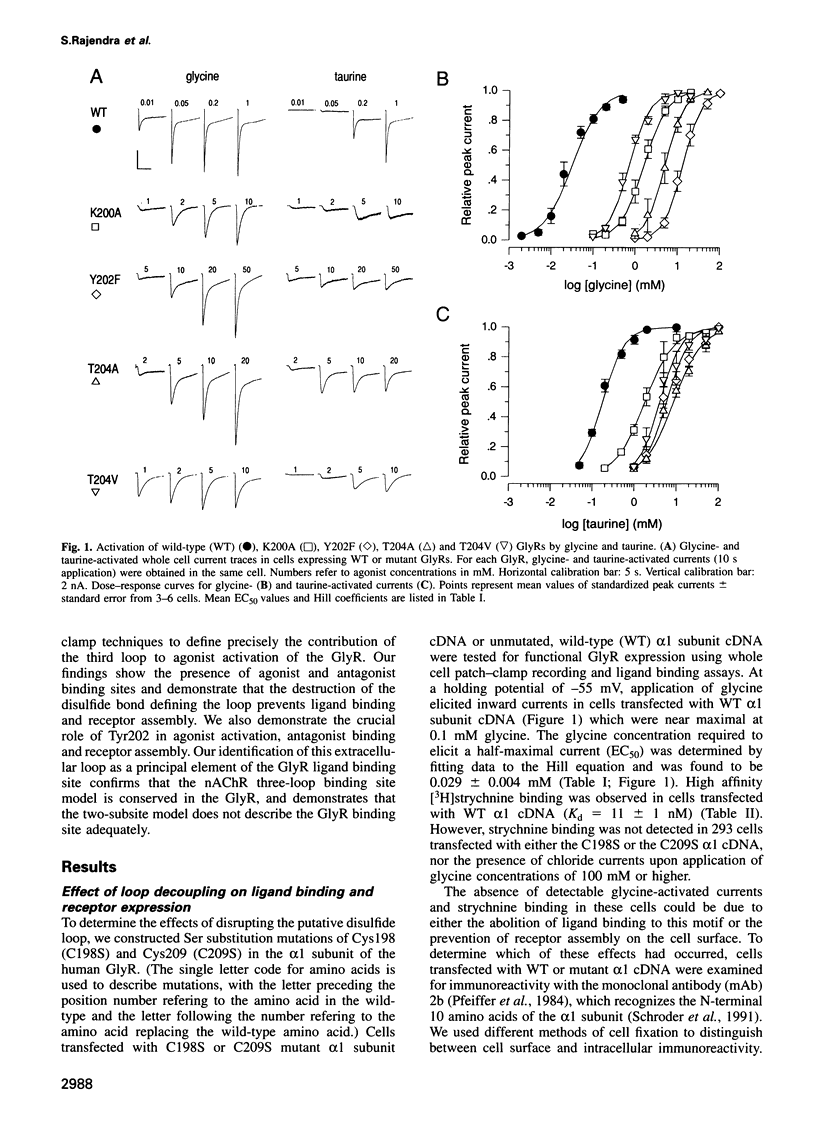
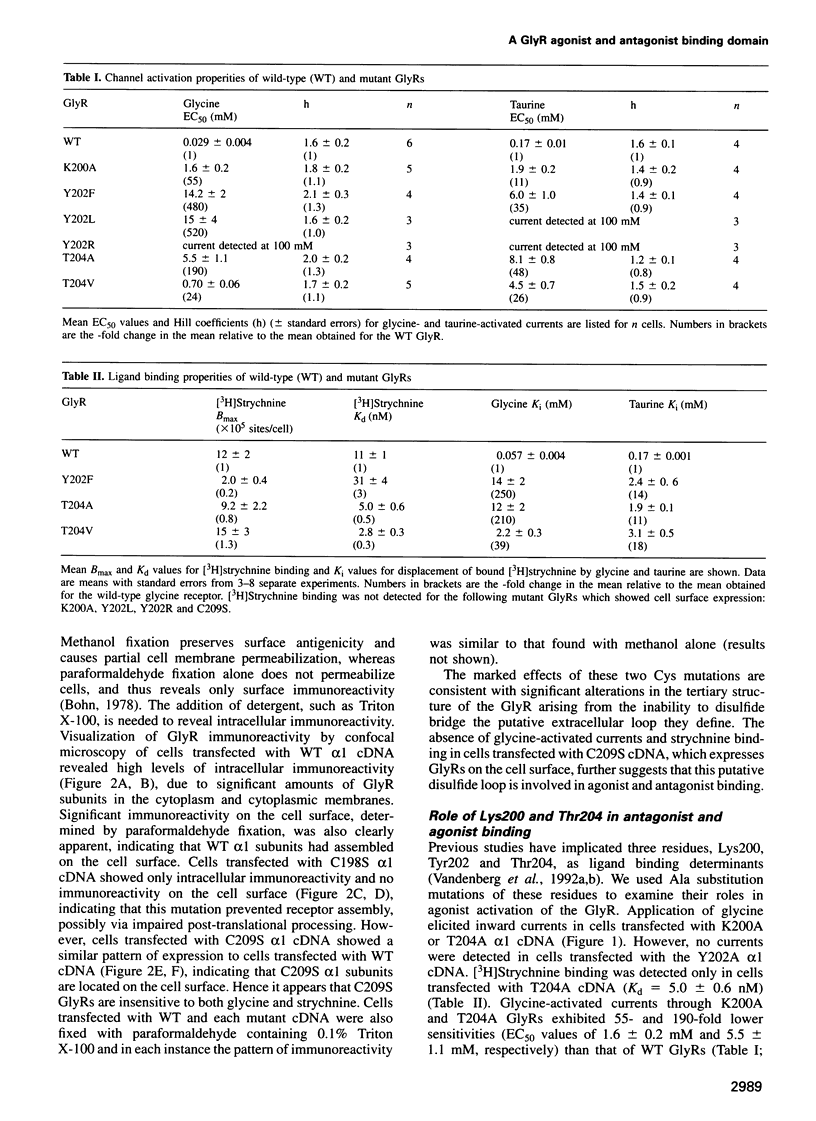
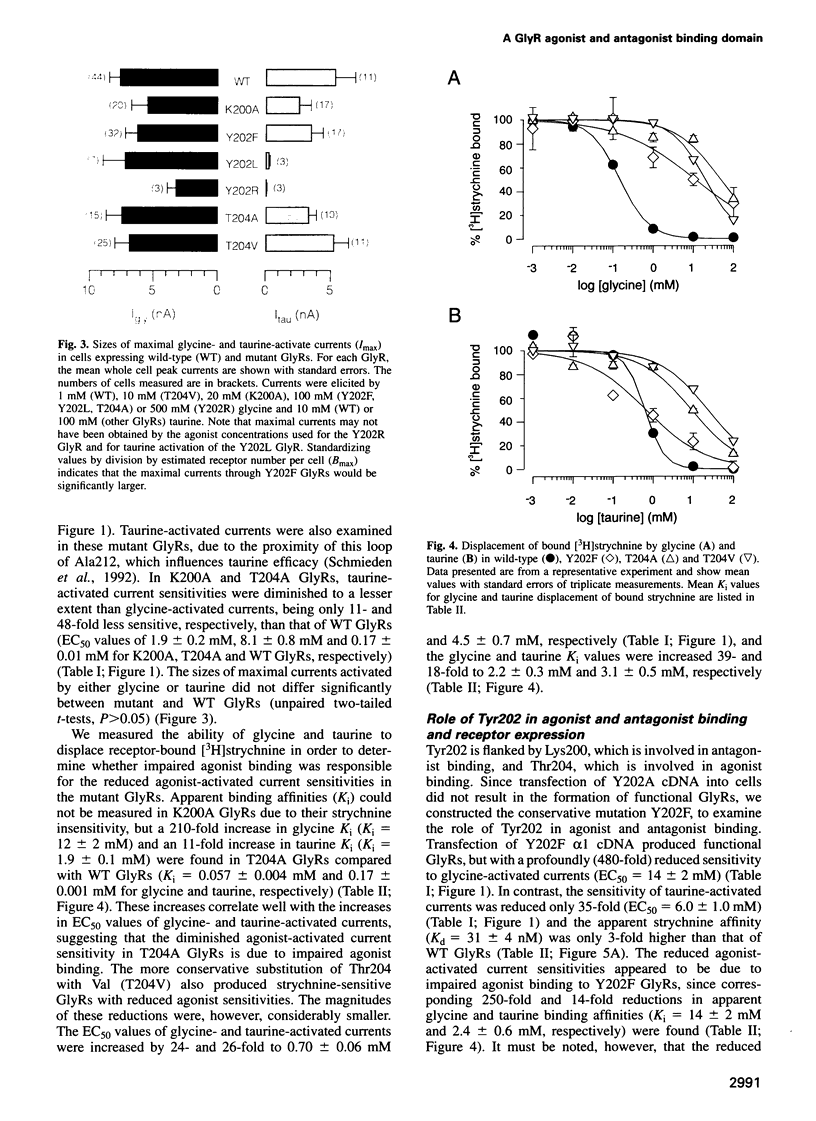
Abstract
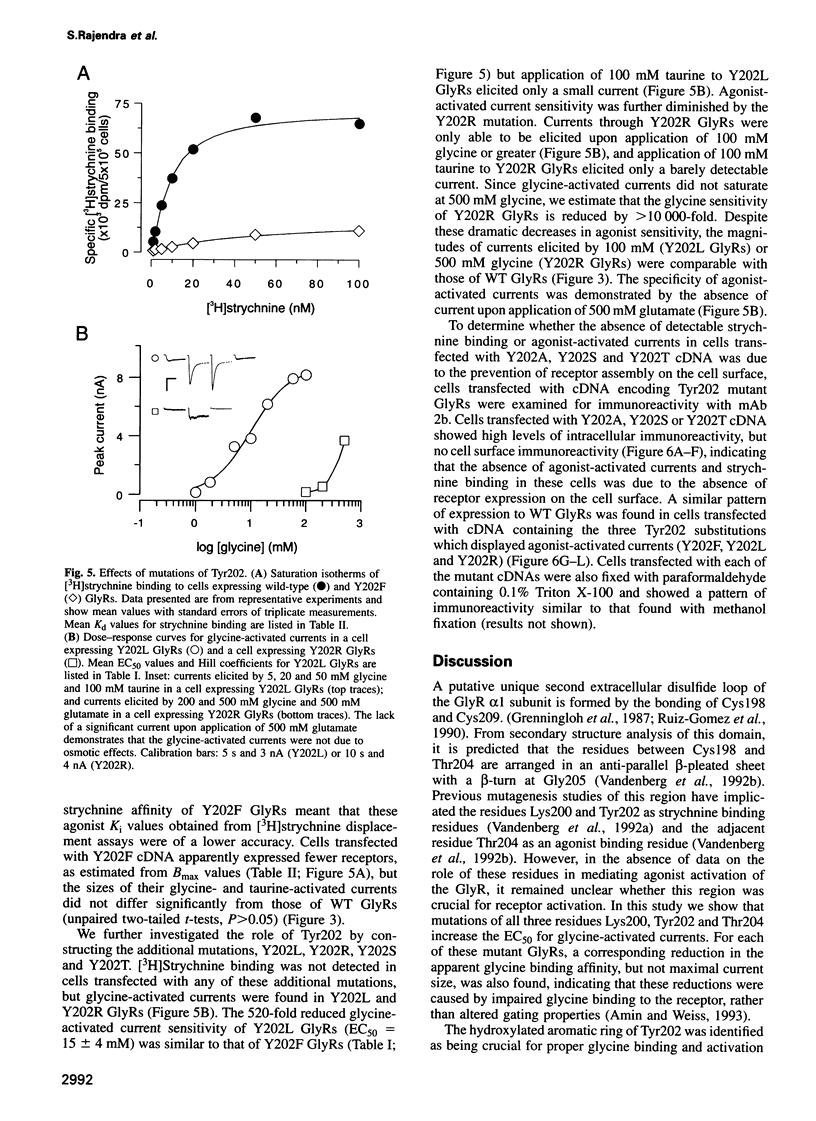
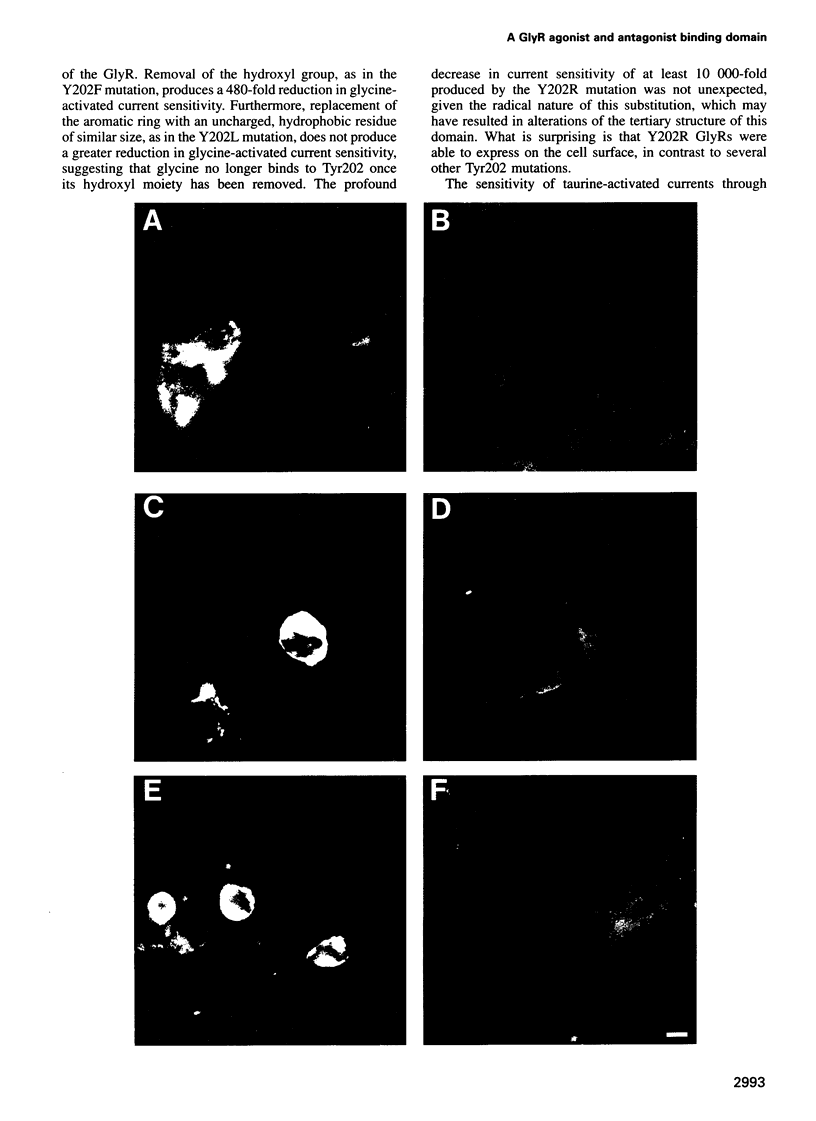
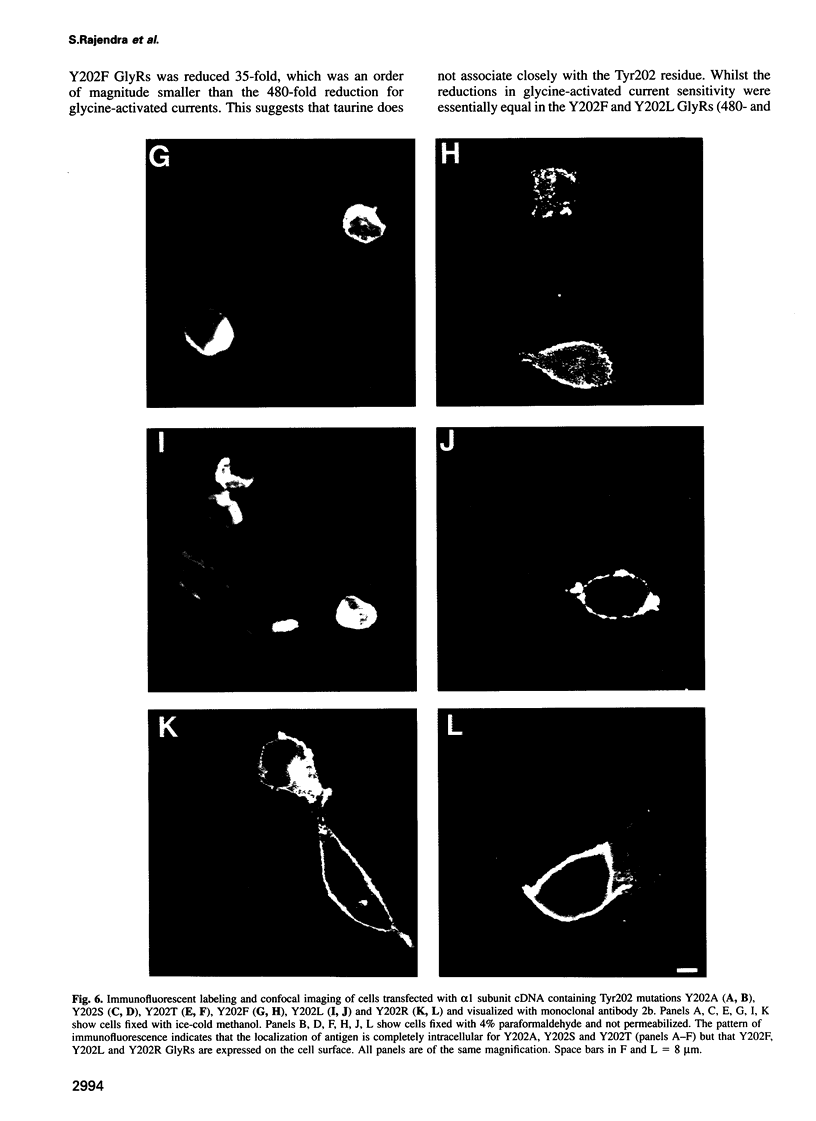
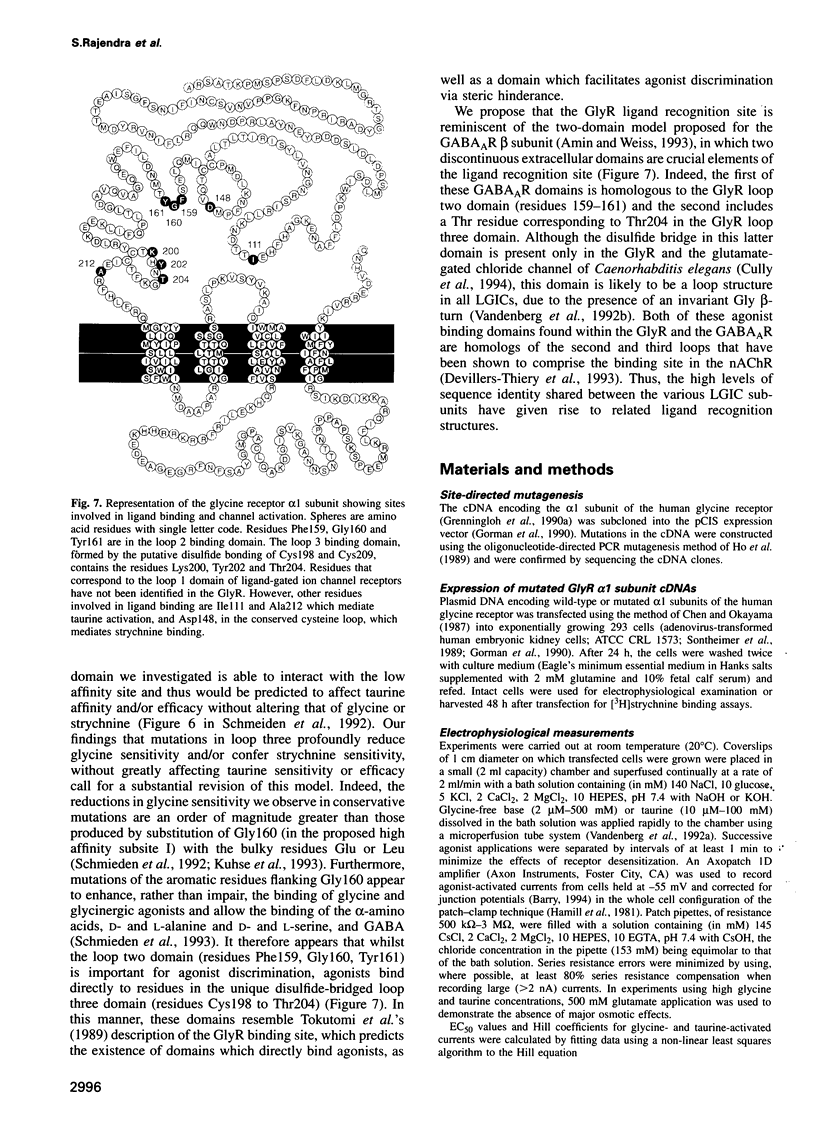
A loop structure, formed by the putative disulfide bridging of Cys198 and Cys209, is a principal element of the ligand binding site in the glycine receptor (GlyR). Disruption of the loop's tertiary structure by Ser mutations of these Cys residues either prevented receptor assembly on the cell surface, or created receptors unable to be activated by agonists or to bind the competitive antagonist, strychnine. Mutation of residues Lys200, Tyr202 and Thr204 within this loop reduced agonist binding and channel activation sensitivities by up to 55-, 520- and 190-fold, respectively, without altering maximal current sizes, and mutations of Lys200 and Tyr202 abolished strychnine binding to the receptor. Removal of the hydroxyl moiety from Tyr202 by mutation to Phe profoundly reduced agonist sensitivity, whilst removal of the benzene ring abolished strychnine binding, thus demonstrating that Tyr202 is crucial for both agonist and antagonist binding to the GlyR. Tyr202 also influences receptor assembly on the cell surface, with only large chain substitutions (Phe, Leu and Arg, but not Thr, Ser and Ala) forming functional receptors. Our data demonstrate the presence of a second ligand binding site in the GlyR, consistent with the three-loop model of ligand binding to the ligand-gated ion channel superfamily.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amin J., Weiss D. S. GABAA receptor needs two homologous domains of the beta-subunit for activation by GABA but not by pentobarbital. Nature. 1993 Dec 9;366(6455):565–569. doi: 10.1038/366565a0. [DOI] [PubMed] [Google Scholar]
- Barry P. H. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J Neurosci Methods. 1994 Jan;51(1):107–116. doi: 10.1016/0165-0270(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Bohn W. A fixation method for improved antibody penetration in electron microscopical immunoperoxidase studies. J Histochem Cytochem. 1978 Apr;26(4):293–297. doi: 10.1177/26.4.77869. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cully D. F., Vassilatis D. K., Liu K. K., Paress P. S., Van der Ploeg L. H., Schaeffer J. M., Arena J. P. Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature. 1994 Oct 20;371(6499):707–711. doi: 10.1038/371707a0. [DOI] [PubMed] [Google Scholar]
- Devillers-Thiéry A., Galzi J. L., Eiselé J. L., Bertrand S., Bertrand D., Changeux J. P. Functional architecture of the nicotinic acetylcholine receptor: a prototype of ligand-gated ion channels. J Membr Biol. 1993 Nov;136(2):97–112. doi: 10.1007/BF02505755. [DOI] [PubMed] [Google Scholar]
- Grenningloh G., Pribilla I., Prior P., Multhaup G., Beyreuther K., Taleb O., Betz H. Cloning and expression of the 58 kd beta subunit of the inhibitory glycine receptor. Neuron. 1990 Jun;4(6):963–970. doi: 10.1016/0896-6273(90)90149-a. [DOI] [PubMed] [Google Scholar]
- Grenningloh G., Rienitz A., Schmitt B., Methfessel C., Zensen M., Beyreuther K., Gundelfinger E. D., Betz H. The strychnine-binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors. Nature. 1987 Jul 16;328(6127):215–220. doi: 10.1038/328215a0. [DOI] [PubMed] [Google Scholar]
- Grenningloh G., Schmieden V., Schofield P. R., Seeburg P. H., Siddique T., Mohandas T. K., Becker C. M., Betz H. Alpha subunit variants of the human glycine receptor: primary structures, functional expression and chromosomal localization of the corresponding genes. EMBO J. 1990 Mar;9(3):771–776. doi: 10.1002/j.1460-2075.1990.tb08172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Kuhse J., Laube B., Magalei D., Betz H. Assembly of the inhibitory glycine receptor: identification of amino acid sequence motifs governing subunit stoichiometry. Neuron. 1993 Dec;11(6):1049–1056. doi: 10.1016/0896-6273(93)90218-g. [DOI] [PubMed] [Google Scholar]
- Kuhse J., Schmieden V., Betz H. A single amino acid exchange alters the pharmacology of neonatal rat glycine receptor subunit. Neuron. 1990 Dec;5(6):867–873. doi: 10.1016/0896-6273(90)90346-h. [DOI] [PubMed] [Google Scholar]
- Langosch D., Thomas L., Betz H. Conserved quaternary structure of ligand-gated ion channels: the postsynaptic glycine receptor is a pentamer. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7394–7398. doi: 10.1073/pnas.85.19.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricq A. V., Peterson A. S., Brake A. J., Myers R. M., Julius D. Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science. 1991 Oct 18;254(5030):432–437. doi: 10.1126/science.1718042. [DOI] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Furutani Y., Hirose T., Asai M., Inayama S., Miyata T., Numa S. Primary structure of alpha-subunit precursor of Torpedo californica acetylcholine receptor deduced from cDNA sequence. Nature. 1982 Oct 28;299(5886):793–797. doi: 10.1038/299793a0. [DOI] [PubMed] [Google Scholar]
- Pan Z. H., Bähring R., Grantyn R., Lipton S. A. Differential modulation by sulfhydryl redox agents and glutathione of GABA- and glycine-evoked currents in rat retinal ganglion cells. J Neurosci. 1995 Feb;15(2):1384–1391. doi: 10.1523/JNEUROSCI.15-02-01384.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer F., Simler R., Grenningloh G., Betz H. Monoclonal antibodies and peptide mapping reveal structural similarities between the subunits of the glycine receptor of rat spinal cord. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7224–7227. doi: 10.1073/pnas.81.22.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Gómez A., Fernández-Shaw C., Morato E., Marvizón J. C., Vázquez J., Valdivieso F., Mayor F., Jr Sulfhydryl groups modulate the allosteric interaction between glycine binding sites at the inhibitory glycine receptor. J Neurochem. 1991 May;56(5):1690–1697. doi: 10.1111/j.1471-4159.1991.tb02069.x. [DOI] [PubMed] [Google Scholar]
- Ruiz-Gómez A., Morato E., García-Calvo M., Valdivieso F., Mayor F., Jr Localization of the strychnine binding site on the 48-kilodalton subunit of the glycine receptor. Biochemistry. 1990 Jul 31;29(30):7033–7040. doi: 10.1021/bi00482a012. [DOI] [PubMed] [Google Scholar]
- Schmieden V., Grenningloh G., Schofield P. R., Betz H. Functional expression in Xenopus oocytes of the strychnine binding 48 kd subunit of the glycine receptor. EMBO J. 1989 Mar;8(3):695–700. doi: 10.1002/j.1460-2075.1989.tb03428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieden V., Kuhse J., Betz H. Agonist pharmacology of neonatal and adult glycine receptor alpha subunits: identification of amino acid residues involved in taurine activation. EMBO J. 1992 Jun;11(6):2025–2032. doi: 10.1002/j.1460-2075.1992.tb05259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieden V., Kuhse J., Betz H. Mutation of glycine receptor subunit creates beta-alanine receptor responsive to GABA. Science. 1993 Oct 8;262(5131):256–258. doi: 10.1126/science.8211147. [DOI] [PubMed] [Google Scholar]
- Schofield P. R., Darlison M. G., Fujita N., Burt D. R., Stephenson F. A., Rodriguez H., Rhee L. M., Ramachandran J., Reale V., Glencorse T. A. Sequence and functional expression of the GABA A receptor shows a ligand-gated receptor super-family. Nature. 1987 Jul 16;328(6127):221–227. doi: 10.1038/328221a0. [DOI] [PubMed] [Google Scholar]
- Schröder S., Hoch W., Becker C. M., Grenningloh G., Betz H. Mapping of antigenic epitopes on the alpha 1 subunit of the inhibitory glycine receptor. Biochemistry. 1991 Jan 8;30(1):42–47. doi: 10.1021/bi00215a007. [DOI] [PubMed] [Google Scholar]
- Sigel E., Baur R., Kellenberger S., Malherbe P. Point mutations affecting antagonist affinity and agonist dependent gating of GABAA receptor channels. EMBO J. 1992 Jun;11(6):2017–2023. doi: 10.1002/j.1460-2075.1992.tb05258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontheimer H., Becker C. M., Pritchett D. B., Schofield P. R., Grenningloh G., Kettenmann H., Betz H., Seeburg P. H. Functional chloride channels by mammalian cell expression of rat glycine receptor subunit. Neuron. 1989 May;2(5):1491–1497. doi: 10.1016/0896-6273(89)90195-5. [DOI] [PubMed] [Google Scholar]
- Tokutomi N., Kaneda M., Akaike N. What confers specificity on glycine for its receptor site? Br J Pharmacol. 1989 Jun;97(2):353–360. doi: 10.1111/j.1476-5381.1989.tb11961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg R. J., French C. R., Barry P. H., Shine J., Schofield P. R. Antagonism of ligand-gated ion channel receptors: two domains of the glycine receptor alpha subunit form the strychnine-binding site. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1765–1769. doi: 10.1073/pnas.89.5.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg R. J., Handford C. A., Schofield P. R. Distinct agonist- and antagonist-binding sites on the glycine receptor. Neuron. 1992 Sep;9(3):491–496. doi: 10.1016/0896-6273(92)90186-h. [DOI] [PubMed] [Google Scholar]
- Vandenberg R. J., Rajendra S., French C. R., Barry P. H., Schofield P. R. The extracellular disulfide loop motif of the inhibitory glycine receptor does not form the agonist binding site. Mol Pharmacol. 1993 Jul;44(1):198–203. [PubMed] [Google Scholar]