Abstract
Background
The Mechanistic Target of Rapamycin (mTOR) pathway is a major regulator of cell immunity and metabolism. mTOR is a well-known suppressor of tissue rejection in organ transplants, however, it has other non-immune functions including in the cardiovascular system, where it is a regulator of heart hypertrophy and locally, in coated vascular stents, inhibits vascular wall cell growth and hence neointimal formation/restenosis. Because the mTOR pathway plays major roles in normal cell growth, metabolism and survival, we hypothesized that inhibiting it with rapamycin, prior to an acute myocardial ischemia-reperfusion injury (IRI), would confer cardioprotection by virtue of slowing down cardiac function and metabolism.
Methods
Yorkshire pigs received orally either placebo or 4 mg/day rapamycin for 7 days before the IRI. All animals underwent median sternotomy and the mid-left anterior descending coronary artery was occluded for 60 min followed by 120 min of reperfusion. Left ventricular pressure-volume data was collected throughout the operation. The ischemic and infarcted areas were determined by monastral blue and triphenyltetrazolium chloride staining, respectively and plasma cardiac troponin I concentration. mTOR kinase activities were monitored in remote cardiac tissue by western blotting with specific antibodies against specific substrates phosphorylating sites.
Results
Rapamycin pre-treatement impaired endothelial-dependent vasorelaxation, attenuated cardiac function during IRI, and increased myocardial necrosis. Western blotting confirmed effective inhibition of myocardial mTOR kinase activities.
Conclusions
Pre-treatment of healthy pigs with rapamycin prior to acute myocardial IRI is associated with decreased cardiac function and higher myocardial necrosis.
Keywords: Cardioprotection, Reperfusion Injury, mTOR, Pharmacology, Cardiac Function
Introduction
The Mechanistic Target of Rapamycin (mTOR) is an atypical member of the Phosphatidylinositol 3-Kinase family with several upstream signals, consisting of two independent mTOR complexes (mTORC) that regulate a myriad of cellular processes. mTOR is a master regulator of cellular metabolism and growth despite an ever-expanding role in the cellular economy (reviewed in 1, 2). The first identified mTOR inhibitor, rapamycin, is a Food and Drug Administration approved drug to prevent transplant rejection, stent-induced coronary restenosis and is a powerful pharmacological tool to study mTOR function 3. While mTORC1 is a major regulator of cellular proliferation, trophysm, protein and RNA synthesis, mTORC2 has a role in cell survival, proliferation and cytoskeleton organization.
Cardiac mTOR is a well-established regulator of myocardial hypertrophy as demonstrated repeatedly in rodents using both genetic and pharmacological approached modulating mTOR activities (reviewed in 4, 5). The importance of mTOR in another common clinical problem, acute myocardial ischemia-reperfusion injury (IRI) remains poorly defined (briefly reviewed in 6, 7). IRI, an unavoidable consequence of a reperfusion of an ischemic tissue by artery re-opening after a variable period of occlusion is common in cardiac medical interventions as in coronary arteries thrombolytic therapy, percutaneous coronary intervention, and during cardiothoracic surgeries with cardioplegia arrest. Thus, determination of the role of a master regulator of cell metabolism/growth like mTOR in IRI is clinically relevant. We hypothesized that inhibiting mTOR activities with rapamycin, prior to an acute myocardial IRI, would confer cardioprotection by virtue of slowing down cardiac function and metabolism.
This study addresses the effects of a 7-day rapamycin treatment (mTOR inhibition) healthy pig model of an IRI that shares many of the cardiovascular characteristics observed in humans (reviewed in 5).
Material and Methods
Experimental Design
Twelve intact male Yorkshire swine (Parsons Research, Amherst, MA) were fed a restricted diet (3–5% of total body weight; ~500 g/once a day) of Teklad miniswine diet # 8753 (Harlan Laboratories, South Easton, MA), and unlimited access to water. Upon reaching a weight of 16 – 19 kg, they were divided into two groups. The control group (n = 5) continued their normal chow diets while the treatment group (n = 7) received 4 mg of oral rapamycin daily (Rapamune@, Wyeth Pharmaceuticals, Philadelphia, PA). Experiments were approved by the Institutional Animal Care and Use Committee at Lifespan Corp. Animals were cared for in accordance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the ‘Guide for the Care and Use of Laboratory Animals8.
Ischemia-Reperfusion Injury Surgical Protocol
After one week receiving either no drug or rapamycin, all animals were sedated with telazol (5 mg/kg IM) before endotracheal intubation and ventilation. Anesthesia was maintained with a gas mixture of 1.5 – 2.0 L/min of O2 and 0.75 – 3.0% isoflurane. At the time of median sternotomy, a phenylephrine drip (0.25 μg/kg/min) was started to prevent isoflurane-induced hypotension, and a heparin bolus of 80 U/kg was administered. The LAD was occluded 3 mm distal to the origin of the second diagonal branch by a Rummel tourniquet, or approximately one-third of the entire length of the LAD proximal to the apex in cases of atypical variation of the diagonals. After 60 min of ischemia, the tourniquet was released and the myocardium reperfused for 120 min. During the course of the operation, a single lidocaine dose of 1.5 mg/kg IV was given if three or more premature ventricular complexes were occurring over one min. Sustained ventricular tachycardia or fibrillation was managed with 50 J electric cardioversion with internal paddles until a perfusing rhythm was sustained. At the end of reperfusion, the LAD was re-occluded, the ascending aorta cross-clamped, and monastral blue pigment (Engelhard Corp., Louisville, KY) was injected into the aortic root to demarcate the area at risk (AAR). The heart was excised and then sectioned into four, 1 cm-thick axial slices perpendicular to the LAD from the apex to the point of LAD occlusion. Tissue from the second slice distal to the point of occlusion was separated into the remote (non-ischemic) left ventricle (RLV) as well as the left ventricular area at risk (AAR), as demarcated by the blue staining. A small, transmural myocardial sample from the AAR immediately adjacent to the NV was submerged in cold Krebs solution and placed on ice for microvessel studies to be conducted on the same day. The remaining tissue from the second circumferential slice was snap frozen in liquid nitrogen for molecular studies of protein expression. The remaining three axial sections were used for quantification of myocardial infarct size as described below. The surgeons performing the IRI protocol and researchers processing and analyzing the tissues were blinded from the animal treatments.
Quantification of Myocardial Infarct Size
The remaining three, 1 cm left ventricle (LV) sections (including septum) were processed for 2,3,5-triphenyltetrazolium (TTC) and the percentage of infarcted area determined as previously described 9, 10.
Pressure -Volume Loop Analysis
A multi-segment pressure-volume (PV) conductance catheter (Millar Instruments Inc., Houston, TX) was placed into the LV via the right common carotid arterial sheath under fluoroscopic guidance and was used in conjunction with the MPVS-Ultra Foundation System hardware and software (ADInstruments, Colorado Springs, CO). PV loops were obtained at baseline immediately before occlusion of the LAD, and at 30 min intervals throughout 60 min of ischemia and 120 min of reperfusion.
Cardiac Cell Proliferation
Immunofluorescence from formalin-fixed, paraffin-embedded LV samples were processed as previously described 10 after antigen retrieval (DAKO retrieval; Dako North America, Carpinteria, CA) and 1 mM EGTA (Boston BioProducts, Ashland, MA) for 20 min at 95°C and an anti Ki67 antibody (Epitomics, Burlingame, CA) was used at 1:200 dilution. Ten low power fields (LPF) were blindly and randomly recorded for each animal, in duplicate, and averaged. Data is presented as number of positive nuclei/LPF.
Microvessel Studies
Coronary arterioles (80 – 180 μm diameter) were isolated, placed in a microvessel chamber and pre-contracted to 30–60% of baseline diameter with U46619. Vasorelaxation responses to endothelial-dependent (adenosine diphosphate, ADP) and endothelial-independent (sodium nitroprusside, SNP) drugs were recorded as previously described 10, 11.
Molecular Studies
Myocardial lysates were prepared in Radio-Immunoprecipitation Assay (RIPA) buffer (Boston BioProducts) as previously described12. Sixty micrograms of RIPA soluble myocardial lysates from a remote left ventricular (RLV) area were fractionated by SDS-PAGE using a 4–12% Bis-Tris gel (Invitrogen) and transferred to PVDF membranes (Millipore, Bedford, MA). Using an automated western blot processor (Precision Biosystems, Mansfield, MA), membranes were incubated with antibodies against mTOR, p-mTOR, p-S6, P70S6K, p-70S6K, LC3A, LC3B, α-tubulin (all from Cell Signaling, Danvers, MA), and S6 (Santa Cruz Biotechnology, Santa Cruz, CA) at dilutions recommended by the manufacturer, followed by the appropriate horse radish peroxidase-linked secondary antibodies (Jackson ImmunoResearch, West Grove, PA), visualized via enhanced chemiluminescense (ECL) and recorded with a digital imaging system (G-Box, Syngene, Cambridge, England). Raw data were collected as arbitrary light units, averaged and quantified microdensitometrically using ImageJ 1.40g (National Institutes of Health, Bethesda, MD).
Blood Draws and Serum/Plasma Studies
Rapamycin Blood Levels
Blood was drawn under general anesthesia for serum/plasma analysis during the operation through a central venous access before median sternotomy. Rapamycin blood concentrations were obtained by immunoassay (Architect System, Abbott Laboratories, Abbott Park, IL) at the Rhode Island Hospital Toxicology Laboratory.
Plasma Cardiac Troponin I (cTnI)
Plasma was obtained by centrifugation and a 1:1 dilution was used for an enzyme-linked immunosorbent assay (ELISA) determination of cTnI according to the manufacture recommended protocol (Life Diagnostics Inc., West Chester, PA).
Statistical Analysis
Results were reported as mean ± standard error of the mean (SEM) and p < 0.05 was considered significant. Microvessel response and PV loop parameters were analyzed with a two-way analysis of variance (ANOVA) using GraphPad Prism 5.0 Software (GraphPad Software Inc., San Diego, CA). Unpaired Student’s t-test was used for comparison between two groups for analysis of area at risk (AAR), infarct size, serum chemistry, and Western blot quantification.
Results
Experimental Model
Rapamycin levels were significantly higher in treated animals vs. controls [(26.8 ± 1.94 ng/ml) vs. below detectable level (< 0.2 ng/ml) 0.00 ± 0.00; p<0.001]. There were no observed differences in behavior, eating or drinking patterns between groups during the acclimatization time (30 days) and all animals completed the protocol.
Cardiac Function
Mean and maximum LV pressures (p = 0.03 and p = 0.007, respectively), maximum power (p = 0.014), pressure at maximum contractility (P@ LV dP/dT max) (p < 0.001), and left ventricular maximum filling (dV/dT max) (p = 0.004) were all significantly decreased in the rapamycin-treated animals as compared to controls except for left ventricular maximum contractility (dP/dT Max) (p = 0.079).
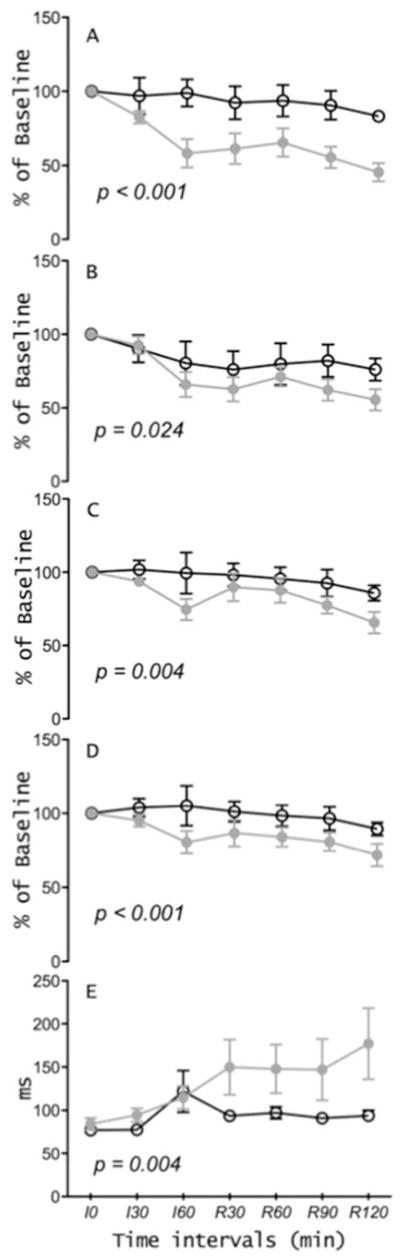
Preservation of cardiac function throughout ischemia-reperfusion, as measured by the percentage of baseline measurement of stroke work, cardiac output, stroke volume, and ejection fractions were all significantly attenuated in rapamycin-treated animals (figures 1A–D) and the isovolumetric LV relaxation time constant (Tau) prolonged in the rapamycin group as compared to controls (figure 1E).
Figure 1.
Preservation of hemodynamic parameters in controls and in the rapamycin-treated animals. Left ventricle isovolumetric relaxation (Tau) at 30 min intervals during the 60 minutes ischemia (I) and 120 minutes reperfusion (R).
Microvessel Reactivity
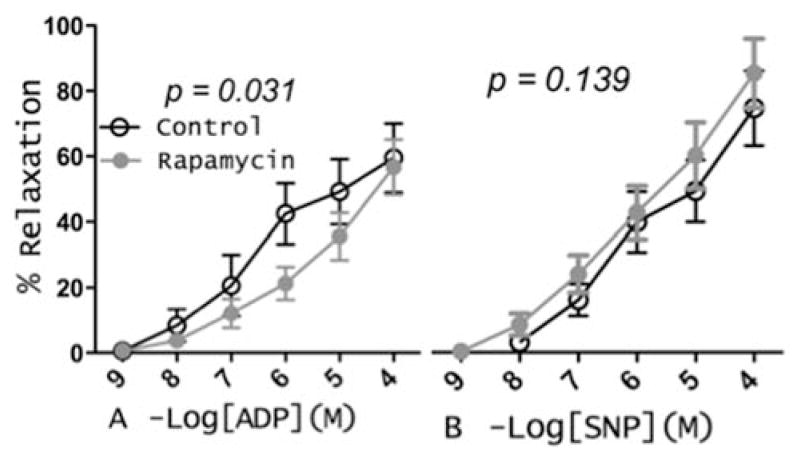
Microvessel reactivity differed significantly between groups with impairment in endothelium-dependent relaxation to ADP but not to SNP in the rapamycin group as compared to controls (figure 2A and B).
Figure 2.
Coronary arterioles were pre-contracted with U46619 and dose-response curves to the endothelium-dependent vasodilator adenosine diphosphate (ADP) and the endothelium-independent vasodilator sodium nitroprusside (SNP) were obtained.
Electrical Cardioversion and Myocardial Infarction/Necrosis
Electromechanical instability was exacerbated in the rapamycin group requiring more intraoperative electrical cardioversion for no-perfusing sustained ventricular tachycardia and/or ventricular fibrillation than controls [1.6 ± 0.75 cardioversions in the rapamycin group vs. 8.71 ± 2.71 in controls (figure 3A)]. AAR was not significantly different between groups (45.9 ± 4.72 % in controls vs. 48.62 ± 2.67 % in the rapamycin-treated. (figure 3B). Control mean infarcted area (7.33 ± 4.68 %) was about half of the rapamycin treated group (13.86 ± 3.03 %) (figure 3C).
Figure 3.
(A) intraoperative electrical conversion, area at risk (B), infarct size (C) in the LV territory distal to the point of LAD occlusion and plasma levels of cardiac troponin I at the end of the IRI protocol.
Plasma cTnI levels (ng/ml) were 0.169 ± 0.068 in control versus 0.383 ± 0.043 in the rapamycin group (figure 3D).
Cardiac Cell Proliferation
Unspecified Cardiac cell proliferation (cardiomyocytes, endothelial, smooth muscle, and fibroblasts) as measured by immunofluorescent staining for Ki67 positive nuclei was significantly lower in the RLV of the rapamycin-treated as compared to controls (23.67 ± 1.45 vs. 10.40 ± 2.25, p = 0.006).
Myocardial mTORC1 and 2 Activities
Western blots recorded significant decrease expression and phosphorylation of downstream substrates of mTORC1, [p-P70S6K(S371) and p-S6(S235/6)] and mTORC2 [ p-AKT (S473) and PKCα (S657)]. The phospho/total ratios were also significantly decreased (Appendix). Expression of P(S2448)- and total mTOR were consistently lower in the heart of rapamycin-treated pigs.
Autophagosome Levels
Expression of the autophagosome markers LC3A II and LC3B II were similar between the 2 groups. Rapamycin or chloroquine treated H9C2 rat neonatal cardiomyocytes were used as positive controls to demonstrate that changes in the expression of LC3A/B II were detectable (Appendix).
Comment
In this study we pre-treated healthy pigs toward the higher end of monotherapeutic rejection treatment levels of rapamycin 13 for one week prior to the acute IRI protocol in an attempt to determine the role of chronic inhibition of basal levels of mTOR in IRI. There were three main reasons that lead us to this study design. Firstly, in small-animal studies, genetic or pharmacologic blocking or inactivating mTOR has yielded inconsistent findings with regard to cardioprotection in the setting of myocardial injury14–16; secondly, activation of the reperfusion injury salvage kinases (including mTOR) is cardioprotective 17, and finally, determining the effects of rapamycin in a healthy pig model will set a baselines for future studies in pig models of human diseases. This study demonstrated that treatment with rapamycin prior to an acute myocardial IRI was detrimental as measured by several parameters, perhaps the most clinically relevant being the higher levels of plasma cTnI in the rapamycin treated group, and the doubling of the myocardial infarct size from 7% to 14% in the AAR and the higher number of intra-operative electrical cardioversion attempts required to revert non-perfusing ventricular arrhythmias (tachycardias and fibrillations) to normal sinus rhythm.
Several functional hemodynamic parameters were measured throughout ischemia and reperfusion. Of note is that the baseline values for all of these functional parameters did not greatly differ at the time point I – 0, just prior to occlusion of the LAD. This suggests that one week of treatment with rapamycin did not have a noticeable effect on the heart at basal condition (i.e., in the absence of ischemia-reperfusion). However, during ischemia and reperfusion these parameters diverged significantly with attenuated hemodynamics in the rapamycin-treated animals. For each of these parameters, the morphology of the curve delineates the largest insult in the rapamycin-treated animals to occur during ischemia, with cardiac function failing to recover to levels comparable to controls despite an apparent leveling-off during reperfusion. Since rapamycin mimics caloric restriction and inhibits mTOR (it is also known to be trophic to the heart), 1week of rapamycin treatment may have caused a mild myocardial hypotrophy that was revealed only during the IRI protocol but not during unstressed conditions. Because the mTOR pathway regulates distinct cellular functions, including cell growth, proliferation, and protein turnover, in addition to inhibiting autophagy18, rapamycin-induced mTOR inhibition below basal levels has broad and complex cellular consequences. Studies showed that FK506 binding protein (FKBP)12, highly abundant in pig hearts, forms a complex with rapamycin before inhibiting mTORC1. Additional studies also showed that rapamycin modifies cardiac ryanodine receptor (RYR) activities by competing and interfering with FKBP12-RYR2 interactions19. In fact, studies with FKBP12 conditional cardiac overexpression as well as knockout mice reveal the appearance of cardiac arrhythmias via regulation of voltage-gated sodium channels (review in 20). A mechanism for how rapamycin-treated pigs may develop larger myocardial infarction areas as compared to control pigs is not clear at this time, but inhibition of survival kinases that include AKT and P70S6K during IRI models are associated with an increase in myocardial infarction areas (review in 17). In addition, the possible displacement of FKBP1 from the RYR2 receptors by rapamycin-FKBP1 binding may lead to dysfunctional intracellular Ca2+ regulation during reperfusion and further injury (review in 20). Interestingly, isoproterenol-induced ectopy in dog heart wedge preparations was increased dramatically in presence of rapamycin while rapamycin alone did not induce arrhythmias 21. Thus, perhaps an interaction between rapamycin and the phenylephrine used in our protocol to limit anesthesia-induced hypotension may have contributed to our observed increase in electromechanical instability.
A retrospective clinical follow up study of 115 transplanted patients receiving long-term rapamycin treatments failed to show an increase in cardiovascular disease, however, there was a trend towards twice as much coronary artery disease (defined as myocardial infarction, need for percutaneous coronary intervention, stroke, aortic aneurysm, pulmonary thromboembolism, and sudden cardiac death) 22.
Microvascular relaxation is well known to be impaired in patients after acute myocardial ischemia23. In this experiment we measured endothelium-dependent and endothelium-independent coronary microvessel reactivity. Though the later showed no change between groups, endothelium-dependent relaxation to ADP was significantly impaired in the rapamycin-treated animals as compared to the controls. Although these results may be consequences of the effects of rapamycin on endothelial and smooth muscle cells protein synthesis inhibition, it is likely related to rapamycin-induced chronic mTOR inhibition, AKT inactivation (lower phosphorylation of AKT S473) that is necessary for eNOS activation, and endothelial NO production leading to endothelial-dependent relaxation 24. Although there was also a trend for improved endothelial-independent relaxation in the rapamycin group, we currently lack a proposed mechanism should this difference be significant in further studies. These results are more in line with Jabs et al. who showed that rats treated with rapamycin for 1 week develop significant aortic vascular dysfunction in both endothelial-dependent and independent vasorelaxation that were associated with higher levels of reactive oxygen species 25.
The efficiency of rapamycin treatment was recorded biochemically as shown by the decrease phosphorylation of substrates reflecting inhibition of mTORC1 (mTOR, P70S6K, and S6) and mTORC2 (PKCα and AKT). Again, although a decrease the phosphorylated forms were anticipated, the concomitant decrease in total mTOR, S6 and P70S6K was not. In fact, mTOR inhibition is associated with a decrease in transcription and/or translation of a number of proteins leading to their lower steady state levels 26, 27. Furthermore, as determined by Ki67 staining, a cell cycle marker, 1-week of rapamycin treatment inhibited cell proliferation as would be expected after mTOR inhibition, which has been the rationale behind the use of rapamycin-coated stents to reduce smooth muscle growth and neointimal formation.
Our results suggest that pre-treatment with rapamycin prior to acute myocardial IRI is detrimental to both cardiac function as well as myocardial survival in healthy pigs. It remains to be determined if an in vivo autophagic flux 28 was induced by rapamycin treatment, but there was no evidence of autophagosome accumulation as LC3AII and LC3BII levels were similar between controls and the rapamycin treated group. It worthwhile to clarify that higher levels of LC3A/B II does not necessarily reflect an increase in autophagy since autophagic activity is also dependent on autophagosome-lysosome fusion (autolysosome formation) that can be inhibited under some conditions such as with the administration of chloroquine (Appendix Figures 1–3) and a high fat diet 29. In addition, chloroquine treated cells had higher levels of LC3A/B II (autophagosome accumulation, Appendix Figures 1–3) but decreased autophagic activity due to inhibition of lysosomal activity, lysosomal autophagosome fusion, and less autolysosome formation 30. The attenuated hemodynamics and microvascular function observed in the current study are likely the effects of rapamycin resulting from mTOR inhibition, regardless of whether or not autophagic flux was altered.
It is prudent to discuss the potential clinical implications of these preliminary findings as rapamycin is commonly used at doses comparable to those in the current study for both induction and maintenance immunosuppression after solid organ transplantation. Our findings do suggest that patients taking rapamycin may be at risk for worse outcomes, specifically in regard to infarct size and hemodynamic function after an acute cardiac event. This is especially concerning if one considers the increased risk of such events with patient taking rapamycin.
Limitations
This study has important limitations, in particular, the small number of animals in each group making it is difficult to reach statistical significance when data has high variability. For example, trends toward larger infarct size and more electromechanical instability in the rapamycin treated animals may have reached significance in a larger study. Additional limitations are the single dose/short treatment of rapamycin (4mg/day for 7 days) as compared to clinical use of rapamycin in transplant patients which are longer (several months to years) and the target therapeutic blood levels of rapamycin about 1/3 of the levels detected in our study. Finally, the studies were performed in healthy normal animals while important co-morbidities are associated with patients receiving repamycin treatment.
Supplementary Material
Acknowledgments
We would like to thank the Rhode Island Hospital animal research facility personnel, clinical and toxicology laboratories and Dr. D. Terentyev for pointing to the potential interactions between rapamycin and RYR2.
Funding provided by grants from the American Heart Association Grant-in-Aid Program (11GRNT5250000, CB), National Heart, Lung, and Blood Institute (R01HL46716, R01HL69024, and R01HL85647, FWS), NIH Training grant 5T32-HL094300 (NYE), NIH Training grant 5T32-HL076134 (ADL), and from the Thoracic Surgery Foundation for Research and Education Fellowship (ADL).
Abbreviations and Acronyms
- AAR
Area at risk
- ADP
adenosine diphosphate
- AKT
Protein kinase B
- ANOVA
Analysis of variance
- cTnI
Cardiac troponin I
- dP/dT Max
left ventricular maximum contractility
- dV/dT max
Left ventricular maximum filling
- ECL
Enhanced chemiluminescense (ECL)
- EGTA
ethylene glycol tetraacetic acid
- ELISA
Enzyme-linked immunosorbent assay
- FKBP12
Peptidyl-prolyl cis-trans isomerase 1A
- IRI
Ischemia-reperfusion injury
- LAD
Left anterior descending
- LC3 A/B
Microtubule-associated proteins 1A/1B light chain 3A/B
- LPF
low power fields
- LV
Left ventricle
- mTOR
Mechanistic target of rapamycin
- mTORC
Mechanistic target of rapamycin complex
- P@ LV dP/dT max
Pressure at maximum contractility
- PKC
Protein kinase C
- PVDF
polyvinylidene difluoride (PVDF)
- PV
Pressure-volume
- RIPA
Radio-Immunoprecipitation Assay
- RISK
Survival kinases
- RLV
Remote left ventricular area
- RYR
Ryanodine receptor
- S6
Ribosomal protein S6
- SEM
Standard error of the mean
- SNP
sodium nitroprusside
- Tau
Relaxation time constant
- TTC
2,3,5-triphenyltetrazolium
Footnotes
Disclosures and Freedom of Investigation
There are no disclosures to report. The authors had full control of the design of the study, methods used, outcome parameters and results, analysis of data and production of the written report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dazert E, Hall MN. Mtor signaling in disease. Curr Opin Cell Biol. 2011;23:744–755. doi: 10.1016/j.ceb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Laplante M, Sabatini DM. Mtor signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: A new generation of mtor inhibitors. Nat Rev Drug Discov. 2011;10:868–880. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- 4.Aoyagi T, Matsui T. Phosphoinositide-3 kinase signaling in cardiac hypertrophy and heart failure. Curr Pharm Des. 2011;17:1818–1824. doi: 10.2174/138161211796390976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balasubramanian S, Johnston RK, Moschella PC, Mani SK, Tuxworth WJ, Jr, Kuppuswamy D. Mtor in growth and protection of hypertrophying myocardium. Cardiovasc Hematol Agents Med Chem. 2009;7:52–63. doi: 10.2174/187152509787047603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hwang SK, Kim HH. The functions of mtor in ischemic diseases. BMB Rep. 2011;44:506–511. doi: 10.5483/bmbrep.2011.44.8.506. [DOI] [PubMed] [Google Scholar]
- 7.Chong ZZ, Shang YC, Maiese K. Cardiovascular disease and mtor signaling. Trends Cardiovasc Med. 2011;21:151–155. doi: 10.1016/j.tcm.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guide for the care and use of laboratory animals. Washington (DC): 2011. [Google Scholar]
- 9.Bianchi C, Wakiyama H, Faro R, Khan T, McCully JD, Levitsky S, Szabo C, Sellke FW. A novel peroxynitrite decomposer catalyst (fp-15) reduces myocardial infarct size in an in vivo peroxynitrite decomposer and acute ischemia-reperfusion in pigs. Ann Thorac Surg. 2002;74:1201–1207. doi: 10.1016/s0003-4975(02)03953-x. [DOI] [PubMed] [Google Scholar]
- 10.Osipov RM, Bianchi C, Clements RT, Feng J, Liu Y, Xu SH, Robich MP, Wagstaff J, Sellke FW. Thrombin fragment (tp508) decreases myocardial infarction and apoptosis after ischemia reperfusion injury. Ann Thorac Surg. 2009;87:786–793. doi: 10.1016/j.athoracsur.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 11.Feng J, Chu LM, Robich MP, Clements RT, Khabbaz KR, Hagberg R, Liu Y, Osipov RM, Sellke FW. Effects of cardiopulmonary bypass on endothelinb-induced contraction and signaling in human skeletal muscle microcirculation. Circulation. 2010;122:S150–155. doi: 10.1161/CIRCULATIONAHA.109.928226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glazer HP, Osipov RM, Clements RT, Sellke FW, Bianchi C. Hypercholesterolemia is associated with hyperactive cardiac mtorc1 and mtorc2 signaling. Cell Cycle. 2009;8:1738–1746. doi: 10.4161/cc.8.11.8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clinic M. Sirolimus level. Mayo Medical Laboratories; Dec, 2011. [Google Scholar]
- 14.Zhang D, Contu R, Latronico MV, Zhang J, Rizzi R, Catalucci D, Miyamoto S, Huang K, Ceci M, Gu Y, Dalton ND, Peterson KL, Guan KL, Brown JH, Chen J, Sonenberg N, Condorelli G. Mtorc1 regulates cardiac function and myocyte survival through 4e-bp1 inhibition in mice. J Clin Invest. 2010;120:2805–2816. doi: 10.1172/JCI43008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill JA. Autophagy in cardiac plasticity and disease. Pediatr Cardiol. 2011;32:282–289. doi: 10.1007/s00246-010-9883-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sciarretta S, Hariharan N, Monden Y, Zablocki D, Sadoshima J. Is autophagy in response to ischemia and reperfusion protective or detrimental for the heart? Pediatr Cardiol. 2011;32:275–281. doi: 10.1007/s00246-010-9855-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hausenloy DJ, Yellon DM. Reperfusion injury salvage kinase signalling: Taking a risk for cardioprotection. Heart Fail Rev. 2007;12:217–234. doi: 10.1007/s10741-007-9026-1. [DOI] [PubMed] [Google Scholar]
- 18.Weichhart T. Mammalian target of rapamycin: A signaling kinase for every aspect of cellular life. Methods Mol Biol. 2012;821:1–14. doi: 10.1007/978-1-61779-430-8_1. [DOI] [PubMed] [Google Scholar]
- 19.Zissimopoulos S, Seifan S, Maxwell C, Williams AJ, Lai FA. Disparities in the association of the ryanodine receptor and the fk506-binding proteins in mammalian heart. J Cell Sci. 2012;125:1759–1769. doi: 10.1242/jcs.098012. [DOI] [PubMed] [Google Scholar]
- 20.Calderon-Sanchez E, Rodriguez-Moyano M, Smani T. Immunophilins and cardiovascular complications. Curr Med Chem. 2011;18:5408–5413. doi: 10.2174/092986711798194379. [DOI] [PubMed] [Google Scholar]
- 21.Katra RP, Oya T, Hoeker GS, Laurita KR. Ryanodine receptor dysfunction and triggered activity in the heart. Am J Physiol Heart Circ Physiol. 2007;292:H2144–2151. doi: 10.1152/ajpheart.00924.2006. [DOI] [PubMed] [Google Scholar]
- 22.Watorek E, Szymczak M, Boratynska M, Patrzalek D, Klinger M. Cardiovascular risk in kidney transplant recipients receiving mammalian target of rapamycin inhibitors. Transplant Proc. 2011;43:2967–2969. doi: 10.1016/j.transproceed.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Xu X, Li J, Simons M, Laham RJ, Sellke FW. Expression of vascular endothelial growth factor and its receptors is increased, but microvascular relaxation is impaired in patients after acute myocardial ischemia. J Thorac Cardiovasc Surg. 2001;121:735–742. doi: 10.1067/mtc.2001.112340. [DOI] [PubMed] [Google Scholar]
- 24.Dudzinski DM, Igarashi J, Greif D, Michel T. The regulation and pharmacology of endothelial nitric oxide synthase. Annu Rev Pharmacol Toxicol. 2006;46:235–276. doi: 10.1146/annurev.pharmtox.44.101802.121844. [DOI] [PubMed] [Google Scholar]
- 25.Jabs A, Gobel S, Wenzel P, Kleschyov AL, Hortmann M, Oelze M, Daiber A, Munzel T. Sirolimus-induced vascular dysfunction. Increased mitochondrial and nicotinamide adenosine dinucleotide phosphate oxidase-dependent superoxide production and decreased vascular nitric oxide formation. J Am Coll Cardiol. 2008;51:2130–2138. doi: 10.1016/j.jacc.2008.01.058. [DOI] [PubMed] [Google Scholar]
- 26.Huo Y, Iadevaia V, Yao Z, Kelly I, Cosulich S, Guichard S, Foster LJ, Proud CG. Stable isotope-labelling analysis of the impact of inhibition of the mammalian target of rapamycin on protein synthesis. Biochem J. 2012;444:141–151. doi: 10.1042/BJ20112107. [DOI] [PubMed] [Google Scholar]
- 27.Thoreen CC, Chantranupong L, Keys HR, Wang T, Gray NS, Sabatini DM. A unifying model for mtorc1-mediated regulation of mrna translation. Nature. 2012;485:109–113. doi: 10.1038/nature11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwai-Kanai E, Yuan H, Huang C, Sayen MR, Perry-Garza CN, Kim L, Gottlieb RA. A method to measure cardiac autophagic flux in vivo. Autophagy. 2008;4:322–329. doi: 10.4161/auto.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez-Navarro JA, Cuervo AM. Dietary lipids and aging compromise chaperone-mediated autophagy by similar mechanisms. Autophagy. 2012:8. doi: 10.4161/auto.20649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma X, Liu H, Foyil SR, Godar RJ, Weinheimer CJ, Hill JA, Diwan A. Impaired autophagosome clearance contributes to cardiomyocyte death in ischemia/reperfusion injury. Circulation. 2012;125:3170–3181. doi: 10.1161/CIRCULATIONAHA.111.041814. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.