Abstract
Background
Plasma glucose levels are tightly regulated within a narrow physiologic range. Insulin-mediated glucose uptake by tissues must be balanced by the appearance of glucose from nutritional sources, glycogen stores, or gluconeogenesis. In this regard, a common pathway regulating both glucose clearance and appearance has not been described. The metabolism of glucose to produce ATP is generally considered to be the primary stimulus for insulin release from beta-cells. Similarly, gluconeogenesis from phosphoenolpyruvate (PEP) is believed to be the primarily pathway via the cytosolic isoform of phosphoenolpyruvate carboxykinase (PEPCK-C). These models cannot adequately explain the regulation of insulin secretion or gluconeogenesis.
Scope of review
A metabolic sensing pathway involving mitochondrial GTP (mtGTP) and PEP synthesis by the mitochondrial isoform of PEPCK (PEPCK-M) is associated with glucose-stimulated insulin secretion from pancreatic beta-cells. Here we examine whether there is evidence for a similar mtGTP-dependent pathway involved in gluconeogenesis. In both islets and the liver, mtGTP is produced at the substrate level by the enzyme succinyl CoA synthetase (SCS-GTP) with a rate proportional to the TCA cycle. In the beta-cell PEPCK-M then hydrolyzes mtGTP in the production of PEP that, unlike mtGTP, can escape the mitochondria to generate a signal for insulin release. Similarly, PEPCK-M and mtGTP might also provide a significant source of PEP in gluconeogenic tissues for the production of glucose. This review will focus on the possibility that PEPCK-M, as a sensor for TCA cycle flux, is a key mechanism to regulate both insulin secretion and gluconeogenesis suggesting conservation of this biochemical mechanism in regulating multiple aspects of glucose homeostasis. Moreover, we propose that this mechanism may be more important for regulating insulin secretion and gluconeogenesis compared to canonical nutrient sensing pathways.
Major conclusions
PEPCK-M, initially believed to be absent in islets, carries a substantial metabolic flux in beta-cells. This flux is intimately involved with the coupling of glucose-stimulated insulin secretion. PEPCK-M activity may have been similarly underestimated in glucose producing tissues and could potentially be an unappreciated but important source of gluconeogenesis.
General Significance
The generation of PEP via PEPCK-M may occur via a metabolic sensing pathway important for regulating both insulin secretion and gluconeogenesis.
Keywords: PEPCK-M, mitochondrial GTP, anaplerosis, Succinyl Coenzyme A Synthetase, mitochondrial metabolism, insulin secretion, gluconeogenesis, Diabetes Mellitus
1. Introduction
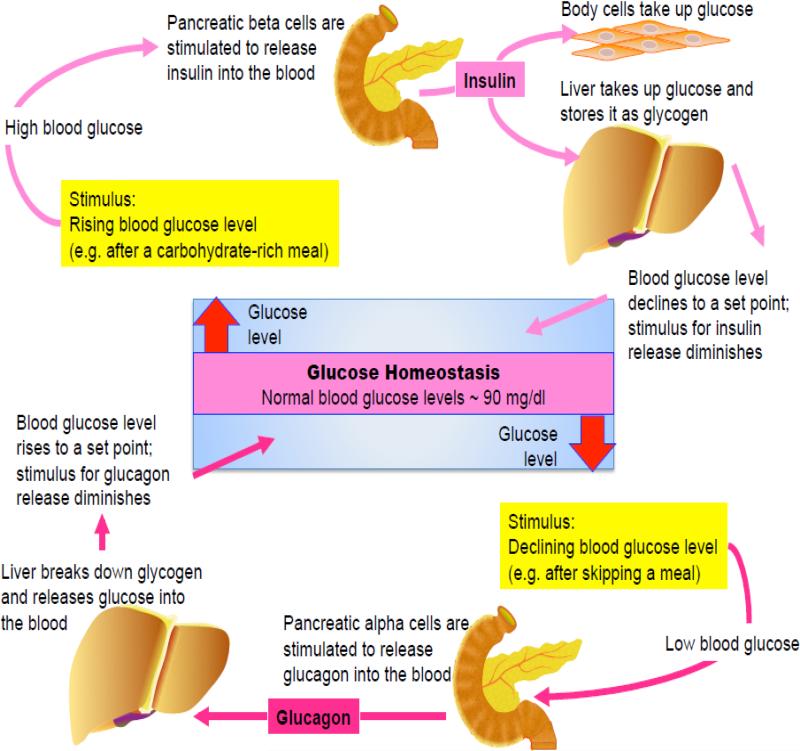
The body regulates blood glucose levels to maintain glucose homeostasis. Together the rates of glucose entry and clearance from the circulation establish blood glucose levels. In the absence of exogenous nutritional sources an organism preserves normal blood glucose levels through a combination of glycogenolysis (glycogen breakdown) and gluconeogenesis (de novo glucose production) [1]. An increase in glucose levels results in insulin secretion from the pancreas and clears blood glucose acutely by promoting tissue glucose uptake and suppressing glucose production [2, 3] (Fig. 1).
Figure 1. Glucose homeostasis.
Plasma glucose levels are normally maintained within a relatively narrow range and are derived from three main sources: intestinal absorption, gluconeogenesis and glycogenolysis. Hormonal control is the most important mediator of plasma glucose. Acute glucoregulatory mechanisms that can affect plasma glucose levels within minutes involve insulin and glucagon. An increase in blood glucose levels provides the stimulus for insulin secretion. Insulin decreases blood glucose acutely by promoting tissue glucose uptake, followed by suppression of gluconeogenesis in both the liver and kidney as well as glycogenolysis in liver. A decrease in blood glucose levels results in the secretion of glucagon. Glucagon only acts on liver and stimulates glucose release, by initiating glycogenolysis. It does not act on the kidney.
While glucose production and insulin secretion oppose each other, at a cellular level, these two distinct cellular processes share metabolic and biochemical features. Namely the biochemical reaction in which oxaloacetate (OAA) is decarboxylated to phosphoenolpyruvate (PEP) by phosphoenolpyruvate carboxykinase (PEPCK) (EC number 4.1.1.32) is central to both processes. This shared biochemistry leads to similarities in how these processes are regulated. This reaction requires GTP and is essential to support phosphoenolpyruvate (PEP) synthesis for insulin secretion as well as gluconeogenesis [4-7]. PEPCK exists in two isoforms: a cytosolic (PEPCK-C) and a mitochondrial (PEPCK-M) form [8]. Most research focused on the function and regulation of PEPCK-C, and glucose production is most commonly ascribed to this isoform. Curiously, the first function of PEPCK-M in mammals was found in glucose-stimulated insulin secretion (GSIS), a function on the diametrically opposite side of glucose production [7, 9]. In the pancreatic β-cell, PEP synthesis by PEPCK-M couples mitochondrial metabolism to insulin release in a mechanism distinct from ATP production by oxidative phosphorylation. Mitochondrial PEP (mtPEP) synthesis is coupled to the TCA cycle via the production of mitochondrial GTP (mtGTP) that is produced by substrate-level synthesis via the enzyme succinyl-CoA synthetase (SCS) in the TCA cycle. Consequently, both glucose production and glucose clearance could be determined, at least in part, by a common mitochondrial metabolic pathway.
Flux through this metabolic “tachometer” derived from mtGTP production by the TCA cycle followed by mtGTP hydrolysis by PEPCK-M may be a common reaction to both β-cells and gluconeogenic tissues. This review will focus on this potential duality of function where PEPCK-M senses TCA cycle flux through mtGTP production.
2. Glucose Homeostasis
Many important functions of the body depend on glucose. Throughout the day plasma glucose concentrations remain within a relative narrow window from about 3 to 8 mM [10, 11]. However, nutrition is variable and stored glucose (in the form of glycogen) is a finite supply that can be depleted. In order to maintain glucose homeostasis, glucose that is leaving the circulation must be balanced by the addition of glucose to the system (Fig. 1). Insulin is the hormone that largely determines the rate of clearance of glucose into peripheral tissues at rest. The source of the glucose, on the other hand varies. During meals, intestinal absorption is a major contributor but during fasting and exercise glucose comes primarily from two other pathways: gluconeogenesis (de novo glucose production in kidney and liver) and glycogenolysis (glycogen breakdown in muscle and liver) [1, 12, 13]. Only the liver and kidney can release glucose into the blood because they have the enzyme glucose-6-phosphatase [14]. Gluconeogenesis is a continuous process that supports both glycogen synthesis as well as endogenous glucose production. Interestingly, even in the immediate post-absorptive state when glycogen levels are high, gluconeogenesis still contributes about half of endogenous glucose production [15]. During a prolonged fast as glycogen stores become progressively depleted then the relative contribution of gluconeogenesis approaches 100%. It is particularly important to note that inappropriately elevated gluconeogenic flux is associated with and may be causal for diabetes mellitus [16, 17].
Gluconeogenesis and glycolysis share many of the same enzymes that catalyze reversible reactions lying close to their equilibrium. However, three glycolytic reactions (hexokinase/glucokinase, phosphofructokinase, and pyruvate kinase) are exchanged with kinetically more favorable reactions (glucose-6-phosphatase, fructose-1,6-biphosphatase, and PEPCK, respectively) during gluconeogenesis. The reactions of gluconeogenesis occur predominantly in the cytosol with the exception of glucose-6-phosphatase (lumen of the ER) and PEPCK that can either take place in the cytosol (PEPCK-C) or the mitochondrial matrix (PEPCK-M). Interestingly, to some degree both gluconeogenesis and glycolysis occur simultaneously in the liver with significant cycling of plasma glucose into the mitochondria and back out [18-20].
The hormone insulin has a key role in normal glucose homeostasis opposing gluconeogenesis. Many of the features of the control of glucose homeostasis under basal conditions can be explained by a simple feedback loop between the liver and the pancreatic β-cell in the islets of Langerhans (Fig. 1). Endogenous glucose production supplies glucose through a combination of gluconeogenesis and glycogenolysis to support energy demand in peripheral tissues. If supply outstrips demand, then plasma glucose levels begin to rise. β-cells metabolically sense glucose as well as other nutrient levels and release insulin into the blood stream. Insulin decreases blood glucose acutely by promoting tissue glucose uptake, suppressing gluconeogenesis in both the liver and kidney as well as inhibiting glycogenolysis and promoting glycogen synthesis in liver [2, 3].
3. Sensing TCA cycle flux
Mitochondria are intimately involved in both processes of glucose sensing and gluconeogenesis. The TCA cycle is the “central wheel” of energy metabolism and is the final common pathway for the aerobic oxidation of fuel molecules. However, biosynthetic intermediates can leave the TCA cycle and be converted to products such as glucose, nucleotides, lipids, or non-essential amino acids [6]. The removal of TCA cycle intermediates is called cataplerosis (of Greek origin, kata=”downward” and plerotikos= “to fill”). If carbons are removed from the TCA cycle, they must be replaced. The replenishment of TCA cycle intermediates is termed anaplerosis (of Greek origin, ana=”up” and plerotikos= “to fill”) [6]. To maintain the appropriate balance of carbon flow in the TCA cycle for various metabolic processes, cataplerosis and anaplerosis must be maintained in equilibrium. If this does not occur, the cycle either accumulates or loses metabolites, either of which could impair TCA flux when excessive. Thus, anaplerosis and cataplerosis must be balanced so that during different physiologic conditions (e.g. fasting, feeding or exercise), the pool size of TCA intermediates remains consistent [21]. How, then, do mitochondria know how much is coming in and going out?
A key insight was obtained from the molecular characterization of a rare disease of familial hypoglycemia, known as Hyperinsulinemia-Hyperammonemia (HI/HA) syndrome. This autosomal dominant disease is associated with a gain-of-function mutation in the mitochondrial enzyme glutamate dehydrogenase (GDH) [22, 23]. GDH is a key net entry point for glutamate into the TCA cycle. Glutamate is oxidatively deaminated to produce anaplerotic α-ketoglutarate and free ammonia. Unrestrained glutamate entry into the TCA cycle is detrimental in the patients due to excessive amino acid catabolism that increases levels of ammonia as a toxic by-product. This process is normally tightly regulated by opposing allosteric signals: mtGTP (inhibitory) and leucine (activating). As long as there are adequate levels of TCA flux then mtGTP will prevent the excessive entry of anaplerotic glutamate by inhibiting GDH. Leucine is one of the essential amino acids and its levels generally only increase following a protein-rich meal. Activation of GDH by leucine provides a mechanism of redirecting the carbons from amino acids into other metabolic pathways during times of excess. The balance of mtGTP and leucine regulation is lost in patients with HI/HA due to a mutation in GDH that impairs the ability of mtGTP to turn off the enzyme [22, 23]. During protein-rich meals, leucine potently over-activates the enzyme. Consequently, glutamate catabolism increases inappropriately and results in excessive insulin secretion with hypoglycemia. While it may not be surprising that excessive TCA flux in β-cells could promote insulin secretion, the characterization of these mutations identified mtGTP as a key metabolic regulatory signal in the mitochondria [9, 22, 23].
3.1. Mitochondrial GTP
The synthesis of ATP in the mitochondria by oxidative phosphorylation is well characterized. Here TCA cycle metabolism provides reducing equivalents (e.g. NADH and FADH2) that donate electrons to the electron transport chain with molecular oxygen as the final acceptor at complex IV. Electron transport then pumps protons out of the matrix generating a proton motive force across the inner mitochondria. This provides the driving force for ATP synthesis as protons translocate back into the matrix through complex V along their electrochemical gradient. There are other pathways that allow the protons to “leak” back into the matrix so the efficiency of oxidative phosphorylation depends, in part, on the tightness of coupling of the proton motive force to ATP synthesis. ATP generated in the matrix is then rapidly and efficiently transported out into the cytosol in exchange for ADP.
Mitochondrial GTP (mtGTP) synthesis is quite different. First, mtGTP is formed by substrate-level phosphorylation of mitochondrial GDP via GTP-forming succinyl-CoA synthetase (SCS-GTP) [24]. Synthesis rates of mtGTP are directly related to flux through the TCA cycle (at least for the SCS step where approximately one molecule of mtGTP is built per one molecule of glucose) but independent of electron transport and oxidative phosphorylation. In contrast, the TCA cycle generates little mitochondrial ATP (mtATP) by the ATP forming isoform of SCS (SCS-ATP). Oxidative phosphorylation generates the majority of mtATP, which is controlled by the transmembrane mitochondrial potential (ΔΨ). The ΔΨ controls important mitochondrial functions, such as protein import, heat formation, free-radical generation, ion transport, in addition to generating ATP. Consequently, the yield of mtATP from one glucose molecule can in theory vary between 1 and 29 [9]. This is particularly relevant in pancreatic beta-cells that are known to have a large proportion of the membrane potential uncoupled from oxidative phosphorylation [25]. In this manner, mtGTP is better poised to be an indicator of TCA cycle flux than ATP generation in a mechanism that is metaphorically similar to the tachometer of an engine [9].
Considered within the framework of GDH regulation, if the intermediates of the TCA cycle are becoming depleted, then GTP levels will drop reducing the restraint on GDH. As noted above, this is evident in the disease Hyperinsulinemia Hyperammonemia syndrome, where mutations in GDH lacking the GTP “off-switch” become profoundly hypoglycemic from excessive amino acid induced insulin secretion. Similarly, glucose deprivation depletes TCA cycle flux and intermediates and reduces mtGTP, consequently relieving inhibition of GDH and enhances GDH activation by the amino acid leucine. In mice expressing the mutant human GDH, amino acids are much more potent insulin secretagogues. Even in normal mouse islets, the longer the duration of hypoglycemia in run down experiments leads to a greater responsivity of insulin secretion to amino acids suggesting that this is a normal physiologic mechanism regulated by mtGTP [26]. If needed, glutamate carbons can replenish the TCA cycle, and in the process will generate more mtGTP leading to feedback inhibition of GDH. Such a feedback circuit limits amino acid catabolism via GDH as long as sufficient glucose is present to maintain levels of TCA cycle intermediates that permit adequate TCA cycle flux and mtGTP production. For amino acids to be oxidized, there must be net loss of the amino group as ammonia or urea. This mechanism may also indirectly favor glucose and fatty acids over amino acids as oxidative substrate since ammonialysis catalyzed by GDH is required not just for anaplerosis, but also for complete amino acid oxidation This preserves the amino acid pools and keeps ammonia levels low. Thus, GDH is a major regulatory site for ammonialysis, whereas mtGTP is a major regulator.
Another unique contrast with mitochondrial ATP is that transport of GTP or GDP into or out of the mitochondria is very slow [27, 28]. Yeast lack a mechanism to synthesize mtGTP and therefore are dependent on a mitochondrial GTP transporter [29]. To date, no transporter has been identified in higher eukaryotes and any measurable transport activity is orders of magnitude slower than ATP [27, 28]. This has important implications for mtGTP. First, since it is not in equilibrium with the cytosolic pool, then it can act as a mitochondrial metabolic sensor. Second, there must either be a mechanism to hydrolyze GTP back to GDP or another GDP-independent mechanism to metabolize succinyl-CoA to succinate. Otherwise, GDP would become rate-limiting for the TCA flux.
3.2. Regeneration of mitochondrial GDP by the mitochondrial GTP cycle
Mitochondrial GTP is synthesized in the TCA cycle via succinyl-CoA synthetase (SCS) [24]. This mitochondrial matrix enzyme catalyzes the reversible reaction: Succinyl-CoA to succinate and CoA with the generation of a purine triphosphate in the process:
Initially it was believed that, in contrast to the ADP-dependent reaction observed in single celled organisms, the reaction was entirely GDP-dependent in higher organisms. Later two isoforms of SCS were identified in the mitochondrial matrix – one ATP forming (SCS-ATP), the other GTP (SCS-GTP) forming [30]. Succinyl-CoA synthetase is a heterodimer, with a common α subunit (SUCLG1) and two distinct β subunits encoded by separate nuclear genes that impart the GDP (SUCLG2) and ADP (SUCLA2) nucleotide specificity. The β subunits are highly homologous and the enzymatic characteristics of the two isoforms are similar differing primarily in their nucleotide specificity. Both isoforms work in parallel in the mitochondria with varying SCS-GTP/SCS-ATP ratios. There is a preponderance of the GDP-specific isoform in synthetic tissues (e.g. liver and kidney) while the ADP-specific isoform can be more commonly found in oxidative tissues (e.g. brain and skeletal muscle) [24, 31]. As a consequence, the stoichiometry at which GTP is made by the TCA cycle is dependent on the ratio of SCS-GTP/SCS-ATP. If ADP and GDP are not limiting and there is no other source of GTP, then GTP production will be proportional to the TCA cycle [9]. At high SCS-GTP/SCS-ATP ratios this would approach a maximum of one GTP per turn of the TCA cycle. The “gain” of the signal will be reduced by increasing amounts of the ATP isoform. Thus, in principle, the mtGTP production rates can be tuned with respect to the TCA flux in a tissue-specific manner.
There may be additional advantages to having two parallel pathways for succinate synthesis besides just changing the GTP production rate. For instance, if GDP becomes rate-limiting during states of high metabolic flux, then a switch to the SCS-ATP isoform could maintain the TCA cycle flux since the adenine nucleotide transporter is very efficient. Secondly, since both isoforms catalyze reversible reactions, the phosphate potential of ATP/ADP and GTP/GDP could be distributed across both isoforms. For example, if GTP/GDP concentrations are high relative to ATP/ADP, then succinate + GTP + CoA may react in reverse via SCS-GTP to make succinyl-CoA + GDP + Pi. The succinyl-CoA formed by the reverse reaction may then contribute to the forward reaction through SCS-ATP to convert ADP to ATP. In this manner, the two isoforms may perform a function akin to the nucleotide diphosphokinase reaction (NDPK). Thus, if equilibration across the SCS isoforms is sufficiently fast relative to GTP consumption, then the ATP/ADP and GTP/GDP could be functionally linked. Additionally, metabolites such as succinate that enter distal in the TCA cycle to SCS are not known to directly generate mtGTP. However, reverse flux of SCS-ATP could generate succinyl-CoA without the need for a pyruvate cycling pathway. Consequently, forward flux through SCS-GTP could then provide a source of mtGTP. Presently, there is no experimental evidence we are aware of to support bi-directional fluxes across SCS isoforms in intact cells.
Prior to the identification of the ATP and GTP isoforms of SCS in mammalian cells, a mitochondrial matrix NDPK was proposed as a mechanism to return mtGTP back to the GDP state by the transfer of the γ-phosphate to ADP forming ATP [32]. Indeed, initial data supported the presence of this activity in isolated mitochondria and it appeared to co-immunoprecipitate with SCS [33]. Subsequent studies now suggest that mitochondrial NDPK is primarily localized to the intermembrane space. Given that SCS-ATP and SCS-GTP together catalyze the NDPK reaction, then in the presence of contaminating levels of CoA and phosphate, the phosphate transfer might be falsely attributed to NDPK. The mitochondrial isoform of NDPK (nm23-H4) is homohexameric with two faces that bind to cardiolipin [34, 35]. Recently it has been described as an enzyme that transfers cardiolipin between leaflets of the inner and outer mitochondrial membrane as well as performing an NDPK function [36]. Interestingly, it also associates with OPA-1, the intermembrane GTPase required for fusion of mitochondrial membranes and cristae remodeling. Here it may convert ATP from oxidative phosphorylation into GTP needed for these functions. Regardless, NDPK activity in mitochondria is two orders of magnitude lower than SCS such that, even if it were in the matrix, it would not be likely to significantly alter the nucleotide ratios.
Neither NDPK nor the combined enzymatic activities of SCS-ATP and SCS-GTP adequately explain why mtGTP is synthesized in the first place. If there is already an ATP-specific SCS isoform then why go to the trouble of making GTP if it will be only used to make ATP? Identification of a significant matrix GTPase could potentially explain its purpose. PEPCK is a GTPase that decarboxylates OAA to form GDP and PEP. Hahn and Novak first proposed in 1975 that the cytosolic form of the enzyme might function as a GTPase to regenerate mtGDP in brown adipose [37]. Brown adipose, which has high activities of PEPCK, is the tissue for non-shivering thermogenesis and in a transmitochondrial, heat-generating futile cycle. They proposed that excessive mtGTP made by SCS-GTP would be transported out of mitochondria and hydrolyzed by PEPCK-C in the cycle. The cytosolic GDP would be then returned to the matrix to support further TCA flux. However, as mentioned above, lack of a mitochondrial GTP/GDP transporter makes this cycle less likely. There may be other mitochondrial processes besides PEP synthesis such as formation of iron sulfur complexes and mitochondrial protein synthesis that are dependent on mtGTP [38]. A few other mitochondrial GTPases of unknown function have been identified [39] suggesting there may be other possible roles for this metabolic signal. In metabolically active cells like liver and beta-cells expressing high levels of SCS-GTP these other GTPases may be quantitatively much less significant. For instance, as high as 30-40% of glycolysis flows into the PEPCK-M pathway in islets [7]. With the remaining glycolytic flux going into PDH, then virtually all of the predicted mtGTP synthesis would support the PEPCK-M reaction.
Drahota et al. identified very high specific activities of PEPCK-M in brown adipose [40]. In addition, they reported high rates of PEP export from mitochondria supplied with malate and alpha-ketoglutarate. They proposed that a PEP cycle involving pyruvate carboxylase (PC), PEPCK-M and pyruvate kinase might facilitate uncoupling by reducing the mitochondrial phosphate potential. Here, PEP would leave the mitochondria via the citrate isocitrate carrier (CIC) in exchange for malate and could be inhibited by 1,2,3-benzenetricarboxylate. In this PEP cycle, ATP consumed by PC and GTP used by PEPCK-M in the mitochondria would generate a single ATP in the cytosol. Such a cycle would help maintain high TCA flux rates by restoring the mitochondrial GDP pool for use by SCS-GTP. Lambeth et al. also proposed a cycle in which PEPCK-M and PEPCK-C collaborate to provide mtGDP for the succinyl-CoA synthetase step while transferring a high energy phosphate to the cytosol [24]. In this mechanism PEPCK-M consumes mtGTP from SCS-GTP to make PEP. PEP is transported out of the mitochondria where it serves as a high-energy phosphosphate donor to generate GTP in the cytosol via PEPCK-C (working in the reverse direction). Cytosolic malate dehydrogenase (cytMDH) then reduces OAA to malate that can return to the matrix to be oxidized back to OAA by the mitochondrial isoform completing the cycle. [24]. Such a PEP cycle would only be possible in tissues that contain PEPCK-C, an enzyme that is less widely expressed compared to PEPCK-M. With the exception of possibly brown fat, reversed flux (in the direction of OAA synthesis) by PEPCK-C would be counterproductive for gluconeogenesis and glyceroneogenesis.
Thus, a mitochondrial GTP cycle where SCS-GTP and PEPCK-M interact to generate and hydrolyze mtGTP respectively appears feasible. Possible functions of the cycle include 1) transferring the ATP and/or GTP phosphate potential to the cytosol, 2) regenerating GDP to maintain TCA flux rates, 3) energy consuming futile cycling, and 4) increasing cataplerotic flux. It is worth noting that tissues with high rates of anaplerosis (e.g. liver, kidney, islets and heart) express significant levels of PEPCK-M and SCS-GTP. In principle, linking mtPEP synthesis to mtGTP synthesis may secure an appropriate TCA cycle intermediate pool before draining carbon into cataplerotic pathways. Regulating TCA cycle pool size is important or it could grow indefinitely. The mtGTP sensing PEPCK-M-driven PEP cycle provides a cataplerotic pathway to balance anaplerosis. In general, these are all homeostatic functions not directly tied to the generation of a metabolic signal or product per se.
4. Glucose “sensing” in the mitochondria of pancreatic β-cells
The canonical model for glucose-stimulated insulin secretion is dependent on the metabolic generation of ATP. According to this model, glucose is metabolized by glycolysis and the TCA cycle to generate ATP that increases the cytosolic ATP/ADP ratio that in turn closes the KATP channel. KATP channel closure depolarizes the plasma membrane and activates voltage-gated calcium channels. As calcium floods into the cytoplasm, fusion of insulin granules with the plasma membrane is triggered so that insulin can be released. However, it has become increasingly more evident that this mechanism cannot explain the entirety of metabolism-coupled insulin secretion. Notably, mice that have the KATP channel knocked out still experience glucose-stimulated insulin secretion that is coupled to cytosolic calcium oscillations [41, 42]. This, among other observations have lead a number of investigators to search for alternative mechanisms to link glucose metabolism with insulin release.
4.1. Metabolic cycling in insulin secretion
Without other carbon additions (anaplerosis) or losses (cataplerosis), the TCA cycle consumes as much OAA as it produces. Pyruvate from glycolysis enters the TCA cycle either via pyruvate dehydrogenase (PDH) reaction or the pyruvate carboxylase (PC) reaction. While the oxidative PDH reaction generates acetyl-CoA, the ATP-dependent PC reaction is anaplerotic and consumes ATP to generate OAA. Considering the canonical view that mitochondrial ATP stimulates insulin secretion, it was somewhat surprising that the mitochondria of the pancreatic beta-cells express high levels of PC. This is because increased glycolytic carbon entry via PC would lead to ATP consumption rather than production [43-45]. In addition, since the fluxes of PC and PDH are roughly equivalent, then about half the carbon that could go towards oxidation would be diverted into an anaplerotic/cataplerotic cycle [46]. How then does PC flux increase insulin secretion if not through ATP production? NMR isotopomeric analysis of the flux of carbons in β-cell lines has demonstrated the strongest correlation of insulin secretion with pyruvate cycling and not with ATP [47-49]. A pyruvate cycle requires reactions that first carboxylate pyruvate followed by the decarboxylation back to pyruvate through a different reaction. Since β-cells do not express PEPCK-C [50], it was initially assumed that malic enzyme was the only metabolic pathway for the decarboxylation of malate into pyruvate. In that regard the anaplerotic pyruvate cycling has been postulated from multiple laboratories to be a critical component of the mechanism regulating GSIS [48, 49, 51-58]. Several cycles (Fig. 2) have been proposed to account for the observed cycling of carbons to pyruvate including (for review, see Ref. [59]):
pyruvate-malate cycling
pyruvate-citrate cycling
pyruvate-isocitrate cycling (isocitrate-α-ketoglutarate shuttle)*
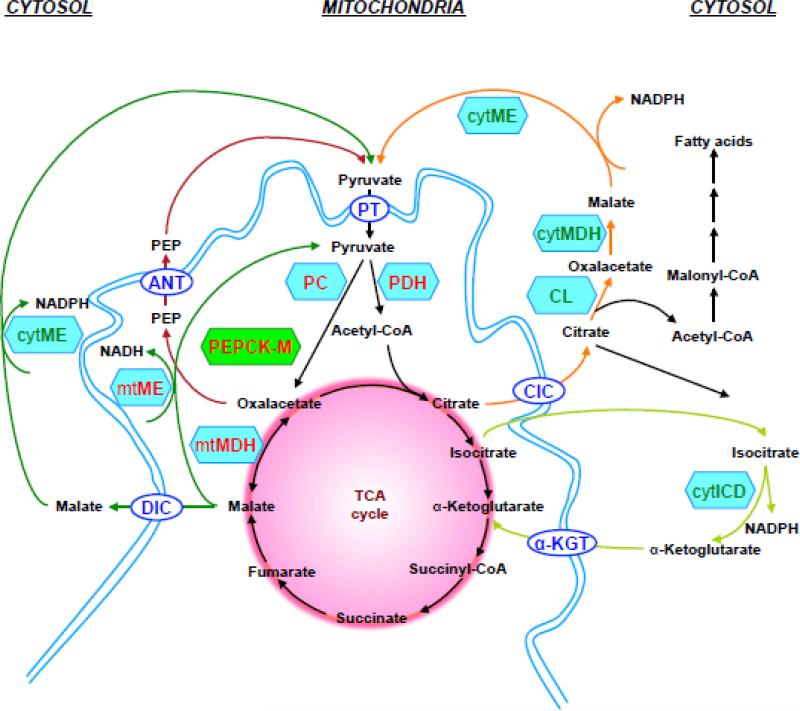
Figure 2. Pyruvate and PEP cycling pathways.
Pyruvate enters the mitochondrion via the pyruvate transporter (PT) and then enters the TCA cycle via the PDH-catalysed reaction that forms acetyl-CoA or via an anaplerotic reaction catalysed by PC. Several cycles have been proposed to account for the observed cycling of carbons to pyruvate which are following:
Pyruvate-malate cycle (dark green): OAA is converted to malate and converted back to pyruvate via mitochondrial NAD-dependent or cytosolic NADP-dependent malic enzyme (ME). Malate exits the mitochondrion via the dicarboxylate carrier (DIC).
Pyruvate-citrate cycle (orange): OAA is converted to citrate, which exits the mitochondrion via citrate/isocitrate carrier (CIC) and is converted to OAA and acetyl-CoA through citrate lyase (CL) reaction. Acetyl-CoA is converted to malonyl-CoA and long-chain acyl-CoA, while malate dehydrogenase (MDH) converts OAA into malate and further to pyruvate by cytosolic ME.
Pyruvate-isocitrate cycle (light green): Citrate can be converted to isocitrate and exits the mitochondrion via CIC. In the cytosol isocitrate is converted to α-ketoglutarate by NADP-dependent isocitrate dehydrogenase (ICDc) which can then re-enter mitochondrial metabolism by α-ketoglutarate transporter (α-KGT).
PEP cycle (red): OAA is converted to PEP by PEPCK-M and exits the mitochondrion via CIC or the adenine nucleotide transporter (ANT) (in exchange for ADP) in the inner mitochondrial membrane. PEP in the cytosol is converted to pyruvate by pyruvate kinase (PK).
Each of these cycles increases cytosolic NADPH via the activity of either malic enzyme or isocitrate dehydrogenase. While an increase in the NADPH/NADP ratio may be important for glucose-stimulated insulin secretion, interestingly it does not appear to play a role in amino acid-stimulated insulin secretion [60]. *Of note, the pyruvate-isocitrate cycle is not actually a true pyruvate cycle, but rather an extramitochondrial loop of the TCA cycle since it does not require net cycling through pyruvate. In this shuttle citrate or isocitrate exit the mitochondria and are oxidized by the cytolosic isoform of isocitrate dehydrogenase before returning to the TCA cycle as α-ketoglutarate but not as pyruvate. The NMR methods used to identify pyruvate cycling require that there is a net flux of carbon out of the mitochondria and back into pyruvate and therefore the pyruvate-isocitrate cycle would not be detected by this method. For this reason has been more appropriately defined as an isocitrate-α-ketoglutarate shuttle [61]. This does not rule out a role for cytoplasmic metabolism of isocitrate in insulin secretion, but it does rule out a direct connection between the observed pyruvate cycling and this pathway. The shuttle could, however, occur in tandem with a true pyruvate cycle such as the pyruvate-malate or pyruvate citrate cycle. Regardless, all these pyruvate cycles are all dependent upon malic enzyme whose role in insulin secretion remains unresolved [52, 53, 58].
It is particularly relevant to note, that at the time NMR isotopomer modeling techniques were developed to measure pyruvate cycling, malic enzyme was assumed to be the sole path back to pyruvate (i.e. PEPCK flux of carbons to PEP and then back to pyruvate were assumed to be absent) [49]. As will be discussed below, this is not the case. Thus, any estimate of pyruvate cycling by these techniques includes an indistinguishable component of PEP cycling. So, in addition to the pyruvate-malate and pyruvate-citrate cycle, the PEP cycle can also be explained by the high rates of PC flux in β-cells. It will be, therefore, important in future studies to distinguish the individual malic enzyme and PEPCK contributions when considering the relationship between pyruvate cycling and insulin secretion.
4.2. The mitochondrial GTP cycle in energy sensing
As noted above, mtGTP was implicated in insulin secretion by human mutations in GDH. Insulin secreting cell lines were also noted to have both ATP and GTP-specific isoforms of SCS in comparable amounts [31]. The possibility that mtGTP synthesis rates might be directly linked to glucose-stimulated insulin secretion was first demonstrated by individually silencing the ATP- and GTP-specific isoforms of SCS in insulin secreting cell lines and rodent islets [9]. The hypothesis was that silencing one isoform would redirect metabolic flux through the other isoform. This would impact mitochondrial GTP synthesis rates primarily since ATP synthesis is predominantly performed by oxidative phosphorylation. Indeed, silencing the two isoforms had the predicted effects on SCS enzyme activities but did not lead to a reduction in oxygen consumption, NAD(P)H levels or mitochondrial membrane potential. However, there was a strong association between the rate of mtGTP synthesis and insulin secretion through a mechanism that involved cytosolic calcium. Surprisingly cellular mitochondrial ATP synthesis rates, ATP levels and ATP/ADP ratios did not correlate with GSIS. These data support the idea that β-cells sense glucose metabolism, at least in part, by the coupling of mtGTP production to the TCA flux rate. However, given that mtGTP is trapped in the mitochondrial matrix, another mechanism is needed to communicate the flux signal to the cytosol.
4.3. Anaplerosis meets the mtGTP cycle: The PEP cycle
The enzyme PEPCK-M lies at the intersection between anaplerosis and the mtGTP cycle. Since PEPCK-M consumes both anaplerotic OAA and mtGTP to produce PEP it could integrate TCA cycle and anaplerotic metabolism with insulin secretion. Because of its dependence upon mtGTP, maximal mitochondrial PEP cycle flux is limited to TCA cycle flux through SCS-GTP. This may in part explain the observation that the best correlation of “pyruvate” cycling with insulin secretion includes both PC and TCA flux [48]. The discovery of PEPCK-M in the pancreatic β-cell and identification of its crucial role in insulin secretion provided the first unique metabolic role for the enzyme [7]. Here, PEPCK-M plays an important part in the process of metabolic sensing and insulin secretion. Islet glucose metabolism generates mtGTP by SCS-GTP that is proportional to TCA cycle flux [9]. While mtGTP is trapped in the mitochondrial matrix due to the lack of a transporter [29], PEPCK-M can then utilize mtGTP to convert OAA into mtPEP. Mitochondrial PEP can then escape the mitochondria to cycle back into pyruvate completing a full PEP cycle (Fig. 2) [7]. Both cellular PEP concentrations as well as flux through PEPCK-M increase at rates proportional to the glucose concentration. It is a particularly high metabolic flux with rates as high as 40% of the glycolytic rate in islets. Silencing PEPCK-M prevents insulin secretion in response to glucose as well as reduces PEPCK-M flux.
Considering that glycolysis also produces PEP, it is still unclear how PEP derived from the mitochondria goes on to stimulate insulin release in the cytosol. One may speculate that mtPEP is the molecule that transfers the “energy-sensing” signal from the mitochondria into the cytosol where it can be used for other signaling pathways (by transferring its phospho group to generate ATP or GTP) or through other biosynthetic processes. Indeed, PEPCK-M synthesis of PEP in the mitochondria increases PEP export from the mitochondria [62]. This transport is carrier-dependent and occurs primarily by the CIC or in exchange for ADP by the adenine nucleotide transporter (ANT) in the inner mitochondrial membrane [63]. A PEP/pyruvate transporter has also been postulated [64]. Since the synthesis of mtGTP depends on substrate flux, biosynthetic processes in the cytosol using mitochondrial derived PEP would be also dependent on substrate availability. However, the export of mtPEP into the cytosol also affects the cellular energy balance via regulation of mitochondrial Ca2+ levels that control TCA cycle flux rates. Mitochondrial free Ca2+ activates PDH, isocitrate dehydrogenase and α-ketoglutarate dehydrogenase [65]. Given that the mitochondrial matrix buffers intramitochondrial Ca2+ by forming an insoluble calcium-phosphate complex that depends on the accompanying Pi accumulation, the release of intramitochondrial free Ca2+ is a function of intramitochondrial Pi and ATP levels as well as PEP [66]. Studies show that PEP regulates both mitochondrial calcium transport and ATP/ADP exchange [57, 66-73].
There could be distinct advantages to having a metabolic signal dependent upon both anaplerotic and oxidative metabolism. For instance, a dependence on anaplerosis would prevent fatty acid oxidation by itself from contributing solely to insulin secretion. Dependence on mtGTP would limit the maximal mtPEP production rate to the TCA cycle rate and ensure ample ATP and TCA intermediates to maintain normal mitochondrial and cellular function. In the absence of activation of GDH by leucine from a protein meal, mtGTP would prevent glutamate, the most abundant intracellular amino acid, from stimulating insulin secretion inappropriately. Thus, substrate level synthesis of mtGTP by SCS-GTP provides energy for PEP synthesis in the mitochondrial matrix by PEPCK-M and thereby link PC flux with TCA cycle flux to stimulate insulin release [7].
5. PEPCK-M
PEPCK was first found in chicken liver (where only the mitochondrial isoform is present) and believed to fix carbon dioxide by the conversion of pyruvate into OAA. This reaction was called Wood-Werkman pathway [74]. The first published reference of PEPCK occurred in 1953 by Utter and Kurahashi [75] with the title, “Mechanism of Action of Oxalacetate Carboxylase from Liver.” Later the enzyme was given the name phosphoenolpyruvate carboxylase [76]. Interestingly, the first and only isolated isoform of PEPCK was PEPCK-M from chicken liver [77] but later most studies have focused upon PEPCK-C.
5.1. PEPCK-M expression and activity
Humans express PEPCK-M in most tissues but especially in liver and kidney [78]. The pancreas also expresses PEPCK-M but unlike liver and kidney, PEPCK-C is not expressed [50, 79-81]. Much emphasis has been placed on the relative enzyme activities of the PEPCK isoforms as a reflection of their contribution to gluconeogenesis. Human livers show equal distribution of PEPCK activity, whereas in the liver of chickens almost all PEPCK activity is attributed to PEPCK-M but in the kidney of chickens both isoforms are equally active. Guinea pig liver and kidney has about 80% PEPCK-M activity. While perhaps the most cited references suggest that the rat and the mouse have a PEPCK-C/PEPCK-M ratio of 9 to 1 [76], some authors [82-85] detected more than 10% PEPCK-M activity in rodent liver. These discrepancies may be due to the differences in the enzymatic activity assay used (Table 1 and reviewed in [7]).
Table 1.
Summary of experimental procedures used to measure PEPCK activity with percentage of PEPCK-M activity to total PEPCK activity measured in rat liver
| Wiese TJ et al (1991) [174] | Söling H et al. (1970) [173] | MacDonal d MJ and Chang CM (1985) [172] | Cornell NW et al. (1986) [171] | Nordlie RC and Lardy HA (1963) [8] | Ballard FJ and Hanson RW (1967) [85] | Usatenko M (1970) [80] | Heitzman R et al. (1972) [81] | Saggerson D and Evans CJ (1975) [79] | Horn D et al. (1997) [78] | |
|---|---|---|---|---|---|---|---|---|---|---|
| % PEPCK-M | 0 | 0 | 3 | 5 | 6 | 10 | 18 | 25 | 25 | 50 |
| direct/indirect | difference | difference | per gram | direct | direct | direct | direct | direct | ||
| pH | 6.8 | 8 | 7.5 | 6.6 | 8 | 7 | 8.1 | 7.5 | 6.6 | 7.4 |
| ion | Mn2+ Mg2+ | Mn2+ Mg2+ | Mg2+ | Mn2+ | Mg2+ | Mn2+ | Mg2+ | Mg2+ | Mn2+ | Mn2+ |
| nucleotide | dGDP | IDP | ITP | IDP | ITP | IDP | ITP | ITP | IDP | dGDP |
| mitochondrial isolation | Deoxy-cholate | Deoxy-cholate | freeze thaw | Digitonin | freeze thaw | lyophylisation | freeze thaw | sonication | no detergent | |
| leakage | citrate synthase | citrate synthase | no | yes | no | yes | ||||
| reaction | reverse | reverse | forward | CO2 fixation | forward | CO2 fixation | forward | forward | CO2 fixation | reverse |
| inhibitor | NaF | no | no | No | NaF | no | KF | no | Rotenone | Rotenone |
| fed/fasted | fed + fasted | fed + fasted | fed | fed + fasted | fed + fasted | fed | fed | fast |
5.2. PEPCK gene expression at different stages of development
Interestingly, PEPCK-C and PEPCK-M show different expression profiles during development [86, 87] and also gluconeogenic activity in mammal liver. Gluconeogenesis is absent before birth as glucose is provided by the mother [88], and is thought to start with birth [89] as a result of decreasing plasma blood glucose levels and subsequent hormonal changes, namely a decrease in insulin and increase in cAMP, glucagon as well as epinephrine [90, 91]. The prenatal mammalian liver does not express PEPCK-C, thus PEPCK-M is the only PEPCK isoform present before birth [92]. After birth and postnatal development the liver undergoes major metabolic alterations increasing PEPCK-C transcription and protein maturation [93-95]. In contrast to liver, fetal kidney expresses PEPCK-C, which significantly rises after birth [96-98]. Glucocorticoids and acid/base status are also stimuli for postnatal PEPCK-C gene expression in kidney [99].
5.3. The PEPCK gene
Mammals have two different nuclear genes for the two PEPCK isoforms. PCK1 encodes PEPCK-C and resides on human chromosome 20q13.31. PCK2 encodes PEPCK-M and resides on human chromosome 14q11.2 [100]. The human genes for PEPCK-M and PEPCK-C contain the same genetic organization: 10 exons and 9 introns, whereas the length of PEPCK-C (5345 bp) is smaller than PEPCK-M (9839 bp), due to expanded intronic length in PEPCK-M [78, 101]. However, the PEPCK-C and PEPCK-M proteins have a similar molecular weight (69,289 for rat PEPCK-C and 69,522 for chicken PEPCK-M). The human proteins are composed of 622 (PEPCK-C) and 640 (PEPCK-M) amino acids, respectively [76]. Both PEPCKs share 68% identity as well as 82% similarity in their sequence [76, 78, 100].
5.4. The PEPCK protein
While GTP-dependent (in higher eukaryotic species) and ATP-dependent PEPCKs (in bacteria, yeast, C-4 plants) share little sequence homology (< 20%), the active sites and structures are reasonably conserved and suggests an important similarity in function and regulation via enzyme confirmation [102]. Mammal GTP-dependent PEPCK isoforms have nearly identical protein folds [103]. The protein folds as a single domain with an N-terminal lobe and a C-terminal lobe forming an active site that carries out the decarboxylation/carboxylation and phosphoryl transfer half-reactions as well as stabilization of the enolate intermediate [102-109]. The active sites involve reactive cysteine residues located in a P-loop (phosphate-binding loop/kinase-1a motif) [103]. The specific modification of a single cysteine residue inactivates the enzyme [110, 111], which is Cys288 in rat PEPCK-C [103, 111] and the homologous cysteine in chicken PEPCK-M is Cys307 [107]. Oxidative state, cation redistribution, and/or pH influence PEPCK function via P-loop confirmation and therefore suggest that different metabolic states lead to loss or gain of function. Given that, PEPCK contains sulfhydryl groups and its activity depends on critical cysteine residues, PEPCK likely requires another protein to maintain its redox state [76]. A class of proteins termed ferroactivators is one possibility as they enable Fe2+ to stimulate PEPCK-C [112, 113]. These ferroactivators were subsequently identified as catalase and glutathione peroxidase [114, 115]. In bovine erythrocytes a similar ferroactivator protein, a green hemoprotein, was purified [116]. Another PEPCK-C stabilizing protein of 29,000 kDa, p29, was isolated and supports Mg2+-induced activation. This protein was later identified as phosphoglycerate mutase and is highly expressed in the liver [117, 118].
PEPCK-M binds a single divalent cation in tetrahedral coordination in the absence of substrates with Mn2+ being the most activating cation. In the presence of nucleotide an additional cation is bound in form of a cation-nucleotide complex [102]. In the fully ligated state both metal ions are octahedrally coordinated [103]. No large domain movements or conformational changes occur upon binding of GTP [103]. During the catalytic cycle, conformational changes occur at the active site. The most prevalent mobile feature is a 10-residue Ω-loop domain, which functions like an active site lid. Closure of the lid reduces the active site region between the N- and C-terminal lobes and the P-loop, and shifts the location of the bound substrates [105]. The close lid confirmation stabilizes and sequesters the reactive enolate intermediate, places the substrates in the correct position, minimizes non-specific reactions and ensures efficient PEPCK-mediated catalysis [102, 104].
5.5. The PEPCK enzyme – catalytic properties
PEPCK-C and PEPCK-M share similar kinetic characteristics and Michaelis constants for their substrates and products [119] as well as similar pH optima at around 7 [120]. Both PEPCKs are active as a monomer and catalyze the same reaction, namely decarboxylating OAA with the concomitant transfer of the γ-phosphate of GTP (or ITP): OAA + GTP → PEP + GDP + CO2. PEP formation is the preferred direction of both enzymes [76]. The only reported substrates for both PEPCKs are GTP (or GDP) and ITP (or IDP) [76, 121]. Dissociation constants of PEPCK-C, measured by isothermal titration calorimetry, are 0.5 μM for GTP, 4 μM for ITP and for the other nucleotides >100 μM [103].
In addition, both isoforms require two divalent cations: Mg2+ and Mn2+. Other divalent metal ions such as Fe2+, Co2+, Zn2+ and Cd2+ stimulate PEPCK, but to a lesser extent [107, 122]. Noteworthy, PEP carboxylation by PEPCK-C absolutely requires Mn2+, whereas Mg2+ can replace it for OAA decarboxylation [123]. Ash et al. [124] proposed that Mg2+ binds GTP and builds the metal nucleotide substrate, whereas Mn2+ associates with PEPCK-C serving as an activator, although Mg2+ can substitute. The Mg-GTP complex binds and activates PEPCK-C and then decarboxylates OAA ligated to the Mn2+ site of PEPCK-C. The Mg-GTP complex phosphorylates the enolate of pyruvate to PEP [124]. In contrast to PEPCK-C, Mn2+ is the best activator for PEPCK-M in chicken [125], whereas the selectivity of PEPCK-M for Mn2+ was 1000-fold greater than for Mg2+ [126]. Further Mg2+ has been noted to reduce PEPCK-M activity [80]. Both enzymes can randomly bind their substrate, although PEPCK-M usually binds GTP before OAA and has a slow off-rate [107, 122]. Once GTP is bound, it has a high likelihood of progressing to product (PEP, CO2, and GDP) [76]. Whether mitochondrial OAA or mtGTP are rate-determining for PEPCK-M is unclear, but the tight association between mtGTP synthesis and PEP production would suggest mtGTP is rate-limiting [127].
5.6. Regulation of PEPCK-M enzyme expression and activity
The two PEPCK isoforms are regulated differently. While PEPCK-C expression can be robustly stimulated by glucagon and suppressed by insulin, PEPCK-M is not known to be under such hormonal control and appears to be constitutively expressed [76, 78]. Although sequence analysis of the PEPCK-M promoter region revealed several potential regulatory elements, including SREBP, CREB, C/EBP, AP-1, AP-2, and SRY elements [128]. Indeed, hormonal treatment of chicken hepatoma cells does not affect PEPCK-M mRNA expression [76, 129]. Due to the mitochondrial location of PEPCK-M, it is a weaker candidate for rapid transcriptional regulation, and further, no post-translational modifications or allosteric effectors are known. Recently, posttranslational modification of PEPCK to regulate its activity has been suggested for PEPCK-C. Acetylation of PEPCK-C determines its stability as it targets the enzyme for ubiquitylation and subsequent proteasomal degradation, hence deactivates PEPCK-C [130-132]. The regulatory significance of acetylation of the enzyme remains to be established. Moreover, PEPCK-M mRNA half-life is relative long, namely ~50 hours, while the half-life of PEPCK-C mRNA is short (~30 min) and transcriptional changes that affect PEPCK-C mRNA levels occur within 1-2 hours [76]. During the shift to lactation, both PEPCK isoform activities increase in the mammary gland – a tissue with no known gluconeogenic activity, where they may support fat deposition (glyceroneogenesis) and subsequent milk production during lactation. During this time PEPCK-C activity (~11-fold) and expression increase, but in contrast to a dramatic increase in PEPCK-M activity (~43-fold) there are no changes in PEPCK-M expression [133, 134]. Thus at least for PEPCK-M, activity levels do not necessarily parallel changes in gene expression. However, PEPCK-M expression may change under pathological conditions as observed in the diabetes model of the chronically glucose-infused rat [135]. Diabetic rats show elevated plasma insulin and glucagon levels as well as hepatic glucose production accompanied with raised PEPCK-M but decreased PEPCK-C expression. Whether PEPCK-M expression is altered by the combination of insulin and glucagon or some other signal remains to be clarified.
Recent work suggests a possible regulatory mechanism of PEPCK-M that operates at the enzyme level by transitioning between inactive tetrahedral and active octahedral coordination geometry, and represents a previously unappreciated mechanism of regulation [108]. Unlike PEPCK-C, the predominant conformation of the P-loop is the tetrahedral coordination of the reactive cysteine residue (Cys307) to Mn2+. This loop confirmation stabilizes the hyper-reactive cysteine, prevents nucleotide-binding and represents a catalytically incompetent state of the enzyme [107]. Further, pH-rate studies propose that the protonation of this cysteine residue is required for optimal interaction of PEPCK-M with its substrates [126]. Catalytic studies revealed that Zn2+ inhibits PEPCK-M activity in the direction of OAA formation because it favors sulfhydryl ligands and stabilizes the Cys307-coordinated tetrahedral geometry. Further, in vitro PEPCK-M activity studies require β-mercaptoethanol (β-ME), suggesting that the inactive conformation is stabilized without, and diminished with β-mercaptoethanol [111]. Transcriptional control tightly mediates PEPCK-C expression, and since the PEPCK-C protein has a half-life of six hours [136], this mechanism of conformational regulation could also provide a mechanism of immediate regulation of PEPCK-C. Indeed, the requirement of reducing agents in enzymatic assays, such as dithiothreitol (DTT) or β-ME was also noted for PEPCK-C [119]. Importantly, the optimal pH for both PEPCK reactions depends on the divalent metal ion utilized because of subtle different secondary and tertiary enzyme structure at different proton concentrations [107, 119, 125].
In summary, since PEPCK activity relies on the availability of divalent metals as well as OAA and GTP, PEPCK seems to be prone to substrate regulation. Moreover, energy metabolism, redox state, ATP/ADP ratio as well as TCA cycle flux are other factors regulating PEPCK activity [137]. Given the lack of known regulators of PEPCK-M expression, its enzymatic flux is consequently much more susceptible to vary based on product and substrate concentrations. Hormones, such as insulin, glucagon, catecholamines and cortisol may all indirectly regulate PEPCK-M via controlling gluconeogenic substrate availability.
6. Is there a role of PEPCK-M in gluconeogenesis?
Presently, PEPCK-C is generally believed to account for virtually all the gluconeogenesis from mitochondrial-derived precursors, at least in rodents [138]. PEPCK-C is often considered as the only isoform and it is even frequently abbreviated simply as PEPCK. However, as noted above, it is less appreciated that there are actually two distinct isoforms that reside in either the cytosol or the mitochondria [8]. The cytosolic isoform has attracted the majority of attention despite PEPCK-M being the first isoform discovered and 40% of the cellular PEP can be found in the mitochondrial fraction [139-142].
PEPCK-C is well-known for its role in gluconeogenesis and its function has been extensively studied and reviewed [76, 86, 138, 143, 144]. Apart from gluconeogenesis the following functions has been suggested: glyceroneogenesis, cataplerosis/anaplerosis, amino acid synthesis, PEP/pyruvate cycling. Similar functions of both PEPCK enzymes are suggested since they both catalyze the same reaction [76]. The common denominator of both PEPCK enzymes is PEP as a product that may be utilized for biochemical processes. PEP is needed for glucose production but also serves for triglyceride, amino acids and nucleic acid synthesis. The source of PEP for these synthetic processes may be dependent on the tissue, metabolic condition, energy status and precursor availability [100]. While PEPCK-C function is remarkably well studied, the biology of PEPCK-M is poorly investigated. PEPCK-M is highly conserved in all eukaryotes studied, suggesting PEPCK-M an important biological role [138].
An inferior role for PEPCK-M in gluconeogenesis has primarily been supported by in vitro studies [145-148]. Deletion and overexpression studies of PEPCK-C in the liver confirmed a role of PEPCK-C in gluconeogenesis and glyceroneogenesis [137, 145-147, 149, 150]. Surprisingly, though, several genetic models of PEPCK-C deletion maintain normal fasting glucose and glucose turnover rates. This suggests that other mechanism(s) must exist that can support glucose production [146, 147]. Notably, there is no correlation between PEPCK-C mRNA, protein, or activity in humans with diabetes [151]. The current dogma of PEPCK-C as sole regulator of cataplerotic PEP production may need to be recalibrated. Here, we will consider the possibility that PEPCK-M has been overlooked and might have a potential role in gluconeogenesis.
6.1. A hint from PEPCK-C knockout animals
In support for an exclusive role of PEPCK-C in glucose production, homozygous whole-body PEPCK-C knockout mice die within 2-3 days of birth that is associated with hypoglycemia [146, 147]. However, these pups could not be rescued by exogenous glucose and had normal plasma lactate. Interestingly, deletion of glucose-6-phosphatase in mice did not cause death until the age of 10 days. These mice were profoundly hypoglycemic due to diminished gluconeogenesis, but, in contrast, the mice could be rescued by glucose [152]. Noteworthy, given that PEPCK-C is dramatically induced at time of birth [89], it may be possible that these PEPCK-C null mice could survive once they made it through the neonatal phase (for example as a cre/lox mice with 80% reduction at birth and a 90-100 % reduction later on). Neonatal death of homozygous whole-body PEPCK-C knockout animals may indicate other important functions of PEPCK-C.
Based on current models of gluconeogenesis, one would predict hepatic PEPCK-C deficiency would cause hypoglycemia, in particular during states of negative energy balance such as fasting. Surprisingly, heterozygous mice lacking hepatic PEPCK-C (PCK1lox/lox Alb-Cre) remain euglycemic under fed and fasted conditions, although these mice show evidence of hepatic steatosis, insulin resistance and exercise intolerance [145-147]. Even with less than 10% of whole body PEPCK-C activity (a level comparable to endogenous PEPCK-M) mice have normal concentrations and turnover rates of glucose, lactate, and glycerol in vivo [146, 147]. In this setting, the absolute rates of PEP production necessary for gluconeogenesis are not significantly reduced [148]. Only, when PEPCK-C is completely eliminated does a significant reduction in PEP synthesis occur in perfused livers [148]. This degree of PEPCK-C suppression does not occur in vivo and suggests other physiologic mechanisms normally regulate PEPCK flux besides just PEPCK-C expression. In order to explain the observed euglycemia in PEPCK-C knockout animals, extra-hepatic gluconeogenesis was suggested to compensate, whereas the alternative hepatic pathway to glucose production was ruled out due to the assumed lack of PEPCK-M activity in rodent liver [146]. This is supported by the observation that renal gluconeogenesis has an enormous capacity and can completely compensate during the extrahepatic phase of liver transplants [13, 153-155]. Intriguingly, liver-specific PEPCK-C deficient mice develop fatty livers, have reduced TCA cycle flux, and accumulate TCA cycle intermediates indicating that they are indeed PEPCK-C deficient [137, 146, 147, 156].
Both PEPCK isoforms depend on normal mitochondrial function to generate energy and gluconeogenic precursors. However, PEPCK-C knockout animals have varying severity of mitochondrial dysfunction [145, 148, 156]. Notably, the degree of PEPCK-C deficiency in liver-specific knockout mice correlates with the extent of the TCA cycle defect. Complete PEPCK-C loss impairs mitochondrial function with a 20% reduction in O2 consumption and 85% reduction in the TCA cycle flux [148]. Consequently, liver-specific PEPCK-C knockout mice accumulate mitochondrial intermediates (including citrate, succinate, fumarate, malate and aspartate), have a high mitochondrial NADH/NAD+ ratio and severe fatty livers (but normal malonyl CoA). In addition, these mice show changes in expression of metabolic and mitochondrial genes. Recently, these mice have been used to confirm a role of PEPCK-M in gluconeogenesis [145]. However, a 100-fold overexpression of PEPCK-M in livers of these mice did not have any detectable consequence in vivo and just a marginal improvement (~8%) of glucose production from PEP in perfused livers. The interpretation was that PEPCK-M lacks a primary role and only supports PEPCK-C as primary determinant of gluconeogenesis. Another interpretation is that rodent livers do not actually lack PEPCK-M (table 1) but rather the normal levels of PEPCK-M cannot work in the setting of severely impaired mitochondrial function. Of note, β-cells have equally high levels of anaplerotic and cataplerotic metabolism compared to the liver. However, β-cells do not express PEPCK-C [7], and still have properly functioning mitochondria. Thus, there must be something unique to hepatic PEPCK-C null mice causing mitochondrial dysfunction. Accordingly, it is difficult to conclude if changes in gluconeogenesis are caused by the loss of PEPCK-C enzymatic activity per se or because of other metabolic deficiencies. However, in contrast to the perfused livers from these mice, in vivo basal gluconeogenesis is not reduced in different PEPCK-C knockout animals. Regardless, given the dependence of both isoforms on normal mitochondrial function, results from PEPCK-C knockouts do not preclude a significant role for PEPCK-M.
Besides the general view that PEPCK-M is not sufficiently active in rodent liver, a few publications (Table 1) [82-85, 135] suggest contrary and would challenge the above mentioned studies [137, 146, 147]. Surprisingly, in the livers of chronically glucose-infused rats, a model of Type-2 diabetes with inappropriate increases in glucagon and insulin, PEPCK-C was suppressed but PEPCK-M increased in accordance with endogenous glucose production rates [135]. These results point towards an undiscovered role for PEPCK-M in gluconeogenesis [146, 147]. Finally, PEPCK-C and PEPCK-M may operate in parallel, thus PEPCK-M may compensate for PEPCK-C knockout animals as long as mitochondrial function remains intact. Taken together, there does appear to be a potential role for PEPCK-M in gluconeogenesis, but inactivation or inhibition of the mitochondrial isoform would need to be performed to solidify its role.
6.2. Could PEPCK-M participate in Gluconeogenesis?
Given the possibility that PEPCK-M's activity in liver may have been underestimated, is there a possibility that it has a role in gluconeogenesis? Unlike PEPCK-C, PEPCK-M is constitutively expressed, hence could provide a continuous supply of PEP. PEPCK-M is not inducible by glucagon [157] but this also does not rule out a gluconeogenic role. Both PEPCK isoforms convert OAA to PEP [158]. However, a potential limitation of the PEPCK-M pathway is its dependency on mtGTP. One TCA cycle turn makes only one mtGTP. Unless there is an alternative source of mtGTP during states of high demand, SCS-GTP flux would set an upper limit to mtPEP production. Presently the capacity of PEPCK-M to accommodate mtGTP from the TCA cycle in rodent liver is unknown. In contrast, PEPCK-C is not dependent on mtGTP and in principle would have the capacity to produce extra PEP albeit at higher energetic cost (Fig. 5). So while PEPCK-M may support basal needs, during times of high glucose demand, collaboration between the isoforms may likely be required. Thus, a system with two PEPCKs would provide an increased degree of metabolic flexibility.
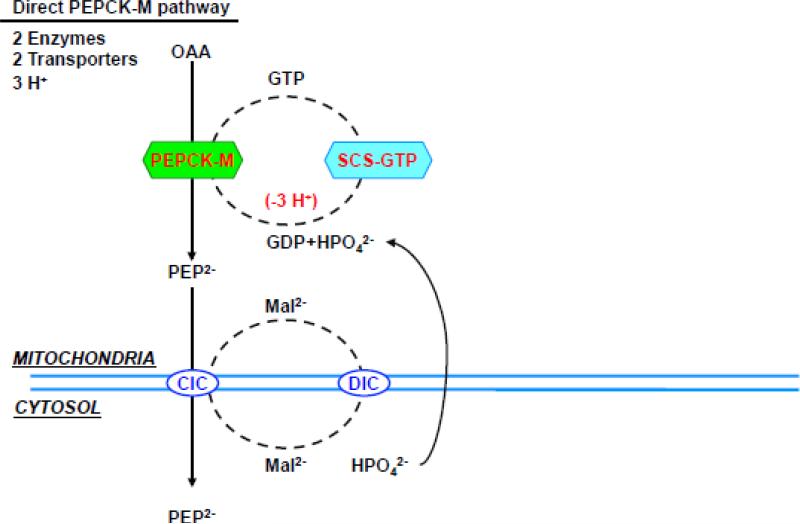
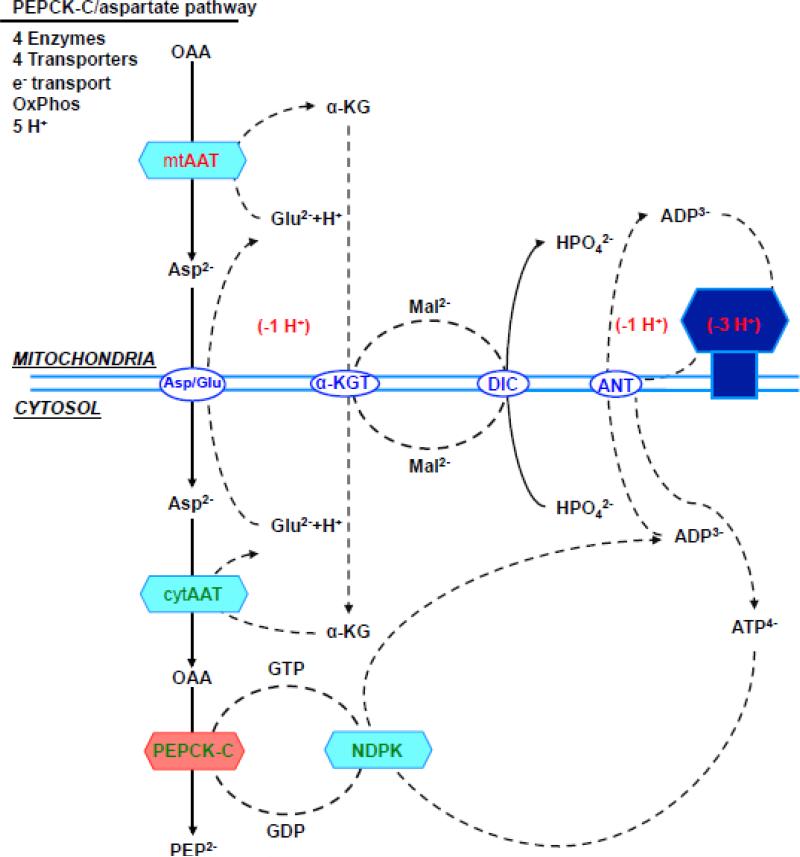
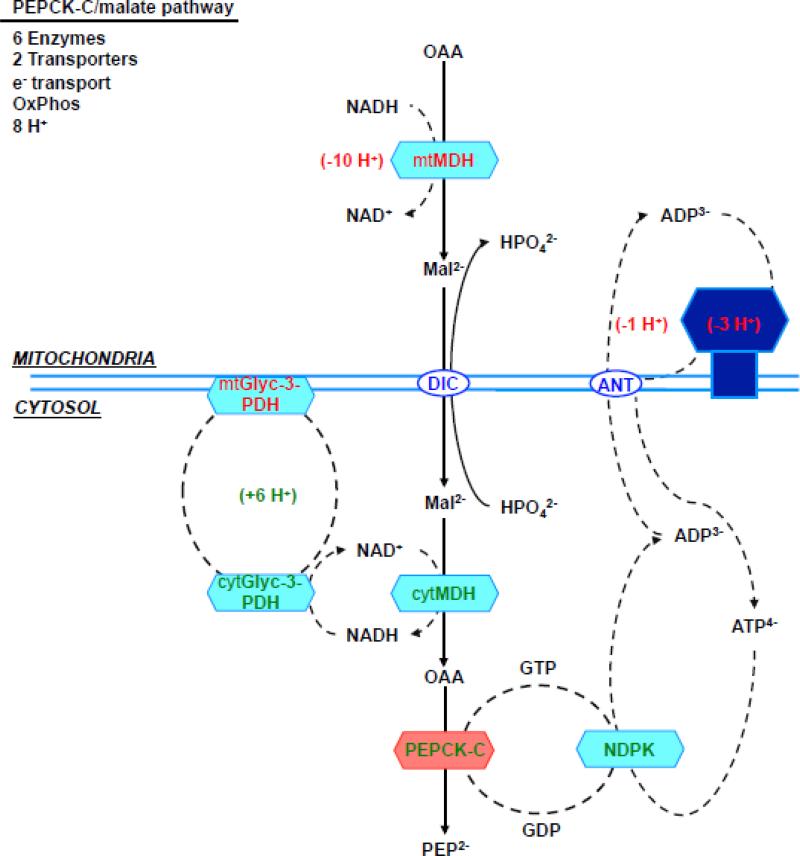
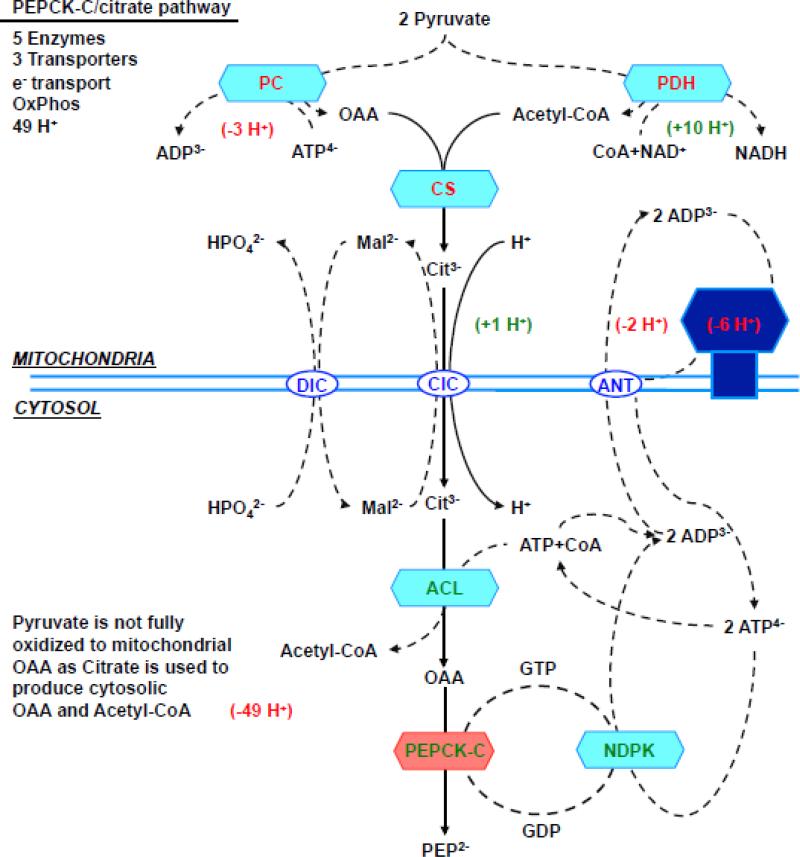
Figure 5. (a) direct PEPCK-M pathway, (b) aspartate/PEPCK-C pathway, (c) malate/PEPCK-C pathway and (d) citrate/PEPCK-C pathway.
Enzymes, transporters and metabolites involved in the 4 metabolic pathways from mitochondrial oxalacetate to cytosolic PEP. The charge valences of the metabolites that are used by the different transporters are listed as superscripts. The accounting is based on the number of protons equivalents generated (in green) or consumed (in red) by each of the metabolic steps. Synthesis of both ATP and GTP within the mitochondria was assumed to be equivalent to 3 protons, the transport of one ATP out of the mitochondria and glutamate into the mitochondria each consume one proton. Oxidation of mitochondrial NADH pumps 10 protons, while cytosolic translocates 6 via the glycerol-3-phosphate step since the malate aspartate shuttle is not available. Abbreviations: OAA, oxaloacetate; PEP, Phosphoenolpyruvate; CIC, citrate isocitrate transporter; DIC, dicarboxylate transporter; Asp, aspartate; αKG, α-ketoglutarate; Asp/Glu, aspartate glutamate transporter; αKGT, α-ketoglutarate transporter; ANT, adenine nucleotide transporter; Mal, malate; Glu, Glutamate; mtAAT, mitochondrial aspartate aminotransferase, cytAAT, cytosolic aspartate aminotransferase; mtMDH, mitochondrial malate dehydrogenase; cytMDH, cytosolic malate dehydrogenase, mtGlyc-3-PDH, mitochondrial glycerol 3 phosphate dehydrogenase; cytGlyc-3-PDH, cytosolic glycerol-3-phosphate dehydrogenase; NDPK, nucleotide diphosphokinase.
In addition to changes in protein expression induced by hormonal signaling, gluconeogenic flux is potentially regulated turning on and off a futile PEP cycle (pyruvate→OAA→PEP→pyruvate) [4, 5, 7, 159]. Here, this anaplerotic/cataplerotic cycling could operate in three ways: 1) redirecting anaplerotic substrates to pyruvate (including substrates that enter the TCA cycle as 4- and 5-carbons such as aspartate, glutamate and glutamine) and 2) consuming ATP that could otherwise be used for gluconeogenesis to buffer substrate flux and 3) providing additional amounts of metabolic cycling to fine tune metabolic flux while ensuring adequate TCA cycle intermediates [4].
6.3. Gluconeogenic Substrates
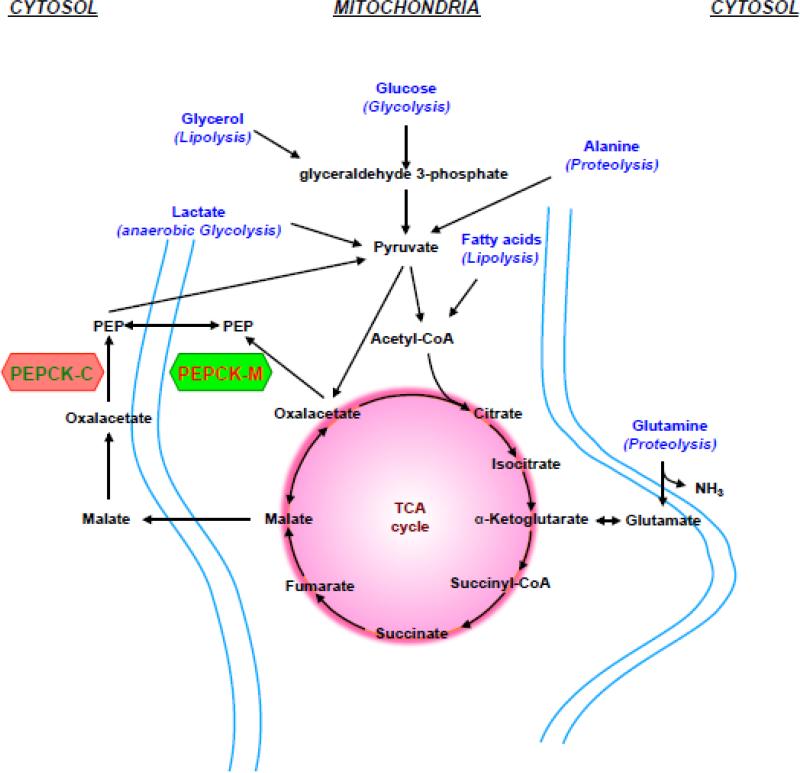
Generally, any metabolite that enters the TCA cycle to build OAA can contribute to glucose production once converted to PEP (Fig. 3). Glucose can be synthesized de novo from mitochondrial-dependent substrates (e.g., lactate, pyruvate, glutamine, alanine, and propionate) or from cytosolic substrates (e.g., glycerol and fructose). All of the mitochondrial gluconeogenic precursors have an absolute requirement for the PEPCK reaction. During each turn of the TCA cycle, citrate synthase consumes as much OAA as is produced by malate dehydrogenase (MDH). Balanced carbon outflow into synthetic pathways (cataplerosis) requires compensation by carbon inflow (anaplerosis) into the TCA cycle pool. Thus, the carbons of acetyl-CoA generated by lipolysis or PDH cannot directly contribute to gluconeogenesis without an additional source of OAA. Unlike fatty acids from triglyceride breakdown, glycerol can contribute to glucose production and enters gluconeogenesis (or glycolysis) via glyceraldehyde-3-phosphate. Pyruvate, lactate, and alanine become anaplerotic substrates when they are carboxylated into OAA by PC. The amino acids alanine and glutamine must first be deaminated in order for them to enter the TCA cycle. Since aminotransferase reactions require a net acceptor of the amide nitrogen, amino acids must be either oxidatively deaminated by GDH to release ammonia or processed in the ornithine cycle to generate urea. Methionine, threonine and the branched chain amino acids are added to the TCA cycle through metabolism to propionyl-CoA. Similarly the final γ oxidation of the occasional odd-chain fatty acids also generates propionyl-CoA. Propionyl-CoA then must be carboxylated by the biotin-dependent enzyme propionyl-CoA carboxylase before it can enter the TCA cycle as succinyl-CoA [20]. Lactate forms in the muscle during anaerobic glycolysis and enters the gluconeogenic pathway via pyruvate and is the main gluconeogenic precursor in the kidney and liver. Noteworthy, the kidney has been suggested to prefer glutamine [160] while the liver may favor alanine as gluconeogenic precursors [13].
Figure 3. Gluconeogenic substrates entering the gluconeogenic pathway.
Graph shows entry point of gluconeogenic substrates, such as lactate, glycerol or amino acids. Both PEPCKs synthesize PEP from OAA that can feed the TCA cycle (anaplerosis) or serve for various biosynthetic processes (cataplerosis), such as gluconoegenesis. Alanine and glutamine are the main amino acids in the blood and arise during starvation from muscular protein breakdown (proteolysis). Likewise, fatty acids and glycerol are released from triglyceride breakdown (lipolysis) during fasting. Unlike glycerol, acetyl-CoA (fatty acid breakdown) does not contribute to cataplerotic OAA or PEP production or other gluconeogenic intermediates. Glycerol and glucose enter via glyceraldehyde 3-phosphate. Lactate forms in the muscle during anaerobic glycolysis and enters the gluconeogenic pathway via pyruvate and is the main gluconeogenic precursor in the kidney and liver.
Each mitochondrial substrate has its own advantages and disadvantages. Based on turnover rates, lactate produced by the Cori cycle is quantitatively the most important gluconeogenic substrate. Given that glucose is the predominant source of lactate, once glycogen stores are depleted, lactate cannot make a net contribution to the glucose pool in the setting of ongoing glucose oxidation. Pyruvate, too, is derived primarily from glucose, but because of rapid equilibration across lactate dehydrogenase (LDH) is about one tenth the concentration of circulating lactate. Alanine is a significant gluconeogenic contributor, but about half the carbon in the glucose-alanine cycle also comes from glucose itself with the remaining coming from amino acids from protein breakdown [12, 15]. Glutamine is the most concentrated circulating amino acid and only a small fraction contains carbons from glucose making it a net contributor to the glucose pool. Some propionate comes from the breakdown of certain amino acids and odd-chain fatty acids, but its significant contribution to gluconeogenesis comes from the gut flora following meals and is an especially important source of glucose for ruminants.
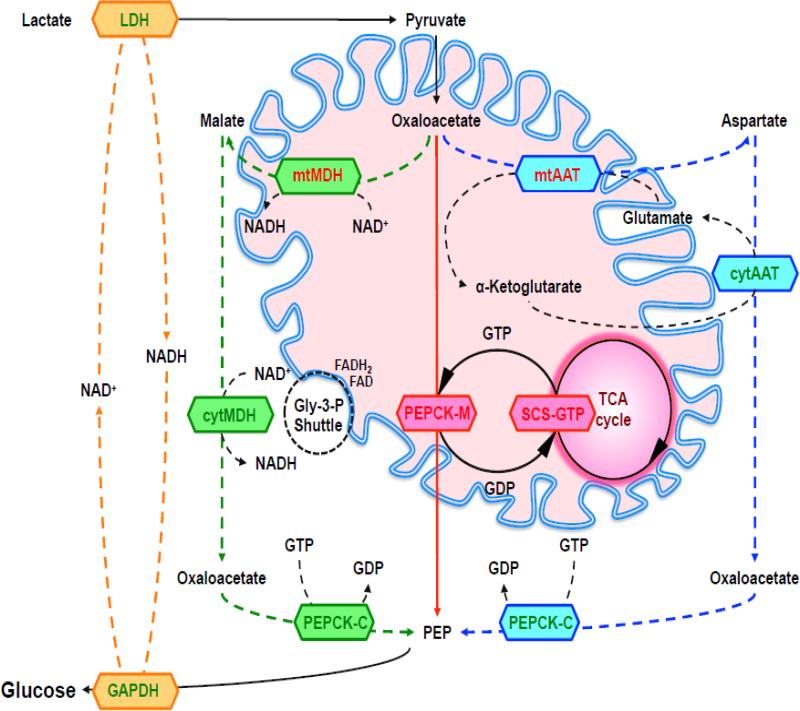
Each mitochondrial substrate may favor one or the other PEPCK isoform in order to provide PEP while maintaining a favorable cytosolic redox. Ultimately, all mitochondrial substrates entering gluconeogenesis oxidize NADH at the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) step. If glucose is made from lactate, NADH for GAPDH is produced in the cytosol by LDH [161]. Therefore, when the substrate for gluconeogenesis is lactate, PEPCK-M is favored since a shift of reducing equivalents from the mitochondria to the cytosol is not required (Fig. 4) [78]. The study from Gstraunthler et al. [162] identifies lactate as a likely substrate for PEPCK-M. Cells from pig kidney cortex (LLC-PK1F+) expressing both PEPCK isoforms grow in a medium containing pyruvate and lactate, whereas cells from opossum kidney (OKGNG+) that express only PEPCK-M could be only maintained in a medium with lactate. This may be explained by the requirement of pyruvate, propionate or alanine to generate cytosolic NADH. In this case, mitochondrial-derived OAA was proposed to be converted to malate in the matrix and shuttled to the cytosol where cytMDH would make both NADH and OAA [76]. Subsequently, PEPCK-C metabolizes cytosolic OAA to PEP, leaving NADH for GAPDH step of gluconeogenesis [76]. Special consideration for alanine depends on whether its amide nitrogen is eventually disposed as ammonia or as urea. While ammonia release (via transamination of α-ketoglutarate into glutamate followed by GDH metabolism) generates mitochondrial NAD(P)H, urea metabolism could increase NADH in the cytosol or mitochondria depending on where the MDH reaction needed for the ornithine cycle occurs. Glutamine catabolism may be advantageous during a prolonged fast as it provides the energy, carbons, as well as mtGTP for efficient mtPEP formation. Again, the mechanism of ammonia disposal may determine whether PEPCK-C or PEPCK-M is favored.
Figure 4. Gluconeogenesis from Lactate.
Lactate as substrate generates NADH via lactate dehydrogenase (LDH) in the cytosol necessary for the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) reaction of gluconeognesis (orange). Mitochondrial oxaloacetate (OAA) has four pathways to cytosolic phosphoenolpyruvate (PEP), whereas three are energetically preferable for gluconeogenesis: The PEPCK-M pathway (red) is the most direct pathway and uses mtGTP produced by succinyl-CoA synthetase (SCS-GTP) in the TCA cycle. The PEPCK-C/aspartate pathway (blue) uses mitochondrial (mtAAT) and cytosolic (cytAAT) transamination reactions via aspartate aminotransferase (AAT) and needs shuttling of glutamate and α-ketoglutarate to generate OAA in the cytosol. OAA is converted by PEPCK-C that hydrolyses cytosolic GTP for the production of cytosolic PEP. The PEPCK-C/malate pathway (green) uses mitochondrial (mtMDH) and cytosolic (cytMDH) malate dehydrogenase (MDH). Mitochondrial MDH converts OAA in malate and malate is transferred to the cytosol, where cytosolic MDH forms OAA. This creates an excess NADH oxidized by the glycerol-3-phosphate shuttle.
6.4. PEPCK-M is the more direct pathway for PEP production
As noted, lactate is quantitatively the most important mitochondrial gluconeogenic substrate. Lactate is continuously produced (even in times of hyper- or hypoinsulinemia) and lactate turnover increases during euglycemic hyperinsulinemic clamps. Since PEPCK-C is suppressed by insulin but PEPCK-M is constitutively expressed, then the mitochondrial isoform is well poised to continuously produce PEP from lactate. In contrast, the release of amino acids into the blood from skeletal muscle is variable and therefore requires the hormone-regulateable adaptation of PEPCK-C gluconeogenesis [78]. Thus, it has been suggested that PEPCK-M is the enzyme for gluconeogenesis from lactate while PEPCK-C is preferred for glucose production from pyruvate and amino acids depending on supply and demand [163]. Taken together, in terms of energetics, enzymatics, and transport, PEPCK-M metabolism of lactate is a more efficient pathway from mitochondrial OAA to cytosolic PEP (Fig. 4). Indeed, a mitochondrial PEPCK location makes sense since the initial step of OAA formation also occurs in the mitochondria. In principle, there are four possible pathways to generate PEP in the cytosol [76] (Fig. 4 and Fig. 5):
PEPCK-M pathway (conversion to PEP via PEPCK-M and SCS-GTP)
PEPCK-C/aspartate pathway (transamination to aspartate by AAT)
PEPCK-C/malate pathway (reduction to malate via MDH)
PEPCK-C/citrate pathway (conversion to citrate by citrate synthase)
In theory, a PEPCK-M pathway (Fig. 5a) would need just two enzymes (SCS-GTP and PEPCK-M) in order to generate mtPEP and is independent of the need for oxidative phosphorylation. The transport of mtPEP into the cytosol via the CIC then occurs with the lowest cumulative energetic cost [7]. In contrast, to deliver OAA in the cytosol for PEPCK-C, the OAA first must be transferred out of the mitochondria. Since OAA lacks a transporter then it is either transaminated to aspartate or reduced to malate.
The PEPCK-C/aspartate pathway (Fig. 5b) is more complex and involves four enzymes, electron transport and oxidative phosphorylation. OAA is first transaminated to aspartate in the mitochondria and subsequently transported into the cytosol where it is transaminated back into OAA via cytosolic aspartate aminotransferase. Continuous transport of glutamate into, and α-ketoglutarate out of the mitochondrion is required for the transamination reaction and therefore the process is restricted by the availability of these other substrates [20]. Four transporters are involved to obtain cytosolic PEP from mitochondrial OAA. This raises the energetic cost to 40% because two additional protons must be delivered to the matrix coupled to the transport of glutamate and ATP.
The PEPCK-C/malate pathway (Fig. 5c) utilizes mtMDH to reduce OAA to malate and is favored by a highly reduced matrix. Malate shuttles to the cytosol where cytMDH oxidizes malate and uses NAD+ to generate OAA and an excess NADH. The PEPCK-C/malate pathway then requires the glycerol-3-phosphate dehydrogenase shuttle in order to balance cytosolic redox. Altogether, it needs four enzymes, energetic expensive shuttles as well as the electron transport and oxidative phosphorylation for a net cost of >60% more energy.
The PEPCK-C/citrate pathway (Fig. 5d), if solely used to generate PEP, is energetically the most expensive pathway to generate PEP in the cytosol. It requires four enzyme reactions (citrate synthase, ATP-citrate lyase, NDPK, PEPCK-C), two transporters (DIC, ANT), electron transport and oxidative phosphorylation. Further, it would cost two pyruvates and two ATPs (for PC and ATP-citrate lyase reaction). Also, pyruvate is not fully oxidized in the TCA cycle and carbons are instead transferred to the cytosol to form OAA and acetyl-CoA. Thus, considering the loss of acetyl-CoA oxidation, then the energetic cost for this pathway is very expensive (~49 protons). Citrate export favors lipid synthesis rather than gluconeogenesis as it generates acetyl-CoA and OAA in the cytosol. Formation of NADPH by malic enzyme could then energize lipid synthesis, but would deprive the cytosol of PEP that could be used for gluconeogenesis.
In summary, the PEPCK-M pathway is in theory the most direct pathway for PEP production in the cytosol. It offers significant metabolic advantages over any described PEPCK-C pathways. These include greater energy efficiency and less dependence upon oxygen consumption - qualities especially favorable for fasting and exercise. This efficiency may explain why PEPCK-M is the only isoform found in the livers of birds of flight that are highly dependent on Cori cycling [163, 164]. At this time, PEPCK-M has not been directly evaluated experimentally as a potential contributor to gluconeogenesis. Here we raise the interesting possibility that it may be more significant than previously considered.
7. PEPCK-M deficiency
Alterations in PEPCK-C gene expression and its metabolic effect have been intensively studied in animal models [24, 137, 146, 147, 165-168]. To date there has been no animal model studied with altered PEPCK-M expression. Human cases of PEPCK-M deficiency were initially reported in some children that died prematurely due to liver failure. Analysis of fibroblasts (which only have PEPCK-M) detected the defect and the residual PEPCK activities were 18 % and 16 % respectively [169-171]. The deficiency was observed with failure to thrive, fasting hypoglycaemia, glucagon insensitivity, lactic acidaemia, hypotonia, hepatomegaly and liver function impairment. Autopsy revealed massive fat deposition in liver and kidneys. However, a later study suggested that the lowered PEPCK-M activity was not the primary defect and that mitochondrial DNA depletion was to blame [172]. The reports of Vidnes and Sovik in 1976 [173-175] describe the phenotype of a boy whose liver lacked PEPCK-C and suffered from persistent hypoglycemia and died in his early childhood. PEPCK-C activity was virtually zero [175]. Interestingly, total hepatic PEPCK activity was normal, and closer investigation revealed that the boy did not have primary PEPCK-C deficiency but rather suppressed PEPCK-C expression due to hyperinsulinema. Predominant α-cells over β-cells and hyperplasia of the islets of the endocrine pancreas suggest the possibility of a hormonal defect rather than PEPCK-C deficiency. Noteworthy, it is uncertain whether a primary gene defect exists or any other defect regulating PEPCK activity (e.g. hormones, another PEPCK regulating protein). In addition, the performed PEPCK activity assays did not distinguish between the two isoforms. However, total PEPCK deficiency (low PEPCK-C plus PEPCK-M activity) is likely to cause severe hypoglycemia and early death [169, 176, 177].
8. Summary and concluding remarks
Since the discovery of PEPCK, the knowledge of its characteristics and biological role has increased tremendously. The enzyme is not exclusively involved in glucose production but may have broader metabolic functions in “balancing” the TCA cycle. Indeed, it plays an important role in cataplerosis of TCA cycle intermediates and is required for gluconeogenesis and glyceroneogenesis. In the past, PEPCK (mainly PEPCK-C) was shown to play a pivotal role as a regulator of both carbohydrate and lipid metabolism. Prior to the cloning of the genes of each isoform, it was not appreciated that each PEPCK isoform comes from two distinct nuclear genes, from different chromosomes with different sequences and catalytic properties. The cytosolic isoform has had considerable attention in the livers of most studied animals – the rat and the mouse – because of reports of insignificant PEPCK-M [8, 76, 89, 137, 146, 147, 178-181]. This conclusion, as well as strong hormonal transcriptional regulation led to considerable early enthusiasm primarily for PEPCK-C.
Nevertheless, mammalian livers express two PEPCK isoforms (PEPCK-C and PEPCK-M). Both catalyze the metabolism of OAA into PEP using GTP as a phosphodonor [76]. As the two isoforms are highly homologous and depend upon GTP hydrolysis, the function, as well as regulation, of PEPCK-M has been assumed to be similar to PEPCK-C [100, 138]. At the present time, the metabolic contribution of PEPCK-M to gluconeogenesis and glyceroneogenesis remains unknown. Upon reconsideration, PEPCK-C may be favored to convert pyruvate and amino acids to glucose during fasting while PEPCK-M may favor lactate from the Cori cycle [76]. Another way to interpret the lack of hormonal regulation of PEPCK-M is that it is constitutively active, unlike PEPCK-C that disappears when insulin is elevated. It is tempting to speculate that PEPCK-M provides the foundation of gluconeogenesis while PEPCK-C contributes additional capacity to augment glucose production in times of need.
The regulation of PEPCK-M may be explained not by expression but rather by its dependency upon mtGTP. It is well disposed to regulate cataplerosis when there is adequate TCA flux. In pancreatic β-cells this mitochondrial metabolic flux pathway couples TCA cycle flux with anaplerotic flux to trigger insulin secretion. In the gluconeogenic tissues we raise the hypothesis that a similar pathway exists. Thus the mtGTP/PEPCK-M pathway may be a significant determinant of endogenous glucose production that is not turned off by hormonal regulation. Such “unresponsiveness” may ensure a continuous level of PEP synthesis. In the background of “basal” PEPCK-M activity the hormonally regulated PEPCK-C pathway can be turned on and off as needed without a risk of hypoglycemia. This is supported by the observation that even a 90 % reduction of whole-body or 100% hepatic PEPCK-C activity did not result in changes in glycemia until the mice were stressed [146]. A continuous supply of PEP by PEPCK-M, even in the presence of high insulin, may also be important for other biosynthetic pathways, such as glycogen or triglycerides. Noteworthy, half of the postprandial glycogen synthesis still comes from gluconeogenesis when insulin levels are increased (and PEPCK-C suppressed) [18, 182, 183]. Further, healthy humans derive 50% of endogenous glucose production from gluconeogenesis even in the immediate post-absorptive phase when glycogen stores are not depleted [184]. Theoretically, having a background pathway for continuous PEP production by PEPCK-M protects against hypoglycemia, which could be caused by complete cessation of gluconeogenesis. The PEPCK-C pathways expand the overall capacity for gluconeogenesis as needed in response to hormonal and metabolic cues. However, PEPCK-C expression may only have minimal control over basal rates of gluconeogenesis. Given that humans have a much higher relative PEPCK-M activity [185], the specter is raised that the mitochondrial isoform could play an even more significant role in normal or pathologic, insulin-independent, fasting and fed glucose production.
In summary, both PEPCK isoforms convert TCA cycle OAA into PEP as they consume GTP. PEP can re-enter the TCA cycle (anaplerosis, “PEP cycle”) [7], or exit the TCA cycle (cataplerosis) to ultimately feed carbons for a number of downstream process, such as gluconeogenesis and glyceroneogenesis [158]. Overall metabolic milieu and type of carbons entering the TCA cycle may ultimately determine the relative activities of each isoform. While PEPCK-M ensures continuous cataplerotic PEP production, PEPCK-C may augment gluconeogenic capacity when needed. As this mitochondrial mtGTP/PEPCK-M pathway may regulate the two faces of glucose homeostastis – glucose clearance and glucose production - we envision PEPCK-M and SCS-GTP as a “metabolic tachometer” that uses mtGTP to “sense” TCA cycle flux. The role of PEPCK-M in gluconeogenesis needs to be confirmed.
Highlights.
► Mitochondrial GTP (mtGTP) is produced at a rate proportional to TCA cycle flux.
► PEPCK-M activity is dependent on mtGTP and thus linked to TCA cycle flux.
► A mtGTP cycle between the enzymes SCS-GTP and PEPCK-M generates mitochondrial PEP.
► The mtGTP cycle couples glucose metabolism to insulin secretion.
► Mitochondrial PEP (mtPEP) generated by PEPCK-M may be a significant source of gluconeogenic flux.
Acknowledgment
We would like to acknowledge grant support from the NIH NIDDK R01 DK092606, K08 DK080142 and the American Diabetes Association (7-12-BS-092).
Abbreviations
- AAT
aspartate aminotransferase
- ANT
adenine nucleotide transporter
- Asp
aspartate
- Asp/Glu
aspartate glutamate transporter
- αKG
alpha-ketoglutarate
- αKGT
alpha-ketoglutarate transporter
- CIC
citrate isocitrate transporter
- cytAAT
cytosolic aspartate aminotransferase
- cytGlyc-3-PDH
cytosolic glycerol-3- phosphate dehydrogenase
- cytMDH
cytosolic malate dehydrogenase
- DIC
dicarboxylate transporter
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GDH
glutamate dehydrogenase
- GSIS
glucose-stimulated insulin secretion
- GTP
Guanosine-5'-triphosphate
- Glu
Glutamate
- Glyc-3-PDH
glycerol-3-phosphate dehydrogenase
- IDP
inositol-1, 4-diphosphate
- ITP
inositol 1,4,5,-tris-phosphate
- LDH
lactate dehydrogenase
- Mal
malate
- MDH
malate dehydrogenase
- mtAAT
mitochondrial aspartate aminotransferase
- mtGlyc-3-PDH
mitochondrial glycerol-3-phosphate dehydrogenase
- mtGTP
mitochondrial GTP
- mtMDH
mitochondrial malate dehydrogenase
- mtPEP
mitochondrial PEP
- NDPK
nucleotide diphosphokinase
- OAA
oxaloacetate
- PC
pyruvate carboxylase
- PDH
pyruvate dehydrogenase
- PEP
phosphoenolpyruvate
- PEPCK
phosphoenolpyruvate carboxikinase
- PEPCK-C
cytosolic PEPCK
- PEPCK-M
mitochondrial PEPCK
- PK
pyruvate kinase
- SCS
succinyl coenzyme A synthetase
- SCS-ATP
ATP-forming SCS SCS-GTP
- SCS
GTP-forming
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Landau BR, et al. Contributions of gluconeogenesis to glucose production in the fasted state. J Clin Invest. 1996;98(2):378–85. doi: 10.1172/JCI118803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cersosimo E, Garlick P, Ferretti J. Insulin regulation of renal glucose metabolism in humans. Am J Physiol. 1999;276(1 Pt 1):E78–84. doi: 10.1152/ajpendo.1999.276.1.E78. [DOI] [PubMed] [Google Scholar]
- 3.Meyer C, et al. Effects of physiological hyperinsulinemia on systemic, renal, and hepatic substrate metabolism. Am J Physiol. 1998;275(6 Pt 2):F915–21. doi: 10.1152/ajprenal.1998.275.6.F915. [DOI] [PubMed] [Google Scholar]
- 4.Jones JG, et al. Hepatic anaplerotic outflow fluxes are redirected from gluconeogenesis to lactate synthesis in patients with Type 1a glycogen storage disease. Metabolic engineering. 2009;11(3):155–62. doi: 10.1016/j.ymben.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Magnusson I, et al. Noninvasive tracing of Krebs cycle metabolism in liver. The Journal of biological chemistry. 1991;266(11):6975–84. [PubMed] [Google Scholar]
- 6.Owen OE, Kalhan SC, Hanson RW. The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem. 2002;277(34):30409–12. doi: 10.1074/jbc.R200006200. [DOI] [PubMed] [Google Scholar]
- 7.Stark R, et al. Phosphoenolpyruvate cycling via mitochondrial phosphoenolpyruvate carboxykinase links anaplerosis and mitochondrial GTP with insulin secretion. J Biol Chem. 2009;284(39):26578–90. doi: 10.1074/jbc.M109.011775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nordlie RC, Lardy HA. Mammalian liver phosphoneolpyruvate carboxykinase activities. J Biol Chem. 1963;238:2259–63. [PubMed] [Google Scholar]
- 9.Kibbey RG, et al. Mitochondrial GTP regulates glucose-stimulated insulin secretion. Cell Metab. 2007;5(4):253–64. doi: 10.1016/j.cmet.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Consoli A, et al. Determination of Krebs cycle metabolic carbon exchange in vivo and its use to estimate the individual contributions of gluconeogenesis and glycogenolysis to overall glucose output in man. J Clin Invest. 1987;80(5):1303–10. doi: 10.1172/JCI113206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wahren J, Felig P, Hagenfeldt L. Physical exercise and fuel homeostasis in diabetes mellitus. Diabetologia. 1978;14(4):213–22. doi: 10.1007/BF01219419. [DOI] [PubMed] [Google Scholar]
- 12.Gerich JE. Physiology of glucose homeostasis. Diabetes Obes Metab. 2000;2(6):345–50. doi: 10.1046/j.1463-1326.2000.00085.x. [DOI] [PubMed] [Google Scholar]
- 13.Stumvoll M, et al. Human kidney and liver gluconeogenesis: evidence for organ substrate selectivity. Am J Physiol. 1998;274(5 Pt 1):E817–26. doi: 10.1152/ajpendo.1998.274.5.E817. [DOI] [PubMed] [Google Scholar]
- 14.Stumvoll M, et al. Renal glucose production and utilization: new aspects in humans. Diabetologia. 1997;40(7):749–57. doi: 10.1007/s001250050745. [DOI] [PubMed] [Google Scholar]
- 15.Gerich JE. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med. 2010;27(2):136–42. doi: 10.1111/j.1464-5491.2009.02894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gastaldelli A, et al. Separate contribution of diabetes, total fat mass, and fat topography to glucose production, gluconeogenesis, and glycogenolysis. The Journal of clinical endocrinology and metabolism. 2004;89(8):3914–21. doi: 10.1210/jc.2003-031941. [DOI] [PubMed] [Google Scholar]
- 17.Maggs DG, et al. Metabolic effects of troglitazone monotherapy in type 2 diabetes mellitus. A randomized, double-blind, placebo-controlled trial. Annals of internal medicine. 1998;128(3):176–85. doi: 10.7326/0003-4819-128-3-199802010-00002. [DOI] [PubMed] [Google Scholar]
- 18.Roden M, et al. The roles of insulin and glucagon in the regulation of hepatic glycogen synthesis and turnover in humans. The Journal of clinical investigation. 1996;97(3):642–8. doi: 10.1172/JCI118460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roden M, Petersen KF, Shulman GI. Nuclear magnetic resonance studies of hepatic glucose metabolism in humans. Recent progress in hormone research. 2001;56:219–37. doi: 10.1210/rp.56.1.219. [DOI] [PubMed] [Google Scholar]
- 20.Berg JM, Tymoczko JL, Stryer L. Biochemistry (6th ed.) 6th Edition W. H. Freeman; New York: 2006. [Google Scholar]
- 21.Gibala MJ, et al. Tricarboxylic acid cycle intermediate pool size and estimated cycle flux in human muscle during exercise. Am J Physiol. 1998;275(2 Pt 1):E235–42. doi: 10.1152/ajpendo.1998.275.2.E235. [DOI] [PubMed] [Google Scholar]
- 22.Kelly A, Stanley CA. Disorders of glutamate metabolism. Mental retardation and developmental disabilities research reviews. 2001;7(4):287–95. doi: 10.1002/mrdd.1040. [DOI] [PubMed] [Google Scholar]
- 23.MacMullen C, et al. Hyperinsulinism/hyperammonemia syndrome in children with regulatory mutations in the inhibitory guanosine triphosphate-binding domain of glutamate dehydrogenase. The Journal of clinical endocrinology and metabolism. 2001;86(4):1782–7. doi: 10.1210/jcem.86.4.7414. [DOI] [PubMed] [Google Scholar]
- 24.Lambeth DO, et al. Expression of two succinyl-CoA synthetases with different nucleotide specificities in mammalian tissues. J Biol Chem. 2004;279(35):36621–4. doi: 10.1074/jbc.M406884200. [DOI] [PubMed] [Google Scholar]
- 25.Wikstrom JD, et al. A novel high-throughput assay for islet respiration reveals uncoupling of rodent and human islets. PloS one. 2012;7(5):e33023. doi: 10.1371/journal.pone.0033023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li C, et al. Regulation of leucine-stimulated insulin secretion and glutamine metabolism in isolated rat islets. The Journal of biological chemistry. 2003;278(5):2853–8. doi: 10.1074/jbc.M210577200. [DOI] [PubMed] [Google Scholar]
- 27.McKee EE, et al. Origin of guanine nucleotides in isolated heart mitochondria. Biochemical and Biophysical Research Communications. 1999;257(2):466–72. doi: 10.1006/bbrc.1999.0489. [DOI] [PubMed] [Google Scholar]
- 28.McKee EE, et al. Guanine nucleotide transport by atractyloside-sensitive and -insensitive carriers in isolated heart mitochondria. American journal of physiology. Cell physiology. 2000;279(6):C1870–9. doi: 10.1152/ajpcell.2000.279.6.C1870. [DOI] [PubMed] [Google Scholar]
- 29.Vozza A, et al. Identification of the mitochondrial GTP/GDP transporter in Saccharomyces cerevisiae. The Journal of biological chemistry. 2004;279(20):20850–7. doi: 10.1074/jbc.M313610200. [DOI] [PubMed] [Google Scholar]
- 30.Johnson JD, et al. Genetic evidence for the expression of ATP- and GTP-specific succinyl-CoA synthetases in multicellular eucaryotes. J Biol Chem. 1998;273(42):27580–6. doi: 10.1074/jbc.273.42.27580. [DOI] [PubMed] [Google Scholar]
- 31.Kowluru A. Adenine and guanine nucleotide-specific succinyl-CoA synthetases in the clonal beta-cell mitochondria: implications in the beta-cell high-energy phosphate metabolism in relation to physiological insulin secretion. Diabetologia. 2001;44(1):89–94. doi: 10.1007/s001250051584. [DOI] [PubMed] [Google Scholar]
- 32.Kadrmas EF, Ray PD, Lambeth DO. Apparent ATP-linked succinate thiokinase activity and its relation to nucleoside diphosphate kinase in mitochondrial matrix preparations from rabbit. Biochimica et biophysica acta. 1991;1074(3):339–46. doi: 10.1016/0304-4165(91)90083-s. [DOI] [PubMed] [Google Scholar]
- 33.Kowluru A, Tannous M, Chen HQ. Localization and characterization of the mitochondrial isoform of the nucleoside diphosphate kinase in the pancreatic beta cell: evidence for its complexation with mitochondrial succinyl-CoA synthetase. Archives of biochemistry and biophysics. 2002;398(2):160–9. doi: 10.1006/abbi.2001.2710. [DOI] [PubMed] [Google Scholar]
- 34.Tokarska-Schlattner M, et al. The nucleoside diphosphate kinase D (NM23-H4) binds the inner mitochondrial membrane with high affinity to cardiolipin and couples nucleotide transfer with respiration. The Journal of biological chemistry. 2008;283(38):26198–207. doi: 10.1074/jbc.M803132200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlattner U, et al. Mitochondrial kinases and their molecular interaction with cardiolipin. Biochimica et biophysica acta. 2009;1788(10):2032–47. doi: 10.1016/j.bbamem.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 36.Schlattner U, et al. Dual function of mitochondrial Nm23-H4 protein in phosphotransfer and intermembrane lipid transfer: a cardiolipin-dependent switch. The Journal of biological chemistry. 2013;288(1):111–21. doi: 10.1074/jbc.M112.408633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hahn P, Novak M. Development of brown and white adipose tissue. J Lipid Res. 1975;16(2):79–91. [PubMed] [Google Scholar]
- 38.Amutha B, et al. GTP is required for iron-sulfur cluster biogenesis in mitochondria. J Biol Chem. 2008;283(3):1362–71. doi: 10.1074/jbc.M706808200. [DOI] [PubMed] [Google Scholar]
- 39.Pagliarini DJ, et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134(1):112–23. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drahota Z, et al. Phosphoenolpyruvate shuttle--transport of energy from mitochondria to cytosol. FEBS letters. 1983;157(2):347–9. doi: 10.1016/0014-5793(83)80573-0. [DOI] [PubMed] [Google Scholar]
- 41.Szollosi A, et al. Glucose stimulates Ca2+ influx and insulin secretion in 2-week-old beta-cells lacking ATP-sensitive K+ channels. J Biol Chem. 2007;282(3):1747–56. doi: 10.1074/jbc.M609875200. [DOI] [PubMed] [Google Scholar]
- 42.Szollosi A, Nenquin M, Henquin JC. Overnight culture unmasks glucose-induced insulin secretion in mouse islets lacking ATP-sensitive K+ channels by improving the triggering Ca2+ signal. J Biol Chem. 2007;282(20):14768–76. doi: 10.1074/jbc.M701382200. [DOI] [PubMed] [Google Scholar]
- 43.Ashcroft SJ, Randle PJ. Enzymes of glucose metabolism in normal mouse pancreatic islets. Biochem J. 1970;119(1):5–15. doi: 10.1042/bj1190005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacDonald MJ. Feasibility of a mitochondrial pyruvate malate shuttle in pancreatic islets. Further implication of cytosolic NADPH in insulin secretion. J Biol Chem. 1995;270(34):20051–8. [PubMed] [Google Scholar]
- 45.MacDonald MJ. Influence of glucose on pyruvate carboxylase expression in pancreatic islets. Arch Biochem Biophys. 1995;319(1):128–32. doi: 10.1006/abbi.1995.1274. [DOI] [PubMed] [Google Scholar]
- 46.MacDonald MJ. Estimates of glycolysis, pyruvate (de)carboxylation, pentose phosphate pathway, and methyl succinate metabolism in incapacitated pancreatic islets. Arch Biochem Biophys. 1993;305(2):205–14. doi: 10.1006/abbi.1993.1413. [DOI] [PubMed] [Google Scholar]
- 47.Cline GW, et al. Rates of insulin secretion in INS-1 cells are enhanced by coupling to anaplerosis and Kreb's cycle flux independent of ATP synthesis. Biochemical and Biophysical Research Communications. 2011;415(1):30–5. doi: 10.1016/j.bbrc.2011.09.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cline GW, et al. 13C NMR isotopomer analysis of anaplerotic pathways in INS-1 cells. J Biol Chem. 2004;279(43):44370–5. doi: 10.1074/jbc.M311842200. [DOI] [PubMed] [Google Scholar]
- 49.Lu D, et al. 13C NMR isotopomer analysis reveals a connection between pyruvate cycling and glucose-stimulated insulin secretion (GSIS). Proc Natl Acad Sci U S A. 2002;99(5):2708–13. doi: 10.1073/pnas.052005699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.MacDonald MJ, Chang C. Do Pancreatic Islets Contain Significant Amounts of Phosphoenolpyruvate Carboxykinase or Ferroactivator Activity? Diabetes. 1985;34:246–250. doi: 10.2337/diab.34.3.246. [DOI] [PubMed] [Google Scholar]
- 51.Newgard CB, et al. Stimulus/secretion coupling factors in glucose-stimulated insulin secretion: insights gained from a multidisciplinary approach. Diabetes. 2002;51(Suppl 3):S389–93. doi: 10.2337/diabetes.51.2007.s389. [DOI] [PubMed] [Google Scholar]
- 52.Pongratz RL, et al. Cytosolic and mitochondrial malic enzyme isoforms differentially control insulin secretion. J Biol Chem. 2007;282(1):200–7. doi: 10.1074/jbc.M602954200. [DOI] [PubMed] [Google Scholar]
- 53.Guay C, et al. A role for ATP-citrate lyase, malic enzyme, and pyruvate/citrate cycling in glucose-induced insulin secretion. J Biol Chem. 2007;282(49):35657–65. doi: 10.1074/jbc.M707294200. [DOI] [PubMed] [Google Scholar]
- 54.Heart E, et al. Role for malic enzyme, pyruvate carboxylation, and mitochondrial malate import in glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab. 2009;296(6):E1354–62. doi: 10.1152/ajpendo.90836.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Joseph JW, et al. The mitochondrial citrate/isocitrate carrier plays a regulatory role in glucose-stimulated insulin secretion. J Biol Chem. 2006;281(47):35624–32. doi: 10.1074/jbc.M602606200. [DOI] [PubMed] [Google Scholar]
- 56.Pongratz RL, Kibbey RG, Cline GW. Investigating the roles of mitochondrial and cytosolic malic enzyme in insulin secretion. Methods Enzymol. 2009;457:425–50. doi: 10.1016/S0076-6879(09)05024-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ronnebaum SM, et al. A pyruvate cycling pathway involving cytosolic NADP-dependent isocitrate dehydrogenase regulates glucose-stimulated insulin secretion. J Biol Chem. 2006;281(41):30593–602. doi: 10.1074/jbc.M511908200. [DOI] [PubMed] [Google Scholar]
- 58.Ronnebaum SM, et al. Silencing of cytosolic or mitochondrial isoforms of malic enzyme has no effect on glucose-stimulated insulin secretion from rodent islets. J Biol Chem. 2008;283(43):28909–17. doi: 10.1074/jbc.M804665200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jensen MV, et al. Metabolic cycling in control of glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab. 2008;295(6):E1287–97. doi: 10.1152/ajpendo.90604.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Odegaard ML, et al. The mitochondrial 2-oxoglutarate carrier is part of a metabolic pathway that mediates glucose- and glutamine-stimulated insulin secretion. The Journal of biological chemistry. 2010;285(22):16530–7. doi: 10.1074/jbc.M109.092593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palmieri F. The mitochondrial transporter family (SLC25): physiological and pathological implications. Pflugers Arch. 2004;447(5):689–709. doi: 10.1007/s00424-003-1099-7. [DOI] [PubMed] [Google Scholar]
- 62.Garber AJ, Ballard FJ. Phosphoenolpyruvate synthesis and release by mitochondria from guinea pig liver. J Biol Chem. 1969;244(17):4696–703. [PubMed] [Google Scholar]
- 63.Passarella S, et al. The role of mitochondrial transport in energy metabolism. Mitochondrion. 2003;2(5):319–43. doi: 10.1016/S1567-7249(03)00008-4. [DOI] [PubMed] [Google Scholar]
- 64.Satrustegui J, Pardo B, Del Arco A. Mitochondrial transporters as novel targets for intracellular calcium signaling. Physiol Rev. 2007;87(1):29–67. doi: 10.1152/physrev.00005.2006. [DOI] [PubMed] [Google Scholar]
- 65.Denton RM, et al. Regulation of mammalian pyruvate dehydrogenase. Mol Cell Biochem. 1975;9(1):27–53. doi: 10.1007/BF01731731. [DOI] [PubMed] [Google Scholar]
- 66.Peng CF, et al. Factors that influence phosphoenolpyruvate-induced calcium efflux from rat liver mitochondria. Biochem Biophys Res Commun. 1974;56:134–41. doi: 10.1016/s0006-291x(74)80325-6. [DOI] [PubMed] [Google Scholar]
- 67.Boquist L. NADP-linked dismutation and concentrations of citrate, cytosolic free Ca2+ and phosphoenolpyruvate in islet B-cells stimulated with glucose. Biochem Int. 1987;14(3):531–8. [PubMed] [Google Scholar]
- 68.Deaciuc IV, D'Souza NB, Miller HI. A novel mechanism for Ca(2+)-dependent regulation of hepatic gluconeogenesis: stimulation of mitochondrial phosphoenolpyruvate synthesis by Ca2+. Int J Biochem. 1992;24(1):129–32. doi: 10.1016/0020-711x(92)90238-v. [DOI] [PubMed] [Google Scholar]
- 69.Shug AL, Shrago E. Inhibition of phosphoenolpyruvate transport via the tricarboxylate and adenine nucleotide carrier systems of rat liver mitochondria. Biochem Biophys Res Commun. 1973;53(2):659–65. doi: 10.1016/0006-291x(73)90712-2. [DOI] [PubMed] [Google Scholar]
- 70.Chudapongse P. Further studies on the effect of phosphoenolpyruvate on respiration-dependent calcium transport by rat heart mitochondria. Biochim Biophys Acta. 1976;423(2):196–202. doi: 10.1016/0005-2728(76)90178-x. [DOI] [PubMed] [Google Scholar]
- 71.Sul HS, Shrago E, Shug AL. Relationship of phosphoenolpyruvate transport, acyl coenzyme A inhibition of adenine nucleotide translocase and calcium ion efflux in guinea pig heart mitochondria. Arch Biochem Biophys. 1976;172(1):230–7. doi: 10.1016/0003-9861(76)90071-0. [DOI] [PubMed] [Google Scholar]
- 72.Roos I, Crompton M, Carafoli E. The effect of phosphoenolpyruvate on the retention of calcium by liver mitochondria. FEBS Lett. 1978;94:418–21. doi: 10.1016/0014-5793(78)80990-9. [DOI] [PubMed] [Google Scholar]
- 73.Szabadkai G, Duchen MR. Mitochondria: the hub of cellular Ca2+ signaling. Physiology (Bethesda) 2008;23:84–94. doi: 10.1152/physiol.00046.2007. [DOI] [PubMed] [Google Scholar]
- 74.Wood HG, Werkman CH. The utilization of CO(2) by the propionic acid bacteria. Biochem J. 1938;32(7):1262–71. doi: 10.1042/bj0321262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Utter MF, Kurahashi K. Mechanism of Action of Oxalacetate Carboxylase from Liver. J. Am. Chem. Soc. 1953;75:785–787. [Google Scholar]
- 76.Hanson RW, Patel YM. Phosphoenolpyruvate carboxykinase (GTP): the gene and the enzyme. Adv Enzymol Relat Areas Mol Biol. 1994;69:203–81. doi: 10.1002/9780470123157.ch6. [DOI] [PubMed] [Google Scholar]
- 77.Hebda CA, Nowak T. The purification, characterization, and activation of phosphoenolpyruvate carboxykinase from chicken liver mitochondria. J Biol Chem. 1982;257(10):5503–14. [PubMed] [Google Scholar]
- 78.Modaressi S, et al. Human mitochondrial phosphoenolpyruvate carboxykinase 2 gene. Structure, chromosomal localization and tissue-specific expression. Biochem J. 1998;333( Pt 2):359–66. doi: 10.1042/bj3330359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hedeskov CJ, Capito K. Pancreatic islet metabolism of pyruvate and other potentiators of insulin release. Effects of starvation. . Horm Metab Res. 1980;10:8–13. [PubMed] [Google Scholar]
- 80.Hedeskov CJ, Capito K, Thams P. Phosphoenolpyruvate carboxykinase in mouse pancreatic islets ATP-induced changes in sensitivity to Mn2+ activation. Biochem Biophys Acta. 1984;791:37–44. doi: 10.1016/0167-4838(84)90278-4. [DOI] [PubMed] [Google Scholar]
- 81.MacDonald MJ, et al. Lack of glyconeogenesis in pancreatic islets: expression of gluconeogenic enzyme genes in islets. Horm Metab Res. 1992;24(4):158–60. doi: 10.1055/s-2007-1003284. [DOI] [PubMed] [Google Scholar]
- 82.Horn DB, et al. Alterations in key gluconeogenic regulators with age and endurance training. Metabolism. 1997;46(4):414–9. doi: 10.1016/s0026-0495(97)90058-5. [DOI] [PubMed] [Google Scholar]
- 83.Saggerson D, Evans CJ. The activities and intracellular distribution of nicotinamide-adenine dinucleotide phosphate-malate dehydrogenase, phosphoenolpyruvate carboxykinase and pyruvate carboxylase in rat, guinea-pig and rabbit tissues. The Biochemical journal. 1975;146(2):329–32. doi: 10.1042/bj1460329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Usatenko MS. Hormonal regulation of phosphoenolpyruvate carboxykinase activity in liver and kidney of adult animals and formation of this enzyme in developing rabbit liver. Biochem Med. 1970;3(4):298–310. doi: 10.1016/0006-2944(70)90030-x. [DOI] [PubMed] [Google Scholar]
- 85.Heitzman RJ, Herriman ID, Mallinson CB. Some effects of glucocorticoids on the subcellular distribution of the activities of citrate synthase and phosphoenolpyruvate carboxykinase in livers of rats and cows. FEBS Lett. 1972;20(1):19–21. doi: 10.1016/0014-5793(72)80006-1. [DOI] [PubMed] [Google Scholar]
- 86.Hanson RW, Reshef L. Glyceroneogenesis revisited. Biochimie. 2003;85(12):1199–205. doi: 10.1016/j.biochi.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 87.Reshef L, Hanson RW, Ballard FJ. A possible physiological role for glyceroneogenesis in rat adipose tissue. J Biol Chem. 1970;245(22):5979–84. [PubMed] [Google Scholar]
- 88.Ballard FJ, Oliver IT. Carbohydrate Metabolism In Liver From Foetal And Neonatal Sheep. Biochem J. 1965;95:191–200. doi: 10.1042/bj0950191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ballard FJ, Hanson RW. Phosphoenolpyruvate carboxykinase and pyruvate carboxylase in developing rat liver. Biochem J. 1967;104(3):866–71. doi: 10.1042/bj1040866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Benvenisty N, Reshef L. Developmental expression and modification of genes. Biol Neonate. 1987;52(2):61–9. doi: 10.1159/000242685. [DOI] [PubMed] [Google Scholar]
- 91.Girard JR, et al. Fuels, hormones, and liver metabolism at term and during the early postnatal period in the rat. J Clin Invest. 1973;52(12):3190–200. doi: 10.1172/JCI107519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Arinze IJ. On the development of phosphoenolpyruvate carboxykinase and gluconeogenesis in guinea pig liver. Biochem Biophys Res Commun. 1975;65(1):184–9. doi: 10.1016/s0006-291x(75)80077-5. [DOI] [PubMed] [Google Scholar]
- 93.McGrane MM, et al. Metabolic effects of developmental, tissue-, and cell-specific expression of a chimeric phosphoenolpyruvate carboxykinase (GTP)/bovine growth hormone gene in transgenic mice. J Biol Chem. 1990;265(36):22371–9. [PubMed] [Google Scholar]
- 94.van Roon MA, et al. Accumulation of carbamoylphosphate-synthetase and phosphoenolpyruvate-carboxykinase mRNA in embryonic rat hepatocytes. Evidence for translational control during the initial phases of hepatocyte-specific gene expression in vitro. Eur J Biochem. 1988;178(1):191–6. doi: 10.1111/j.1432-1033.1988.tb14443.x. [DOI] [PubMed] [Google Scholar]
- 95.Benvenisty N, Reshef L. Developmental acquisition of DNase I sensitivity of the phosphoenolpyruvate carboxykinase (GTP) gene in rat liver. Proc Natl Acad Sci U S A. 1987;84(5):1132–6. doi: 10.1073/pnas.84.5.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Eisenberger CL, et al. Differential regulation of the rat phosphoenolpyruvate carboxykinase gene expression in several tissues of transgenic mice. Mol Cell Biol. 1992;12(3):1396–403. doi: 10.1128/mcb.12.3.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mencher D, Reshef L. Effect of triamcinolone on renal and hepatic phosphoenolpyruvate carboxykinase in the newborn rat. Changes in the rate of synthesis of the enzyme and in the activity of its translatable messenger RNA. Eur J Biochem. 1979;94(2):581–9. doi: 10.1111/j.1432-1033.1979.tb12928.x. [DOI] [PubMed] [Google Scholar]
- 98.Zorzoli A, Turkenkopf IJ, Mueller VL. Gluconeogenesis in developing rat kidney cortex. Biochem J. 1969;111(2):181–5. doi: 10.1042/bj1110181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alleyne GA, Scullard GH. Renal metabolic response to acid base changes. I. Enzymatic control of ammoniagenesis in the rat. J Clin Invest. 1969;48(2):364–70. doi: 10.1172/JCI105993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Beale EG, Harvey BJ, Forest C. PCK1 and PCK2 as candidate diabetes and obesity genes. Cell Biochem Biophys. 2007;48(2-3):89–95. doi: 10.1007/s12013-007-0025-6. [DOI] [PubMed] [Google Scholar]
- 101.Beale EG, et al. Rat hepatic cytosolic phosphoenolpyruvate carboxykinase (GTP). Structures of the protein, messenger RNA, and gene. J Biol Chem. 1985;260(19):10748–60. [PubMed] [Google Scholar]
- 102.Carlson GM, Holyoak T. Structural insights into the mechanism of phosphoenolpyruvate carboxykinase catalysis. J Biol Chem. 2009;284(40):27037–41. doi: 10.1074/jbc.R109.040568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dunten P, et al. Crystal structure of human cytosolic phosphoenolpyruvate carboxykinase reveals a new GTP-binding site. J Mol Biol. 2002;316(2):257–64. doi: 10.1006/jmbi.2001.5364. [DOI] [PubMed] [Google Scholar]
- 104.Johnson TA, Holyoak T. Increasing the conformational entropy of the Omega-loop lid domain in phosphoenolpyruvate carboxykinase impairs catalysis and decreases catalytic fidelity. Biochemistry. 2010;49(25):5176–87. doi: 10.1021/bi100399e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sullivan SM, Holyoak T. Enzymes with lid-gated active sites must operate by an induced fit mechanism instead of conformational selection. Proc Natl Acad Sci U S A. 2008;105(37):13829–34. doi: 10.1073/pnas.0805364105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stiffin RM, et al. Differential inhibition of cytosolic PEPCK by substrate analogues. Kinetic and structural characterization of inhibitor recognition. Biochemistry. 2008;47(7):2099–109. doi: 10.1021/bi7020662. [DOI] [PubMed] [Google Scholar]
- 107.Holyoak T, Sullivan SM, Nowak T. Structural insights into the mechanism of PEPCK catalysis. Biochemistry. 2006;45(27):8254–63. doi: 10.1021/bi060269g. [DOI] [PubMed] [Google Scholar]
- 108.Sullivan SM, Holyoak T. Structures of rat cytosolic PEPCK: insight into the mechanism of phosphorylation and decarboxylation of oxaloacetic acid. Biochemistry. 2007;46(35):10078–88. doi: 10.1021/bi701038x. [DOI] [PubMed] [Google Scholar]
- 109.Chen CY, Sato Y, Schramm VL. Isotope trapping and positional isotope exchange with rat and chicken liver phosphoenolpyruvate carboxykinases. Biochemistry. 1991;30(17):4143–51. doi: 10.1021/bi00231a006. [DOI] [PubMed] [Google Scholar]
- 110.Lewis CT, Haley BE, Carlson GM. Formation of an intramolecular cystine disulfide during the reaction of 8-azidoguanosine 5'-triphosphate with cytosolic phosphoenolpyruvate carboxykinase (GTP) causes inactivation without photolabeling. Biochemistry. 1989;28(24):9248–55. doi: 10.1021/bi00450a003. [DOI] [PubMed] [Google Scholar]
- 111.Makinen AL, Nowak T. A reactive cysteine in avian liver phosphoenolpyruvate carboxykinase. J Biol Chem. 1989;264(21):12148–57. [PubMed] [Google Scholar]
- 112.Bentle LA, Lardy HA. P-enolpyruvate carboxykinase ferroactivator. Purification and some properties. J Biol Chem. 1977;252(4):1431–40. [PubMed] [Google Scholar]
- 113.Bentle LA, Snoke RE, Lardy HA. A protein factor required for activation of phosphoenolpyruvate carboxykinase by ferrous ions. J Biol Chem. 1976;251(10):2922–8. [PubMed] [Google Scholar]
- 114.Merryfield ML, Kramp DC, Lardy HA. Purification and characterization of a rat liver ferroactivator with catalase activity. J Biol Chem. 1982;257(8):4646–54. [PubMed] [Google Scholar]
- 115.Punekar NS, Lardy HA. Phosphoenolpyruvate carboxykinase ferroactivator 1. Mechanism of action and identity with glutathione peroxidase. J Biol Chem. 1987;262(14):6714–9. [PubMed] [Google Scholar]
- 116.Chee PP, Lardy HA. Isolation from erythrocytes of a green hemoprotein with ferroactivator activity. J Biol Chem. 1981;256(8):3865–70. [PubMed] [Google Scholar]
- 117.Brinkworth RI, et al. Mn2+-sensitive and -insensitive forms of phosphoenolpyruvate carboxykinase (GTP). J Biol Chem. 1981;256(21):10795–802. [PubMed] [Google Scholar]
- 118.Hoppner W, et al. Is the p29 protein involved in the rapid regulation of phosphoenolpyruvate carboxykinase (GTP)? J Biol Chem. 1991;266(26):17257–60. [PubMed] [Google Scholar]
- 119.Sato A, Suzuki T, Kochi H. Purification and characterization of cytosol-specific phosphoenolpyruvate carboxykinase from chicken liver. J Biochem. 1986;100(3):671–8. doi: 10.1093/oxfordjournals.jbchem.a121759. [DOI] [PubMed] [Google Scholar]
- 120.Holten DD, Nordlie RC. Comparative Studies Of Catalytic Properties Of Guinea Pig Liver Intra- And Extramitochondrial Phosphoenolpyruvate Carboxykinases. Biochemistry. 1965;4:723–31. doi: 10.1021/bi00880a018. [DOI] [PubMed] [Google Scholar]
- 121.Barzu O, et al. Nucleotide specificity of pyruvate kinase and phosphoenolpyruvate carboxykinase. Biochim Biophys Acta. 1976;452(2):406–12. doi: 10.1016/0005-2744(76)90190-x. [DOI] [PubMed] [Google Scholar]
- 122.Hlavaty JJ, Nowak T. Characterization of the second metal site on avian phosphoenolpyruvate carboxykinase. Biochemistry. 2000;39(6):1373–88. doi: 10.1021/bi991692a. [DOI] [PubMed] [Google Scholar]
- 123.Goto Y, et al. Purification and characterization of cytosol phosphoenolpyruvate carboxykinase from bullfrog (Rana catesbeiana) liver. J Biochem. 1979;86(1):71–8. [PubMed] [Google Scholar]
- 124.Ash DE, et al. Mammalian and avian liver phosphoenolpyruvate carboxykinase. Alternate substrates and inhibition by analogues of oxaloacetate. J Biol Chem. 1990;265(13):7377–84. [PubMed] [Google Scholar]
- 125.Lee MH, Hebda CA, Nowak T. The role of cations in avian liver phosphoenolpyruvate carboxykinase catalysis. Activation and regulation. J Biol Chem. 1981;256(24):12793–801. [PubMed] [Google Scholar]
- 126.Holyoak T, Nowak T. pH Dependence of the reaction catalyzed by avian mitochondrial phosphoenolpyruvate carboxykinase. Biochemistry. 2004;43(22):7054–65. doi: 10.1021/bi049707e. [DOI] [PubMed] [Google Scholar]
- 127.Ishihara N, Kikuchi G. Studies on the functional relationship between the phosphopyruvate synthesis and the substrate level phosphorylation in guinea-pig liver mitochondria. Biochim Biophys Acta. 1968;153(4):733–48. doi: 10.1016/0005-2728(68)90001-7. [DOI] [PubMed] [Google Scholar]
- 128.Suzuki M, et al. Cloning and reporter analysis of human mitochondrial phosphoenolpyruvate carboxykinase gene promoter. Gene. 2004;338(2):157–62. doi: 10.1016/j.gene.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 129.Savon S, Hakimi P, Hanson RW. Expression of the genes for the mitochondrial and cytosolic forms of phosphoenolpyruvate carboxykinase in avian liver during development. Biol Neonate. 1993;64(1):62–8. doi: 10.1159/000243972. [DOI] [PubMed] [Google Scholar]
- 130.Lin YY, et al. Protein acetylation microarray reveals that NuA4 controls key metabolic target regulating gluconeogenesis. Cell. 2009;136(6):1073–84. doi: 10.1016/j.cell.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jiang W, et al. Acetylation regulates gluconeogenesis by promoting PEPCK1 degradation via recruiting the UBR5 ubiquitin ligase. Molecular cell. 2011;43(1):33–44. doi: 10.1016/j.molcel.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xiong Y, et al. Regulation of glycolysis and gluconeogenesis by acetylation of PKM and PEPCK. Cold Spring Harbor symposia on quantitative biology. 2011;76:285–9. doi: 10.1101/sqb.2011.76.010942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jones DH, Raymer DM, Schoelen SL. The activity of phosphoenolpyruvate carboxykinase throughout the lactation cycle of the guinea pig mammary gland. Proc Soc Exp Biol Med. 1989;192(1):16–22. doi: 10.3181/00379727-192-42948. [DOI] [PubMed] [Google Scholar]
- 134.Agca C, et al. Cloning and characterization of bovine cytosolic and mitochondrial PEPCK during transition to lactation. Physiological genomics. 2002;11(2):53–63. doi: 10.1152/physiolgenomics.00108.2001. [DOI] [PubMed] [Google Scholar]
- 135.Jamison RA, et al. Hyperglucagonemia precedes a decline in insulin secretion and causes hyperglycemia in chronically glucose-infused rats. American journal of physiology. Endocrinology and metabolism. 2011;301(6):E1174–83. doi: 10.1152/ajpendo.00175.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hopgood MF, Ballard FJ. Synthesis and degradation of phosphoenolpyruvate carboxylase in rat liver and adipose tissue. Changes during a starvation-re-feeding cycle. The Biochemical journal. 1973;134(2):445–53. doi: 10.1042/bj1340445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Burgess SC, et al. Impaired tricarboxylic acid cycle activity in mouse livers lacking cytosolic phosphoenolpyruvate carboxykinase. J Biol Chem. 2004;279(47):48941–9. doi: 10.1074/jbc.M407120200. [DOI] [PubMed] [Google Scholar]
- 138.Hanson RW. Thematic minireview series: a perspective on the biology of phosphoenolpyruvate carboxykinase 55 years after its discovery. J Biol Chem. 2009;284(40):27021–3. doi: 10.1074/jbc.R109.040519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Utter MF, Kurahashi K. Mechanism of action of oxalacetic carboxylase. J Biol Chem. 1954;207(2):821–41. [PubMed] [Google Scholar]
- 140.Utter MF, Kurahashi K. Purification of oxalacetic carboxylase from chicken liver. J Biol Chem. 1954;207(2):787–802. [PubMed] [Google Scholar]
- 141.Utter MF, Kurahashi K, Rose IA. Some properties of oxalacetic carboxylase. J Biol Chem. 1954;207(2):803–19. [PubMed] [Google Scholar]
- 142.Siess EA, et al. Effect of glucagon on metabolite compartmentation in isolated rat liver cells during gluconeogenesis from lactate. The Biochemical journal. 1977;166(2):225–35. doi: 10.1042/bj1660225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hanson RW, Garber AJ. Phosphoenolpyruvate carboxykinase. I. Its role in gluconeogenesis. Am J Clin Nutr. 1972;25(10):1010–21. doi: 10.1093/ajcn/25.10.1010. [DOI] [PubMed] [Google Scholar]
- 144.Hanson RW, Reshef L. Regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression. Annu Rev Biochem. 1997;66:581–611. doi: 10.1146/annurev.biochem.66.1.581. [DOI] [PubMed] [Google Scholar]
- 145.Mendez-Lucas A, et al. PEPCK-M expression in mouse liver potentiates, not replaces, PEPCK-C mediated gluconeogenesis. Journal of hepatology. 2013 doi: 10.1016/j.jhep.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.She P, et al. Mechanisms by which liver-specific PEPCK knockout mice preserve euglycemia during starvation. Diabetes. 2003;52(7):1649–54. doi: 10.2337/diabetes.52.7.1649. [DOI] [PubMed] [Google Scholar]
- 147.She P, et al. Phosphoenolpyruvate carboxykinase is necessary for the integration of hepatic energy metabolism. Mol Cell Biol. 2000;20(17):6508–17. doi: 10.1128/mcb.20.17.6508-6517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Burgess SC, et al. Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Cell metabolism. 2007;5(4):313–20. doi: 10.1016/j.cmet.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sun Y, et al. Phosphoenolpyruvate carboxykinase overexpression selectively attenuates insulin signaling and hepatic insulin sensitivity in transgenic mice. J Biol Chem. 2002;277(26):23301–7. doi: 10.1074/jbc.M200964200. [DOI] [PubMed] [Google Scholar]
- 150.Valera A, et al. Transgenic mice overexpressing phosphoenolpyruvate carboxykinase develop non-insulin-dependent diabetes mellitus. Proc Natl Acad Sci U S A. 1994;91(19):9151–4. doi: 10.1073/pnas.91.19.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Samuel VT, et al. Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with Type 2 Diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(29):12121–6. doi: 10.1073/pnas.0812547106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lei KJ, et al. Glucose-6-phosphatase dependent substrate transport in the glycogen storage disease type-1a mouse. Nat Genet. 1996;13(2):203–9. doi: 10.1038/ng0696-203. [DOI] [PubMed] [Google Scholar]
- 153.Cersosimo E, Garlick P, Ferretti J. Renal substrate metabolism and gluconeogenesis during hypoglycemia in humans. Diabetes. 2000;49(7):1186–93. doi: 10.2337/diabetes.49.7.1186. [DOI] [PubMed] [Google Scholar]
- 154.Stumvoll M, et al. Effects of glucagon on renal and hepatic glutamine gluconeogenesis in normal postabsorptive humans. Metabolism. 1998;47(10):1227–32. doi: 10.1016/s0026-0495(98)90328-6. [DOI] [PubMed] [Google Scholar]
- 155.Stumvoll M, et al. Role of glutamine in human carbohydrate metabolism in kidney and other tissues. Kidney Int. 1999;55(3):778–92. doi: 10.1046/j.1523-1755.1999.055003778.x. [DOI] [PubMed] [Google Scholar]
- 156.Hakimi P, et al. Phosphoenolpyruvate carboxykinase and the critical role of cataplerosis in the control of hepatic metabolism. Nutr Metab (Lond) 2005;2:33. doi: 10.1186/1743-7075-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Croniger CM, et al. Mini-Series: Modern Metabolic Concepts, Phosphoenolpyruvate Carboxykinase Revisited, II. Control of PEPCK-C Gene Expression. Biochemistry and Molecular Biology Education. 2002;30(6):353–362. [Google Scholar]
- 158.Yang J, Kalhan SC, Hanson RW. What is the metabolic role of phosphoenolpyruvate carboxykinase? J Biol Chem. 2009;284(40):27025–9. doi: 10.1074/jbc.R109.040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jones JG, et al. Measurement of gluconeogenesis and pyruvate recycling in the rat liver: a simple analysis of glucose and glutamate isotopomers during metabolism of [1,2,3-(13)C3]propionate. FEBS Lett. 1997;412(1):131–7. doi: 10.1016/s0014-5793(97)00764-3. [DOI] [PubMed] [Google Scholar]
- 160.Meyer C, et al. Renal substrate exchange and gluconeogenesis in normal postabsorptive humans. Am J Physiol Endocrinol Metab. 2002;282(2):E428–34. doi: 10.1152/ajpendo.00116.2001. [DOI] [PubMed] [Google Scholar]
- 161.Söling HD, Kleineke J. Gluconeogenesis: Its Regulation in Mammalian Species. John Wiley & Sons, Inc.; New York: 1976. pp. 369–462. [Google Scholar]
- 162.Gstraunthaler G, Holcomb H, Curthoys NP. Subcellular localization of PEPCK and metabolism of gluconeogenic substrains of renal cell lines. J. Amer. Soc. Nephr. 1993;4:887. doi: 10.1152/ajpcell.1995.268.2.C449. [DOI] [PubMed] [Google Scholar]
- 163.Watford M, et al. The unique role of the kidney in gluconeogenesis in the chicken. The significance of a cytosolic form of phosphoenolpyruvate carboxykinase. J Biol Chem. 1981;256(19):10023–7. [PubMed] [Google Scholar]
- 164.Soling HD, et al. Relationship between intracellular distribution of phosphoenolpyruvate carboxykinase, regulation of gluconeogenesis, and energy cost of glucose formation. Eur J Biochem. 1973;37(2):233–43. doi: 10.1111/j.1432-1033.1973.tb02980.x. [DOI] [PubMed] [Google Scholar]
- 165.Olswang Y, et al. A mutation in the peroxisome proliferator-activated receptor gamma-binding site in the gene for the cytosolic form of phosphoenolpyruvate carboxykinase reduces adipose tissue size and fat content in mice. Proc Natl Acad Sci U S A. 2002;99(2):625–30. doi: 10.1073/pnas.022616299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Franckhauser S, et al. Increased fatty acid re-esterification by PEPCK overexpression in adipose tissue leads to obesity without insulin resistance. Diabetes. 2002;51(3):624–30. doi: 10.2337/diabetes.51.3.624. [DOI] [PubMed] [Google Scholar]
- 167.Hakimi P, et al. Overexpression of the cytosolic form of phosphoenolpyruvate carboxykinase (GTP) in skeletal muscle repatterns energy metabolism in the mouse. J Biol Chem. 2007;282(45):32844–55. doi: 10.1074/jbc.M706127200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hanson RW, Hakimi P. Born to run; the story of the PEPCK-Cmus mouse. Biochimie. 2008;90(6):838–42. doi: 10.1016/j.biochi.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hommes FA, et al. Two cases of phosphoenolpyruvate carboxykinase deficiency. Acta Paediat. Scand. 1976;65:233–240. doi: 10.1111/j.1651-2227.1976.tb16543.x. [DOI] [PubMed] [Google Scholar]
- 170.Clayton PT, et al. Mitochondrial phosphoenolpyruvate carboxykinase deficiency. Eur J Pediatr. 1986;145(1-2):46–50. doi: 10.1007/BF00441851. [DOI] [PubMed] [Google Scholar]
- 171.Robinson BH, Taylor J, Kahler S. Mitochondrial phosphoenolpyruvate carboxykinase deficiency in a child with lacticacidemia, hypotonia and failure to thrive. Am. J. Hum. Genet. 1979;31:60A. [Google Scholar]
- 172.Leonard JV, et al. Mitochondrial phosphoenolpyruvate carboxykinase deficiency. Europ. J. Pediat. 1991;150:198–199. doi: 10.1007/BF01963566. [DOI] [PubMed] [Google Scholar]
- 173.Vidnes J. Gluconeogenesis in infancy and childhood. I. A method for the study of the in vivo gluconeogenesis from alanine and glycerol. Scand J Clin Lab Invest. 1976;36(4):347–56. doi: 10.1080/00365517609055270. [DOI] [PubMed] [Google Scholar]
- 174.Vidnes J, Sovik O. Gluconeogenesis in infancy and childhood. II. Studies on the glucose production from alanine in three cases of persistent neonatal hypoglycaemia. Acta Paediatr Scand. 1976;65(3):297–305. doi: 10.1111/j.1651-2227.1976.tb04889.x. [DOI] [PubMed] [Google Scholar]
- 175.Vidnes J, Sovik O. Gluconeogenesis in infancy and childhood. III. Deficiency of the extramitochondrial form of hepatic phosphoenolpyruvate carboxykinase in a case of persistent neonatal hypoglycaemia. Acta Paediatr Scand. 1976;65(3):307–12. doi: 10.1111/j.1651-2227.1976.tb04890.x. [DOI] [PubMed] [Google Scholar]
- 176.Matsuo M, et al. Hepatic phosphoenolpyruvate carboxykinase deficiency: a neonatal case with reduced activity of pyruvate carboxylase. J Inherit Metab Dis. 1989;12(3):336–7. doi: 10.1007/BF01799232. [DOI] [PubMed] [Google Scholar]
- 177.Fiser RH, Jr, Melsher HL, Fisher DA. Hepatic phosphoenolpyruvate carboxykinase (PEPCK) deficiency: a new cause of hypoglycaemia in childhood. Pediat. Res. 1974;8:432. [Google Scholar]
- 178.Cornell NW, et al. Subcellular location of phosphoenolpyruvate carboxykinase in hepatocytes from fed and starved rats. J Nutr. 1986;116(6):1101–8. doi: 10.1093/jn/116.6.1101. [DOI] [PubMed] [Google Scholar]
- 179.MacDonald MJ, Chang CM. Pancreatic islets contain the M2 isoenzyme of pyruvate kinase. Its phosphorylation has no effect on enzyme activity. Mol Cell Biochem. 1985;68(2):115–20. doi: 10.1007/BF00219375. [DOI] [PubMed] [Google Scholar]
- 180.Soling HD, et al. Regulation of gluconeogenesis in the guinea pig liver. Eur J Biochem. 1970;16(2):289–302. doi: 10.1111/j.1432-1033.1970.tb01084.x. [DOI] [PubMed] [Google Scholar]
- 181.Wiese TJ, Lambeth DO, Ray PD. The intracellular distribution and activities of phosphoenolpyruvate carboxykinase isozymes in various tissues of several mammals and birds. Comp Biochem Physiol B. 1991;100(2):297–302. doi: 10.1016/0305-0491(91)90378-q. [DOI] [PubMed] [Google Scholar]
- 182.Moore MC, et al. Sources of carbon for hepatic glycogen synthesis in the conscious dog. The Journal of clinical investigation. 1991;88(2):578–87. doi: 10.1172/JCI115342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Taylor R, et al. Direct assessment of liver glycogen storage by 13C nuclear magnetic resonance spectroscopy and regulation of glucose homeostasis after a mixed meal in normal subjects. The Journal of clinical investigation. 1996;97(1):126–32. doi: 10.1172/JCI118379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Petersen KF, et al. Contribution of net hepatic glycogenolysis to glucose production during the early postprandial period. The American journal of physiology. 1996;270(1 Pt 1):E186–91. doi: 10.1152/ajpendo.1996.270.1.E186. [DOI] [PubMed] [Google Scholar]
- 185.Diesterhaft M, Shrago E, Sallach HJ. Human Liver Phosphoenolpyruvate Carboxykinase: Evidence for a Separate Mitochondrial and Cytosol Enzyme. Biochemical Medicine. 1971;5:297–303. [Google Scholar]