/RANKL expression by the cells [62].
These studies raise the interesting question as to whether inflammation not only induces bone resorption, but also may impair osteoblast differentiation and/or function in vivo. The impact of local inflammation on osteoblast maturation and function was recently determined using a murine model of arthritis [63]. Inflammation was induced in mice using the K/BxN model of serum transfer arthritis and the number, state of differentiation, and function of osteoblasts were evaluated within sites of articular erosion. Histological sections from ankles of arthritic mice showed that although osteoblast-lineage cells were present at sites of inflammation and erosion, the majority of these cells lacked markers of maturation (alkaline phosphatase and osteocalcin). Dynamic histomorphometry demonstrated that less mineralized bone is formed at bone surfaces adjacent to inflammation compared to surfaces adjacent to normal bone marrow. This finding indicates that bone formation is reduced at sites of inflammation, where formation should be enhanced, due to bone loss at these sites. Overall, these experiments support the hypothesis that factors within the inflamed joint impede bone formation at arthritic sites by inhibiting osteoblast maturation.
Matzelle et al. subsequently demonstrated that the complete resolution of inflammation within the joint does allow osteoblast activity to resume at sites of articular bone loss [64]. Using the same K/BxN model of serum transfer arthritis, inflammation and bone erosion were induced in wild type mice and inflammation was then allowed to resolve completely. At the peak of inflammation and production of proinflammatory cytokines, bone erosions and cortical breaks were evident in articular bone. However, after inflammation resolution, the presence of mature osteocalcin-expressing osteoblasts was demonstrated at sites of eroded bone. In addition, dynamic histomorphometry demonstrated that as inflammation resolved, osteoblast activity and bone formation at eroded surfaces significantly increased, indicating, “repair” of erosions. Taken together, these findings demonstrate that osteoblast-mediated bone formation can resume and bone erosions can be repaired once inflammation has completely abated.
In this same study, the impact of inflammation on the expression of Wnt agonists and antagonists in synovial tissue was evaluated. During peak inflammation, mRNA expression of the Wnt antagonists sFRP1 and sFRP2 was induced in ankle joint synovium, whereas mRNA expression of the Wnt agonist Wnt10b was suppressed below levels of non-arthritic mice. As inflammation resolved, sFRP1 and sFRP2 mRNA expression decreased and Wnt10b mRNA expression increased. These findings support the hypothesis that inflammation may inhibit osteoblast differentiation through modulation of the Wnt signaling pathway. In fact, this hypothesis was put forth in a prior study using a blocking antibody to DKK1 in a TNF-driven model of inflammatory arthritis [65]. In this model, blockade of DKK1 from arthritis onset was shown to prevent erosive joint destruction. In addition, sites at which bone erosion would typically be seen were converted to sites of periosteal bone formation, suggesting that DKK1 may play a key regulatory role in determining the outcome for bone in arthritis. Importantly, treatment with the anti-DKK1 antibody did not lower the level of inflammation in arthritic mice; thus despite the presence of synovitis, treatment still prevented bone erosions. TNF-α was shown to induce DKK1 expression in vitro and in vivo. It has also been shown that DKK1, in turn, elevates SOST expression levels, which would further inhibit Wnt signaling [66].
These data have been supported by studies examining the role of DKK1 in human disease. Clinically, DKK1 serum levels are significantly increased in RA subjects compared to healthy controls. Additionally, TNF-α blockade in RA patients has been shown to reduce elevated DKK1 levels to a near normal range [26]. Sclerostin has also been shown to contribute to the persistence of bone erosions in inflammatory arthritis. In arthritic TNF-transgenic mice, sclerostin blockade was initiated after the onset of inflammation and was shown to block the progression of bone and cartilage destruction. Importantly, the combination of anti-TNF and anti-sclerostin antibodies increased periarticular osteoblast numbers and partially reversed the focal bone erosions, suggesting that combination therapy may repair the joint destruction involved in inflammatory arthritis [67].
Clinical Considerations
Prevention of bone erosions is still an important endpoint for the treatment of RA. Bone erosions have been correlated with more severe disease and with long-term disability in RA patients [44]. Furthermore, studies in animal models of RA have clearly shown a close relationship between cartilage loss and erosion of subchondral bone, which provides the “scaffold” for cartilage. In arthritic RANKL-deficient mice, protection from subchondral bone loss resulted in partial protection from cartilage loss, due to preservation of the supporting subchondral bone [51]. It is also likely that changes in mechanical forces that result from bone erosion contribute to loss of articular cartilage. The functional importance of the healing of existing bone erosions, however, is a separate issue.
It is clear from careful studies of RA patients treated with potent anti-inflammatory biologic agents that although inflammation is clinically controlled and the erosive process is slowed or even halted, healing of bone erosions is not commonly observed. Finzel et al. evaluated the effects of TNF or IL-6 antagonist therapy on repair of bone erosions in RA using high-resolution microcomputed tomography [68, 69]. After 12 months of therapy, the dimensions of individual bone erosions decreased, but complete healing was not observed. These studies demonstrate that initiation of new bone formation in RA can occur, but repair of erosions is quite limited. Studies from our laboratory, previously discussed [63, 64], suggest that one reason for this may be the persistence of joint inflammation despite the appearance of “clinical remission.” Indeed, several studies using high-resolution radiographic techniques have demonstrated the persistence of inflammation in such settings [54]. If persistent inflammation is responsible for lack of erosion healing, then healing of bone erosions could be considered as a surrogate marker for more complete control of inflammation in clinical trials of new therapeutic agents. Alternatively, however, chronic inflammation in the bone marrow space in diseases such as RA may lead to significant changes in cellular composition, including a decrease in the mesenchymal pool of osteoblast precursor cells available to ultimately form bone to repair erosions.
Bone Formation in Ankylosing Spondylitis
Although the inflammatory milieu in RA leads to bone loss, inflammation in rheumatic diseases can also promote bone formation, as is seen in AS and other spondyloarthropathies [70]. AS is a rheumatic disease characterized by chronic inflammation that primarily affects the spine and sacroiliac joints, although peripheral joints are also commonly involved. Inflammation in the AS spine occurs outside of the joint synovium at sites of entheses, areas of contact between tendons or ligaments and bone. Over time, bone deposition accrues at inflamed enthesial sites, ultimately resulting in syndesmophyte formation, fused facet joints, back pain, and reduced mobility (Figure 1B).
The Wnt antagonists DKK1 and sclerostin have been implicated in the pathogenesis of bone accrual in AS. The role of DKK1 in ankylosis of the spine has been studied in mouse models of inflammation. In the TNF transgenic model, neutralization of DKK1 promoted ankylosis and the fusion of sacroiliac joints [71]. Recently, the proteoglycan-induced spondylitis mouse model (PG1Sp) was used to evaluate the contribution of Wnt signaling to ankylosis [72]. In these mice, inflammation was induced for 12 weeks, resulting in excessive bone matrix formation at the periphery of intervertebral discs. The mRNA expression levels of DKK1 and SOST in the spine of the mice were reduced by 49% and 63% respectively, compared with control mice.
In contrast to patients with RA, where DKK1 levels have been shown to be elevated, DKK1 levels in AS are below normal levels compared to healthy controls. As DKK1 is an inhibitor of Wnt signaling and thus an inhibitor of bone formation, lower DKK1 levels would permit bone formation. Functional activity of DKK1 has been investigated by capture ELISA with its receptor LRP6 in samples of serum from 65 AS patients [27]. Patients with higher functional levels of DKK1 showed less syndesmophyte formation in the spine than patients with lower DKK1 activity. This suggests that lower levels or decreased activity of DKK1 may contribute to the excessive bone formation seen in AS, and that DKK1 may serve as a biomarker for osteophyte formation.
Sclerostin may also contribute to the bone changes seen in AS. Sclerostin expression in periarticular bone from AS patients was found to be significantly reduced compared to healthy patients. Additionally, patients with AS were shown to have reduced serum levels of sclerostin compared to healthy individuals, and radiographic progression of bone formation in AS has been associated with decreasing levels of sclerostin [73]. These findings support the hypothesis that sclerostin may also act as a biomarker for predicting progression of bone formation in AS patients. Taken together, these data support a role for aberrant Wnt signaling in the pathogenesis of bone accrual in AS.
In addition to the Wnt signaling pathway, the BMP pathway may also contribute to bone formation in AS. The BMP pathway was studied in the DBA/1 model of spontaneous arthritis, in which mice develop ankylosing enthesitis [39]. At various stages of enthesitis, immunohistochemistry revealed the presence of BMP2, BMP6, and BMP7 proteins, while gene transfer of noggin, a BMP antagonist, inhibited the progression of ankylosis in these mice. Activation of BMP signaling was also demonstrated in AS patients by the presence of nuclear phosphorylation of Smads1/5 in enthesial biopsies from the Achilles tendon [39]. Additionally, serum concentrations of BMP2 and BMP4 were higher in AS patients with spinal fusion compared to patients without spinal fusion or healthy controls [74]. These studies suggest that BMP signaling may play a role in the process of periosteal and syndesmophyte bone formation in AS.
The effect of cytokines on the excessive bone formation in AS is still under active investigation. Although TNF antagonists reduce the inflammation and symptoms associated with AS, they do not significantly impede the progression of new bone formation, leaving the question as to which cytokines are indeed responsible for bone accrual in this and related diseases [75, 76]. Several studies have identified IL-23 as a cytokine involved in the pathogenesis of AS. Increased serum levels of IL-23 are found in AS patients and polymorphisms in the IL-23 receptor have been linked to this disorder [77, 78]. Therefore, investigators have examined the potential role of IL-23 in inflammation and bone formation in spondyloarthropathies. Sherlock et al. have demonstrated a role for the cytokines IL-23 and IL-22 in the development of enthesitis and bone formation, respectively in a mouse overexpression model [79]. Interestingly, the authors demonstrated that IL-23 overexpression alone promotes both enthesitis and spondylitis. Indeed, after 6 days of IL-23 overexpression, severe enthesitis was observed and after 18 days new bone formation was seen within the mouse entheses. Through imaging techniques and by isolating cells at the entheses, a population of CD3+CD4−CD8− T cells was identified that express the IL-23 receptor. Upon stimulation with IL-23, these T cells are induced to express inflammatory genes as well as IL-22, a cytokine involved in bone formation. IL-22 was shown to upregulate mRNA expression for factors that promote osteoblast differentiation, including Wnt10b, BMP4, and alkaline phosphatase genes. Overall, these studies provide early evidence for the hypothesis that neutralization of IL-23 may alleviate not only the inflammation associated with AS, but also the bone accrual that is so disabling for patients with this disease [80].
Conclusion
The effects of proinflammatory cytokines on the osteoclast and osteoblast are numerous and are still being revealed. It is clear, however, that these cytokines disturb the tightly regulated balance between bone formation and resorption. Diseases that may serve as prototypes for the impact of inflammation on bone include RA, in which bone is eroded at articular sites, and AS, in which bone formation occurs at enthesial sites. Cytokines expressed in the inflamed synovium in RA, including TNF-α, IL-1, IL-6 and others, induce osteoclastogenesis through the upregulation of RANKL and through direct effects on osteoclast differentiation and function. In addition, the inflammation inhibits osteoblast maturation and new bone formation at least in part through modulation of the Wnt signaling pathway. Evidence exists that the Wnt signaling pathway is also modulated by proinflammatory cytokines in AS, while IL-23 has recently emerged as a cytokine that appears to contribute to enthesitis and new bone formation in this disease. While current therapeutic strategies aim to reduce the inflammation associated with rheumatic diseases, effects on promoting repair of damaged articular bone in RA or preventing progression of bone formation in AS have yet to be fully realized. Further elucidation of the pathways and factors mediating these processes may lead to the development of novel agents that will impact bone in these diseases.
Footnotes
Conflict of Interest
R Baum declares no conflicts of interest.
EM Gravallese has received research grants from Eli Lilly and Abbott and is a consultant for Abbott.
Human and Animal Rights and Informed Consent
All studies by the authors involving animal and/or human subjects were performed after approval by the appropriate institutional review boards. When required, written informed consent was obtained from all participants.
References
Papers of particular interest, published recently, have been highlighted as:
•• Of major importance
• Of importance
- 1.Sims NA, Gooi JH. Bone remodeling: Multiple cellular interactions required for coupling of bone formation and resorption. Semin Cell Dev Biol. 2008;19(5):444–51. doi: 10.1016/j.semcdb.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 2.Kular J, et al. An overview of the regulation of bone remodelling at the cellular level. Clin Biochem. 2012;45(12):863–73. doi: 10.1016/j.clinbiochem.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 3.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289(5484):1504–8. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 4.Matsuo K, Irie N. Osteoclast-osteoblast communication. Arch Biochem Biophys. 2008;473(2):201–9. doi: 10.1016/j.abb.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 5.Hayden JM, Mohan S, Baylink DJ. The insulin-like growth factor system and the coupling of formation to resorption. Bone. 1995;17(2):93S–98S. doi: 10.1016/8756-3282(95)00186-h. [DOI] [PubMed] [Google Scholar]
- 6.Tang Y, et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. 2009;15(7):757–65. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin TJ, et al. Communication between ephrinB2 and EphB4 within the osteoblast lineage. Adv Exp Med Biol. 2010;658:51–60. doi: 10.1007/978-1-4419-1050-9_6. [DOI] [PubMed] [Google Scholar]
- 8.Zhao C, et al. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4(2):111–21. doi: 10.1016/j.cmet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Walker EC, et al. Cardiotrophin-1 is an osteoclast-derived stimulus of bone formation required for normal bone remodeling. J Bone Miner Res. 2008;23(12):2025–32. doi: 10.1359/jbmr.080706. [DOI] [PubMed] [Google Scholar]
- 10.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 11.Komori T. Regulation of skeletal development by the Runx family of transcription factors. J Cell Biochem. 2005;95(3):445–53. doi: 10.1002/jcb.20420. [DOI] [PubMed] [Google Scholar]
- 12.Biskobing DM, Fan X, Rubin J. Characterization of MCSF-induced proliferation and subsequent osteoclast formation in murine marrow culture. J Bone Miner Res. 1995;10(7):1025–32. doi: 10.1002/jbmr.5650100706. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi N, Udagawa N, Suda T. A new member of tumor necrosis factor ligand family, ODF/OPGL/TRANCE/RANKL, regulates osteoclast differentiation and function. Biochem Biophys Res Commun. 1999;256(3):449–55. doi: 10.1006/bbrc.1999.0252. [DOI] [PubMed] [Google Scholar]
- 14.Yasuda H, et al. Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology. 1998;139(3):1329–37. doi: 10.1210/endo.139.3.5837. [DOI] [PubMed] [Google Scholar]
- 15.Ma YL, et al. Catabolic effects of continuous human PTH (1--38) in vivo is associated with sustained stimulation of RANKL and inhibition of osteoprotegerin and gene-associated bone formation. Endocrinology. 2001;142(9):4047–54. doi: 10.1210/endo.142.9.8356. [DOI] [PubMed] [Google Scholar]
- 16.Blackwell KA, Raisz LG, Pilbeam CC. Prostaglandins in bone: bad cop, good cop? Trends Endocrinol Metab. 2010;21(5):294–301. doi: 10.1016/j.tem.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang H, He X. Wnt/beta-catenin signaling: new (and old) players and new insights. Curr Opin Cell Biol. 2008;20(2):119–25. doi: 10.1016/j.ceb.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Regard JB, et al. Wnt signaling in bone development and disease: making stronger bone with Wnts. Cold Spring Harb Perspect Biol. 2012;4(12) doi: 10.1101/cshperspect.a007997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monroe DG, et al. Update on Wnt signaling in bone cell biology and bone disease. Gene. 2012;492(1):1–18. doi: 10.1016/j.gene.2011.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bodine PV, et al. The Wnt antagonist secreted frizzled-related protein-1 controls osteoblast and osteocyte apoptosis. J Cell Biochem. 2005;96(6):1212–30. doi: 10.1002/jcb.20599. [DOI] [PubMed] [Google Scholar]
- 21.Yao W, et al. Overexpression of secreted frizzled-related protein 1 inhibits bone formation and attenuates parathyroid hormone bone anabolic effects. J Bone Miner Res. 2010;25(2):190–9. doi: 10.1359/jbmr.090719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bodine PV, et al. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocrinol. 2004;18(5):1222–37. doi: 10.1210/me.2003-0498. [DOI] [PubMed] [Google Scholar]
- 23.Pinzone JJ, et al. The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood. 2009;113(3):517–25. doi: 10.1182/blood-2008-03-145169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morvan F, et al. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res. 2006;21(6):934–45. doi: 10.1359/jbmr.060311. [DOI] [PubMed] [Google Scholar]
- 25.Li J, et al. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone. 2006;39(4):754–66. doi: 10.1016/j.bone.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 26.Wang SY, et al. Circulating Dickkopf-1 is correlated with bone erosion and inflammation in rheumatoid arthritis. J Rheumatol. 2011;38(5):821–7. doi: 10.3899/jrheum.100089. [DOI] [PubMed] [Google Scholar]
- 27.Heiland GR, et al. High level of functional dickkopf-1 predicts protection from syndesmophyte formation in patients with ankylosing spondylitis. Ann Rheum Dis. 2012;71(4):572–4. doi: 10.1136/annrheumdis-2011-200216. [DOI] [PubMed] [Google Scholar]
- 28.Li X, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280(20):19883–7. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- 29.Balemans W, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet. 2001;10(5):537–43. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- 30.van Lierop AH, et al. Van Buchem disease: clinical, biochemical, and densitometric features of patients and disease carriers. J Bone Miner Res. 2013;28(4):848–54. doi: 10.1002/jbmr.1794. [DOI] [PubMed] [Google Scholar]
- 31.Hamersma H, Gardner J, Beighton P. The natural history of sclerosteosis. Clin Genet. 2003;63(3):192–7. doi: 10.1034/j.1399-0004.2003.00036.x. [DOI] [PubMed] [Google Scholar]
- 32.Li X, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23(6):860–9. doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- 33.Winkler DG, et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003;22(23):6267–76. doi: 10.1093/emboj/cdg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Costa AG, Bilezikian JP. Sclerostin: therapeutic horizons based upon its actions. Curr Osteoporos Rep. 2012;10(1):64–72. doi: 10.1007/s11914-011-0089-5. [DOI] [PubMed] [Google Scholar]
- 35.Spatz JM, et al. Sclerostin antibody inhibits skeletal deterioration due to reduced mechanical loading. J Bone Miner Res. 2013;28(4):865–74. doi: 10.1002/jbmr.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li X, et al. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res. 2009;24(4):578–88. doi: 10.1359/jbmr.081206. [DOI] [PubMed] [Google Scholar]
- 37.Padhi D, et al. Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res. 2011;26(1):19–26. doi: 10.1002/jbmr.173. [DOI] [PubMed] [Google Scholar]
- 38.Chen G, Deng C, Li YP. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8(2):272–88. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lories RJ, Derese I, Luyten FP. Modulation of bone morphogenetic protein signaling inhibits the onset and progression of ankylosing enthesitis. J Clin Invest. 2005;115(6):1571–9. doi: 10.1172/JCI23738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li P, et al. Systemic tumor necrosis factor alpha mediates an increase in peripheral CD11bhigh osteoclast precursors in tumor necrosis factor alpha-transgenic mice. Arthritis Rheum. 2004;50(1):265–76. doi: 10.1002/art.11419. [DOI] [PubMed] [Google Scholar]
- 41.Kotake S, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103(9):1345–52. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lam J, et al. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000;106(12):1481–8. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walsh NC, Gravallese EM. Bone remodeling in rheumatic disease: a question of balance. Immunol Rev. 2010;233(1):301–12. doi: 10.1111/j.0105-2896.2009.00857.x. [DOI] [PubMed] [Google Scholar]
- 44.Scott DL. Prognostic factors in early rheumatoid arthritis. Rheumatology (Oxford) 2000;39:24–9. doi: 10.1093/oxfordjournals.rheumatology.a031490. [DOI] [PubMed] [Google Scholar]
- 45.Gravallese EM, et al. Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am J Pathol. 1998;152(4):943–51. [PMC free article] [PubMed] [Google Scholar]
- 46.Bromley M, Woolley DE. Chondroclasts and osteoclasts at subchondral sites of erosion in the rheumatoid joint. Arthritis Rheum. 1984;27(9):968–75. doi: 10.1002/art.1780270902. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki Y, et al. Osteoclast-like cells in murine collagen induced arthritis. J Rheumatol. 1998;25(6):1154–60. [PubMed] [Google Scholar]
- 48.Romas E, et al. Expression of osteoclast differentiation factor at sites of bone erosion in collagen-induced arthritis. Arthritis Rheum. 2000;43(4):821–6. doi: 10.1002/1529-0131(200004)43:4<821::AID-ANR12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 49.Kuratani T, et al. Induction of abundant osteoclast-like multinucleated giant cells in adjuvant arthritic rats with accompanying disordered high bone turnover. Histol Histopathol. 1998;13(3):751–9. doi: 10.14670/HH-13.751. [DOI] [PubMed] [Google Scholar]
- 50.Gravallese EM, et al. Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum. 2000;43(2):250–8. doi: 10.1002/1529-0131(200002)43:2<250::AID-ANR3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 51.Pettit AR, et al. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am J Pathol. 2001;159(5):1689–99. doi: 10.1016/S0002-9440(10)63016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Redlich K, et al. Osteoclasts are essential for TNF-alpha-mediated joint destruction. J Clin Invest. 2002;110(10):1419–27. doi: 10.1172/JCI15582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moller Dohn U, et al. Erosive progression is minimal, but erosion healing rare, in patients with rheumatoid arthritis treated with adalimumab. A 1 year investigator-initiated follow-up study using high-resolution computed tomography as the primary outcome measure. Ann Rheum Dis. 2009;68(10):1585–90. doi: 10.1136/ard.2008.097048. [DOI] [PubMed] [Google Scholar]
- 54•.Dohn UM, et al. No overall progression and occasional repair of erosions despite persistent inflammation in adalimumab-treated rheumatoid arthritis patients: results from a longitudinal comparative MRI, ultrasonography, CT and radiography study. Ann Rheum Dis. 2011;70(2):252–8. doi: 10.1136/ard.2009.123729. [DOI] [PubMed] [Google Scholar]
- 55.Haavardsholm EA, et al. Magnetic resonance imaging findings in 84 patients with early rheumatoid arthritis: bone marrow oedema predicts erosive progression. Ann Rheum Dis. 2008;67(6):794–800. doi: 10.1136/ard.2007.071977. [DOI] [PubMed] [Google Scholar]
- 56.Hetland ML, et al. MRI bone oedema is the strongest predictor of subsequent radiographic progression in early rheumatoid arthritis. Results from a 2-year randomised controlled trial (CIMESTRA). Ann Rheum Dis. 2009;68(3):384–90. doi: 10.1136/ard.2008.088245. [DOI] [PubMed] [Google Scholar]
- 57.Gilbert L, et al. Expression of the osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2alpha A) is inhibited by tumor necrosis factor-alpha. J Biol Chem. 2002;277(4):2695–701. doi: 10.1074/jbc.M106339200. [DOI] [PubMed] [Google Scholar]
- 58.Wei S, et al. IL-1 mediates TNF-induced osteoclastogenesis. Journal of Clinical Investigation. 2005;115(2):282–290. doi: 10.1172/JCI23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jilka RL, et al. Osteoblast programmed cell death (apoptosis): modulation by growth factors and cytokines. J Bone Miner Res. 1998;13(5):793–802. doi: 10.1359/jbmr.1998.13.5.793. [DOI] [PubMed] [Google Scholar]
- 60.Stashenko P, et al. Interleukin-1 beta is a potent inhibitor of bone formation in vitro. J Bone Miner Res. 1987;2(6):559–65. doi: 10.1002/jbmr.5650020612. [DOI] [PubMed] [Google Scholar]
- 61.Hengartner NE, et al. IL-1beta inhibits human osteoblast migration. Mol Med. 2013;19:36–42. doi: 10.2119/molmed.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu XH, et al. Cross-talk between the interleukin-6 and prostaglandin E(2) signaling systems results in enhancement of osteoclastogenesis through effects on the osteoprotegerin/receptor activator of nuclear factor-{kappa}B (RANK) ligand/RANK system. Endocrinology. 2005;146(4):1991–8. doi: 10.1210/en.2004-1167. [DOI] [PubMed] [Google Scholar]
- 63.Walsh NC, et al. Osteoblast function is compromised at sites of focal bone erosion in inflammatory arthritis. J Bone Miner Res. 2009;24(9):1572–85. doi: 10.1359/jbmr.090320. [DOI] [PubMed] [Google Scholar]
- 64••.Matzelle MM, et al. Resolution of inflammation induces osteoblast function and regulates the Wnt signaling pathway. Arthritis Rheum. 2012;64(5):1540–50. doi: 10.1002/art.33504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diarra D, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007;13(2):156–63. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- 66.Heiland GR, et al. Neutralisation of Dkk-1 protects from systemic bone loss during inflammation and reduces sclerostin expression. Ann Rheum Dis. 2010;69(12):2152–9. doi: 10.1136/ard.2010.132852. [DOI] [PubMed] [Google Scholar]
- 67••.Chen XX, et al. Sclerostin inhibition reverses systemic, periarticular and local bone loss in arthritis. Ann Rheum Dis. 2013;72(10):1732–6. doi: 10.1136/annrheumdis-2013-203345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Finzel S, et al. Repair of bone erosions in rheumatoid arthritis treated with tumour necrosis factor inhibitors is based on bone apposition at the base of the erosion. Ann Rheum Dis. 2011;70(9):1587–93. doi: 10.1136/ard.2010.148395. [DOI] [PubMed] [Google Scholar]
- 69••.Finzel S, et al. Interleukin-6 receptor blockade induces limited repair of bone erosions in rheumatoid arthritis: a micro CT study. Ann Rheum Dis. 2013;72(3):396–400. doi: 10.1136/annrheumdis-2011-201075. [DOI] [PubMed] [Google Scholar]
- 70.Lories RJ, Schett G. Pathophysiology of new bone formation and ankylosis in spondyloarthritis. Rheum Dis Clin North Am. 2012;38(3):555–67. doi: 10.1016/j.rdc.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 71••.Uderhardt S, et al. Blockade of Dickkopf (DKK)-1 induces fusion of sacroiliac joints. Ann Rheum Dis. 2010;69(3):592–7. doi: 10.1136/ard.2008.102046. [DOI] [PubMed] [Google Scholar]
- 72.Haynes KR, et al. Excessive bone formation in a mouse model of ankylosing spondylitis is associated with decreases in Wnt pathway inhibitors. Arthritis Res Ther. 2012;14(6):R253. doi: 10.1186/ar4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Appel H, et al. Altered skeletal expression of sclerostin and its link to radiographic progression in ankylosing spondylitis. Arthritis Rheum. 2009;60(11):3257–62. doi: 10.1002/art.24888. [DOI] [PubMed] [Google Scholar]
- 74.Chen HA, et al. Association of bone morphogenetic proteins with spinal fusion in ankylosing spondylitis. J Rheumatol. 2010;37(10):2126–32. doi: 10.3899/jrheum.100200. [DOI] [PubMed] [Google Scholar]
- 75.van der Heijde D, et al. Assessment of radiographic progression in the spines of patients with ankylosing spondylitis treated with adalimumab for up to 2 years. Arthritis Res Ther. 2009;11(4):R127. doi: 10.1186/ar2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schett G, et al. Tumor necrosis factor alpha and RANKL blockade cannot halt bony spur formation in experimental inflammatory arthritis. Arthritis Rheum. 2009;60(9):2644–54. doi: 10.1002/art.24767. [DOI] [PubMed] [Google Scholar]
- 77.Mei Y, et al. Increased serum IL-17 and IL-23 in the patient with ankylosing spondylitis. Clin Rheumatol. 2011;30(2):269–73. doi: 10.1007/s10067-010-1647-4. [DOI] [PubMed] [Google Scholar]
- 78.Duan Z, et al. Interleukin-23 receptor genetic polymorphisms and ankylosing spondylitis susceptibility: a meta-analysis. Rheumatol Int. 2012;32(5):1209–14. doi: 10.1007/s00296-010-1769-7. [DOI] [PubMed] [Google Scholar]
- 79••.Sherlock JP, et al. IL-23 induces spondyloarthropathy by acting on ROR-gammat+ CD3+CD4-CD8- entheseal resident T cells. Nat Med. 2012;18(7):1069–76. doi: 10.1038/nm.2817. [DOI] [PubMed] [Google Scholar]
- 80.Sherlock JP, Buckley CD, Cua DJ. The critical role of interleukin-23 in spondyloarthropathy. Mol Immunol. 2013;57(1):38–43. doi: 10.1016/j.molimm.2013.06.010. [DOI] [PubMed] [Google Scholar]