Abstract
PURPOSE
Cognitive impairment after critical illness is common and debilitating. We developed a cognitive therapy program for critically ill patients and assessed the feasibility and safety of administering combined cognitive and physical therapy early during a critical illness.
METHODS
We randomized 87 medical and surgical ICU patients with respiratory failure and/or shock in a 1:1:2 manner to three groups: usual care, early once-daily physical therapy, or early once-daily physical therapy plus a novel, progressive, twice-daily cognitive therapy protocol. Cognitive therapy included orientation, memory, attention, and problem solving exercises, and other activities. We assessed feasibility outcomes of the early cognitive plus physical therapy intervention. At 3-months, we also assessed cognitive, functional and health-related quality of life outcomes. Data are presented as median [interquartile range] or frequency (%).
RESULTS
Early cognitive therapy was a delivered to 41/43 (95%) of cognitive plus physical therapy patients on 100% [92–100%] of study days beginning 1.0 [1.0–1.0] day following enrollment. Physical therapy was received by 17/22 (77%) of usual care patients, by 21/22 (95%) of physical therapy only patients and 42/43 (98%) of cognitive plus physical therapy patients on 17% [10–26%], 67% [46–87%] and 75% [59–88%] of study days, respectively. Cognitive, functional and health-related quality of life outcomes did not differ between groups at 3-month follow-up.
CONCLUSIONS
This pilot study demonstrates that early rehabilitation can be extended beyond physical therapy to include cognitive therapy. Future work to determine optimal patient selection, intensity of treatment and benefits of cognitive therapy in the critically ill is needed.
Keywords: Delirium, cognitive impairment, brain injury, critical illness, rehabilitation, early ambulation, barriers
INTRODUCTION
Each year, millions who survive critical illness are left with newly acquired cognitive impairment and/or functional disability [1–4]. To date, interventional trials have focused on early physical and occupational therapy with a higher proportion of patients maintaining baseline functional status, a shortening of delirium duration and a decreased risk of death or rehospitalization [5–7]. Following discharge from the intensive care unit (ICU) combined cognitive and physical therapy was associated with improved executive functioning (e.g., problem solving, carrying out multi-step tasks) [8]. Cognitive therapy protocols that can be administered during the earliest stages of a critical illness have not been developed and the feasibility of initiating cognitive therapy early during critical illness has not previously been studied.
We hypothesized that combined early cognitive and physical/occupational therapy in conjunction with outpatient cognitive therapy would be feasible and safe to deliver and would establish methods for ICU rehabilitation extending beyond conventional early physical/occupational therapy. Therefore, we first developed a cognitive therapy protocol that could be implemented early during a critical illness. We then conducted a randomized controlled trial to establish the feasibility and safety of combining early cognitive therapy and early physical/occupational therapy with prolonged (up to 12 weeks) outpatient cognitive therapy in critically ill adult medical and surgical patients. The study protocol has been previously described [9].
METHODS
Setting and Participants
We recruited study participants from the Medical and Surgical Intensive Care Units (MICU and SICU, respectively) at the Vanderbilt University Medical Center (Nashville, TN). The institutional review board at Vanderbilt University approved the study and the trial was registered with clinicaltrials.gov (NCT01270269). Prior to any study interventions, written consent was obtained from patients or their authorized surrogates (with re-consent obtained, when indicated, once patients were cognitively able).
Eligibility Criteria
We screened the MICU and SICU censuses daily to identify adult patients (≥18 years old) being treated for respiratory failure and/or septic, cardiogenic or hemorrhagic shock who resided within 120 miles of Nashville, TN. We excluded patients who had already been critically ill for >72 hours since the opportunity to administer early cognitive and physical therapy had passed, those who had been in the ICU for more than 5 days in the previous 30 days, those were unlikely to benefit from rehabilitation targeting acute declines in cognitive or functional status due to a moribund state, severe pre-existing dementia or physical disability in activities of daily living (e.g., bathing, dressing, etc.), or were unlikely to continue the intervention in the outpatient setting due to active substance abuse, active psychiatric disorder or homelessness. After obtaining informed consent, but prior to randomization, patients or their surrogates completed the Short Form of the Informant Questionnaire of Cognitive Decline in the Elderly (IQCODE) [10] and the Katz Activities of Daily Living (ADL) questionnaire [11], respectively, to detect pre-existing dementia and physical disability not evident during the initial screening process. These questionnaires demonstrate good agreement when completed by either the patient or surrogates [12, 13]. We excluded those scoring >3.3 on the IQCODE or >3 on the Katz ADL.
Study Procedures
We used a computer-generated permuted-block randomization scheme to determine intervention group allocation, which was printed on tri-folded forms placed in consecutively numbered, sealed, opaque envelopes that were not opened until after informed consent was obtained and inclusion and exclusion criteria were verified. We assigned patients in a 1:1:2 manner to one of three groups: usual care, early physical therapy only or early cognitive plus physical therapy.
Each day, all mechanically ventilated patients were managed with coordinated spontaneous awakening and spontaneous breathing trials [14]. Twice daily, we assessed each patient’s level of consciousness using the Richmond Agitation-Sedation Scale (RASS) [15] and delirium status using the Confusion Assessment Method for the ICU (CAM-ICU) [16].
Usual care patients received physical therapy after it was ordered by the treating clinicians and per the routine hospital treatment protocol and schedule (typically 1–2 sessions per week). No cognitive therapy interventions were performed as part of usual care. As described in detail previously (and in the supplementary appendix), patients in the early physical therapy only group underwent a once-daily physical therapy session, and patients in the cognitive plus physical therapy group received twice-daily 20-minute cognitive therapy sessions along with a once-daily physical therapy session [9].
Inpatient Cognitive Therapy
An interdisciplinary team of critical care physicians, nurses and neuropsychologists developed the in-hospital cognitive therapy protocol by compiling a series of exercises we deemed to be both cognitively challenging and diverse in nature. These exercises focused on orientation, memory, attention and problem solving (see Figure 1) [9]. The cognitive therapy protocol was guided by the patient’s RASS assessment immediately preceding the session and advanced patients through a progressively more difficult series of exercises delivered by the interdisciplinary study team that included physicians and nurses.
Figure 1.
Inpatient intervention protocols began within 24 hours of study enrollment and continued until hospital discharge for the cognitive therapy intervention or until the patient had independently ambulated more than 200 feet and required no assistance in performing activities of daily living (ADLs) on two consecutive study days for the physical/occupational therapy intervention. The study protocol has been described in detail previously.[9] Briefly, cognitive therapy was delivered in 20-minute sessions, twice each day. We chose exercises that targeted neurocognitive domains commonly impaired in survivors of critical illness including orientation (e.g., orientation exercises), memory/attention (e.g., digit span forward, digit span reverse, noun recall, paragraph recall and letter-number sequences), delayed memory (e.g., digit span reverse), and problem solving/processing speed (e.g., matrix puzzles, ‘real world’ exercises and pattern recognition). Physical therapy was delivered in a single session each day. RASS, Richmond Agitation-Sedation Scale; ROM, Range of Motion; ADL, Activities of Daily Living
Inpatient Physical and Occupational Therapy
Physical and occupational therapy interventions, modeled after those previously described in the literature [6, 7, 17] and the existing ICU mobility protocol at our institution (but with an emphasis on early and more frequent delivery), advanced patients from passive range of motion exercises to independent ambulation, guided by the patient’s RASS and were titrated to allow patients to reach their ‘maximal functional milestone’ as rapidly as possible (see Figure 1) under the guidance of physical and occupational therapists [9].
Outpatient Cognitive Therapy
Patients randomized to cognitive plus physical therapy during the hospitalization who demonstrated either impaired executive functioning or impaired functional mobility at the time of hospital discharge were instructed they would continue cognitive therapy on an outpatient basis in the form of a 12-week, 6-session, in-home, cognitive therapy program using Goal Management Training (GMT)[18–20] GMT is an established, protocolized cognitive rehabilitation program that specifically targets executive functioning, a domain of significant importance for independent functioning [8, 18–21]. Patients who were discharged to a nursing home or rehabilitation facility received GMT once they returned home, with ‘make-up’ sessions performed to complete as many of the six in-home GMT sessions as possible within the 12-week follow-up period.
Feasibility Outcome Assessment
The primary aim of this study was to establish the feasibility of delivering cognitive therapy early during critical illness. Therefore, we recorded data on the number of patients to whom the cognitive therapy protocol was delivered, the number of days of cognitive therapy performed, the timing of initiation of cognitive therapy, exercises performed during each cognitive therapy session and the duration of each session.
Secondary feasibility outcomes were the number of patients who received physical therapy, the number of days of physical therapy received, timing of initiation of physical therapy, exercises performed during each physical therapy session and duration of these sessions. Finally, we also recorded the number of outpatient GMT sessions performed by eligible patients who were randomized to the cognitive plus physical therapy group.
Follow-up Outcomes Assessment
While the main purpose of the study was to gather feasibility data, researchers blinded to all study interventions and details regarding the patient’s hospital course collected data on cognitive, functional and health-related quality of life (HRQOL) outcome measures at 3 months following hospital discharge. Patients were instructed not to disclose details regarding study interventions they had received.
Additionally, we collected secondary clinical outcomes data including days free of delirium and coma, days free of mechanical ventilation, ICU and hospital lengths of stay and mortality. Finally, we assessed executive functioning, global cognitive status and functional mobility at the time of hospital discharge.
Data and Study Management
We collected all study data using an electronic data capture tool hosted at Vanderbilt University (REDCap, project-redcap.org) [22]. An independent monitoring committee reviewed safety data following the enrollment of 30 and 60 patients, respectively.
Statistical Analyses
We enrolled participants until 60 had been discharged from the hospital. This sample size was chosen based on our overarching objective of obtaining feasibility data for both the inpatient and outpatient interventions as well as the resource availability of the research team. Thus, the study was not powered to allow determination of the efficacy of the interventions. Nevertheless, we collected follow-up outcome data to inform future early cognitive rehabilitation trials. We chose the total achievement score on the Tower Test from the Delis-Kaplan Executive Function System [23], at 3-month follow-up as the primary follow-up outcome. Secondary follow-up outcomes included results from a battery of cognitive, functional and HRQOL outcomes assessed at 3-month follow-up.
We analyzed all data with an intention-to-treat approach. Feasibility outcomes were analyzed using descriptive statistics. Follow-up outcomes data were analyzed using the Kruskal-Wallis test or the Pearson chi-square test to compare groups with respect to cognitive, functional and HRQOL outcomes. Analysis of ICU length of stay, survival and other time-to-event outcomes were performed using the Kaplan-Meier product limit method and log-rank testing. We used R (version 2.13, www.r-project.org) for all statistical analyses. Data are presented as median [interquartile range] unless otherwise noted.
RESULTS
Between February 2011 and April 2012, 931 patients met inclusion criteria, and 844 met at least one exclusion criteria (Figure 2). We therefore randomized a total of 87 patients: 22 to usual care, 22 to physical therapy only and 43 to cognitive plus physical therapy.
Figure 2.
Screening, randomization, and participant follow-up
a Unable to physically complete any follow-up testing (i.e., could not move discs for the Tower Test or hold a pencil to complete MMSE testing)
b CAM-ICU positive on the day of hospital discharge
ICU = intensive care unit
At baseline, the three treatment groups were similar with regard to age, cognitive status (i.e., IQCODE score), functional status (i.e., ADL and IADL status) and admission diagnoses (Table 1). The usual care group included more female patients than the other treatment groups, and more patients were enrolled from the MICU in the physical therapy only group.
Table 1.
Baseline Demographics of Study Population
| Characteristica | Usual Care (n=22) | Physical Therapy (n=22) | Cognitive + Physical Therapy (n=43) |
|---|---|---|---|
| Age (years) | 60 [51–69] | 62 [48–67] | 62 [54–69] |
| Sex (% female) | 64% (14) | 41% (9) | 35% (15) |
| Race (% white) | 86% | 95% | 93% |
| Education Level (years) | 14 [12–14] | 12 [11–14] | 12 [12–14] |
| APACHE II b | 27.0 [17.5–31.0] | 21.5 [20.0–28.8] | 25 [19.5–29.5] |
| SOFA b | 10.0 [8.0–11.8] | 8.0 [6.0–10.0] | 9.0 [6.0–11.0] |
| Mechanically Ventilated at enrollment | 100% (22) | 78% (17) | 77% (33) |
| ICU Type (% Medical ICU) | 55% (12) | 86% (19) | 51% (22) |
| Diagnosis at ICU Admission | |||
| Sepsis/ARDS/Pneumonia | 50% (11) | 55% (12) | 67% (29) |
| Abdominal Surgery | 23% (5) | 5% (1) | 16% (7) |
| Other Surgery | 0% (0) | 0% (0) | 7% (3) |
| Airway Protection | 14% (3) | 23% (5) | 0% (0) |
| Cirrhosis/GI bleeding | 0% (0) | 5% (1) | 7% (3) |
| CHF/Arrhythmia/Cardiogenic Shock | 9% (2) | 0% (0) | 0% (0) |
| Other | 5% (1) | 14% (3) | 2% (1) |
| IQCODE at Enrollment c | 3.0 [3.0–3.1] | 3.0 [3.0–3.2] | 3.0 [3.0–3.1] |
| Katz ADL at Enrollment d | 0 [0-0] | 0 [0-0] | 0 [0-0] |
APACHE II, Acute Physiologic and Chronic Health Evaluation, version II; SOFA, Sequential Organ Failure Assessment; IQCODE, Informant Questionnaire on Cognitive Decline in the Elderly; ADL, Activities of Daily Living
Data are median [interquartile range] for continuous values and % (number) for categorical values.
Calculated from data collected around 24-hours of ICU admission
Screening tool for cognitive impairment. Patients with an IQCODE score >3.3 were excluded from participation.
Screening tool for physical disability. Patients with a Katz ADL score > 3 were excluded from participation.
Feasibility of Cognitive Therapy Protocol
Of the 43 patients randomized to the cognitive plus physical therapy group, 41 (95%) received cognitive therapy on at least one study day. One patient in this group died prior to receiving any intervention and one refused participation in cognitive therapy exercises.
A majority of patients received the cognitive therapy protocol on all days while mechanically ventilated, in the ICU, and in the hospital, respectively (Table 2). The first cognitive therapy session was delivered 1.0 [1.0–1.0] day after study enrollment and 3.0 [2.0–4.0] days after ICU admission.
Table 2.
Feasibility Outcomes
| Usual Care (n=22) | Physical Therapy (n=22) | Cognitive + Physical Therapy (n=43) | |
|---|---|---|---|
| Received Cognitive Therapy, n/N(%) a,b | |||
| during mechanical ventilation | 32/33 (97%) | ||
| during ICU stay | 39/41 (95%) | ||
| during hospitalization | 41/43 (95%) | ||
|
| |||
| Median percent [IQR] of study days where cognitive therapy was received a,c | |||
| during mechanical ventilation | 100% [100–100%] | ||
| during ICU stay | 100% [100–100%] | ||
| during hospitalization | 100% [92–100%] | ||
|
| |||
| Received Physical Therapy, n/N (%) | |||
| during mechanical ventilation | 5/17 (29%) | 16/17 (94%) | 33/33 (100%) |
| during ICU stay | 10/21 (48%) | 18/19 (95%) | 40/41 (98%) |
| during hospitalization | 17/22 (77%) | 21/22 (95%) | 42/43 (98%) |
|
| |||
| Median percent [IQR] of study days where physical therapy was received | |||
| during mechanical ventilation | 0% [0–13%] | 71% [67–100%] | 100% [86–100%] |
| during ICU stay | 0% [0–20%] | 96% [63–100%] | 89% [60–100%] |
| during hospitalization | 17% [10–26%] | 67% [46–87%] | 75% [59–88%] |
only patients in the Cognitive + Physical Therapy group received cognitive therapy
Data indicate the number of patients in each clinical status with ≥1 study day alert enough to receive study interventions. For example, only 33 patients randomized to the Cognitive + Physical Therapy group were mechanically ventilated, all received physical therapy but only 32 received cognitive therapy
Data indicate days where the patient was eligible to receive cognitive therapy protocol (i.e., RASS -3 or more alert for the cognitive therapy protocol)
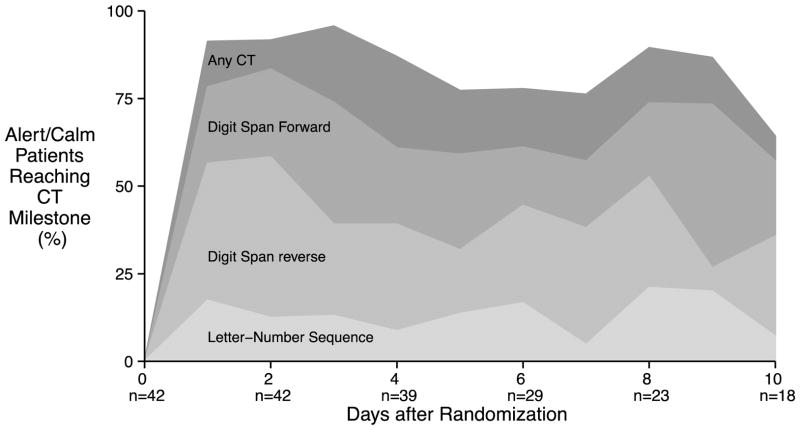
We chose three exercises—digit span forward, digit span backwards, and letter-number sequences—representing the second, fifth and eight exercises in the protocol to illustrate how patients progressed through the cognitive therapy protocol. At least once during the study, 34/43 (79%) patients correctly performed at least one forward digit span (i.e., repeated four digits in the forward direction), 30/43 (70%) correctly performed at least one reverse digit span (i.e., repeated three digits in the reverse direction) and 19/43 (44%) correctly performed at least one letter-number sequence (i.e., repeated a string of two letters and numbers arranged in numerical then alphabetical order). These milestones were achieved 1.5 [1.0–3.0] day, 3.0 [1.2–6.8] days and 5.0 [3.0–9.0] days after enrollment, respectively, and while they represented significant progress for our patients, they are regularly achieved by healthy, non-critically ill persons.[24] The proportion of eligible patients (i.e., those alive, in the hospital and RASS −1 to +1) who achieved each of these milestones on each of the first 10 days of the study is presented in Figure 3. Patients performed 20 [20–20] minutes of cognitive therapy during each of the twice-daily sessions.
Figure 3.
Proportion of hospitalized, alert (RASS −1 to +1) patients meeting cognitive therapy milestones on each of the first 10 days of the study. Each of the shaded areas represent the proportion of patients who performed at least one problem correctly for each milestone exercise on a given study day
Feasibility of the Physical and Occupational Therapy Protocol
Though a large majority of patients across all three groups received physical therapy at least once during their hospitalization, physical therapy was delivered much less often to patients in the usual care group (Table 2). Usual care patients received physical therapy on roughly one out of every six days. Conversely, patients in both intervention groups underwent physical therapy on almost all days while mechanically ventilated, in the ICU and overall during the study (Table 2). The first physical therapy session was received 3.0 [2.0–6.0] days after study enrollment in usual care group, 1.0 [1.0–1.0] day after study enrollment in the physical therapy only group and 1.0 [1.0–1.8] after study enrollment in the cognitive plus physical therapy group.
Patients in both intervention groups (physical therapy only and cognitive plus physical therapy) routinely progressed through the physical therapy protocol (e.g., sat at the edge of the bed, stood and ambulated; see Supplementary Appendix for time to achievement of these milestones).
During hospitalization, four patients in the cognitive plus physical therapy group withdrew. Out of the 28 surviving patients in this group who remained enrolled in the study, 26 (82%) were cognitive impaired or physically disabled at the time of hospital discharge and, therefore, qualified for in-home cognitive therapy (GMT). During the 12-week outpatient phase, five additional patients died and three more withdrew. Thus, 18 patients in the cognitive plus physical therapy group survived and remained in the study during the full 12-week follow-up period. Of these patients, 17 (94%) received at least one GMT session, with one patient unable to receive GMT due to a prolonged (>12 weeks) stay at a rehabilitation facility. Overall, patients received 6 [2–6] out of the possible six in-home GMT sessions.
Tolerability and Safety of Cognitive and Physical Therapy Interventions
Patients completed 613/785 (78%) of possible cognitive therapy sessions. Of the sessions that were not completed, most were due to patient or family refusal and no cognitive therapy session was stopped for patient safety concerns (see Table S3). Of the seven patients who withdrew from the study, all were randomized to cognitive plus physical therapy. No unifying reason for withdraws, or a specific time point during which withdrawal from the study occurred, was present (see Table S4).
Combined, patients in the physical therapy only and cognitive plus physical therapy groups completed 543/651 (83%) of the possible physical therapy sessions. As with the cognitive therapy sessions, the most common reason for not completing a physical therapy session was patient or family refusal. Only 21/543 (4%) of the physical therapy sessions were halted for safety concerns such as hypoxemia or tachycardia. No inadvertent removal of endotracheal tubes or vascular catheters occurred during the physical therapy sessions. One adverse event (acute back pain accompanied by hypertensive urgency) occurred during a physical therapy session, but did not preclude participation in subsequent study interventions.
Follow-up Outcomes
At 3-month follow-up, 44 (82%) of the 54 living patients were assessed, including 12 of (92%) 13 in the usual care group, 14 of (93%) 15 in the physical therapy only group, and 18 of (69%) 26 in the cognitive plus physical therapy group. Follow-up occurred less frequently in the cognitive plus physical therapy group because of withdrawals.
At the 3-month follow-up, the total achievement score on the Tower Test, the primary follow-up outcome, was not different between groups (p=0.20, Table 3). Similarly, secondary follow-up outcome measures of executive functioning, global cognition, functional mobility, ADL status, IADL status and HRQOL status did not differ between the three groups at 3-month follow-up (Table 3).
Table 3.
3-Month Follow-up Outcomes
| Usual Care (n=12) | Physical Therapy (n=14) | Cognitive + Physical Therapy (n=18) | P | |
|---|---|---|---|---|
| Primary Follow-up Outcome | ||||
| Tower Test (Executive Functioning)a | 10.0 [8.8–12.2] | 11.0 [11.0–12.0] | 10.0 [8.0–11.0] | .20 |
| Secondary Follow-up Outcomes | ||||
| DEX (Executive Functioning)b | 17.5 [8.5–28.8] | 10.0 [5.0–17.0] | 9.0 [2.0–17.5] | .08 |
| MMSE (Global Cognition)c | 28.0 [26.8–29.0] | 29.0 [27.0–30.0] | 29.0 [27.9–29.8] | .64 |
| TUG (Functional Mobility)d | 8.0 [7.5–13.5] | 10.0 [8.0–13.0] | 11.0 [9.0–13.0] | .79 |
| Katz ADL (Activities of Daily Living)e | 0 [0–0] | 0 [0–1] | 0 [0–2] | .69 |
| FAQ (Instrumental Activities of Daily Living)f | 2.5 [0.8–5.5] | 2.0 [0.0–4.8] | 1.0 [0.0–3.8] | .67 |
| EQ-5D VAS (Health Related Quality of Life)g | 75 [61–86] | 80 [62–89] | 75 [54–80] | .44 |
DEX, Dysexecutive Questionnaire [35]; MMSE, Mini-mental State Exam [36]; TUG, Timed Up-and-Go Test [37]; ADL, Activities of Daily Living [11]; FAQ, Functional Activities Questionnaire [38]; EQ-5D, European Quality of Life-5 Dimensions Visual Analog Scale [39].
Tower achievement score ‘normal’ range is 7–13. Higher scores indicate better functioning
Dysexecutive questionnaire scores range from 0 to 80. Lower scores indicate better functioning
Mini-mental state scores range from 0–30. Lower scores indicating worse functioning
TUG time in seconds. Less than 10 seconds is normal, longer than 20 seconds is indicative of impaired functional mobility and longer than 30 seconds indicates disability. Two patients were unable to complete the TUG secondary to physical weakness and another refused to complete this portion of the outcomes testing
Katz ADL scores range from 0–12, with lower scores indicating better functioning.
FAQ scores range from 0–30, with lower scores indicating better functioning
EQ-5D visual analog scale score, where 0 indicates the “worst imaginable health state” and 100 indicates “best imaginable health state”
Secondary in-hospital clinical outcomes, including delirium/coma-free days, ventilator-free days, ICU and hospital lengths of stay, mortality, and cognitive or functional outcomes assessed at hospital discharge did not differ between groups (see Table S5, Table S6 and Table S7).
DISCUSSION
Our results demonstrate that administration of a combined interdisciplinary cognitive and physical therapy intervention beginning during the early stages of a critical illness is feasible and safe. Nearly all patients were able to undergo cognitive and physical therapy, according to randomization treatment group assignment, beginning within 72 hours of ICU admission. Patients were able to undergo cognitive therapy on nearly every study day including while on mechanical ventilation, while critically ill in the ICU, while on the hospital ward following ICU discharge and after returning home. Likewise, physical therapy was administered on a majority of study days, regardless of clinical status. Among 65 intervention patients for a total of 1156 cognitive and physical therapy sessions, we noted no events that required cessation of cognitive therapy sessions and less than 4% of physical therapy sessions required early termination for pre-defined safety criteria.
Our study’s overarching aim was to establish the proof-of-concept that combined cognitive and physical therapy for critically ill patients could be administered. Because our goal was to evaluate feasibility and safety, our study was not powered to detect meaningful changes in follow-up outcomes. A post-hoc power analysis revealed that we had only 34% power to detect a clinically meaningful 1.5-point change (e.g., one-half standard deviation) in the Tower Test score at 3-month follow-up. Thus the effects of our rehabilitation interventions on these outcomes remain inconclusive. Building on the idea that combined cognitive and physical therapy can be feasibly delivered; future studies should focus on efficacy. These studies will necessarily be larger and should explore key questions related to this type of early cognitive intervention, including defining the specific types of cognitive therapy that may be efficacious, the ideal time to initiate cognitive therapy, as well as the duration and intensity of therapy to facilitate improved long-term cognitive outcomes among survivors of critical illness.
Cognitive interventions, such as ours, have been studied in outpatient populations of healthy elderly[25–27], those with mild cognitive impairment [25, 28, 29], those with Alzheimer’s disease [30–32], and in non-critically ill, hospitalized elderly patients [33, 34]. This study was the first to examine the feasibility of cognitive therapy in critically ill intensive care unit patients.
Previously, the RETURN study established the feasibility of post-hospital discharge cognitive and physical/functional rehabilitation and reported improved executive functioning and IADL status following the 3-month intervention [8]. The current ACT-ICU study extended a cognitive therapy intervention into the ICU using a variety of cognitive exercises for up to 40 minutes each day and also provided outpatient cognitive rehabilitation. Although we provided patients with the same outpatient cognitive therapy intervention (but no physical/functional intervention) in the current ACT-ICU study, we did not find a similar improvement in executive functioning or IADL status. Nonetheless, the current study provides additional feasibility data for performing outpatient cognitive therapy with the GMT program. Future work is needed to determine which components of post-ICU interventions (e.g., cognitive, physical/functional or combined) may translate to improved outcomes for survivors of critical illness.
Strengths of the ACT-ICU study included the randomized controlled trial design, inclusion of both medical and surgical ICU patients, use of standardized sedation and ventilator management across all three groups, twice-daily delirium monitoring, blinded assessment of all outcomes, and delivery of interventions by an interdisciplinary team. Limitations include a small sample size, limiting our ability to draw conclusions regarding this intervention’s effect on cognitive and functional outcomes, enrollment at a single center, which limits the generalizability of our findings; inability to blind patients or those performing the interventions, which may introduce bias into our results; and the inability to provide cognitive therapy while patients were in rehabilitation facilities or nursing homes, which reduced the overall dose of the cognitive intervention for these patients.
In conclusion, these data suggest that a combined cognitive and physical therapy intervention is feasible to deliver and was safe for critically ill patients to undergo during the earliest stages of ICU care but the long-term effects of this type of intervention remain inconclusive and are in need of further study. This pilot study provides evidence that conventional ICU rehabilitation programs can be extended beyond physical and occupational therapy to include cognitive therapy.
Supplementary Material
Take Home Message.
Cognitive therapy is feasible during the earliest stages of critical illness, yet long-term effects of this intervention remain inconclusive. Future work is needed to establish the optimal patient population, intensity of early cognitive therapy and efficacy of cognitive therapy in the critically ill.
Acknowledgments
We thank the ACT-ICU trial study personnel (Lindsay Anderson, Andrea Antone, Amy Kiehl, Jessica McCurley, Janine Reter, Michael Santoro, Cayce Strength and Joyce Okahashi); the members of the data and safety monitoring board (Terry Clemmer, Sumi Misra and Lorraine Ware); and the staff of the Medical and Surgical Intensive Care Units at the Vanderbilt University Medical Center for their invaluable participation in the ACT-ICU trial.
Funding/Support:
Dr. Brummel was supported by the National Heart Lung and Blood Institute of the National Institutes of Health (NIH) under award number T32HL087738 and is supported by the Vanderbilt Clinical and Translational Scholars Program and the National Institute on Aging of the NIH under award number R03AG045095. Dr. Jackson is supported by the National Institute on Aging of the NIH under award number K23AG031322. Dr. Girard is supported by the National Institute on Aging of the NIH under award number K23AG034257. Dr. Pandharipande is supported by the VA Clinical Science Research and Development Service (VA Career Development Award) and the National Heart Lung and Blood Institute of the NIH under award number R01HL111111. Dr. Hughes is supported by a Foundation for Anesthesia Education and Research Mentored Research Training Grant. Dr. Ely is supported by the VA Clinical Science Research and Development Service (VA MERIT Review Award) and the National Institute on Aging of the NIH under award numbers R01AG027472 and R01AG035117. Dr. Gill was supported by K24AG021507, K07AG043587 and P30AG021342. Drs. Girard, Dittus and Ely are supported by the Veterans Affairs Tennessee Valley Geriatric Research, Education and Clinical Center (GRECC). This work is also supported by the National Center for Advancing Translational Science under award number UL1TR000445. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute, the National Institute on Aging, the National Center for Advancing Translational Science or the National Institutes of Health.
Footnotes
Potential financial conflict of interest:
Dr. Ely has received research grants and/or honoraria from Hospira, Orion, and Abbott. Dr. Girard has received honoraria from Hospira. Dr. Pandharipande has received a research grant from Hospira and honoraria from Hospira, and Orion Pharma. Ms. Pun has received honoraria from Hospira. Ms. Boehm has received honoraria from Hospira. Dr. Gill has received honoraria from Novartis. The other authors report no financial disclosures.
References
- 1.Hopkins RO, Weaver LK, Collingridge D, Parkinson RB, Chan KJ, Orme JF., Jr Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171:340–347. doi: 10.1164/rccm.200406-763OC. [DOI] [PubMed] [Google Scholar]
- 2.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herridge MS, Tansey CM, Matte A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, Kudlow P, Cook D, Slutsky AS, Cheung AM. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 4.Barnato AE, Albert SM, Angus DC, Lave JR, Degenholtz HB. Disability among elderly survivors of mechanical ventilation. Am J Respir Crit Care Med. 2011;183:1037–1042. doi: 10.1164/rccm.201002-0301OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morris PE, Griffin L, Berry M, Thompson C, Hite RD, Winkelman C, Hopkins RO, Ross A, Dixon L, Leach S, Haponik E. Receiving early mobility during an intensive care unit admission is a predictor of improved outcomes in acute respiratory failure. AmJMed Sci. 2011;341:373–377. doi: 10.1097/MAJ.0b013e31820ab4f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris PE, Goad A, Thompson C, Taylor K, Harry B, Passmore L, Ross A, Anderson L, Baker S, Sanchez M, Penley L, Howard A, Dixon L, Leach S, Small R, Hite RD, Haponik E. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36:2238–2243. doi: 10.1097/CCM.0b013e318180b90e. [DOI] [PubMed] [Google Scholar]
- 7.Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, Spears L, Miller M, Franczyk M, Deprizio D, Schmidt GA, Bowman A, Barr R, McCallister KE, Hall JB, Kress JP. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson JC, Ely EW, Morey MC, Anderson VM, Denne LB, Clune J, Siebert CS, Archer KR, Torres R, Janz D, Schiro E, Jones J, Shintani AK, Levine B, Pun BT, Thompson J, Brummel NE, Hoenig H. Cognitive and physical rehabilitation of intensive care unit survivors: Results of the RETURN randomized controlled pilot investigation*. Crit Care Med. 2012;40:1088–1097. doi: 10.1097/CCM.0b013e3182373115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brummel NE, Jackson JC, Girard TD, Pandharipande PP, Schiro E, Work B, Pun BT, Boehm L, Gill TM, Ely EW. A combined early cognitive and physical rehabilitation program for people who are critically ill: the activity and cognitive therapy in the intensive care unit (ACT-ICU) trial. Physical Therapy. 2012;92:1580–1592. doi: 10.2522/ptj.20110414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19:1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 11.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychological function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 12.Santos-Eggimann B, Zobel F, Berod AC. Functional status of elderly home care users: do subjects, informal and professional caregivers agree? J Clin Epidemiol. 1999;52:181–186. doi: 10.1016/s0895-4356(98)00155-3. [DOI] [PubMed] [Google Scholar]
- 13.Pisani MA, Inouye SK, McNicoll L, Redlich CA. Screening for preexisting cognitive impairment in older intensive care unit patients: use of proxy assessment. J Am Geriatr Soc. 2003;51:689–693. doi: 10.1034/j.1600-0579.2003.00215.x. [DOI] [PubMed] [Google Scholar]
- 14.Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, Taichman DB, Dunn JG, Pohlman AS, Kinniry PA, Jackson JC, Canonico AE, Light RW, Shintani AK, Thompson JL, Gordon SM, Hall JB, Dittus RS, Bernard GR, Ely EW. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 15.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, Tesoro EP, Elswick RK. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 16.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, Truman B, Speroff T, Gautam S, Margolin R, Hart RP, Dittus R. Delirium in mechanically ventilated patients: validity and reliability of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 17.Bailey P, Thomsen GE, Spuhler VJ, Blair R, Jewkes J, Bezdjian L, Veale K, Rodriquez L, Hopkins RO. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35:139–145. doi: 10.1097/01.CCM.0000251130.69568.87. [DOI] [PubMed] [Google Scholar]
- 18.Levine B, Robertson IH, Clare L, Carter G, Hong J, Wilson BA, Duncan J, Stuss DT. Rehabilitation of executive functioning: an experimental-clinical validation of goal management training. J Int Neuropsychol Soc. 2000;6:299–312. doi: 10.1017/s1355617700633052. [DOI] [PubMed] [Google Scholar]
- 19.Levine B, Schweizer TA, O’Connor C, Turner G, Gillingham S, Stuss DT, Manly T, Robertson IH. Rehabilitation of executive functioning in patients with frontal lobe brain damage with goal management training. Front HumNeurosci. 2011;5:9. doi: 10.3389/fnhum.2011.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krasny-Pacini A, Chevignard M, Evans J. Goal Management Training for rehabilitation of executive functions: a systematic review of effectivness in patients with acquired brain injury. Disabil Rehabil. 2013 doi: 10.3109/09638288.2013.777807. ePub ahead of print. [DOI] [PubMed] [Google Scholar]
- 21.Gross AL, Rebok GW, Unverzagt FW, Willis SL, Brandt J. Cognitive predictors of everyday functioning in older adults: results from the ACTIVE Cognitive Intervention Trial. J Gerontol B Psychol Sci Soc Sci. 2011;66:557–566. doi: 10.1093/geronb/gbr033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS): Examiner’s manual. Psychological Corporation; San Antonio: 2001. [Google Scholar]
- 24.WAIS-III and WMS-III Technical Manual. The Psychological Corporation; San Antonio: 1997. [Google Scholar]
- 25.Martin M, Clare L, Altgassen AM, Cameron MH, Zehnder F. Cognition-based interventions for healthy older people and people with mild cognitive impairment. Cochrane Database Syst Rev. 2011:CD006220. doi: 10.1002/14651858.CD006220.pub2. [DOI] [PubMed] [Google Scholar]
- 26.Tardif S, Simard M. Cognitive stimulation programs in healthy elderly: a review. International journal of Alzheimer’s disease. 2011;2011:378934. doi: 10.4061/2011/378934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, Morris JN, Rebok GW, Smith DM, Tennstedt SL, Unverzagt FW, Willis SL. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gates NJ, Sachdev PS, Fiatarone Singh MA, Valenzuela M. Cognitive and memory training in adults at risk of dementia: a systematic review. BMC geriatrics. 2011;11:55. doi: 10.1186/1471-2318-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jean L, Bergeron ME, Thivierge S, Simard M. Cognitive intervention programs for individuals with mild cognitive impairment: systematic review of the literature. Am J Geriatr Psychiatry. 2010;18:281–296. doi: 10.1097/JGP.0b013e3181c37ce9. [DOI] [PubMed] [Google Scholar]
- 30.Aguirre E, Hoare Z, Streater A, Spector A, Woods B, Hoe J, Orrell M. Cognitive stimulation therapy (CST) for people with dementia--who benefits most? Int J Geriatr Psychiatry. 2013;28:284–290. doi: 10.1002/gps.3823. [DOI] [PubMed] [Google Scholar]
- 31.Orrell M, Woods B, Spector A. Should we use individual cognitive stimulation therapy to improve cognitive function in people with dementia? BMJ. 2012;344:e633. doi: 10.1136/bmj.e633. [DOI] [PubMed] [Google Scholar]
- 32.Woods B, Aguirre E, Spector AE, Orrell M. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev. 2012;2:CD005562. doi: 10.1002/14651858.CD005562.pub2. [DOI] [PubMed] [Google Scholar]
- 33.Inouye SK, Bogardus ST, Jr, Charpentier PA, Leo-Summers L, Acampora D, Holford TR, Cooney LM., Jr A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 34.Cheng CM, Chiu MJ, Wang JH, Liu HC, Shyu YI, Huang GH, Chen CC. Cognitive stimulation during hospitalization improves global cognition of older Taiwanese undergoing elective total knee and hip replacement surgery. J Adv Nurs. 2012;68:1322–1329. doi: 10.1111/j.1365-2648.2011.05842.x. [DOI] [PubMed] [Google Scholar]
- 35.Bodenburg S, Dopslaff N. The Dysexecutive Questionnaire advanced: item and test score characteristics, 4-factor solution, and severity classification. J Nerv Ment Dis. 2008;196:75–78. doi: 10.1097/NMD.0b013e31815faa2b. [DOI] [PubMed] [Google Scholar]
- 36.Folstein MF, Folstein SE, McHugh PR. Mini-mental state a practical method for grading the cognitive state of patients for the clinician. J Psychiat Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 37.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 38.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 39.EuroQOL. EuroQol--a new facility for the measurement of health-related quality of life. The EuroQol Group. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.