Abstract
Plasmodium vivax is the most widely distributed human malaria parasite. Despite its importance, both clinical and basic research has been hampered by lack of a convenient in vitro culture system, in part due to the parasite’s infection preference of reticulocytes rather than mature erythrocytes. The use of reticulocyte-producing hematopoietic stem cell culture has been proposed for the maintenance of the parasite, but good numbers of reticulocytes and P. vivax parasites sufficient for practical use in research have been difficult to produce from this system. Here, we report an improved method of hematopoietic stem cell culture for P. vivax infection, which requires less time and produces higher or equivalent percentage of reticulocytes than previously reported systems. Reticulocytes were cultured from cryopreserved erythroblasts that were frozen after 8 days-cultivation of purified CD34+ cells from human umbilical cord blood. This method of production allowed the recovery of reticulocytes in a shorter time than with continuous stem cell culture. We obtained a relatively high percentage of peak reticulocyte production by using co-cultivation with a mouse stromal cell line. Using P. vivax mature stage parasites obtained from infected Aotus monkeys, we observed substantial numbers (up to 0.8 % of the total number of the cells) of newly invaded reticulocytes 24 hours after initial cultivation. The addition of fresh reticulocytes after 48 hours culture, however, did not result in significant increase of second cycle reticulocyte invasion. Assays of invasion inhibition with specific antibodies were successful with this system, demonstrating potential for study of biological processes as well as the conditions necessary for long-term maintenance of P. vivax in vitro.
1. Introduction
Plasmodium vivax research is limited by the lack of practical in vitro culture system. One of the obstacles to this system is that P. vivax mainly infects reticulocytes, which constitute only a small percentage of the red blood cells in adult donor blood [1]. Attempts to culture the parasites in vitro have therefore included provision of reticulocytes from Aotus monkeys [2], from human hemochromatosis patients [3] and human cord blood [4, 5, 6]. However, it is difficult to maintain these cultures for more than a few days and the sources for reticulocytes are not widely available.
Hematopoietic stem cells (HSCs) can be a useful source for reticulocytes when the cells are cultured in a condition that directs erythrocyte development [7]. Panichakul et al. [8] reported an in vitro culture system of P. vivax using reticulocytes obtained from HSCs. The system produced up to 0.5% of reticulocytes in the HSC culture and the percentage of P. vivax-infected cells that could be obtained was low (0.0013% maximum). Better yield of reticulocytes from HSC culture may be needed for P. vivax research. Also, to obtain reticulocytes from a HSC culture system takes 14 days, which can be very demanding, especially considering new reticulocytes need to be continuously added to the cultures for successful parasite infection. Noulin et al. (9) recently reported use of cryopreserved hematopoietic stem cells to produce reticulocytes for infection of P. vivax parasites from infected patients. They achieved relatively high percentage of reticulocytes which could be infected by P. vivax parasites. However, detailed information about subsequent status of the infected parasites, particularly the percentages of each stage during the culture was not demonstrated and infection with P. vivax parasites experimentally amplified in Aotus monkeys was not examined.
Here, we report an improved method for HSC cultures to produce reticulocytes for in vitro infection of P. vivax parasites. By using cryopreserved erythroblasts developed from CD34+ human cord blood, and adding to the culture a mouse stromal cell line, reticulocytes are consistently obtained within 7 to 8 days in high percentage (15–20 %). Using these reticulocytes we demonstrate that P. vivax can efficiently infect these cells, allowing laboratory investigation of parasite host cell invasion.
2. Materials and Methods
2.1. Hematopoietic stem cell (HSC) culture
Purified CD34+ cells from human cord blood (AllCells, Emeryville, CA) were grown in Iscove’s modified Dulbecco’s medium (IMDM, Cedarlane) containing L-glutamine (4 mM, Invitrogen), penicillin and streptomycin (50 U/ml and 50 μg/ml, respectively, Invitrogen), monothioglycerol (160 μM), transferrin (120 μg/ml), insulin (10 μg/ml), ferrous nitrate (90 ng/ml), ferrous sulfate (900 ng/ml) and bovine serum albumin (10 mg/ml, StemCell Technologies, Vancouver, BC, Canada). All reagents are from Sigma-Aldrich unless otherwise specified. The culture medium was supplemented during day 0–8 (stage 1, to obtain erythroblasts) with Stem Cell Factor (100 ng/ml, R&D Systems), hydrocortisone (1 μM, Sigma-Aldrich), human recombinant IL-3 (5 ng/ml), and erythropoietin (EPO, 3 U/ml, StemCell Technologies) (stage 1 medium). Cultures were started at 1×104 cells/ml and maintained below 2×106 cells/ml until day 8. At this point, erythroblasts were either cryopreserve or maintained at 5×104 cells/ml and layered onto a confluent mouse stromal cell line (MS-5, kindly provided by Dr. Sugimoto, Niigata University) in medium only with EPO (stage 2, day 9–12 from initial CD34+ cells culture) (stage 2 medium). MS-5 cells were maintained in α Minimum Essential Medium (α MEM, Invitrogen) with 10 % fetal calf serum until used for HSC co-culture. After day 12, cells were maintained in medium without the cytokines (stage 3 medium). All cultures were incubated in a CO2-incubator with 5 % CO2 at 37°C.
For cryopreservation of erythroblasts, cells from the culture on day 8 were suspended in IMDM, mixed with an equal volume of 20 % DMSO in fetal bovine serum (both from ATCC), placed in a freezing container (Mr. Frosty, Nalgene) and gradually frozen at −80 °C overnight. The frozen cells were transferred to liquid nitrogen for permanent storage.
When required, cryopreserved erythroblasts were thawed, washed once with IMDM, and suspended in stage 1 medium and incubated overnight for recovery. On the next day, the cells were suspended in stage 2 medium and layered on MS-5 for three days. The cells were then maintained in stage 3 medium until addition of parasites.
2.2. P. vivax infection and development in Aotus monkeys
All procedures were performed in agreement with the National Institutes of Health Guidelines under a protocol approved by the National Institute of Allergy and Infectious Diseases Animal Care and Use Committee and housed in compliance with the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals [10]. P. vivax (AMRU-I strain) [11] infections of splenectomized Aotus nancymaae monkeys were established under ketamine sedation by intravenous inoculation of approximately 5 × 106 P. vivax parasitized Aotus red blood cells. Parasitemia was followed by daily thin blood smears prepared using approximately 10 μl of blood drawn from the saphenous vein using insulin syringes. An estimated 10,000 red blood cells (RBCs) were counted from each thin smear to express the parasitemia as percentage of parasitized red blood cells. At the desired parasitemia between 1 and 3 ml of blood was drawn in 10 % heparin from the femoral vein of sedated animals.
2.3. P. vivax propagation in reticulocytes
P. vivax infected RBCs (iRBCs) freshly obtained from Aotus monkeys were washed once with McCoy’s 5A medium (Invitrogen) and suspended in the same medium containing 25% of human type-AB serum (Interstate Blood Bank, complete medium). Depending on parasite stages, cells were either immediately used or incubated overnight in a CO2-incubator with 5 % CO2 at 37°C to allow parasite maturation. Mature stage P. vivax parasites were enriched with magnetic column (MACS separation column, Miltenyi Biotec Inc.) according to the manufacturer’s instruction. Purified parasites were mixed (at 1:10 proportion) with cells from HSC culture (14–15 days) washed once with McCoy’s 5A medium, suspended in the complete medium described above, and incubated on 24-well tissue culture plate (Nunc) in 1 ml medium per well at 1.0 × 106 cells/ml in a CO2 incubator at 37°C with 5 % CO2.
For the addition of reticulocytes 48 hours post-infection (PI), half the volume in the well was mixed with equal numbers of erythropoietic cells from HSC culture, which were washed once with McCoy’s 5A medium and suspended in 0.5 ml complete medium.
2.4. Reticulocyte, enucleated cell and total cell counting
Cultured reticulocytes and all enucleated cells were stained with New Methylene Blue solution (Reticulocyte Stain) and Giemsa staining (both from Sigma-Aldrich), respectively, according to the manufacturer’s instruction. For reticulocyte staining 3 volumes of cultured reticulocytes were mixed with 2 volumes of Reticulocyte Stain solution, incubated for 10 minutes and observed under a light microscope after making thin smear on a glass slide. For enucleated cell staining, thin smear of the cells were fixed with methanol, stained in 1:5 dilution of Giemsa staining solution in water for 20 minutes and observed under a light microscope. Total cell concentration was determined by counting on hemocytometer (Hausser Scientific). Pictures of stained cells were taken with CCD camera and imaging software (Olympus MicroSuite, Olympus, Center Valley, PA).
2.5. Immunofluorescence assay (IFA)
Cells were collected, washed with phosphate buffered saline (PBS) twice and smeared on glass slides. The cells were dried in the air and fixed with methanol at −20 °C for 15 minutes. After drying, the cells were blocked with 5 % skim milk in PBS for one hour at room temperature, followed by incubation with a mixture of a rat monoclonal antibody against human glycophorin A (YTH89.1, Abcam) and a rabbit polyclonal antibody against P. vivax MSP-1 19 kD fragment (obtained through the MR4 as part of the BEI Resources Repository, NIAID, NIH: Plasmodium vivax PvMSP-1/19 Rabbit Antiserum, MRA-16, deposited by JH Adams), both diluted to 1:200 in PBS containing 0.1 % Tween 20 (TPBS), for one hour at room temperature. The cells were washed twice with TPBS, incubated for one hour with a mixture of secondary antibodies (Alexa Fluor 594 anti-rat IgG and Alexa Fluor 488 anti-rabbit IgG, both diluted to 1:400 in TPBS), then washed twice with TPBS. The cells were covered with a cover slip with anti-fade reagent (ProLong Gold, Life Technologies) and analyzed with a UV microscope and imaging system (Olympus MicroSuite, Olympus, Center Valley, PA).
2.5. Flow cytometry
Cells were washed twice with FSB (PBS with 2 % fetal bovine serum and 0.1 % sodium azide) and incubated with fluorescent dye-conjugated antibodies (either FITC- or phycoerythrin- labeled, BD Biosciences) for 30 minutes on ice in the dark. After washing twice with FSB, stained cells were analyzed with a flow cytometer (BD FACSCalibur System, BD Biosciences).
2.6. Invasion inhibition assay
Inhibition of P. vivax merozoite invasion into cultured reticulocytes was assayed by adding anti-FY6 monoclonal antibody [12] (2C3, from Dr. Yves Colin, Inserm, Paris, France) and a control monoclonal antibody against band 3 (A-6, Santa Cruz Biotechnology) to the culture medium at final concentrations of 13 ng/ml (anti-FY6) and 2 μg/ml (anti-band 3), respectively. For an antibody against PvRII [13], the antibody or rabbit pre-immune serum were mixed in the medium (both at 5 μg/ml final concentration). Concentrations of anti-FY6 and anti-band 3 antibodies were determined by measuring light absorbance at 280 nm and the concentrations of anti-PvRII and pre-immune serum were determined using the BCA protein assay method (Pierce). The cells were stained with Giemsa 24 hours after the inoculation for microscopic examination.
3. Results
To obtain substantial numbers of reticulocytes for P. vivax infection, we modified a previously reported method of HSC culture for production of reticulocytes [7] (Figure 1). In order to shorten the culture period of the original method, we cryopreserved erythroblasts after culturing the stem cells for 8 days and followed the rest of the culture schedule after thawing the cryopreserved cells. We did not find any detectable differences in morphology and erythrocyte surface marker expression between the cells with cryopreservation/thawing processes and those without cryopreservation (Supplemental Figure S1). Moreover, reticulocyte percentages on the time course were similar between the cells with these two different processes (Supplemental Figure S2), demonstrating that our modification in the protocol does not affect quality and quantity of reticulocytes produced by culturing the HSCs.
Figure 1.
Schematic presentation of in vitro erythropoietic cell culture with human CD34+ HSCs. Ages of the culture are described as both days after the initiation of the culture and those after thawing cryopreserved erythroblasts.
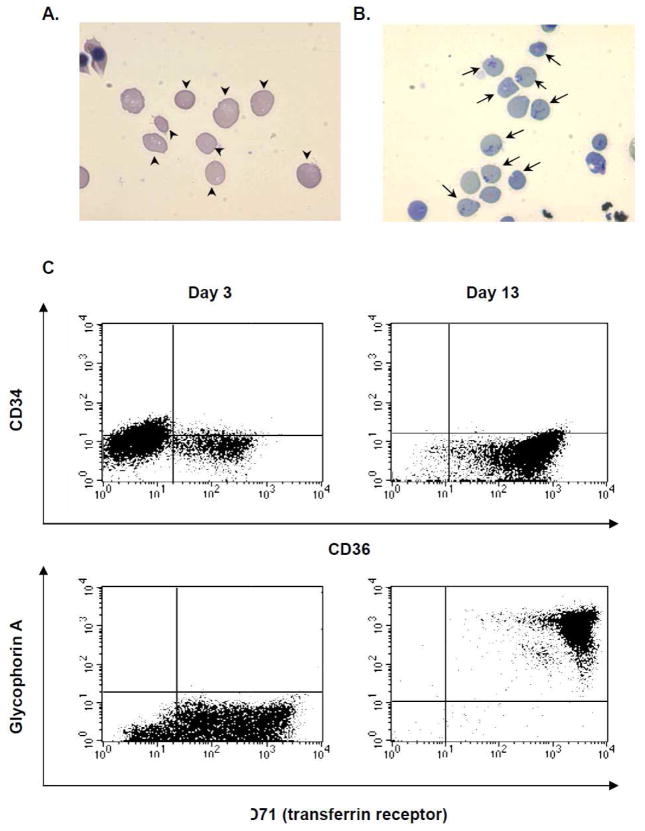
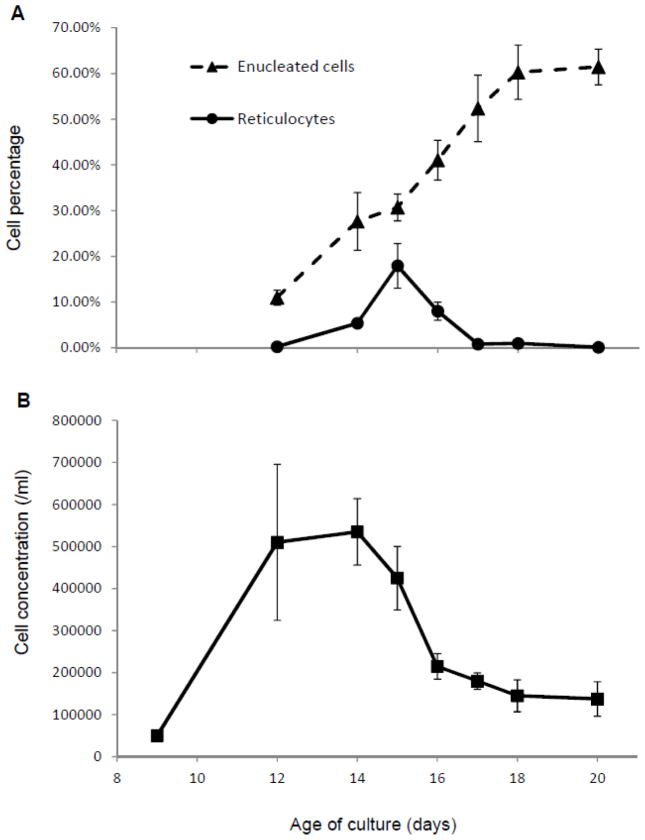
When purified CD34+ HSCs were cultured for reticulocyte production with a mouse stromal cell line (MS-5), a much higher percentage (around 20 % of total number of the cells) of reticulocytes compared to adult red blood cells was observed (Figure 2A) around 15 days after the beginning of the HSC culture. Characteristic reticular morphology was observed when the cells were stained with new methylene blue staining solution (Figure 2B). Erythropoietic differentiation of the cultured HSCs was confirmed by flow cytometry analysis of two time points showing an increase of cells expressing the surface erythroblast marker CD36, erythrocyte markers glycophorin A and transferring receptor on day 13 (Figure 2C). PCR analysis was performed [14] to identify the genotype of Duffy blood group locus of the HSC donor and confirm that the donor’s genotype was not Duffy-negative (homozygous Fy alleles) (data not shown). Four independent batches of the HSC culture were developed and key indicators on the time course were measured (Figure 3). The highest percentages of reticulocytes are observed on day 15 of HSC culture (Figure 3A), while average enucleated cell percentages show that enucleated cells accumulate until 18 days in culture when approximately 60 % of the cells become enucleated (Figure 3A). Average total cell concentration starts declining after 14 days in culture (Figure 3B). These results suggest that reticulocytes should be collected at day 15 of the HSC culture.
Figure 2.
Morphology and surface protein expression of erythropoietic cells cultured from HSCs. Images of the cells on day 15 stained with Giemsa (A) and New Methylene Blue (B). Arrow heads in (A) indicate enucleated cells and arrows in (B) indicate reticulocytes with characteristic patterns. (C) Surface expression of the marker proteins on the 3 day-old (left panels) and 13 days-old (right panels) cells in the culture was measured by using flow cytometry with specific antibodies against markers for HSCs (CD34) and erythroblasts (CD36) (upper panels), and erythrocytes (glycophorin A and CD71) (lower panels).
Figure 3.
Enucleated cell and reticulocyte percentages (A), and total cell concentrations (B) during the HSC culture. Slides were stained with Giemsa or New Methylene Blue to count enucleated cells or reticulocytes, respectively, under a light microscope and obtain percentages in total cell numbers. At least 200 of total cells were counted for each point to obtain the percentages. Total cell concentrations were calculated based on counts with hemocytometer. Values and error bars represent averages and standard errors based on four independent cultures.
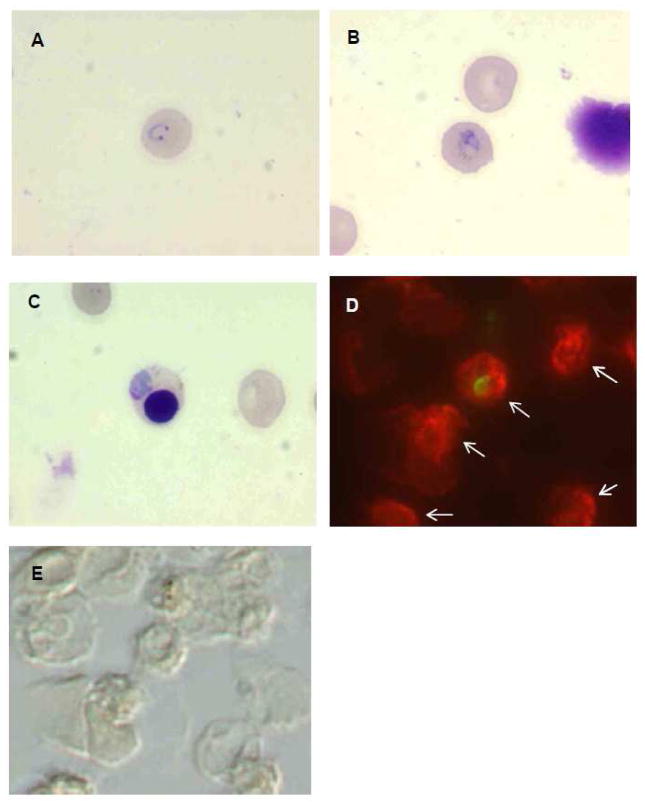
To examine whether P. vivax could infect reticulocytes produced by this method, mature stage parasites from infected Aotus monkeys were enriched with magnetic columns and added to HSC cultures containing a high percentage of reticulocytes. Upon microscopic examination of Giemsa-stained slides, healthy-appearing early stage parasites were observed at 24 hours PI (Figure 4A). Subsequently, later stage parasites were also observed at 48 and 72 hours PI in enucleated cells (Figure 4B) and occasionally in nucleated erythroblasts (Figure 4C), although numbers of the infected erythroblasts were less than those of infected enucleated cells. The infected cells 24 hours PI were fixed and stained with the anti-human glycophorin A antibody, which binds human erythrocytes but not Aotus cells (Figure S4), and the anti-P. vivax MSP antibody, which binds intraerythrocytic stages including ring stages. The image of immunostaining demonstrates that the ring stage parasites found 24 hours PI originated from the parasite infection of reticulocytes from HSC culture, not of the carry-over Aotus RBCs from the blood drawn (Figure 4 D and E).
Figure 4.
Images of P. vivax parasites infected on cultured erythropoietic cells. Thin smears were made on glass slides, stained with Giemsa and observed under microscope (A–C), or fixed and immuno-stained with antibodies (D and E). A and B, P. vivax-infected reticulocytes 24 and 48 hours PI, respectively. C, P. vivax-infected erythroblast 48 hours PI. D, UV-microscope image of P. vivax-infected reticulocyte stained with anti-P. vivax MSP-1 (green) and anti-human glycophorin A (red). E, differential interference image of the same field as E. Human erythropoietic cells which stained red are indicated by arrows.
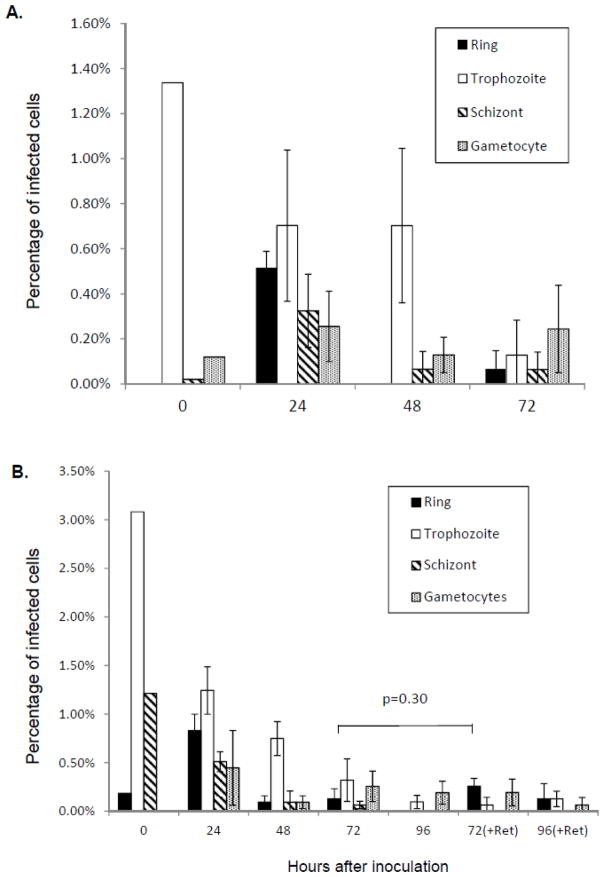
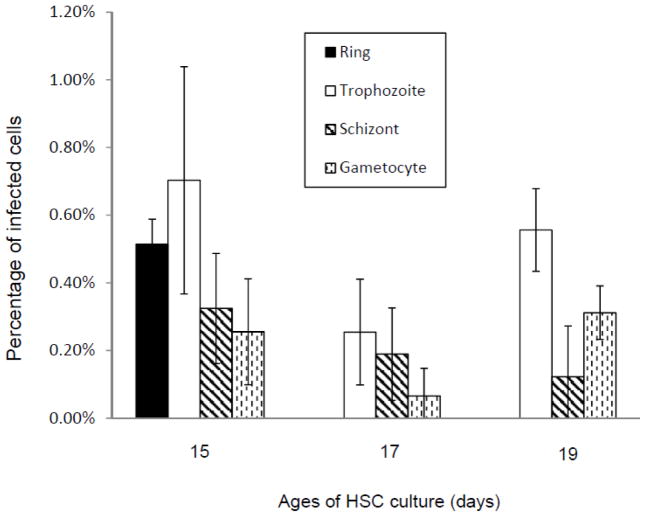
Percentages of P. vivax-infected cells were calculated for different parasite stages for each time point based on the microscopic counting of Giemsa-stained slides (Figure 5A and 5B). A substantial percentage of ring stage parasites were observed at 24 hours PI (Figure 5A and 5B, 24 h), indicating that the parasite invaded reticulocytes produced from the HSC culture. However, any subsequent increase in the mature stages (trophozoites and schizonts) was not observed later and total parasitemia decreased during cultivation (Figure 5A and B). Gametocytes were consistently present throughout the cultivation (Figure 5A and B), which were likely carried over from Aotus monkey blood and enriched together with mature stages after magnetic column enrichment since they contained good amount of hemozoin crystals.
Figure 5.
Percentages of P. vivax-infected cells for each parasite stage without (A) or with (B) additional fresh reticulocytes at 48 hours after the inoculation of P. vivax parasite on HSC culture reticulocytes. In (B), equal numbers of freshly prepared HSC cells with 14 days culture were added to the infected reticulocytes at 48 hours PI. 72 (+Ret) and 96 (+Ret) are the numbers of samples with the additional reticulocytes, while 72 and 96 are the ones without any additional reticulocytes. Percentages in the total number of cells were calculated based on microscopic counts of Giemsa-stained slides. At least 500 total cells were counted for each sample. Values and error bars represent averages and standard errors of triplicate wells. Error bars are not included with the data for 0 h after inoculation since they are based on observation of single Giemsa-slide of enriched parasites used for the inoculation. A p-value based on Student’s two-sided t-test for the difference between percentages of ring stage parasites without and with additional reticulocytes is shown in (B).
Though we observed that P. vivax parasites infected the reticulocytes from the HSC culture, no significant number of ring stage parasites resulted from the second cycle infection (Figure 5A, 72h PI). Since freshly developed reticulocytes mature and become erythrocytes after around 48 hours, the low percentage of second-cycle infection might be due to a reduced number of reticulocytes present at the time of the merozoite release around 48h PI. To enhance the second-cycle infection, approximately equal numbers of freshly prepared reticulocytes from HSC culture were added to the infected reticulocytes 48 hours PI (Figure 5B, 72h and 96h +Ret). When fresh reticulocytes were added at 48 hours PI, we observed higher ring stage percentages were observed than when we did not add reticulocytes (Figure 5B, 72h and 72h (+Ret); 96h and 96h (+Ret)). However, statistical analysis (Student’s t-test, two-sided) of ring stage percentages at 72 hours PI does not conclude significances between cases with or without additional reticulocytes (P=0.30), suggesting that addition of fresh reticulocytes may not contribute to enhancement of the second-cycle infection.
P. vivax parasites may infect slightly older or younger cells than the ones at the peak reticulocyte percentage. To examine this possibility, cells from the HSC culture with different ages (15, 17 and 19 days) were obtained simultaneously and inoculated with the same preparation of enriched mature stages of P. vivax parasites. Percentages of P. vivax-infected cells for different parasite stages at 24 and 48 hours PI were calculated based on microscopic counting of Giemsa-stained slides (Figure 6). A relatively high percentage of reticulocytes (more than 10 %) were observed with the cells from the 15 days old HSC culture, while less than 1 % of reticulocytes were found with those from the 17 or 19 days old culture (data not shown). A substantial percentage of ring stages was observed only with the cells of the 15 days old culture at 24 h PI, and the percentage of trophozoite stages at 48 h PI is also substantially higher than those with the cells of older ages. These results indicate that among the cells from different ages of HSC culture, only the one with highest percentage of reticulocytes is susceptible to P. vivax parasite invasion.
Figure 6.
Percentages of P. vivax-infected cells after inoculation of P. vivax parasites on cultured erythropoietic cells with different ages. Percentages of the total number of cells 24 hours after the inoculation were calculated based on microscopic counting of Giemsa-stained slides. At least 500 total cells were counted for each time point. Values and error bars represent averages and standard errors of triplicate wells.
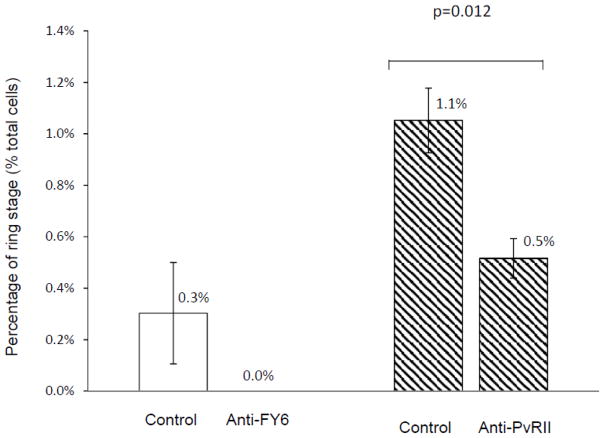
To test parasite reticulocyte invasion rates and inhibition using reticulocytes from HSC, a monoclonal antibody (2C3) against FY6 epitope of the Duffy antigen protein [12] was added to the medium when P. vivax parasites were inoculated to cultured reticulocytes. FY6 epitope resides in the region of the protein which is critical for its binding to Duffy binding protein of P. vivax during the invasion process of the parasite [12]. Percentage for ring stage parasite at 24 hours PI were quantitated for the sample incubated with the anti-FY6 antibody or a control (anti-band 3) antibody (Figure 7, left). Ring stages were observed only with the cells incubated with the control antibody, suggesting that invasion was blocked with the anti-FY6 antibody. A similar invasion inhibition assay was performed with a rabbit polyclonal antibody against PvRII, which specifically recognized the receptor-binding domain of P. vivax Duffy binding protein [13]. Similarly, higher percentage of ring stage parasites were observed in the P. vivax-inoculated reticulocytes with the control serum (Figure 7, right, control), but 50% less ring stage parasites were observed with the anti-PvRII antibody (Figure 7B, right, anti-PvRII). These results demonstrate that reticulocytes produced from HSC culture can be used for reticulocyte-invasion blocking assays for P. vivax parasites to evaluate candidate molecules involved in the mechanism of host cell infection by the parasite.
Figure 7.
Inhibition of P. vivax parasite invasion on HSC culture reticulocytes with a mouse monoclonal antibody (2C3) against the parasite-binding domain of host receptor (FY6) (left) or a rabbit polyclonal antibody against the host-binding domain of the parasite protein (PvRII) (right). An antibodies against FY6 of Duffy antigen protein (kindly provided by Dr. Yves Colin Aronovicz [9]) (at 13 μg/ml for the final concentration) or a control (an antibody against band 3 protein at 100 μg/ml for the final concentration) (left), or an antibody against PvRII (kindly provided by Dr. Chetan E Chitnis [10]) or a control (rabbit pre-immune serum) (right) (both at 5 μg/ml for the final concentrations) were mixed in the medium when P. vivax parasite was inoculated on the reticulocytes from 15 days-old HSC culture. Percentages in the total numbers of the cells were calculated 24 hours after the inoculation of P. vivax parasites based on microscopic counting of Giemsa-stained slides. At least 500 total cells were counted for each sample. Values and error bars represent averages and standard errors of triplicate wells. A p-value based on Student’s two-sided t-test is shown.
4. Discussion
To improve HSC cultures for P. vivax in vitro studies, we cryopreserved erythroblasts and employed a co-culture with a mouse stromal cell line that increased the yield of reticulocyte production to approximately 20 % within 7 days of the culture. In addition, numbers of hematopoietic stem cells increase more than 300 times the initial number of CD34+ purified cells before cryopreservation (data not shown), making the culture easier to prepare since we can store a higher number of erythroblasts even with a much smaller number of initial CD34+ cells. More than 1.0 × 106 times the blood cells would be necessary for direct purification of reticulocytes from cord blood [5] to yield the same number of reticulocytes than CD34+ HSCs.
Based on the experiments with HSC culture of different ages (Figure 6), P. vivax parasites did not invade the cells from the 17- or 19-day old cultures. Since the 17-day old culture should contain a substantial number of early stages of erythrocytes that recently developed from reticulocytes, the results demonstrate that reticulocytes quickly lose their susceptibility to P. vivax infection during their development in the HSC culture. The results also suggest that, in order for the HSC culture to be susceptible to P. vivax infection, the culture should be 15-day old or younger. In addition to the differential staining of human and Aotus red blood cells (Fig. 4E), these results ensure that ring stage parasites observed in the cultured cells 24 hours PI (Figure 5 and 6) did not originate from the carryover of infected Aotus RBCs with very early stages of the parasite, since the same purified P. vivax parasites were used for the 17- or 19-day old HSC culture cells.
Purified recombinant human EPO is one of the essential cytokines in the culture medium for the HSC culture for production of reticulocytes. It is widely available from commercial vendors though it is indeed expensive in large quantities. Based on our data (Figure S3), the culture produces comparable reticulocyte percentages and cell concentrations using one fourth (0.75 U/ml) of the concentration of EPO originally reported [7], making the system more affordable for its use for research. This may be important for assays such as invasion inhibition assays (such as Fig. 7) or growth inhibition assays with chemical compounds where this system would need to produce a greater quantity of reticulocytes.
In this paper, we demonstrate the novel use of stem cell-derived reticulocytes to measure the effects of specific antibodies on reticulocyte invasion rate of P. vivax parasites (Fig. 7). A similar assay was performed by Russell et al. using directly purified reticulocytes from cord blood cells [5]; although both assays appear to work well, the assay in this study has the advantage of being able to produce more reticulocytes from stem cells from a single donor, enabling a more uniform assay condition. This may be particularly important when we compared the effects of different antibodies on P. vivax reticulocyte invasion, since changes in blood donor can dramatically affect the results of these comparisons.
In our study, we observed only limited P. vivax growth in reticulocytes from HSC culture beyond 48 hour after the parasite invasion. Since reasonable percentage (0.5–0.8 %) of the newly invaded cells with P. vivax parasite were observed 24 hours PI (Fig. 5A and B), other factors may be needed for growth and development into later stages of the parasite in vitro. In a previous report, hematopoietic cells from HSC culture were infected with P. vivax parasites from human patients for longer time periods (60 and 85 days) [8]. The difference between parasite sources (P. vivax-infected patient blood in the previous report while infected Aotus monkeys in our study) can be a factor causing this discrepancy. However, it should be noted that active growth of the parasites was not observed with the system in the previous report, since the percentages of the infected cells were extremely low (0.0002–0.0012 %) and a continuous increase in the number of the parasite was not observed in the time course [8]. In another study, P. vivax parasites were developed into later stages and re-invaded into reticulocytes after the initial cycle. In this study, P. vivax parasites grown in Aotus monkeys were infected on reticulocytes from a human hemochromatosis patient [3]. Here we see that it is possible that reticulocytes produced in the hematopoietic stem cell culture contain little or none of some important factors for growth of mature stages of P. vivax parasites. Comparative proteomic analyses between reticulocytes from HSC culture and those produced in vivo may provide important information about essential factors for active P. vivax growth and development in reticulocytes.
Supplementary Material
Highlights.
Modified stem cell culture produced reticulocytes with higher efficiency.
Aotus-grown Plasmodium vivax infection on cultured reticulocytes was demonstrated.
Invasion inhibition was quantitated using P. vivax infection on cultured reticulocytes.
Selective infection on cultured reticulocytes was demonstrated using immunostaining.
Acknowledgments
This study was partly supported by National Institutes of Health grant (1R03AI079475-01) and also partly by the Intramural Research Program of the NIH, NIAID. We thank Dr. Yves Colin Aronovicz for providing anti-FY6 monoclonal antibody and Dr. Louis H. Miller for critical advice for the study. We also thank Sarah Kaslow, Theresa Aguirre, Faith Sentz, and Ahlin Bruce for support on Aotus research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mons B. Preferential invasion of malarial merozoites into young red blood cells. Blood Cells. 1990;16:299–312. [PubMed] [Google Scholar]
- 2.Mons B, Collins WE, Skinner JC, van der Star W, Croon JJ, van der Kaay HJ. Plasmodium vivax: in vitro growth and reinvasion in red blood cells of Aotus nancymai. Exp Parasitol. 1988;66:183–188. doi: 10.1016/0014-4894(88)90089-6. [DOI] [PubMed] [Google Scholar]
- 3.Golenda CF, Li J, Rosenberg R. Continuous in vitro propagation of the malaria parasite Plasmodium vivax. Proc Natl Acad Sci U S A. 1997;94:6786–6791. doi: 10.1073/pnas.94.13.6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Udomsangpetch R, Somsri S, Panichakul T, Chotivanich K, Sirichaisinthop J, Yang Z, Cui L, Sattabongkot J. Short-term in vitro culture of field isolates of Plasmodium vivax using umbilical cord blood. Parasitol Int. 2007;56:65–69. doi: 10.1016/j.parint.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Russell B, Suwanarusk R, Borlon C, Costa FT, Chu CS, Rijken MJ, Sriprawat K, Warter L, Koh EG, Malleret B, Colin Y, Bertrand O, Adams JH, D’Alessandro U, Snounou G, Nosten F, Rénia L. A reliable ex vivo invasion assay of human reticulocytes by Plasmodium vivax. Blood. 2011;118:e74–81. doi: 10.1182/blood-2011-04-348748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borlon C, Russell B, Sriprawat K, Suwanarusk R, Erhart A, Renia L, Nosten F, D’Alessandro U. Cryopreserved Plasmodium vivax and cord blood reticulocytes can be used for invasion and short term culture. Int J Parasitol. 2012;42:155–60. doi: 10.1016/j.ijpara.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giarratana MC, Kobari L, Lapillonne H, Chalmers D, Kiger L, Cynober T, Marden MC, Wajcman H, Douay L. Ex vivo generation of fully mature human red blood cells from hematopoietic stem cells. Nat Biotechnol. 2005;23:69–74. doi: 10.1038/nbt1047. [DOI] [PubMed] [Google Scholar]
- 8.Panichakul T, Sattabongkot J, Chotivanich K, Sirichaisinthop J, Cui L, Udomsangpetch R. Production of erythropoietic cells in vitro for continuous culture of Plasmodium vivax. Int J Parasitol. 2007;37:1551–1557. doi: 10.1016/j.ijpara.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Noulin F, Borlon C, van den Eede P, Boel L, Verfaillie CM, D’Alessandro U, Erhart A. Cryopreserved reticulocytes derived from hematopoietic stem cells can be invaded by cryopreserved Plasmodium vivax isolates. PLoS One. 2012;7:e40798. doi: 10.1371/journal.pone.0040798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Research Council. Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research (ILAR) National Academy Press; Washington (DC): 1996. p. 125. [Google Scholar]
- 11.Sullivan JS, Morris CL, Richardson BB, Galland GG, Jennings VM, Kendall J, Collins WE. Adaptation of the AMRU-1 strain of Plasmodium vivax to Aotus and Saimiri monkeys and to four species of anopheline mosquitoes. J Parasitol. 1999;85:672–7. [PubMed] [Google Scholar]
- 12.Wasniowska K, Petit-LeRoux Y, Tournamille C, Le van Kim C, Cartron JP, Colin Y, Lisowska E, Blanchard D. Structural characterization of the epitope recognized by the new anti-Fy6 monoclonal antibody NaM 185-2C3. Transfus Med. 2002;12:205–211. doi: 10.1046/j.1365-3148.2002.00373.x. [DOI] [PubMed] [Google Scholar]
- 13.Singh S, Pandey K, Chattopadhayay R, Yazdani SS, Lynn A, Bharadwaj A, Ranjan A, Chitnis C. Biochemical, biophysical, and functional characterization of bacterially expressed and refolded receptor binding domain of Plasmodium vivax duffy-binding protein. J Biol Chem. 2001;276:17111–17116. doi: 10.1074/jbc.M101531200. [DOI] [PubMed] [Google Scholar]
- 14.Olsson ML, Hansson C, Avent ND, Akesson IE, Green CA, Daniels GL. A clinically applicable method for determining the three major alleles at the Duffy (FY) blood group locus using polymerase chain reaction with allele-specific primers. Transfusion. 1998;38:168–173. doi: 10.1046/j.1537-2995.1998.38298193099.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.