Abstract
Epigenetic alterations of histone proteins and DNA are essential for hippocampal synaptic plasticity and cognitive function, and contribute to the etiology of psychiatric disorders and neurodegenerative diseases. Hippocampal memory formation depends on histone alterations and DNA methylation, and increasing evidence suggests that regulation of these epigenetic processes by modulatory factors such as environmental enrichment, stress, and hormones substantially influences memory function. Recent work from our laboratory suggests that the ability of the sex-steroid hormone 17β-estradiol (E2) to enhance novel object recognition memory consolidation in young adult female mice is dependent on histone H3 acetylation and DNA methylation in the dorsal hippocampus. Our data also suggest that enzymes mediating DNA methylation and histone acetylation work in concert to regulate the effects of E2 on memory consolidation. These findings shed light on the epigenetic mechanisms that influence hormonal modulation of cognitive function, and may have important implications for understanding how hormones influence cognition in adulthood and aging. This review will provide a brief overview of the literature on epigenetics and memory, describe in detail our findings demonstrating that epigenetic alterations regulate E2-induced memory enhancement in female mice, and discuss future directions for research on the epigenetic regulation of E2-induced memory enhancement.
Keywords: histone acetylation, DNA methylation, hippocampus, novel object recognition, estrogen
Introduction
Gene expression is necessary for long-term alterations in central nervous system structure and function. In recent years, it has become increasingly clear that epigenetic mechanisms, which regulate transcriptional access to DNA, play a significant role in the etiology of age-related memory decline, depression, drug addiction, and alcoholism, as well as the pathophysiology of neurodevelopmental (e.g., Rett's syndrome, Fragile X syndrome), psychiatric (e.g., schizophrenia, post-traumatic stress disorder), and neurodegenerative (e.g., Alzheimer's disease) disorders (1-11). Epigenetic alterations do not change the genetic code, but rather regulate the transcription of existing genes by methylating specific cytosine residues on the DNA or modifying the histone proteins around which DNA is supercoiled. In addition to their contributions to disease onset and risk, epigenetic alterations are critically important to controlling the gene expression associated with normal learning, memory, and environmental experience (5, 12-16). As will be discussed below, histone modifications (e.g., acetylation, phosphorylation, methylation) and DNA methylation are necessary for both basic long-term memory formation and the modulatory influences of hormones on memory. Most research on the epigenetics of learning and memory has focused on the hippocampus, largely because deficits in the types of memory subserved by this structure are characteristic of many neuropsychiatric and neurodegenerative diseases. However, recent data support the importance of epigenetic modifications to memory mediated by other brain regions including the amygdala and prefrontal cortex (17-19). Nevertheless, this review will focus on the role of epigenetics alterations in the hippocampus because this brain region has been the focus of the few studies examining the role of epigenetic mechanisms in mediating effects of 17β-estradiol (E2) on memory.
Why is it important to study how epigenetics might influence hormonal regulation of cognition? From a clinical standpoint, drugs that inhibit histone deacetylation have shown promise as potential treatments for cognitive dysfunction a myriad of animal models of neurodegenerative and psychiatric diseases, including Alzheimer's disease, Parkinson's disease, schizophrenia, depression, and traumatic brain injury (5). The prevalence of serious mental illness, including depression, anxiety disorders, schizophrenia, and dementia is nearly double in women compared to men (20-23), suggesting that organizational or activational effects of sex steroid hormones contribute to sex differences in the etiology and/or symptomatology of these illnesses. Because hippocampal dysfunction and cognitive deficits, including memory loss, are common to these mental illnesses (24), pinpointing the contribution of epigenetic alterations to hormonal regulation of cognition is important to the development of novel drugs for disorders in which sex steroid hormones are thought to increase risk. Further, because hormone treatment can elevate the risk of side effects that are harmful (e.g., breast cancer, heart disease) or undesirable (e.g., gynecomastia in men), targeting the epigenetic mechanisms through which hormones influence cognition could lead to safer and more acceptable treatment options for patients.
Many outstanding comprehensive reviews have detailed the epigenetic mechanisms involved in the neurobiology of learning and memory (e.g., (4, 12, 13, 15, 25-27)), and therefore, this literature will be only briefly summarized here. Instead, this review will focus largely on data showing that epigenetic alterations are critical for E2 to enhance hippocampal-dependent novel object recognition memory. These studies from my own laboratory are, thus far, the only research to investigate the roles of epigenetic alterations in hormone-induced memory enhancement. Therefore, this work will be described in some detail. Because the study of epigenetic influences on hormonal regulation of cognition is clearly in its infancy, this review will conclude by considering future directions for this research in the hope of inspiring others to begin studying this important issue.
The epigenetics of hippocampal memory
Within chromosomes, DNA is tightly supercoiled around histone octamers containing two copies each of histones H2A, H2B, H3, and H4 (28) (Fig. 1). Each of these histone proteins has an amino acid tail that can be altered by post-translational modifications including acetylation, phosphorylation, methylation, ubiquitination, and sumoylation. Many of these modifications relax the bonds between histones and DNA, thereby allowing transcription factors access to the DNA. Hippocampal learning, such as contextual fear conditioning, increases the acetylation, phosphorylation, and methylation of histone H3 in the hippocampus (29-31). Of the four core histones, H3 appears to be the most consistently altered by learning and E2 in the hippocampus (29, 32, 33).
Figure 1.
Representation of the histone octamer illustrating the processes of histone acetylation and DNA methylation. Histone acetylation is regulated by histone acetyltransferases (HATs) that add acetyl groups (Ac) to lysine residues (K) on histone tails, and histone deacetylases (HDACs) that remove acetyl groups from lysine residues. During DNA methylation, DNA methyltransferases (DNMTs) add methyl groups to cytosine residues within CpG islands on DNA. Adapted with permission from (58).
Histone acetylation is the most well studied chromatin modification associated with hippocampal learning and memory. Histone acetylation is regulated by histone acetyltransferases (HATs), which add acetyl groups to specific lysine residues, and histone deacetylases (HDACs), which remove these acetyl groups (34) (Fig. 1). The dependence of hippocampal memory and plasticity on HAT activity is supported by reports that mutations of the HATs p300/CBP and PCAF (p300/CBP-associated factor) impair hippocampal long-term potentiation (LTP) and hippocampal-dependent spatial, contextual fear, and novel object recognition memory (35-41). Pharmacological inhibition of HAT activity in the dorsal hippocampus also blocked novel object recognition memory consolidation in wild-type mice (32), providing converging evidence for a role of histone acetylation in memory formation. In contrast to HATs, certain HDACs, like HDAC2 and HDAC3, are potent negative regulators of hippocampal synaptic plasticity and memory formation (42, 43). For example, overexpression of HDAC2 impairs contextual and cued fear conditioning and spatial memory, reduces hippocampal spinogenesis and LTP, and suppresses the expression of proteins necessary for synaptic plasticity including CREB, CaMKIIA, NR2A, NR2B, and β-catenin (42). Such deficits are reversed by HDAC2 knockout or treatment with an HDAC inhibitor (42). HDAC2 knockout also enhances LTP magnitude, accelerates extinction of conditioned fear and taste aversion, and improves prefrontal cortex-dependent attentional set-shifting (44). Further, deletion of HDAC3 in the dorsal hippocampus enhances long-term novel object recognition and object placement memory in mice (43). Systemic or intracranial administration of HDAC inhibitor drugs, such as trichostatin-A (TSA), sodium butyrate (NaB), suberoylanilide hydroxamic acid (SAHA), and RGFP136 also support an essential role for histone acetylation in hippocampal learning and memory. In wild-type rodents, these HDAC inhibitors increase hippocampal histone H3 and H4 acetylation, facilitate LTP, and enhance several forms of hippocampal memory, including contextual fear conditioning, spatial memory, and novel object recognition (33, 42, 43, 45, 46). Moreover, HDAC inhibitors reverse hippocampal memory deficits in mouse models of aging (47) and Alzheimer's disease (8, 48), supporting their possible use for treating cognitive dysfunction associated with aging and neurodegenerative disease. Another potentially promising approach to reducing cognitive dysfunction comes from a recent study showing that an activator of p300/CBP HATs promotes hippocampal neurogenesis and enhances spatial memory in the Morris water maze (49).
In addition to histone modifications, DNA methylation also plays a major role in regulating hippocampal memory consolidation (26, 27, 50-52). DNA methylation generally decreases transcriptional access to DNA, although the functional effects of this gene silencing depend on the genes altered. DNA methylation is catalyzed by DNA (cytosine-5’) methyltransferases (DNMTs) that methylate cytosine residues in CpG islands on DNA (Fig. 1). This methylation serves to reduce transcriptional access to DNA. DNMT1 is a maintenance methyltransferase that transfers established methylation marks from one strand of DNA to the other (53). DNMT3A and DNMT3B are de novo methyltransferases that add new methyl marks to previously unmethylated cytosines (27, 53). The de novo methyltransferases appear to be more involved in hippocampal learning, as illustrated by findings indicating that the expression of DNMT3A and DNMT3B, but not DNMT1, mRNA is increased in the hippocampus by contextual fear learning (51). Contextual fear conditioning also increases the methylation of memory suppressor genes like protein phosphatase 1 (PP1), but decreases the methylation of memory promoting genes like reelin (51). Supporting the importance of DNA methylation in memory formation are recent data showing that genetic deletion of the protein Growth arrest and DNA damage-inducible 45b (Gadd45b), which regulates gene-specific demethylation, enhances late-phase hippocampal LTP, contextual fear memory, and spatial memory (54). Moreover, DNMT inhibitors like 5-aza-2-deoxycytidine (5-AZA) and zebularine prevent induction of hippocampal LTP and contextual fear memory consolidation (50, 51, 55). Interestingly, these effects are blocked by HDAC inhibitors, and the ability of contextual fear conditioning to increase H3 acetylation is blocked by DNMT inhibition (50). These data suggest an important synergy between histone acetylation and DNA methylation in regulating hippocampal memory formation.
E2 and hippocampal memory
E2 has emerged in recent decades as a pivotal modulator of hippocampal function and hippocampal memory. Many extensive reviews on this subject are available (e.g., (56-65)), so I will provide only a succinct description of this work here. The hippocampus is exceptionally sensitive to E2, as demonstrated by seminal work showing that E2 increases CA1 dendritic spine density in naturally cycling or ovariectomized female rats within 24 hours of exposure (66, 67). E2 also promotes neurogenesis in the dentate gyrus of the hippocampus (see (68) for a recent review), suggesting that it regulates multiple aspects of hippocampal morphology essential for long-term memory formation. Moreover, E2 regulates forms of synaptic plasticity thought to underlie learning and memory, including LTP (69-71). Although a positive correlation between high levels of E2 and hippocampal memory formation has been reported in many studies, this association is not universally observed. The relationship between naturally cycling E2 and memory has been somewhat difficult to test due to rapidly changing levels of gonadal hormones in circulation. Some evidence suggests that high levels of E2 during proestrus are associated with enhanced spatial memory and spatial strategy use (72-74), whereas other studies report no effects of cyclic hormone fluctuations on spatial memory, social recognition, or novel object recognition (75-80). The contribution of hippocampally-synthesized E2 to learning and memory is currently unknown, but will be important to assess in future work. Because of the challenges associated with assessing memory within the context of the natural estrous cycle, the vast majority of studies in animal models have been conducted using ovariectomized female rats and mice administered acute or chronic E2 either systemically or directly into the dorsal hippocampus. Generally, exogenous E2 administered to ovariectomized young adult rodents enhances several types of hippocampal-dependent memory, including spatial memory, novel object recognition, social recognition, inhibitory avoidance, and trace eyeblink conditioning (see (57, 63, 81, 82) for reviews). However, not all studies report an E2-induced enhancement in hippocampal memory (e.g. (83, 84)), and comparisons across studies suggest that the beneficial effects of E2 depend on numerous elements of the experimental design, including dose, age at treatment, duration and type of treatment, duration of hormone loss prior to treatment, timing of treatment relative to testing, type of memory tested, and task difficulty (see (57) for a discussion of these issues).
The past few years has seen a proliferation of studies examining acute effects of E2 administered immediately after training to examine effects of E2 on memory consolidation. These studies are quite consistent in showing that E2, and agonists of ERα and ERβ, enhance the consolidation of spatial memory measured in the Morris water maze, spatial memory measured in an object placement task, and novel object recognition memory (33, 80, 85-94). Unlike pre-training treatments, immediate post-training E2 treatments allow effects of E2 on memory consolidation to be pinpointed in the absence of potentially confounding effects on motivation and anxiety. E2 given two or three hours after training does not affect spatial memory or object recognition (87, 93, 94), indicating that E2 influences memory consolidation fairly rapidly after training. Because effects of E2 can be attributed specifically to the memory consolidation phase of memory formation, this design permits more causal links between E2-induced memory enhancement and specific cellular and molecular changes within the hippocampus.
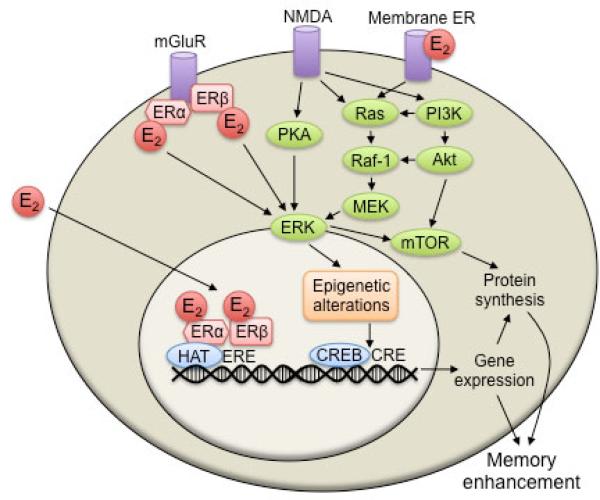
The rapid effects on hippocampal memory consolidation are likely mediated by some combination of estrogen receptors (ERs) and other plasma membrane receptors. ERs of both the classical (ERα and ERβ) and non-classical types (e.g., GPER, Gq-mER) are thought to mediate the effects of E2 in the hippocampus. ERα and ERβ are located throughout the hippocampus in the nuclei, dendritic spines, and axon terminals of pyramidal neurons and interneurons (95-97). ERα and ERβ may mediate the epigenetic effects of E2 in several ways. In their classical mechanism of action, these ERs dimerize upon estrogen binding, and then the hormone-ER complex binds to estrogen response elements (EREs) on DNA to facilitate gene transcription (Fig. 2). ERE-mediated transcription requires coregulator proteins. Many coactivators function as HATs or interact with HATs, whereas some corepressors exhibit HDAC activity (98-100). Therefore, histone acetylation is intricately involved in ERE-mediated gene transcription. However, ERα and ERβ may also exert epigenetic effects by regulating cell-signaling pathways that initiate processes like histone acetylation. In this non-classical mechanism, the ERs translocate to the plasma membrane after binding E2 (101, 102), where they interact with integral membrane proteins like metabotropic glutamate receptors (mGluRs) to rapidly initiate extracellular signal-regulated/mitogen activated protein kinase (ERK/MAPK) signaling and stimulate phosphorylation of the transcription factor cAMP response element-binding protein (CREB) (103, 104) (Fig. 2). This E2/mGluR signaling is essential for E2 and agonists of ERα and ERβ to enhance novel object recognition and object placement memory consolidation (105). Other data support the involvement of putative membrane-bound ERs, including GPER (a.k.a., GPR30, GPER1), in mediating the effects of E2 on ERK signaling and hippocampal memory (87, 106-108) (Fig. 2). Although it is not yet clear which ERs mediate specific cell signaling events, it has become increasingly well accepted that both classical and non-classical ERs facilitate the rapid effects of E2 (109).
Figure 2.
Our current working model of the molecular mechanisms mediating the rapid effects of E2 on memory consolidation. ERα and ERβ could influence memory by binding to coregulators, including HATs, and stimulating estrogen response element (ERE)-mediated gene transcription. Alternatively, E2 may rapidly enhance memory consolidation by triggering interactions between ERs and metabotropic glutamate receptors (mGluRs), NMDA receptor activation, and/or membrane ER activation, all of which can activate ERK and mammalian target of rapamycin (mTOR) signaling in dorsal hippocampal neurons. Activation of ERK then leads to histone H3 acetylation, and potentially the methylation of memory repressor genes like Hdac2, Hdac3, or reelin, causing increased expression of genes that facilitate protein synthesis and memory consolidation. This model is based on my laboratory's findings (32, 33, 87-90, 105), some of which are discussed in more detail in the main text.
The rapid effects of E2 on memory consolidation fit well with data showing that E2 can activate hippocampal cell signaling within minutes. For example, E2 activates numerous cell signaling cascades in the dorsal hippocampus within 5 minutes, including the ERK/MAPK and phosphatidylinositol-3/Akt (PI3K/Akt) pathways (87, 89, 110-114), which play critical roles in hippocampal long-term memory formation (115, 116). Our own work has shown that the ability of E2 to enhance novel object recognition memory consolidation in young and middle-aged ovariectomized mice is dependent on activation of PI3K and ERK in the dorsal hippocampus (87, 89). We have also shown that the E2-induced activation of the p42 isoform of ERK is dependent on initial activation of the upstream kinases PI3K and PKA in the dorsal hippocampus (88-90), suggesting that p42 ERK functions as something of a final common signaling molecule leading to the activation of transcription factors such as CREB (Fig. 2). ERK activation is necessary for other kinases (e.g., PKC) to increase histone H3 acetylation (29), suggesting that ERK can also influence gene transcription by altering chromatin structure. As such, ERK appears to not only activate transcription factors, but also to regulate transcriptional access to DNA via histone acetylation. Given the importance of ERK activation to E2-induced memory enhancement, we reasoned that epigenetic processes influenced by ERK, such as histone acetylation, might be involved in the estrogenic modulation of memory.
E2, epigenetics, and hippocampal memory
Histone acetylation
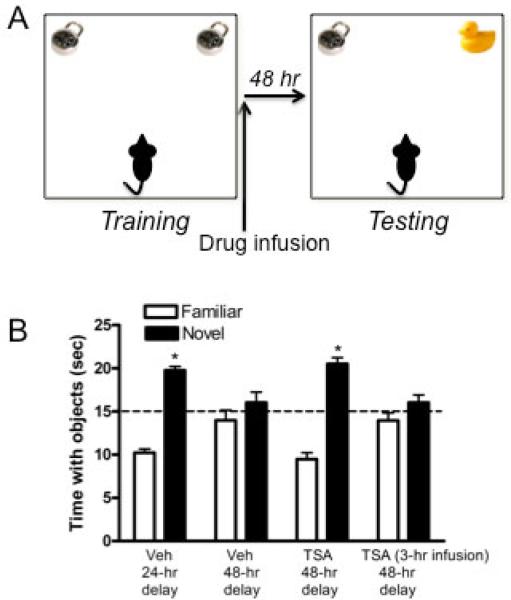
We first tested whether our novel object recognition task was sensitive to epigenetic alterations. Mice first accumulated 30 seconds exploring two identical objects in an open arena (117) (Fig. 3A). Immediately after training, mice were infused with vehicle or the HDAC inhibitor TSA into the dorsal hippocampus. Forty-eight hours later, mice were allowed to explore one novel and one familiar object. Because mice are inherently drawn to novelty, mice who remember the familiar object will spend significantly more time than chance (15 seconds) exploring the novel object (117). As in our previous work (87, 118), vehicle-treated females exhibited intact object recognition memory 24 hours after training, but not 48 hours after training (33) (Fig. 3B). However, mice infused with the HDAC inhibitor TSA into the dorsal hippocampus displayed intact novel object recognition memory 48 hours later (Fig. 3B), suggesting that HDAC inhibition rendered this memory more persistent than normal. This finding is consistent with similar data from male mice (45). Importantly, the effects of HDAC inhibition were limited to a specific window of time after training during which memory consolidation occurs, as indicated by the fact that infusion of TSA delayed three hours after training had no effect on memory consolidation (33) (Fig. 3B).
Figure 3.
(A) Schematic of the novel object recognition testing protocol. Mice accumulate 30 seconds exploring two identical objects in an open arena. Immediately post-training, mice are infused and then returned to their home cage. Retention is tested 24 or 48 hours later by presenting mice with one novel and one familiar object. Mice who remember the familiar object spend more time than chance (15 sec) exploring the novel object. (B) The HDAC inhibitor TSA enhances novel object recognition memory consolidation. Ovariectomized female mice given bilateral infusions of vehicle into the dorsal hippocampus immediately after training spent significantly more time than chance (dashed line at 15 sec; *p < 0.05 relative to chance) with the novel object 24 hours after infusion, but not 48 hours after infusion, suggesting that they did not remember the familiar object for 48 hours. In contrast, mice given bilateral infusions of TSA (16.5 mM/hemisphere) into the dorsal hippocampus immediately after training did spend significantly more time than chance (*p < 0.05) with the novel object 48 hours later. However, this memory enhancement was not observed if TSA infusion was delayed for three hours. These data suggest that histone acetylation enhances novel object memory consolidation. Bars represent the mean ± SEM for each object. Panel B reprinted with permission from (33).
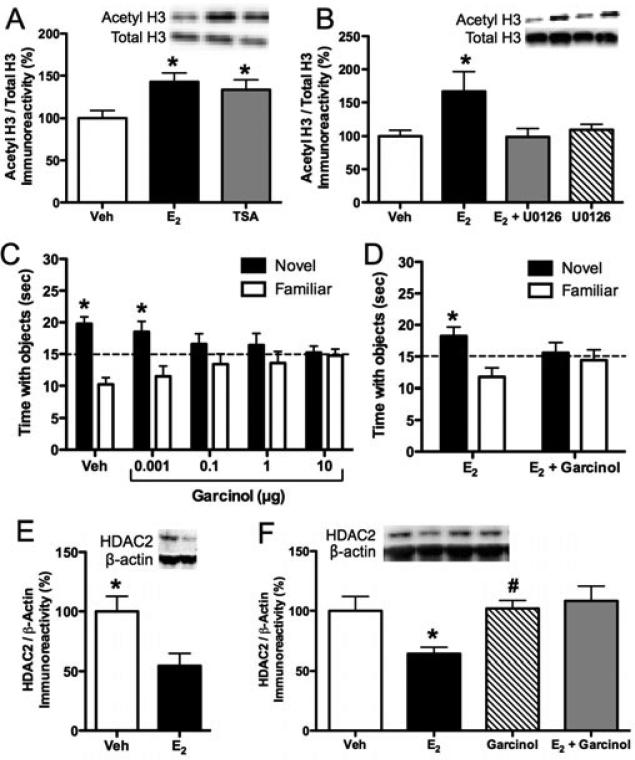
We next examined the effects of E2 or TSA on histone H3 and H4 acetylation 30 minutes after infusion into the dorsal hippocampus. As would be expected from an HDAC inhibitor, TSA significantly increased acetylation of both H3 and H4 (33) (Fig. 4A). However, the effects of E2 were more specific; like contextual fear conditioning (29), E2 increased acetylation of histone H3 (Fig. 4A), but not histone H4 (33). We have replicated this specificity in several studies, and have subsequently shown no effect of E2 on histone H2B in young females (32) and on histones H2A and H2B in middle-aged females (unpublished observations), suggesting that genes associated with histone H3 are particularly important for the functional effects of E2 in the dorsal hippocampus.
Figure 4.
ERK-driven histone H3 acetylation is necessary for E2 to enhance novel object recognition memory consolidation. (A) Bilateral infusion of β-cyclodextrin encapsulated E2 (5 μg/hemisphere) or TSA (16.5 mM/hemisphere) into the dorsal hippocampus significantly increased histone H3 acetylation in the dorsal hippocampus relative to vehicle 30 minutes after infusion (*p < 0.05). (B) Infusion of E2 (10 μg) into the dorsal third ventricle significantly increased histone H3 acetylation in the dorsal hippocampus relative to vehicle 30 minutes after infusion (*p < 0.05). Infusion of the ERK pathway inhibitor U0126 (0.5 μg/hemisphere) into the dorsal hippocampus blocked this increase, but had no effect on H3 acetylation on its own. (C) Mice were given bilateral infusions of vehicle or one of four doses of the HAT inhibitor garcinol into the dorsal hippocampus immediately after novel object recognition training. Mice infused with 0.1, 1, or 10 μg/hemisphere spent no more time than chance with the novel object. In contrast, mice infused with vehicle or 0.001 μg garcinol exhibited a significant preference for the novel object (*p < 0.05 relative to chance), suggesting that all but the lowest dose of garcinol impaired novel object recognition memory consolidation. (D) When this lowest dose (0.001 μg) of garcinol was infused into the dorsal hippocampus with E2, it blocked the effects of E2 on memory, demonstrating that histone acetylation is necessary for E2 to enhance novel object recognition memory consolidation. (E) Bilateral infusion of E2 into the dorsal hippocampus significantly reduced levels of HDAC2 protein in the dorsal hippocampus four hours after infusion (*p < 0.05 relative to vehicle). (F) The E2-induced decrease in HDAC2 protein was blocked by 0.001 μg garcinol, indicating that histone acetylation is necessary for E2 to reduce HDAC2 levels. Bars in all panels represent the mean ± SEM. Insets in panels A, B, E, and F illustrate representative Western blot images. Acetylated H3 protein was normalized to total H3, and HDAC2 protein was normalized to β-actin. Reprinted with permission from (32, 33).
Given that p42 ERK activation in the dorsal hippocampus is necessary for E2 to enhance novel object recognition memory consolidation (87, 89), we next examined whether ERK activation was also necessary for E2 to increase histone H3 acetylation. Mice were given a single intracerebroventricular (ICV) infusion of vehicle or E2 immediately following a bilateral dorsal hippocampal infusion of vehicle or the ERK pathway inhibitor U0126. The rationale for this triple infusion procedure was to allow us to administer E2 to the brain and specifically inhibit signaling in the dorsal hippocampus without having to infuse twice into the dorsal hippocampus in rapid succession and risk damaging hippocampal tissue. U0126 blocked the E2-induced increase in histone H3 acetylation (Fig. 4B), whereas histone H4 acetylation remained unchanged by either drug (33). These data demonstrate that ERK activation in the dorsal hippocampus is necessary for E2 to enhance both memory and histone H3 acetylation.
But is histone H3 acetylation necessary for E2 to enhance novel object recognition memory consolidation? This question was addressed in a subsequent study designed to test whether a HAT inhibitor could prevent E2 from influencing memory and histone acetylation. We used the potent HAT inhibitor garcinol, which had not previously been used in vivo to study the effects of histone acetylation on biological functions. Garcinol is derived from the rind of the Garcinia indica fruit, and is highly permeable to cultured cells (119, 120). We first established a dose of garcinol that did not impair memory on its own using a shorter 24-hour delay between training (33, 118) to ensure that any effects on memory at a longer 48-hour delay were due to a specific interaction between E2 and garcinol, rather than a garcinol-induced blockade of general memory formation. Immediately after novel object recognition training, mice were infused with vehicle or one of four doses of garcinol into the dorsal hippocampus. All but the 0.001 μg dose impaired novel object recognition (Fig. 4C) (32). However, this dose prevented E2 from facilitating novel object recognition memory consolidation (Fig. 4D) (32), suggesting that histone acetylation is necessary for E2-induced memory enhancement. Further, garcinol prevented E2 from increasing histone H3 acetylation, but had no effect on H2B or H4 acetylation (32). Together, these data suggest that acetylation of H3 in the dorsal hippocampus is essential for the beneficial effects of E2 on memory.
E2 can also influence the expression of HDAC proteins in the dorsal hippocampus. As described above, HDAC2 and HDAC3 are detrimental for hippocampal memory formation (42, 43). Consistent with the role of HDAC2 as a negative modulator of memory, E2 significantly decreased levels of HDAC2 protein in the dorsal hippocampus four hours after infusion (Fig. 4E) (32, 33). E2 induced similar reductions of HDAC3 protein in middle-aged females (unpublished observations). In contrast, HDAC1 protein levels in the dorsal hippocampus were not affected by E2 (32, 33), which is consistent with previous work showing a minimal role of HDAC1 in hippocampal memory (42). Interestingly, garcinol completely blocked the E2-induced reduction of HDAC2 protein in the dorsal hippocampus four hours after infusion (Fig. 4F), while having no effects on HDAC1 or HDAC2 on its own (32). These findings suggest that histone acetylation regulates levels of HDAC2 protein in the dorsal hippocampus.
DNA methylation
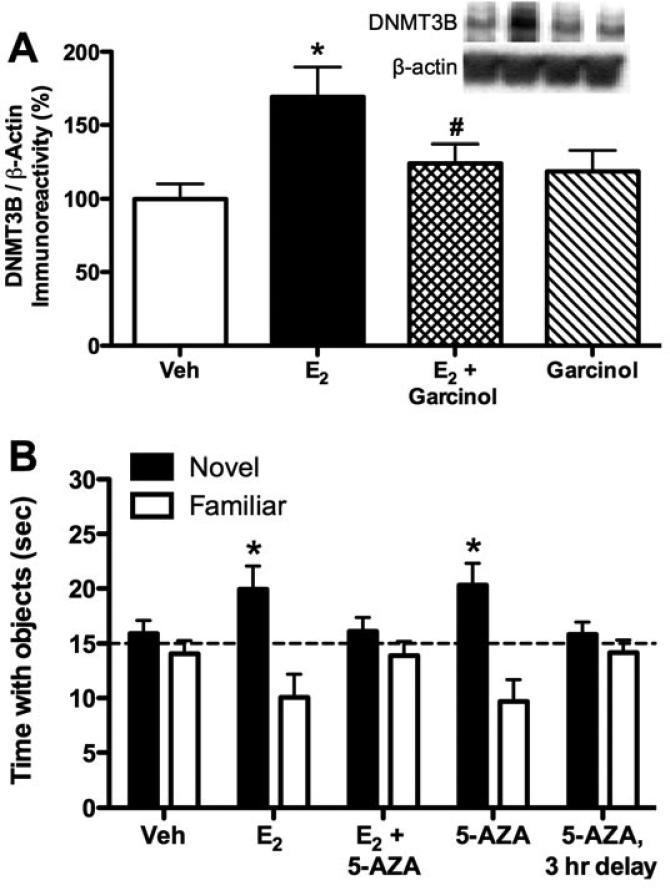
Because learning-induced histone H3 acetylation is blocked by DNMT inhibition (50), we next wondered whether DNA methylation could also regulate the ability of E2 to enhance hippocampal memory. We first examined the effects of E2 on expression of the three DNMT enzymes. mRNA for DNMT3A and DNMT3B, but not DNMT1, in the dorsal hippocampus was increased 45 min after infusion of E2 into the dorsal hippocampus (33). However, only DNMT3B protein was significantly affected by E2, and levels of this de novo methyltransferase were increased by E2 four hours after dorsal hippocampal infusion (Fig. 5A) (32, 33). This result suggests that E2 may preferentially increase DNA methylation at previously unmethylated cytosine residues. Interestingly, the increase in DNMT3B protein was blocked by garcinol (Fig. 5A) (32), suggesting that histone acetylation is necessary for E2 to increase DNMT3B levels.
Figure 5.
DNA methyltransferase enzymes are involved E2-induced memory enhancement. (A) Bilateral infusion of E2 (5 μg/hemisphere) into the dorsal hippocampus significantly increased levels of DNMT3B protein in the dorsal hippocampus relative to vehicle four hours after infusion (*p < 0.05). This increase was blocked by concurrent infusion of garcinol (#p < 0.05 relative to the E2 group), suggesting that histone acetylation is necessary for E2 to increase DNMT3B levels in the dorsal hippocampus. Garcinol alone had no effect on DNMT3B levels. Inset illustrates representative Western blot images. DNMT3B protein was normalized to β-actin. Reprinted with permission from (32). (B) Bilateral infusion of 5-AZA (100 μg/hemisphere) into the dorsal hippocampus administered immediately, but not three hours, after training significantly increased the time spent with the novel object relative to chance (*p < 0.05), suggesting that DNA methyltransferase enzymes regulate novel object recognition within a three-hour time window after training. 5-AZA blocked the memory enhancing effect of E2, indicating that DNMT enzymes regulate the memory-enhancing effects of E2. Reprinted with permission from (33). Bars in both panels represent the mean ± SEM.
We next examined the role of DNA methylation in mediating the effects of E2 on memory. As with histone acetylation, we first tested whether novel object recognition is sensitive to pharmacological manipulations of DNA methylation. Immediately after training, ovariectomized females were infused with vehicle or the DNMT inhibitor 5-AZA into the dorsal hippocampus. 5-AZA significantly enhanced novel object recognition memory consolidation (Fig. 5B) (33), suggesting that DNMTs regulate novel object recognition independent of E2. As with the HDAC inhibitor TSA, the effects of 5-AZA were limited to a brief window of time after training, as an infusion delayed three hours after training had no effect on memory (Fig. 5B) (33). We next found that 5-AZA prevented E2 from facilitating novel object recognition memory (Fig. 5B) (33), suggesting that activation of DNMTs is necessary for E2 to enhance novel object recognition memory consolidation. Although this finding suggests that DNA methylation regulates E2-induced memory enhancement, more definitive conclusions await direct measurement of specific E2-induced changes in DNA methylation.
In summary, our data show that E2-induced increases in dorsal hippocampal his-tone acetylation are specific to histone H3 and are dependent on ERK activation in the dorsal hippocampus. E2 also decreases levels of HDAC2, and possibly HDAC3, protein in the dorsal hippocampus four hours after infusion, and this effect depends on histone acetylation. Further, novel object recognition itself is enhanced by HDAC inhibition and impaired by HAT inhibition, demonstrating that histone acetylation is essential for novel object recognition memory consolidation in ovariectomized females. These findings indicate that ERK-driven histone H3 acetylation in the dorsal hippocampus is necessary for E2 to enhance novel object recognition memory consolidation, possibly through decreased expression of the memory-repressing HDAC2. Our data also suggest that an increase in de novo DNA methylation may be essential for E2 to enhance novel object recognition memory consolidation. The most likely targets of this putative methylation are genes whose expression is detrimental for memory, such as Hdac2, Hdac3, or PP1. The fact that the HAT inhibitor garcinol prevented E2 from increasing DNMT3B levels suggests that histone acetylation may regulate DNA methylation by influencing levels of DNMT enzymes. As such, our data support the notion that the enzymes regulating DNA methylation and histone acetylation work in concert to mediate effects of E2 on the expression of genes that mediate hippocampal memory consolidation. Ongoing work in our laboratory is aimed at identifying these genes.
Future directions
To date, our studies of the epigenetic processes that regulate E2-induced memory enhancement provide a tantalizing glimpse into the complex epigenetic mechanisms through which E2 influences memory. This work has only begun to reveal the countless ways in which chromatin modifications may influence the hormonal regulation of cognition. For example, it is difficult to know at this point whether E2 influences epigenetic mechanisms in a fundamentally different manner from learning itself, or rather enhances the mechanisms already triggered by learning. In the case of post-training treatments, the answer may be the latter, unless learning triggers locally-synthesized E2 that substantively alters how learning influences epigenetic processes during the learning event. Indeed, E2 present during learning (whether locally-synthesized or exogenous) may play a permissive role in facilitating epigenetic alterations during learning that are not possible without E2. This issue will need to be addressed in future studies using aromatase inhibitors to block local E2 synthesis in ovariectomized females. Other future directions for this research are discussed below.
Epigenetics, estradiol, and aging
The loss of estrogens and progestins at menopause significantly increases the risk of memory decline and Alzheimer's disease (AD) in middle-aged women relative to men (23, 121). Although estrogens can enhance hippocampal memory in menopausal women and aging female rodents (57, 122), it has become widely recognized that estrogen treatment must be started during a critical period in middle age to benefit cognitive function in both women and rodent models (123). Indeed, duration of hormone loss has emerged as a critical regulator of the mnemonic response to E2 in middle-aged rats, with delays of five months or more between ovariectomy and treatment preventing E2 from enhancing spatial working memory in tasks such as the radial arm maze and T-maze (124-127). The precise mechanisms underlying this loss of responsiveness are unclear, but are likely due to alterations in the hippocampus. In middle-aged female rats, extended hormone loss prevents E2 from enhancing hippocampal synaptic physiology (128), increasing hippocampal levels of choline acetyltransferase (129), and up-regulating ERα (130). Age-related reductions in ERα and ERβ levels could contribute to this reduced hippocampal responsiveness, as levels of both receptors are significantly decreased in the middle-aged and aged female hippocampus (130-133). One proposed mechanism that may contribute to the etiology of the critical period and, more specifically, the loss of hippocampal ERα and ERβ, is the increased ubiquitination and degradation of ERα and ERβ that occurs in the CA1 region of aged female rat hippocampus (133).
Another mechanism that might contribute to the age-related reduction of classical ERs is increased methylation of ERα and ERβ. The expression of ERα is highly regulated by methylation in specific promoters during early development. For example, methylation of ERα5’ Exon A is increased on post-natal day 10 in male mice, which coincides with a significant reduction in ERα5’ Exon A mRNA expression at this age (134). In support of this potential mechanism, ERβ promoter methylation was increased in the neocortex of middle-aged (9-12 months old), but not young (3-4 months old), female rats, which corresponded with an age-related reduction in ERβ mRNA (135). However, our own preliminary data from female mice indicates that epigenetic processes remain responsive to E2 into middle-age (15-16 months old), where we find that dorsal hippocampal infusion of E2 can still increase histone H3 acetylation, decrease HDAC2 and HDAC3 protein, and increase DNMT3B protein in the dorsal hippocampus (unpublished observations). E2 also enhances object recognition memory in middle-aged female mice (89), so perhaps the mouse hippocampus remains responsive to E2 further into old age than the rat hippocampus. Future studies in aged mice may resolve this issue, as E2 no longer enhances object recognition or activates ERK or PI3K in aged (21 months old) female mice (89). Therefore, methylation changes similar to those observed in middle-aged rats may occur in aged female mice. Regardless of the age of onset, epigenetic alterations are likely to play a major role in the closing of the critical period in females. As such, future research that pinpoints how age-related alterations in chromatin modifications influence the mnemonic response to E2 could be used to develop treatments that reverse these changes, thereby significantly extending the critical period and enhancing the effectiveness of estrogen therapies.
Epigenetics and sex differences
Because all of our own work to date on epigenetics, estrogen, and memory was conducted in females, it will also be necessary to determine if similar epigenetic alterations occur in males in response to estradiol or testosterone. It is also important note that the research reviewed above on the epigenetics of learning and memory has been historically conducted in males. Therefore, research detailing the modulatory influences of ovarian hormones on the epigenetics of learning and memory would add greatly to a literature that has studied epigenetic mechanisms primarily in males. Indeed, it is unknown if the epigenetic response to learning or hormones differs in the male and female hippocampus, so potential sex differences should be examined in future work. Several lines of evidence support the possible existence of such sex differences. For example, the masculinization of brain regions like the bed nucleus of the stria terminalis is regulated by testosterone-induced histone acetylation (136). In adulthood, contextual fear learning increases ERK activation in the ventral hippocampus more in male rats than in gonadally intact females, so epigenetic events downstream from ERK activation, like histone H3 acetylation, may be increased more in males than in females. Consistent with this notion are data showing that histone H3 acetylation in the hippocampus and cortex is higher in male mice than in females on embryonic day 18 and post-natal day 0 (137). In these same brain regions, males also exhibited higher levels of histone H3 methylation than females on post-natal days 0 and 6 (137). DNA methylation may also be sexually dimorphic, given numerous sex differences reported in the patterns of DNA methylation in the neonatal rodent brain (138). Given the dearth of studies examining the epigenetics of sex differences in the adult brain, this topic is ripe for investigation.
Other future directions
In addition to histone acetylation, other histone modifications, such as phosphorylation and methylation, play important roles in regulating memory formation (30, 31, 139). Therefore, the effects of E2 on these processes should be examined in future studies. Much more work will also be needed to identify which promoter regions on key memory genes are altered by epigenetic processes in order to gain a more precise understanding of how gene expression in the hippocampus is altered by E2. Future research should also investigate the roles that epigenetic alterations play in regulating the effects of E2 and related hormones on other forms of hippocampal memory (e.g., spatial and contextual memories), and on other cognitive processes mediated elsewhere in the brain (e.g., the prefrontal cortex and amygdala).
Conclusions
Emerging data on epigenetic mechanisms have already revolutionized the study of cognition and mental illness. Understanding how modulatory factors, such as hormones, regulate the epigenetic code is essential to uncovering the molecular mechanisms that govern psychological processes in both females and males. Such discoveries will open the door to exciting new avenues of research that may lead to novel treatments to reduce the incidence and severity of neurodegenerative and psychiatric disorders.
Acknowledgements
The University of Wisconsin-Milwaukee sponsored the writing of this manuscript and the author's presentation on this material at the 7th International Meeting on Steroids and the Nervous System in Torino, Italy (February 16-20, 2013). The empirical work described in this review was supported by a grant from the National Institute on Aging (AG022525) and the University of Wisconsin-Milwaukee. Thanks to Drs. Marissa Boulware and Ashley Fortress for critical comments on this manuscript.
References
- 1.Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- 2.Day JJ, Sweatt JD. Epigenetic treatments for cognitive impairments. Neuropsychopharmacology. 2011;37:247–260. doi: 10.1038/npp.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shukla SD, Velazquez J, French SW, Lu SC, Ticku MK, Zakhari S. Emerging role of epigenetics in the actions of alcohol. Alcohol Clin Exp Res. 2008;32:1525–1534. doi: 10.1111/j.1530-0277.2008.00729.x. [DOI] [PubMed] [Google Scholar]
- 4.Franklin TB, Mansuy IM. The prevalence of epigenetic mechanisms in the regulation of cognitive functions and behaviour. Curr Opin Neurobiol. 2010;20:441–449. doi: 10.1016/j.conb.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Fischer A, Sananbenesi F, Mungenast A, Tsai LH. Targeting the correct HDAC(s) to treat cognitive disorders. Trends Pharmacol Sci. 2010;31:605–617. doi: 10.1016/j.tips.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Penner MR, Roth TL, Barnes CA, Sweatt JD. An epigenetic hypothesis of aging-related cognitive dysfunction. Front Aging Neurosci. 2010;2(article 9):1–11. doi: 10.3389/fnagi.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pandey SC, Ugale R, Zhang H, Tang L, Prakash A. Brain chromatin remodeling: A novel mechanism of alcoholism. J Neurosci. 2008;281:3729–3737. doi: 10.1523/JNEUROSCI.5731-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kilgore M, Miller CA, Fass DM, Hennig KM, Haggarty SJ, Sweatt JD, Rumbaugh G. Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer's disease. Neuropsychopharmacology. 2010;35:870–880. doi: 10.1038/npp.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mastroeni D, Grover A, Delvaux E, Whiteside C, Coleman PD, Rogers J. Epigenetics changes in Alzheimer's disease: Decrements in DNA methylation. Neurobiol Aging. 2010;31:2025–2037. doi: 10.1016/j.neurobiolaging.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roth TL, Zoladz PR, Sweatt JD, Diamond DM. Epigenetic modification of hippocampal Bdnf DNA in adult rats in an animal model of post-traumatic stress disorder. J Psychiatr Res. 2011;45:919–926. doi: 10.1016/j.jpsychires.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golden SA, Christoffel DJ, Heshmati M, Hodes GE, Magida J, Davis K, Cahill ME, Dias C, Ribeiro E, Ables JL, Kennedy PJ, Robison AJ, Gonzalez-Maeso J, Neve RL, Turecki G, Ghose S, Tamminga CA, Russo SJ. Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nat Med. 2013;19:337–344. doi: 10.1038/nm.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sweatt JD. Experience-dependent epigenetic modifications in the central nervous system. Biol Psychiatry. 2009;65:191–197. doi: 10.1016/j.biopsych.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zovkic IB, Guzman-Karlsson MC, Sweatt JD. Epigenetic regulation of memory formation and maintenance. Learn Mem. 2013;20:61–74. doi: 10.1101/lm.026575.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447:178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- 15.Lattal KM, Wood MA. Epigenetics and persistent memory: Implications for reconsolidation and silent extinction beyond the zero. Nat Neurosci. 2013;16:124–129. doi: 10.1038/nn.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gräff J, Tsai LH. Histone acetylation: Molecular mnemonics on the chromatin. Nat Rev Neurosci. 2013;14:97–111. doi: 10.1038/nrn3427. [DOI] [PubMed] [Google Scholar]
- 17.Miller CA, Gavin CF, White JA, Parrish RR, Honasoge A, Yancey CR, Rivera IM, Rubio MD, Rumbaugh G, Sweatt JD. Cortical DNA methylation maintains remote memory. Nat Neurosci. 2010;13:664–666. doi: 10.1038/nn.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maddox SA, Schafe GE. Epigenetic alterations in the lateral amygdala are required for reconsolidation of a Pavlovian fear memory. Learn Mem. 2011;18:579–593. doi: 10.1101/lm.2243411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maddox SA, Watts CS, Schafe GE. p300/CBP histone acetyltransferase activity is required for newly acquired and reactivated fear memories in the lateral amygdala. Learn Mem. 2013;20:109–119. doi: 10.1101/lm.029157.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grigoriadis S, Robinson GE. Gender issues in depression. Ann Clin Psychiatry. 2007;19:247–255. doi: 10.1080/10401230701653294. [DOI] [PubMed] [Google Scholar]
- 21.Weinstock LS. Gender differences in the presentation and management of social anxiety disorder. J Clin Psychiatry. 1999;60(Suppl 9):9–13. [PubMed] [Google Scholar]
- 22.Huber TJ, Borsutzky M, Schneider U, Emrich HM. Psychotic disorders and gonadal function: Evidence supporting the oestrogen hypothesis. Acta Psychiatr Scand. 2004;109:269–274. doi: 10.1046/j.1600-0447.2003.00251.x. [DOI] [PubMed] [Google Scholar]
- 23.Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JCS. Hormone replacement therapy and incidence of Alzheimer disease in older women. JAMA. 2002;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- 24.Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci. 2011;12:585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barrett RM, Wood MA. Beyond transcription factors: The role of chromatin modifying enzymes in regulating transcription required for memory. Learn Mem. 2008;15:460–467. doi: 10.1101/lm.917508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Day JJ, Sweatt JD. DNA methylation and memory formation. Nat Neurosci. 2010;11:1319–1323. doi: 10.1038/nn.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Day JJ, Sweatt JD. Cognitive neuroepigenetics: A role for epigenetic mechanisms in learning and memory. Neurobiol Learn Mem. 2011;96:2–12. doi: 10.1016/j.nlm.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luger K, Mäder AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 29.Levenson JM, O'Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- 30.Chwang WB, O'Riordan KJ, Levenson JM, Sweatt JD. ERK/MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning. Learn Mem. 2006;13:322–328. doi: 10.1101/lm.152906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta S, Kim SY, Artis S, Molfese DL, Schumacher A, Sweatt JD, Paylor RE, Lubin FD. Histone methylation regulates memory formation. J Neurosci. 2010;30:3589–3599. doi: 10.1523/JNEUROSCI.3732-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Z, Fan L, Fortress AM, Boulware MI, Frick KM. Hippocampal histone acetylation regulates object recognition and the estradiol-induced enhancement of object recognition. J Neurosci. 2012;32:2344–2351. doi: 10.1523/JNEUROSCI.5819-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Z, Fan L, Frick KM. Epigenetic alterations regulate the estradiol-induced enhancement of memory consolidation. Proc Natl Acad Sci USA. 2010;107:5605–5610. doi: 10.1073/pnas.0910578107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang XJ, Seto E. HATs and HDACs: From structure, function and regulation to novel strategies for therapy and prevention. Oncogene. 2007;26:5310–5318. doi: 10.1038/sj.onc.1210599. [DOI] [PubMed] [Google Scholar]
- 35.Korzus E, Rosenfeld MG, Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42:961–972. doi: 10.1016/j.neuron.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wood MA, Kaplan MP, Park A, Blanchard EJ, Oliveira AM, Lombardi TL, Abel T. Transgenic mice expressing a truncated form of CREB-binding protein (CBP) exhibit deficits in hippocampal synaptic plasticity and memory storage. Learn Mem. 2005;12:111–119. doi: 10.1101/lm.86605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maurice T, Duclot F, Meunier J, Naert G, Givalois L, Meffre J, Célérier A, Jacquet C, Copois V, Mechti N, Ozato K, Gongora C. Altered memory capacities and response to stress in p300/CBP-associated factor (PCAF) histone acetylase knockout mice. Neuropsychopharmacology. 2008;33 doi: 10.1038/sj.npp.1301551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oliveira AM, Wood MA, McDonough CB, Abel T. Transgenic mice expressing an inhibitory truncated form of p300 exhibit long-term memory deficits. Learn Mem. 2007;14:564–572. doi: 10.1101/lm.656907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alarcón JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: A model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42:947–959. doi: 10.1016/j.neuron.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 40.Wood MA, Attner MA, Oliveira AM, Brindle PK, Abel T. A transcription factor-binding domain of the coactivator CBP is essential for long-term memory and the expression of specific target genes. Learn Mem. 2006;13:606–617. doi: 10.1101/lm.213906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duclot F, Jacquet C, Gongora C, Maurice T. Alteration of working memory but not in anxiety or stress response in p300/CBP associated factor (PCAF) histone acetylase knockout mice bred on a C57BL/6 background. Neurosci Lett. 2010;475:179–183. doi: 10.1016/j.neulet.2010.03.077. [DOI] [PubMed] [Google Scholar]
- 42.Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, Nieland TJ, Zhou Y, Wang X, Mazitschek R, Bradner JE, DePinho RA, Jaenisch R, Tsai LH. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McQuown SC, Barrett RM, Matheos DP, Post RJ, Rogge GA, Alenghat T, Mullican SE, Jones S, Rusche JR, Lazar MAW, M.A. HDAC3 is a critical negative regulator of long-term memory formation. J Neurosci. 2011;31:764–747. doi: 10.1523/JNEUROSCI.5052-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morris MJ, Mahgoub M, Na ES, Pranav H, Monteggia LM. Loss of histone deacetylase 2 improves working memory and accelerates extinction learning. J Neurosci. 2013;33:6401–6411. doi: 10.1523/JNEUROSCI.1001-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stefanko DP, Barrett RM, Ly AR, Reolon GK, Wood MA. Modulation of long-term memory for object recognition via HDAC inhibition. Proc Natl Acad Sci USA. 2009;106:9447–9452. doi: 10.1073/pnas.0903964106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haettig J, Stefanko DP, Multani ML, Figueroa DX, McQuown SC, Wood MA. HDAC inhibition modulates hippocampus-dependent long-term memory for object location in a CBP-dependent manner. Learn Mem. 2011;18:71–79. doi: 10.1101/lm.1986911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, Agis-Balboa RC, Cota P, Wittnam JL, Gogol-Doering A, Opitz L, Salinas-Riester G, Dettenhofer M, Kang H, Farinelli L, Chen W, Fischer A. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science. 2010;328:753–756. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- 48.Ricobaraza A, Cuadrado-Tejedor M, Perez-Mediavilla A, Frechilla D, Del Rio J, Garcia-Osta A. Phenylbutyrate ameliorates cognitive deficit and reduces tau pathology in an Alzheimer's disease mouse model. Neuropsychopharmacology. 2009;34:1721–1732. doi: 10.1038/npp.2008.229. [DOI] [PubMed] [Google Scholar]
- 49.Chatterjee S, Mizar P, Cassel R, Neidl R, Selvi BR, Mohankrishna DV, Vedamurthy BM, Schneider A, Bousiges O, Mathis C, Cassel JC, Eswaramoorthy M, Kundu TK, Boutillier AL. A novel activator of CBP/p300 acetyltransferases promotes neurogenesis and extends memory duration in adult mice. J Neurosci. 2013;33:10698–10712. doi: 10.1523/JNEUROSCI.5772-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller CA, Campbell SL, Sweatt JD. DNA methylation and histone acetylation work in concert to regulate memory formation and synaptic plasticity. Neurobiol Learn Mem. 2008;89:599–603. doi: 10.1016/j.nlm.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53:857–869. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 52.Mikaelsson MA, Miller CA. The path to epigenetic treatment of memory disorders. Neurobiol Learn Mem. 2011;96:13–18. doi: 10.1016/j.nlm.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 54.Sultan FA, Wang J, Tront J, Liebermann DA, Sweatt JD. Genetic deletion of Gadd45b, a regulator of active DNA demethylation, enhances long-term memory and synaptic plasticity. J Neurosci. 2012;32:17059–17066. doi: 10.1523/JNEUROSCI.1747-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levenson JM, Roth TL, Lubin FD, Miller CA, Huang I-C, Desai P, Malone LM, Sweatt JD. Evidence that DNA (Cytosine-5) methyltransferase regulates synaptic plasticity in the hippocampus. J Biol Chem. 2006;281:15763–15773. doi: 10.1074/jbc.M511767200. [DOI] [PubMed] [Google Scholar]
- 56.Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- 57.Frick KM. Estrogens and age-related memory decline in rodents: What have we learned and where do we go from here? Horm Behav. 2009;55:2–23. doi: 10.1016/j.yhbeh.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frick KM. Building a better hormone therapy?: How understanding the rapid effects of sex steroid hormones could lead to novel therapeutics for age-related memory decline. Behav Neurosci. 2012;126:29–53. doi: 10.1037/a0026660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: A critical review. Front Neuroendocrinol. 2008;29:88–113. doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 60.Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29:219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dumitriu D, Rapp PR, McEwen BS, Morrison JH. Estrogen and the aging brain: An elixir for the weary cortical network. Ann N Y Acad Sci. 2010;1204:104–112. doi: 10.1111/j.1749-6632.2010.05529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Srivastava DP, Waters EM, Mermelstein PG, Kramár EA, Shors TJ, Liu F. Rapid estrogen signaling in the brain: Implications for the fine-tuning of neuronal circuitry. J Neurosci. 2011;31:16056–16063. doi: 10.1523/JNEUROSCI.4097-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Daniel JM. Effects of oestrogen on cognition: What have we learned from basic research? J Neuroendocrinol. 2006;18:787–795. doi: 10.1111/j.1365-2826.2006.01471.x. [DOI] [PubMed] [Google Scholar]
- 64.McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endrocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 65.Ogiue-Ikeda M, Tanabe N, Mukai H, Hojo Y, Murakami G, Tsurugizawa T, Takata N, Kimoto Y, Kawato S. Rapid modulation of synaptic plasticity by estrogens as well as endocrine disrupters in hippocampal neurons. Brain Res Rev. 2008;57:363–375. doi: 10.1016/j.brainresrev.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 66.Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- 67.Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Galea LA, Wainwright SR, Roes MM, Duarte-Guterman P, Chow C, Hamson DK. Sex, hormones, and neurogenesis in the hippocampus: Hormonal modulation of neurogenesis and potential functional implications. J Neuroendocrinol. 2013 doi: 10.1111/jne.12070. [DOI] [PubMed] [Google Scholar]
- 69.Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17ß-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- 70.Gu Q, Moss RL. 17β-estradiol potentiates kainate-induced currents via activation of the cAMP cascade. J Neurosci. 1996;16:3620–3629. doi: 10.1523/JNEUROSCI.16-11-03620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kramár EA, Chen LY, Brandon NJ, Rex CS, Liu F, Gall CM, Lynch G. Cytoskeletal changes underlie estrogen's acute effects on synaptic transmission and plasticity. J Neurosci. 2009;29:12982–12993. doi: 10.1523/JNEUROSCI.3059-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Frick KM, Berger-Sweeney J. Spatial reference memory and neocortical neurochemistry vary with the estrous cycle in C57BL/6 mice. Behav Neurosci. 2001;115:229–237. doi: 10.1037/0735-7044.115.1.229. [DOI] [PubMed] [Google Scholar]
- 73.Pompili A, Tomaz C, Arnone B, Tavares MC, Gasbarri A. Working and reference memory across the estrous cycle of rat: a long-term study in gonadally intact females. Behav Brain Res. 2010;213:10–18. doi: 10.1016/j.bbr.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 74.Korol DL, Malin EL, Borden KA, Busby RA, Couper-Leo J. Shifts in preferred learning strategy across the estrous cycle in female rats. Horm Behav. 2004;45:330–338. doi: 10.1016/j.yhbeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 75.Frye CA. Estrus-associated decrements in a water maze task are limited to acquisition. Physiol Behav. 1995;57:5–14. doi: 10.1016/0031-9384(94)00197-d. [DOI] [PubMed] [Google Scholar]
- 76.Warren SG, Juraska JM. Spatial and non-spatial learning across the rat estrous cycle. Behav Neurosci. 1997;111:259–266. doi: 10.1037//0735-7044.111.2.259. [DOI] [PubMed] [Google Scholar]
- 77.Berry B, McMahan R, Gallagher M. Spatial learning and memory at defined points of the estrous cycle: Effects of performance on a hippocampal-dependent task. Behav Neurosci. 1997;111:267–274. doi: 10.1037//0735-7044.111.2.267. [DOI] [PubMed] [Google Scholar]
- 78.Sutcliffe JS, Marshall KM, Neill JC. Influence of gender on working and spatial memory in the novel object recognition task in the rat. Behav Brain Res. 2007;177:117–125. doi: 10.1016/j.bbr.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 79.Markham JA, Juraska JM. Social recognition memory: Influence of age, sex, and ovarian hormonal status. Physiol Behav. 2007;92:881–888. doi: 10.1016/j.physbeh.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gibbs RB. Estrogen therapy and cognition: A review of the cholinergic hypothesis. Endrocr Rev. 2010;31:224–253. doi: 10.1210/er.2009-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Choleris E, Clipperton-Allen AE, Phan A, Valsecchi P, Kavaliers M. Estrogenic involvement in social learning, social recognition and pathogen avoidance. Front Neuroendocrinol. 2012;33:140–159. doi: 10.1016/j.yfrne.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 83.Galea LAM, Wide JK, Paine TA, Holmes MM, Ormerod BK, Floresco SB. High levels of estradiol disrupt conditioned place preference learning, stimulus response learning and reference memory but have limited effects on working memory. Behav Brain Res. 2001;126:115–126. doi: 10.1016/s0166-4328(01)00255-8. [DOI] [PubMed] [Google Scholar]
- 84.Faivre EJ, Lange CA. Progesterone receptors upregulate Wnt-1 to induce epidermal growth factor receptor transactivation and c-Src-dependent sustained activation of Erk1/2 mitogen-activated protein kinase in breast cancer cells. Mol Cell Biol. 2007;27:466–480. doi: 10.1128/MCB.01539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jacome LF, Gautreaux C, Inagaki T, Mohan G, Alves S, Lubbers LS, Luine V. Estradiol and ERβ agonists enhance recognition memory, and DPN, an ERβ agonist, alters brain monoamines. Neurobiol Learn Mem. 2010;94:488–498. doi: 10.1016/j.nlm.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor beta knockout, mice enhances performance in the object recognition and object placement tasks. Neurobiol Learn Mem. 2008;89:513–521. doi: 10.1016/j.nlm.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal ERK activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lewis MC, Kerr KM, Orr PT, Frick KM. Estradiol-induced enhancement of object memory consolidation involves NMDA receptors and protein kinase A in the dorsal hippocampus of female C57BL/6 mice. Behav Neurosci. 2008;122:716–721. doi: 10.1037/0735-7044.122.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fan L, Zhao Z, Orr PT, Chambers CH, Lewis MC, Frick KM. Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. J Neurosci. 2010;30:4390–4400. doi: 10.1523/JNEUROSCI.4333-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fortress AM, Fan L, Orr PT, Zhao Z, Frick KM. Estradiol-induced object recognition memory consolidation is dependent on activation on mTOR signaling in the dorsal hippocampus. Learn Mem. 2013;20:147–155. doi: 10.1101/lm.026732.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Luine VN, Jacome LF, MacLusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- 92.Gresack JE, Frick KM. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol Biochem Behav. 2006;84:112–119. doi: 10.1016/j.pbb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 93.Packard MG, Teather LA. Posttraining estradiol injections enhance memory in ovariectomized rats: Cholinergic blockade and synergism. Neurobiol Learn Mem. 1997;68:172–188. doi: 10.1006/nlme.1997.3785. [DOI] [PubMed] [Google Scholar]
- 94.Packard MG, Teather LA. Intra-hippocampal estradiol infusion enhances memory in ovariectomized rats. NeuroReport. 1997;8:3009–3013. doi: 10.1097/00001756-199709290-00004. [DOI] [PubMed] [Google Scholar]
- 95.Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- 96.Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- 97.Waters EM, Yildirim M, Janssen WG, Lou WY, McEwen BS, Morrison JH, Milner TA. Estrogen and aging affect the synaptic distribution of estrogen receptor beta-immunoreactivity in the CA1 region of female rat hippocampus. Brain Res. 2011;1379:86–97. doi: 10.1016/j.brainres.2010.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Spencer TE, Jenster G, Burcin MM, Allis CD, Zhou J, Mizzen CA, McKenna NJ, Onate SA, Tsai SY, Tsai MJ, O'Malley BW. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature. 1997;389:194–198. doi: 10.1038/38304. [DOI] [PubMed] [Google Scholar]
- 99.Blanco JC, Minucci S, Lu J, Yang XJ, Walker KK, Chen H, Evans RM, Nakatani Y, Ozato K. The histone acetylase PCAF is a nuclear receptor coactivator. Genes Dev. 1998;12:1638–1651. doi: 10.1101/gad.12.11.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kishimoto M, Fujiki R, Takezawa S, Sasaki Y, Nakamura T, Yamaoka K, Kitagawa H, Kato S. Nuclear receptor mediated gene regulation through chromatin remodeling and histone modifications. Endocr J. 2006;53:157–172. doi: 10.1507/endocrj.53.157. [DOI] [PubMed] [Google Scholar]
- 101.Sheldahl LC, Shapiro RA, Bryant DN, Koerner IP, Dorsa DM. Estrogen induced rapid translocation of estrogen receptor β, but not estrogen receptor α, to the neuronal plasma membrane. Neuroscience. 2008;153:751–761. doi: 10.1016/j.neuroscience.2008.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: Studies of ERα and ERβ expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 103.Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J Neurosci. 2007;27:9941–9950. doi: 10.1523/JNEUROSCI.1647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Boulware MI, Heisler JD, Frick KM. The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. J Neurosci. doi: 10.1523/JNEUROSCI.1716-13.2013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu TW, Chen S, Brinton RD. Membrane estrogen receptors mediate calcium signaling and MAP kinase activation in individual hippocampal neurons. Brain Res. 2011;1379:34–43. doi: 10.1016/j.brainres.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carrer HF, Araque A, Buño W. Estradiol regulates the slow Ca2+-activated K+ current in hippocampal pyramidal neurons. J Neurosci. 2003;23:6338–6344. doi: 10.1523/JNEUROSCI.23-15-06338.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hammond R, Gibbs RB. GPR30 is positioned to mediate estrogen effects on basal forebrain cholinergic neurons and cognitive performance. Brain Res. 2011;1379:53–60. doi: 10.1016/j.brainres.2010.11.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Micevych P, Christensen A. Membrane-initiated estradiol actions mediate structural plasticity and reproduction. Front Neuroendocrinol. 2012;33:331–341. doi: 10.1016/j.yfrne.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bi R, Foy MR, Vouimba R-M, Thompson RF, Baudry M. Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proc Natl Acad Sci USA. 2001;98:13391–13395. doi: 10.1073/pnas.241507698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Manella P, Brinton RD. Estrogen receptor protein interaction with phosphatidylinositol 3-kinase leads to activation of phosphorylated Akt and extracellular signal-regulated kinase 1/2 in the same population of cortical neurons: A unified mechanism of estrogen action. J Neurosci. 2006;26:9437–9447. doi: 10.1523/JNEUROSCI.1443-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wade CB, Dorsa DM. Estrogen activation of cyclic adenosine 5'-monophosphate response element-mediated transcription requires the extracellularly regulated kinase/mitogen-activated protein kinase pathway. Endocrinology. 2003;144:832–838. doi: 10.1210/en.2002-220899. [DOI] [PubMed] [Google Scholar]
- 113.Yokomaku D, Numakawa T, Numakawa Y, Suzuki S, Matsumoto T, Adachi N, Nishio C, Taguchi T, Hatanaka H. Estrogen enhances depolarization-induced glutamate release through activation of phosphatidylinositol 3-kinase and mitogen-activated protein kinase in cultured hippocampal neurons. Mol Endocrinol. 2003;17:831–844. doi: 10.1210/me.2002-0314. [DOI] [PubMed] [Google Scholar]
- 114.Zhao L, Brinton RD. Estrogen receptor α and β differentially regulate intracellular Ca2+ dynamics leading to ERK phosphorylation and estrogen neuroprotection in hippocampal neurons. Brain Res. 2007;1172:48–59. doi: 10.1016/j.brainres.2007.06.092. [DOI] [PubMed] [Google Scholar]
- 115.Adams JP, Sweatt JD. Molecular psychology: Roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol. 2002;42:135–163. doi: 10.1146/annurev.pharmtox.42.082701.145401. [DOI] [PubMed] [Google Scholar]
- 116.Kelly A, Laroche S, Davis S. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. J Neurosci. 2003;12:5354–5360. doi: 10.1523/JNEUROSCI.23-12-05354.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Frick KM, Gresack JE. Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behav Neurosci. 2003;117:1283–1291. doi: 10.1037/0735-7044.117.6.1283. [DOI] [PubMed] [Google Scholar]
- 118.Gresack JE, Kerr KM, Frick KM. Life-long environmental enrichment differentially affects the mnemonic response to estrogen in young, middle-aged, and aged female mice. Neurobiol Learn Mem. 2007;88:393–408. doi: 10.1016/j.nlm.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Balasubramanyam K, Altaf M, Varier RA, Swaminathan V, Ravindran A, Sadhale PP, Kundu TK. Polyisoprenylated benzophenone, garcinol, a natural histone acetyltransferase inhibitor, represses chromatin transcription and alters global gene expression. J Biol Chem. 2004;279:33716–33726. doi: 10.1074/jbc.M402839200. [DOI] [PubMed] [Google Scholar]
- 120.Mantelingu K, Reddy BA, Swaminathan V, Kishore AH, Siddappa NB, Kumar GV, Nagashankar G, Natesh N, Roy S, Sadhale PPR,U, Narayana C, Kundu TK. Specific inhibition of p300-HAT alters global gene expression and represses HIV replication. Chem Biol. 2007;14:645–657. doi: 10.1016/j.chembiol.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 121.Yaffe K, Barnes D, Lindquist K, Cauley J, Simonsick EM, Penninx B, Satterfield S, Harris T, Cummings SR. Endogenous sex hormone levels and risk of cognitive decline in an older biracial cohort. Neurobiol Aging. 2007;28:171–178. doi: 10.1016/j.neurobiolaging.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 122.Duff SJ, Hampson E. A beneficial effect of estrogen on working memory in postmenopausal women taking hormone replacement therapy. Horm Behav. 2000;38:262–276. doi: 10.1006/hbeh.2000.1625. [DOI] [PubMed] [Google Scholar]
- 123.Sherwin BB. Estrogen and cognitive functioning in women: Lessons we have learned. Behav Neurosci. 2012;126:123–127. doi: 10.1037/a0025539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147:607–614. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- 125.Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging. 2000;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- 126.Markowska AL, Savonenko AV. Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. J Neurosci. 2002;22:10985–10995. doi: 10.1523/JNEUROSCI.22-24-10985.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Heikkinen T, Puoliväli J, Tanila H. Effects of long-term ovariectomy and estrogen treatment on maze learning in aged mice. Experimental Gerontology. 2004;39:1277–1283. doi: 10.1016/j.exger.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 128.Smith CC, Vedder LC, Nelson AR, Bredemann TM, McMahon LL. Duration of estrogen deprivation, not chronological age, prevents estrogen's ability to enhance hippocampal synaptic physiology. Proc Natl Acad Sci USA. 2010;107:19543–19548. doi: 10.1073/pnas.1009307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bohacek J, Bearl AM, Daniel JM. Long-term ovarian hormone deprivation alters the ability of subsequent oestradiol replacement to regulate choline acetyltransferase protein levels in hippocampus and prefrontal cortex of middle-aged rats. J Neuroendocrinol. 2008;20:1023–1027. doi: 10.1111/j.1365-2826.2008.01752.x. [DOI] [PubMed] [Google Scholar]
- 130.Bohacek J, Daniel JM. The ability of oestradiol administration to regulate protein levels of oestrogen receptor alpha in the hippocampus and prefrontal cortex of middle-aged rats is altered following long-term ovarian hormone deprivation. J Neuroendocrinol. 2009;21:640–647. doi: 10.1111/j.1365-2826.2009.01882.x. [DOI] [PubMed] [Google Scholar]
- 131.Yamaguchi-Shima N, Yuri K. Age-related changes in the expression of ER-β mRNA in the female rat brain. Brain Res. 2007;1155:34–41. doi: 10.1016/j.brainres.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 132.Adams MM, Fink SE, Shah RA, Janssen WG, Hayashi S, Milner TA, McEwen BS, Morrison JH. Estrogen and aging affect the subcellular distribution of estrogen receptor-α in the hippocampus of female rats. J Neurosci. 2002;22:3608–3614. doi: 10.1523/JNEUROSCI.22-09-03608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang QG, Han D, Wang RM, Dong Y, Yang F, Vadlamudi RK, Brann DW. C terminus of Hsc70-interacting protein (CHIP)-mediated degradation of hippocampal estrogen receptor-alpha and the critical period hypothesis of estrogen neuroprotection. Proc Natl Acad Sci USA. 2011;108:E617–624. doi: 10.1073/pnas.1104391108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Westberry JM, Wilson ME. Regulation of estrogen receptor alpha gene expression in the mouse prefrontal cortex during early postnatal development. Neurogenetics. 2012;13:159–167. doi: 10.1007/s10048-012-0323-z. [DOI] [PubMed] [Google Scholar]
- 135.Westberry JM, Trout AL, Wilson ME. Epigenetic regulation of estrogen receptor beta expression in the rat cortex during aging. NeuroReport. 2011;22:428–432. doi: 10.1097/WNR.0b013e328346e1cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Murray EK, Hien A, de Vries GJ, Forger NG. Epigenetic control of sexual differentiation of the bed nucleus of the stria terminalis. Endocrinology. 2009;150:4241–4247. doi: 10.1210/en.2009-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tsai HW, Grant PA, Rissman EF. Sex differences in histone modifications in the neonatal mouse brain. Epigenetics. 2009;4:47–53. doi: 10.4161/epi.4.1.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McCarthy MM, Auger AP, Bale TL, De Vries GJ, Dunn GA, Forger NG, Murray EK, Nugent BM, Schwarz JM, Wilson ME. The epigenetics of sex differences in the brain. J Neurosci. 2009;29:12815–12823. doi: 10.1523/JNEUROSCI.3331-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kerimoglu C, Agis-Balboa RC, Kranz A, Stilling R, Bahari-Javan S, Benito-Garagorri E, Halder R, Burkhardt S, Stewart AF, Fischer A. Histone-Methyltransferase MLL2 (KMT2B) is required for memory formation in mice. J Neurosci. 2013;33:3452–3464. doi: 10.1523/JNEUROSCI.3356-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]