Abstract
Recent studies on the effect of stress on modulation of fear memory in our laboratory have uncovered endogenous opioid and adrenergic based modulation systems, working in concert, that limit the strengthening or weakening of newly acquired fear memory during consolidation under conditions of mild or intense stress, respectively. The present study sought to determine if similar stress-dependent modulation, mediated by endogenous opioid and adrenergic systems, occurs during reconsolidation of newly retrieved fear memory. Rats underwent contextual fear conditioning followed 24 hr later by reactivation of fear memory; a retention test was administered the next day. Stress was manipulated by varying duration of recall of fear memory during reactivation. In the first experiment, vehicle or the opioid-receptor blocker naloxone was administered immediately after varied durations (30 or 120 sec) of reactivation. The results indicate that 1) reactivation, in the absence of drug, has a marked effect on freezing behavior—as duration of reactivation increases from 30 to 120 sec, freezing behavior and presumably fearinduced stress increases and 2) naloxone, administered immediately after 30 sec (mild stress) or 120 sec (intense stress) of reactivation, enhances or impairs retention, respectively, the next day. In the second experiment, naloxone and the ß-adrenergic blocker propranolol were administered either separately or in combination immediately after 120 sec (intense stress) reactivation. The results indicate that separate administration of propranolol and naloxone impairs retention, while the combined administration fails to do so. Taken together the results of the two experiments are consistent with a protective mechanism, mediated by endogenous opioid and adrenergic systems working in concert, that limits enhancement and impairment of newly retrieved fear memory during reactivation in a stress-dependent manner.
Keywords: reconsolidation, modulation, updating, stress, opioid, adrenergic, retrieval
1. Introduction
Consolidation of fear memory, the process by which newly acquired memories related to fear are stabilized and stored, is not a one-time process; it may recur during subsequent retrieval of the initially consolidated memory by a process referred to as memory reconsolidation (Abel & Lattal, 2001; Sara, 2000). Various post-retrieval pharmacological manipulations previously used to characterize consolidation have been used to determine the cellular and molecular mechanisms underlying reconsolidation. These studies have indicated that, like storage and modulation of newly acquired fear memory during consolidation, de novo protein synthesis (Nader, Schafe, & Le Doux, 2000) and stress-related hormones and transmitters, including glucocorticoids (Cordero, Merino, & Sandi, 1998; Tronel & Alberini, 2007), norepinephrine (Debiec & LeDoux, 2004; Przybyslawski, Roullet, & Sara, 1999), opioids (Meilandt, Barea- Rodriguez, Harvey, & Martinez, Jr., 2004) and acetylcholine (Boccia, Acosta, Blake, & Baratti, 2004), are essential for storage and modulation of newly retrieved fear memory during reconsolidation.
Recent studies on stress-dependent modulation of newly acquired fear memory in our laboratory, using ß-adrenergic and opioid-receptor blockers, have uncovered endogenous adrenergic and opioid based modulation systems, working in concert, that limit the strengthening or weakening of newly acquired fear memory during consolidation under conditions of mild or intense stress, respectively (Schneider et al., 2009; Schneider, Simson, Atapattu, & Kirby, 2011). The present study sought to determine if similar stress-dependent modulation, via adrenergic and opioid modulatory systems working concurrently, occurs during reconsolidation of newly retrieved fear memory. If modulation of newly retrieved memory does indeed parallel modulation of newly acquired memory, then modulation of newly retrieved memory (like modulation of newly acquired memory) should depend on both the level of stress as well as the nature of the receptor blockade accompanying it. Specifically, the following a priori predictions concerning modulation of newly retrieved fear memory can be made:
As with modulation of newly acquired memory, opioid-receptor blockade after mild stress should enhance modulation of newly retrieved memory, while opioid-receptor blockade after intense stress should impair modulation of newly retrieved memory (owing to the blockade-induced loss of a "protective", stress-dependent opioid-based modulation system that normally "limits" enhancement or impairment of memory under conditions of mild and intense stress, respectively).
As with modulation of newly acquired memory, ß-adrenergic receptor blockade after intense stress should impair modulation of newly retrieved memory (owing to the blockade-induced loss of a "protective", stress-dependent adrenergic-based modulation system that normally limits impairment of memory under conditions of intense stress).
As with modulation of newly acquired memory, concurrent opioid and β-adrenergic receptor blockade after intense stress should prevent impairment of modulation of newly retrieved memory suggesting a non-additive interaction between the two modulation systems under conditions of intense stress.
The present study tested these predictions. In contrast to previous experiments on stress-dependent modulation of newly acquired memory in which the level of stress was manipulated shortly after training via stressors such as predator exposure (Diamond et al., 2006), restraint (Klenerova et al., 2003) or forced swim (Schneider et al., 2011), the present study focused on stress-dependent modulation of newly retrieved memory utilizing reactivation of the retrieved fear memory itself (24 hr after training) as the stressor. As an agent of stress, reactivated fear memory (freezing behavior) has been validated in studies using activation of the hypothalamo-pituitary- adrenal axis as a physiological index of stress intensity (Antoniadis & McDonald, 1999). During retrieval of fear-related memory, stress-related hormones and neurotransmitters, including glucocorticoids, norepinephrine (NE) and opioids, act in limbic nuclei, including the amygdala and hippocampus, to regulate the strength of retention (Meilandt et al., 2004; Murchison et al., 2004; Roozendaal, Hahn, Nathan, de Quervain, & McGaugh, 2004; de Quervain, Roozendaal, & McGaugh, 1998). Thus, reactivated fear memory not only meets the criteria of a stressor but produces neurochemical effects (particularly with respect to glucocorticoids, adrenergic and opioid action) consistent with a potential modulator of retention. In the present study pharmacological blockade was initiated immediately after reactivation of fear memory; a retention test to measure memory strength was administered the next day.
2. Material and Methods
2.1. Subjects
The subjects (N = 99) were male Long-Evans hooded rats weighing 240–280 g at the start of the experiment. The rats were housed two to a cage with access to food and water ad libitum. The colony room was maintained at 20 °C and was illuminated on a 12-hr light–dark cycle (lights on at 9:00 a.m.). All experiments were conducted between 10:00 a.m. and 12:00 p.m. at the nadir of the diurnal cycle for glucocorticoids (Krieger, 1974) minimizing the extent to which fluctuations in endogenous levels of stress-dependent hormones influence the results. The experimental protocol was approved by Swarthmore College's Institutional Animal Care and Use Committee and was in compliance with the National Research Council Guide for the Care and Use of Laboratory Animals.
2.2. Apparatus
Rats were trained and tested in a fear conditioning apparatus that consisted of a trough-shaped compartment (42 L × 28 H × 20 W cm at the top; 42 L × 28 H × 8 W cm at the base) with a hinged plastic top and stainless steel plates making up the floor and sidewalls. A constant-current Lafayette Master Shocker (Model 2400)SS; Lafayette, IN) was connected to the floor of the compartment. The training apparatus was located in a quiet, dimly illuminated room, and was cleaned with water followed by acetone before all training and testing trials.
2.3. Drug administration and drug doses
The rats were injected intraperitoneally with vehicle (0.9% saline, 2 ml/kg), the ß-adrenergic antagonist dl-propranolol hydrochloride (10 mg/kg, 2 ml/kg, Sigma Chemical), the opioid antagonist naloxone hydrochloride (3 mg/kg, 2 ml/kg, Sigma Chemical) or a mixture of propranolol (10 mg/kg) and naloxone (3 mg/kg) in a 2 ml/kg injection volume. The doses of propranolol and naloxone chosen were similar to doses that have previously been shown to be effective in studies on memory modulation (McGaugh, Introini-Collison, & Nagahara, 1988; Przybyslawski et al., 1999; Schneider et al., 2009; Schneider et al., 2011).
2.4. Experimental Procedure and Treatment
Two experiments were conducted. The timeline for each experiment was as follows: contextual fear conditioning (Day 1), reactivation of fear memory followed immediately by administration of vehicle or drug (Day 2) and a retention test (Day 3). Contextual fear conditioning consisted of placing rats in a dark compartment for 120 sec followed by a single footshock (1.0 mA, 0.5 sec); reactivation of fear memory consisted of returning the animals to the dark compartment for brief durations (see below for details) in the absence of shock; the retention test consisted of returning the animals to the dark compartment for 6 min in the absence of shock. Freezing behavior, defined by the cessation of movement other than that associated with respiratory function, was monitored by an observer blind to the treatment conditions and served as a measure of fear during reactivation and the retention test.
2.4.1. Experiment 1: Stress-dependent effect of naloxone on newly retrieved fear memory
The first experiment (Experiment 1) was aimed at determining whether activation of the endogenous opioid system, like its effect on modulation of newly acquired fear memory in previous studies (Schneider et al., 2009), limits enhancement and impairment of newly retrieved fear memory under conditions of weak and intense stress, respectively. Specifically, the effect of naloxone on modulation of newly-retrieved (reactivated) fear memory was determined under conditions of mild and intense stress.
Twenty-four hours after training, animals were randomly assigned to two groups and were placed in the conditioning chamber (to reactivate fear memory) for either 30 sec (mild stress) or 120 sec (intense stress) in the absence of shock. Immediately thereafter each group was divided into two subgroups and was administered vehicle (Veh) or naloxone (Nal), thus yielding the following four groups: Veh-30 sec (N=8), Nal-30 sec (N=7), Veh-120 sec (N=8), Nal-120 sec (N=8). The animals were tested for retention the following day. The reactivation durations were based on previous studies in which similar exposure times prior to shock during training increased strength of fear conditioning as a function of exposure time (Fanselow, 1990).
Animals in a non-reactivation (NR) control condition received the same treatment as above with one exception: in lieu of reactivation (i.e., in lieu of exposure to the conditioning chamber) 24 hr after training, the animals were administered vehicle (Veh-NR group, N=6) or naloxone (Nal-NR group, N=6) in a dimly lit room.
2.4.2. Experiment 2: Effect of naloxone and propranolol administered separately or in combination on modulation of newly retrieved fear memory under conditions of intense stress
The second experiment (Experiment 2) was aimed at determining whether concurrent activation of endogenous opioid and adrenergic systems, like its effect on modulation of newly acquired fear memory in previous studies (Schneider et al. 2011), limits impairment of newly retrieved fear memory under conditions of intense stress. Specifically, the effect of naloxone and propranolol, administered separately or in combination, on modulation of newly retrieved fear memory was determined under conditions of intense stress.
Twenty-four hours after training, animals were placed in the conditioning chamber and fear memory was reactivated for 120 sec (intense stress). Immediately thereafter the animals were randomly assigned to one of four conditions that received vehicle (Veh), naloxone (Nal), propranolol (Pro), or a combination of naloxone and propranolol yielding the following four groups: Veh-120 sec (N=8), Pro-120 sec (N=8), Nal-120 sec (N=8), and Nal+Pro-120 sec (N=8), respectively. The animals were tested for retention the following day.
Animals in a non-reactivation (NR) control condition received the same treatment and drug doses, except that 24 hr after training, in lieu of reactivation, they were administered vehicle (Veh), naloxone (Nal), propranolol (Pro) or a combination of naloxone and propranolol in a dimly lit room yielding the following four non-reactivation groups: Veh-NR (N=6), Nal-NR (N=6), Pro-NR (N=6), and Nal+Pro-NR (N=6).
2.5. Statistical Analysis
Data were analyzed with one- or two-way analyses of variance (ANOVA) which, if statistically significant, were followed by pair-wise comparisons (Fisher's Least Significance Difference (LSD) tests); p values (2-tailed) less than 0.05 were taken as statistically significant.
3. Results
3.1. Experiment 1: Stress-dependent effect of naloxone on newly retrieved fear memory
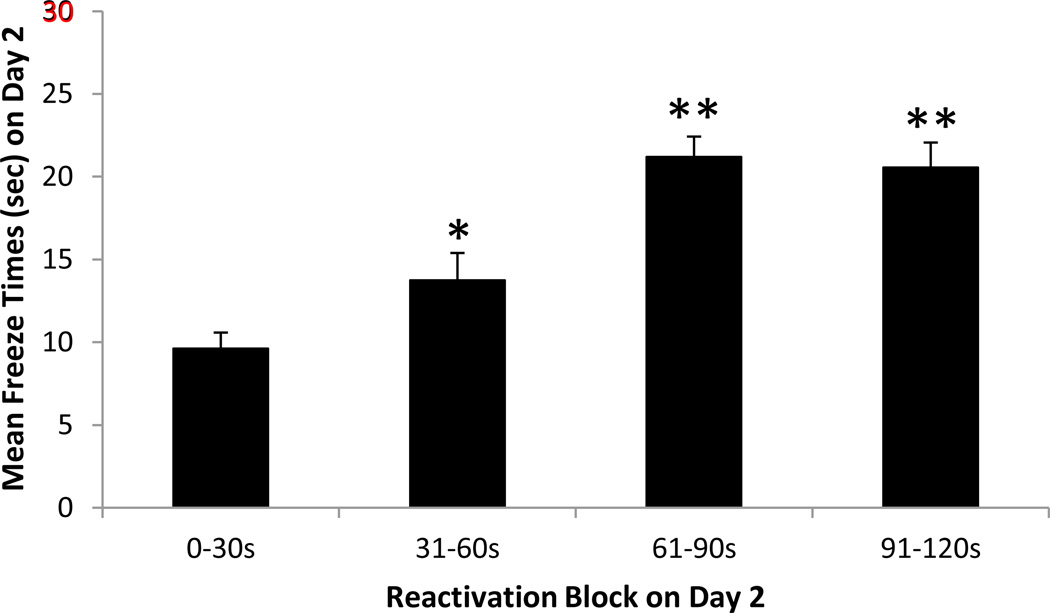
The results of Experiment 1 in regard to the effect of reactivation per se on freezing behavior (Day 2), that is, in regard to the effect of reactivation on freezing behavior prior to injection of vehicle or naloxone, are shown in Figure 1. It can be seen that mean freezing behavior, monitored in 30-sec blocks, increased markedly over the course of the 120-sec reactivation session (F(3,45) = 16.99, p < 0.001); all pair-wise comparisons (except 0–30 sec vs 31–60 sec and 61–90 sec vs 91–120 sec) indicated significant differences (p < 0.05). These results indicate that fear memory---and presumably fear-induced stress---increased during the reactivation session.
Figure 1. Reactivation of fear memory.
Freezing behavior (mean ± SEM) in seconds on the reactivation trial monitored in 30-sec blocks for the 120 sec groups (N = 16). * p < 0.05 vs. 0– 30s group, ** p < 0.01 vs. 0–30s and 31–60s groups by post-hoc Fisher's LSD tests following one-way repeated measures ANOVA.
On the other hand, although freezing behavior increased over the course of the 120-sec reactivation session it did not differ, within a given exposure duration (that is, within the 0–30 sec or within 91–120 sec block), between vehicle and corresponding naloxone groups prior to injections: Veh-30 sec vs Nal-30 sec (10.1 ± 1.7 vs 9.1 ± 0.9; p > 0.05); Veh-120 sec vs Nal-120 sec (20.8 ± 2.5 vs 20.4 ± 1.8; p > 0.05). These results rule out the possibility that differences in freezing behavior prior to injections account for the differences between vehicle and corresponding naloxone groups during the subsequent retention test.
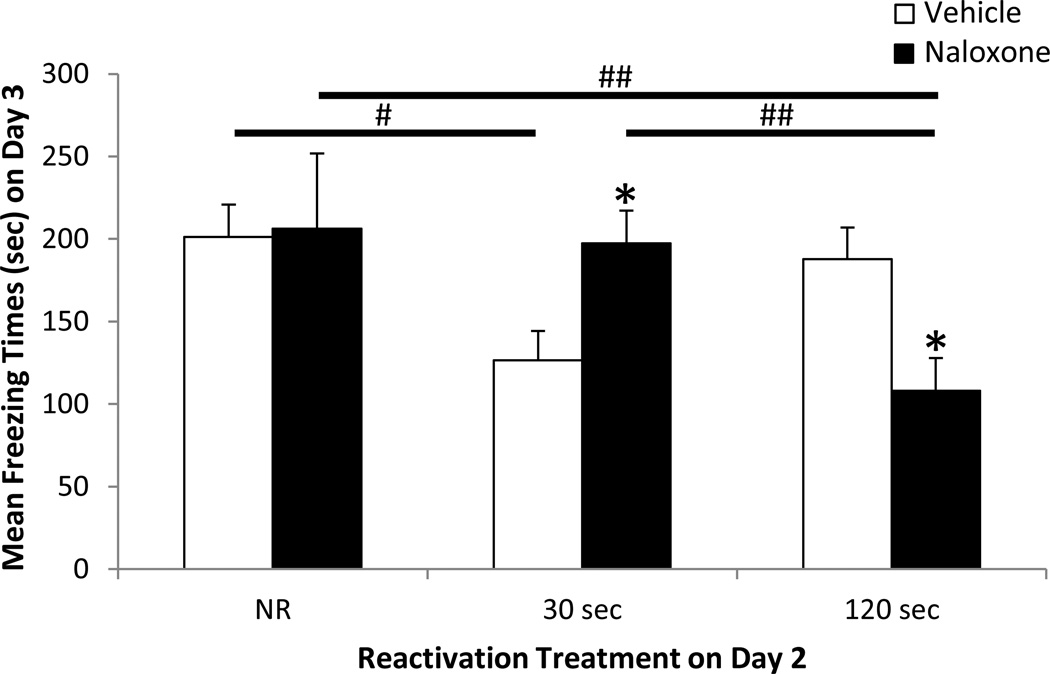
The results of Experiment 1 regarding retention on the subsequent test (Day 3) are shown in Figure 2 and demonstrate a significant drug×reactivation interaction by two-way ANOVA (F(2,37) = 5.40, p < 0.01). It can be seen in the vehicle groups that reactivation of newly retrieved memory itself (i.e., in the absence of pharmacological manipulation) had a marked effect on subsequent retention. As evidenced by decreased mean freezing behavior in the Veh-30 sec condition, 30 sec of reactivation impaired retention relative to non-reactivated vehicle controls (p < 0.05); 120 sec of reactivation (Veh-120 sec) offset the impairment, thereby restoring (i.e., updating) retention to the level of non-reactivated controls (p > 0.05).
Figure 2. Retention of fear memory: Effect of naloxone administered after varied durations of reactivation (30 or 120 sec).
Freezing behavior (mean ± SEM) in seconds during the retention test for the non-reactivation (NR) groups [Vehicle (N = 6), Naloxone (N = 6)], 30 sec reactivation groups [Vehicle (N = 8), Naloxone (N = 7)], and 120 sec reactivation groups [Vehicle (N = 8), Naloxone (N = 8)]. * p < 0.05 vs. corresponding vehicle controls, # p < 0.05 vs. NR controls, ## p < 0.01 vs. NR controls and vs. 30s group by post-hoc Fisher's LSD tests following two-way ANOVA.
A much different picture emerged regarding the effect of reactivation on retention in the presence of naloxone. As shown in Figure 2, compared to vehicle controls, naloxone a) increased retention when administered after 30 sec of reactivation (the period during which reactivation in the absence of naloxone decreased retention), and b) decreased retention when administered after 120 sec of reactivation (the period during which reactivation in the absence of naloxone restored retention). Specifically, mean freezing behavior increased in the Nal-30 sec group relative to Veh-30 sec controls (p < 0.05). In contrast, and in support of a stress-dependent bidirectional effect of naloxone, mean freezing behavior decreased in the Nal-120 sec group relative to its vehicle control (Veh-120 sec, p < 0.05) and its non-reactivation control (Nal- NR, p < 0.01). Importantly, naloxone had no effect on subsequent retention when administered in the absence of reactivation: mean freezing behavior in the Nal-NR group and Veh-NR group did not differ significantly (p > 0.05). The results of Experiment 1 thus indicate that 1) newly retrieved fear memory (and presumably fear-induced stress) increases during reactivation, and 2) naloxone, administered after 30 sec or 120 sec reactivation, strengthens or weakens retention of newly retrieved fear memory, respectively.
3.2. Experiment 2: Effect of naloxone and propranolol administered separately or in combination on modulation of newly retrieved fear memory under conditions of intense stress
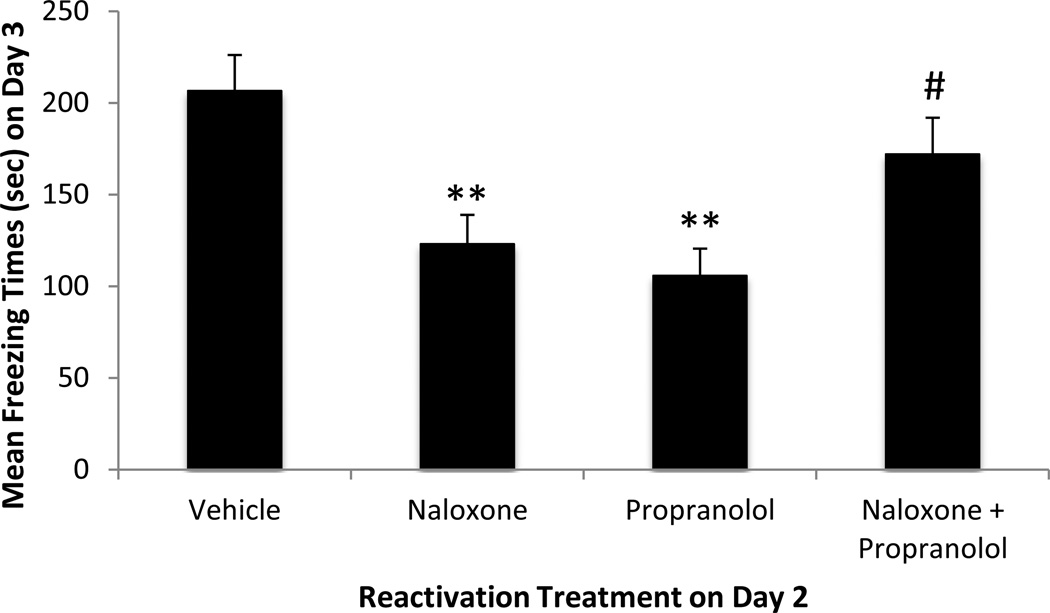
The results of Experiment 2 in regard to retention (Day 3) are shown in Figure 3. It can be seen that separate administration of propranolol or naloxone immediately after 120-sec of reactivation of newly retrieved fear memory decreased subsequent retention (F(3,28) = 6.78, p < 0.01); mean freezing behavior in the naloxone and the propranolol conditions was significantly lower than mean freezing in the vehicle condition (p < 0.01). In contrast, the combined administration of naloxone + propranolol produced no such decrease in retention; mean freezing behavior in the naloxone + propranolol condition did not differ significantly from the vehicle condition (p > 0.05). Importantly, naloxone, propranolol, and naloxone combined with propranolol administered in the non-reactivation controls had no effect on retention: mean freezing behavior in the Nal-NR (189.0 ± 36.3), Pro-NR (170.3 ± 39.3), and Nal+Pro-NR groups (172.8 ± 25.2), did not differ significantly from that of the Veh-NR group (200.3 ± 19.7; F(3,20) = 0.21, p > 0.05). The results of Experiment 2 thus indicate that 1) the ability of ß-adrenergic or opioid-receptor blockade to impair subsequent retention is critically dependent upon reactivation-induced stress and 2) concurrent blockade of endogenous adrenergic- and opioid-based modulation systems under stressful conditions is protective of newly retrieved memory.
Figure 3. Retention of fear memory: Effect of naloxone and propranolol administered separately or in combination after reactivation (120 sec).
Freezing behavior (mean ± SEM) in seconds during the retention test for the Vehicle, Naloxone, Propranolol, Naloxone+Propranolol groups (N = 8 in each group). ** p < 0.01 vs. vehicle group, # p < 0.05 vs. propranolol group by post-hoc Fisher's LSD tests following one-way ANOVA.
4. Discussion
The present findings indicate that exposure to the apparatus during reactivation in the absence of pharmacological manipulation had two effects on retention of fear: 1) an immediate effect—as exposure to the apparatus increased, freezing behavior and presumably retention of fear increased during reactivation, and 2) a delayed effect—as exposure to the apparatus increased, freezing behavior and presumably retention of fear increased, 24 hr later, during a subsequent retention test.
One possible explanation for the immediate effect that is consistent with the literature is based on evidence suggesting that exposure to the apparatus triggers an orienting response (specifically, activation of an attentional network including the hypothalamo-pituitary-adrenal axis) that increases over time (see (Sara, 2000) for detailed discussion). This increase would account not only for the increased retrieval of fear during reactivation but also for the stress-dependent effect that the increased fear presumably has, 24 hr later, on subsequent retention.
A much different picture emerged regarding the delayed effect of reactivation on retention 24 hr later. To account for the delayed results—specifically, that 30 sec exposure impaired subsequent retention while 120 sec offset the impairment—it seems reasonable to assume that during the reactivation experience the newly retrieved fear memory passes through a brief period of lability or updating (see (Alberini, 2011) for detailed discussion). Consequently, because retrieved fear memory during the 30 sec exposure was relatively weak, minimal updating occurred and weak retention was observed the next day; in contrast, because retrieved fear memory during the 120 sec exposure was relatively strong, greater updating occurred and enhanced retention was observed the next day.
Finally, with regard to the effect of reactivation in the presence of pharmacological manipulation on subsequent retention 24 hr later, the results of the two experiments suggest that newly retrieved fear memory during reactivation, like newly acquired fear memory during consolidation (Schneider et al., 2009; Schneider et al., 2011), is modulated by stress-dependent activation of adrenergic and opioid systems. The finding that naloxone enhanced or impaired retention in a stress-dependent manner when administered after reactivation (Experiment 1) — and that its effectiveness was dependent on the duration of reactivation—is consistent with a protective mechanism, mediated by endogenous opioids, that limits enhancement and impairment of newly retrieved memory under mild and intense stress, respectively.
By the same token, the finding that the ß-adrenergic receptor blocker propranolol administered after reactivation impaired retention (Experiment 2)—while the combined administration of propranolol and naloxone failed to do so—is consistent with a protective mechanism mediated by endogenous opioid and adrenergic systems that work in concert to prevent or limit impairment of newly retrieved fear memory under intense stress. Finally, the finding that naloxone (Experiment 1) and naloxone and propranolol (Experiment 2) administered in the absence of reactivation had no effect on retention the next day indicates that the drug effects on retention are critically dependent on reactivation-induced opioid and adrenergic modulation and not merely the result of drug effects alone on subsequent retention.
Previous studies of stress-dependent modulation of newly acquired fear memory utilized forced swim as the stressor during consolidation (Schneider et al., 2009; Schneider et al., 2011); the present study utilized reactivation of newly retrieved fear memory itself as the stressor during reconsolidation. Consequently, exposure to the stressor in the present study entails exposure to a previously conditioned stimulus (CS--the conditioning chamber) in the absence of shock, a procedure akin to extinction training. Although this raises the possibility that extinction, not exposure to stress and reconsolidation, underlies the effect of naloxone and propranolol on modulation of newly retrieved fear memory, the exposure durations to the stressor/CS appear to be too brief to involve extinction (Lee, Milton, & Everitt, 2006) as evidenced by the increase rather than a decrease in freezing behavior (retention) with increasing durations in the absence of drugs. Moreover, the fact that the results of the present study on stress-dependent modulation of newly retrieved fear memory parallel those of our previous studies on stress-dependent modulation of newly acquired fear memory (in which no opportunity for extinction exists) lends further support to reactivation-induced stress determining the present effects.
If the same opioid-adrenergic model used to explain the effect of stress on modulation of newly acquired fear memory applies to the effect of stress on modulation of newly retrieved fear memory, then in the present study, by isolating either modulatory system through pharmacological blockade of the other, the unblocked system acts unopposed to modulate newly retrieved memory. Thus, in the presence of opioid receptor blockade (Experiment 1), the unblocked adrenergic system (the "excitatory" modulation system) acts unopposed; depending on the level of stress, it either activates (mild stress) or over-activates (intense stress) brain sites modulating memory. This would account for the enhancement (after 30 sec of reactivated fear memory) and impairment (after 120 sec of reactivated fear memory) of retention produced by opioid-receptor blockade.
Similarly, in the presence of adrenergic-receptor blockade (Experiment 2), the unblocked opioid system (the "inhibitory" modulation system) acts unopposed to “over-suppress” brain sites modulating memory and impair retention under intense stress (120 sec of reactivated fear memory). In the presence of opioid-receptor blockade under intense stress, the unblocked adrenergic system “over-activates” modulatory brain sites, thus impairing retention. In contrast, the combined administration of propranolol and naloxone, by blocking both modulatory systems, neither over-suppresses nor over-activates modulation sites; consequently, no impairment of retention occurs.
The finding in the present study that propranolol, administered after reactivation, is effective in producing impairment of retention has been demonstrated in previous studies utilizing both aversive (passive avoidance and contextual fear conditioning) and non-aversive (spatial radialmaze training and natural reinforcement) tasks (Milton, Lee, & Everitt, 2008; Muravieva & Alberini, 2010; Przybyslawski et al., 1999). These findings, particularly with regard to the effect of propranolol on retention of a non-aversive task in which animals are not under fear-related stress prior to the administration of propranolol indicate that propranolol’s effectiveness in producing impairment of retention is not limited to aversive conditioning but rather is likely the result of propranolol affecting brain sites shared by reactivation of both aversive and non-aversive tasks. One brain site in which the adrenergic system might act to regulate memory modulation under aversive and non-aversive conditions in the present and previous studies is the hippocampus wherein retrieval of contextual and spatial memory is regulated, at least in part, by the adrenergic system (Murchison et al., 2004; Roozendaal et al., 2004; Schutsky, Ouyang, Castelino, Zhang, & Thomas, 2011). Of course, before conclusions can be drawn with regard to the involvement of specific brain sites in mediating the effect of stress on memory modulation in the present study, local administration of adrenergic and opioid-receptor antagonists into the brain, paralleling the systemic administration studies presented herein, are necessary.
Both adrenergic and opioid-receptor antagonists have been proposed as potential therapies for posttraumatic stress disorder (Albucher & Liberzon, 2002; Pitman et al., 2002), a disorder characterized by persistent and intrusive negative memories of a stressful event. The present and previous results support the efficacy of the two receptor antagonists, administered after newly retrieved as well as newly acquired fear memory, in attenuating pathological memories but caution that their effectiveness may be stress-dependent and enhanced by single, rather than simultaneous, administration.
Highlights.
activation of opioid and adrenergic systems protects newly-retrieved memory
pharmacological blockade of either modulatory system results in loss of protection
concurrent blockade of opioid and adrenergic systems restores protection
Acknowledgements
This work was supported by a grant from the Howard Hughes Medical Institute to Swarthmore College and NIH grant DA 20126 to L. Kirby. We thank Barry Schwartz for his incisive comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: Least Significant Difference (LSD); vehicle (veh); naloxone (nal); propranolol (pro); nonreactivation (NR); analysis of variance (ANOVA); standard error of the mean (SEM)
Contributor Information
Allen M. Schneider, Email: aschnei1@swarthmore.edu.
Peter E. Simson, Email: Simsonpe@Miamioh.edu.
Caitlin M. Daimon, Email: caitlindaimon@gmail.com.
Jakob Mrozewski, Email: j.mrozewski1@gmail.com.
Nicholas M. Vogt, Email: nickvogt1@gmail.com.
John Keefe, Email: jack.keefe@gmail.com.
Lynn G. Kirby, Email: lkirby@temple.edu.
Reference List
- Abel T, Lattal KM. Molecular mechanisms of memory acquisition, consolidation and retrieval. Current Opinion in Neurobiology. 2001;11:180–187. doi: 10.1016/s0959-4388(00)00194-x. [DOI] [PubMed] [Google Scholar]
- Alberini CM. The role of reconsolidation and the dynamic process of long-term memory formation and storage. Front Behav.Neurosci. 2011;5:12. doi: 10.3389/fnbeh.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albucher RC, Liberzon I. Psychopharmacological treatment in PTSD: a critical review. Journal of Psychiatric Research. 2002;36:355–367. doi: 10.1016/s0022-3956(02)00058-4. [DOI] [PubMed] [Google Scholar]
- Antoniadis EA, McDonald RJ. Discriminative fear conditioning to context expressed by multiple measures of fear in the rat. Behavioral Brain Research. 1999;101:1–13. doi: 10.1016/s0166-4328(98)00056-4. [DOI] [PubMed] [Google Scholar]
- Boccia MM, Acosta GB, Blake MG, Baratti CM. Memory consolidation and reconsolidation of an inhibitory avoidance response in mice: effects of i.c.v. injections of hemicholinium-3. Neuroscience. 2004;124:735–741. doi: 10.1016/j.neuroscience.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Cordero MI, Merino JJ, Sandi C. Correlational relationship between shock intensity and corticosterone secretion on the establishment and subsequent expression of contextual fear conditioning. Behavioral Neuroscience. 1998;112:885–891. doi: 10.1037//0735-7044.112.4.885. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129:267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Campbell AM, Park CR, Woodson JC, Conrad CD, Bachstetter AD, et al. Influence of predator stress on the consolidation versus retrieval of longterm spatial memory and hippocampal spinogenesis. Hippocampus. 2006;16:571–576. doi: 10.1002/hipo.20188. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Factors Governing One-Trial Contextual Conditioning. Animal Learning & Behavior. 1990;18:264–270. [Google Scholar]
- Klenerova V, Jurcovicova J, Kaminsky O, Sida P, Krejci I, Hlinak Z, et al. Combined restraint and cold stress in rats: effects on memory processing in passive avoidance task and on plasma levels of ACTH and corticosterone. Behavioral Brain Research. 2003;142:143–149. doi: 10.1016/s0166-4328(02)00401-1. [DOI] [PubMed] [Google Scholar]
- Krieger DT. Food and water restriction shifts corticosterone, temperature, activity and brain amine periodicity. Endocrinology. 1974;95:1195–1201. doi: 10.1210/endo-95-5-1195. [DOI] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Reconsolidation and extinction of conditioned fear: inhibition and potentiation. Journal of Neuroscience. 2006;26:10051–10056. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL, Introini-Collison IB, Nagahara AH. Memory-enhancing effects of posttraining naloxone: involvement of beta-noradrenergic influences in the amygdaloid complex. Brain Research. 1988;446:37–49. doi: 10.1016/0006-8993(88)91294-2. [DOI] [PubMed] [Google Scholar]
- Meilandt WJ, Barea-Rodriguez E, Harvey SA, Martinez JL., Jr Role of hippocampal CA3 mu-opioid receptors in spatial learning and memory. J.Neurosci. 2004;24:2953–2962. doi: 10.1523/JNEUROSCI.5569-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton AL, Lee JL, Everitt BJ. Reconsolidation of appetitive memories for both natural and drug reinforcement is dependent on {beta}-adrenergic receptors. Learning and Memory. 2008;15:88–92. doi: 10.1101/lm.825008. [DOI] [PubMed] [Google Scholar]
- Muravieva EV, Alberini CM. Limited efficacy of propranolol on the reconsolidation of fear memories. Learning and Memory. 2010;17:306–313. doi: 10.1101/lm.1794710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison CF, Zhang XY, Zhang WP, Ouyang M, Lee A, Thomas SA. A distinct role for norepinephrine in memory retrieval. Cell. 2004;117:131–143. doi: 10.1016/s0092-8674(04)00259-4. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Sanders KM, Zusman RM, Healy AR, Cheema F, Lasko NB, et al. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biological Psychiatry. 2002;51:189–192. doi: 10.1016/s0006-3223(01)01279-3. [DOI] [PubMed] [Google Scholar]
- Przybyslawski J, Roullet P, Sara SJ. Attenuation of emotional and nonemotional memories after their reactivation: role of beta adrenergic receptors. Journal of Neuroscience. 1999;19:6623–6628. doi: 10.1523/JNEUROSCI.19-15-06623.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Hahn EL, Nathan SV, de Quervain DJ, McGaugh JL. Glucocorticoid effects on memory retrieval require concurrent noradrenergic activity in the hippocampus and basolateral amygdala. J.Neurosci. 2004;24:8161–8169. doi: 10.1523/JNEUROSCI.2574-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ. Retrieval and reconsolidation: toward a neurobiology of remembering. Learning and Memory. 2000;7:73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- Schneider AM, Simson PE, Atapattu RK, Kirby LG. Stress-dependent impairment of passive-avoidance memory by propranolol or naloxone. Pharmacology, Biochemistry & Behavior. 2011;98:539–543. doi: 10.1016/j.pbb.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider AM, Simson PE, Spiller K, Adelstein J, Vacharat A, Short KR, et al. Stress-dependent enhancement and impairment of retention by naloxone: evidence for an endogenous opioid-based modulatory system protective of memory. Behavioral Brain Research. 2009;205:290–293. doi: 10.1016/j.bbr.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutsky K, Ouyang M, Castelino CB, Zhang L, Thomas SA. Stress and glucocorticoids impair memory retrieval via beta2-adrenergic, Gi/o-coupled suppression of cAMP signaling. Journal of Neuroscience. 2011;31:14172–14181. doi: 10.1523/JNEUROSCI.2122-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronel S, Alberini CM. Persistent disruption of a traumatic memory by postretrieval inactivation of glucocorticoid receptors in the amygdala. Biological Psychiatry. 2007;62:33–39. doi: 10.1016/j.biopsych.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]