Abstract
While subtypes 1 and 2 of the ryanodine receptor (RyR) function as intracellular Ca2+ release channels, little is known about the function of the third subtype (RyR-3), first identified in brain. Myocytes from mice homozygous for a targeted mutation in the RyR-1 gene (dyspedic mice) can now be used for a study on the function of RyR-3, which is predominantly expressed in these cells according to our reverse transcription-polymerase chain reaction analysis. We here demonstrate in these myocytes caffeine-, ryanodine- and adenine nucleotide-sensitive Ca(2+)-induced Ca2+ release with approximately 10 times lower sensitivity to Ca2+ than that of RyR-1. Although RyR-3 does not mediate excitation-contraction coupling of the skeletal muscle type, we propose that RyR-3 may induce intracellular Ca2+ release in response to a Ca2+ rise with a high threshold.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams B. A., Beam K. G. Muscular dysgenesis in mice: a model system for studying excitation-contraction coupling. FASEB J. 1990 Jul;4(10):2809–2816. doi: 10.1096/fasebj.4.10.2165014. [DOI] [PubMed] [Google Scholar]
- Beam K. G., Knudson C. M., Powell J. A. A lethal mutation in mice eliminates the slow calcium current in skeletal muscle cells. Nature. 1986 Mar 13;320(6058):168–170. doi: 10.1038/320168a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Block B. A., Imagawa T., Campbell K. P., Franzini-Armstrong C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J Cell Biol. 1988 Dec;107(6 Pt 2):2587–2600. doi: 10.1083/jcb.107.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coronado R., Morrissette J., Sukhareva M., Vaughan D. M. Structure and function of ryanodine receptors. Am J Physiol. 1994 Jun;266(6 Pt 1):C1485–C1504. doi: 10.1152/ajpcell.1994.266.6.C1485. [DOI] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Inui M. Biochemistry and biophysics of excitation-contraction coupling. Annu Rev Biophys Biophys Chem. 1989;18:333–364. doi: 10.1146/annurev.bb.18.060189.002001. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Ogunbunmi E. M., Dixon M. C., Fleer E. A. Localization of Ca2+ release channels with ryanodine in junctional terminal cisternae of sarcoplasmic reticulum of fast skeletal muscle. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7256–7259. doi: 10.1073/pnas.82.21.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi T., Furutama D., Hakamata Y., Nakai J., Takeshima H., Mikoshiba K. Multiple types of ryanodine receptor/Ca2+ release channels are differentially expressed in rabbit brain. J Neurosci. 1994 Aug;14(8):4794–4805. doi: 10.1523/JNEUROSCI.14-08-04794.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini G., Clementi E., Ceci R., Marziali G., Sorrentino V. Expression of a ryanodine receptor-Ca2+ channel that is regulated by TGF-beta. Science. 1992 Jul 3;257(5066):91–94. doi: 10.1126/science.1320290. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hakamata Y., Nakai J., Takeshima H., Imoto K. Primary structure and distribution of a novel ryanodine receptor/calcium release channel from rabbit brain. FEBS Lett. 1992 Nov 9;312(2-3):229–235. doi: 10.1016/0014-5793(92)80941-9. [DOI] [PubMed] [Google Scholar]
- Iino M. Calcium-induced calcium release mechanism in guinea pig taenia caeci. J Gen Physiol. 1989 Aug;94(2):363–383. doi: 10.1085/jgp.94.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F. A., Erickson H. P., Rousseau E., Liu Q. Y., Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988 Jan 28;331(6154):315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- McPherson P. S., Campbell K. P. The ryanodine receptor/Ca2+ release channel. J Biol Chem. 1993 Jul 5;268(19):13765–13768. [PubMed] [Google Scholar]
- Meissner G. Ryanodine receptor/Ca2+ release channels and their regulation by endogenous effectors. Annu Rev Physiol. 1994;56:485–508. doi: 10.1146/annurev.ph.56.030194.002413. [DOI] [PubMed] [Google Scholar]
- Murayama T., Ogawa Y. Purification and characterization of two ryanodine-binding protein isoforms from sarcoplasmic reticulum of bullfrog skeletal muscle. J Biochem. 1992 Oct;112(4):514–522. doi: 10.1093/oxfordjournals.jbchem.a123931. [DOI] [PubMed] [Google Scholar]
- Nakai J., Imagawa T., Hakamat Y., Shigekawa M., Takeshima H., Numa S. Primary structure and functional expression from cDNA of the cardiac ryanodine receptor/calcium release channel. FEBS Lett. 1990 Oct 1;271(1-2):169–177. doi: 10.1016/0014-5793(90)80399-4. [DOI] [PubMed] [Google Scholar]
- Näbauer M., Callewaert G., Cleemann L., Morad M. Regulation of calcium release is gated by calcium current, not gating charge, in cardiac myocytes. Science. 1989 May 19;244(4906):800–803. doi: 10.1126/science.2543067. [DOI] [PubMed] [Google Scholar]
- Otsu K., Willard H. F., Khanna V. K., Zorzato F., Green N. M., MacLennan D. H. Molecular cloning of cDNA encoding the Ca2+ release channel (ryanodine receptor) of rabbit cardiac muscle sarcoplasmic reticulum. J Biol Chem. 1990 Aug 15;265(23):13472–13483. [PubMed] [Google Scholar]
- Oyamada H., Iino M., Endo M. Effects of ryanodine on the properties of Ca2+ release from the sarcoplasmic reticulum in skinned skeletal muscle fibres of the frog. J Physiol. 1993 Oct;470:335–348. doi: 10.1113/jphysiol.1993.sp019861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyamada H., Murayama T., Takagi T., Iino M., Iwabe N., Miyata T., Ogawa Y., Endo M. Primary structure and distribution of ryanodine-binding protein isoforms of the bullfrog skeletal muscle. J Biol Chem. 1994 Jun 24;269(25):17206–17214. [PubMed] [Google Scholar]
- Percival A. L., Williams A. J., Kenyon J. L., Grinsell M. M., Airey J. A., Sutko J. L. Chicken skeletal muscle ryanodine receptor isoforms: ion channel properties. Biophys J. 1994 Nov;67(5):1834–1850. doi: 10.1016/S0006-3495(94)80665-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzan T., Rizzuto R., Volpe P., Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev. 1994 Jul;74(3):595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- Ríos E., Pizarro G. Voltage sensor of excitation-contraction coupling in skeletal muscle. Physiol Rev. 1991 Jul;71(3):849–908. doi: 10.1152/physrev.1991.71.3.849. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Schneider M. F. Control of calcium release in functioning skeletal muscle fibers. Annu Rev Physiol. 1994;56:463–484. doi: 10.1146/annurev.ph.56.030194.002335. [DOI] [PubMed] [Google Scholar]
- Seok J. H., Xu L., Kramarcy N. R., Sealock R., Meissner G. The 30 S lobster skeletal muscle Ca2+ release channel (ryanodine receptor) has functional properties distinct from the mammalian channel proteins. J Biol Chem. 1992 Aug 5;267(22):15893–15901. [PubMed] [Google Scholar]
- Sutko J. L., Airey J. A., Murakami K., Takeda M., Beck C., Deerinck T., Ellisman M. H. Foot protein isoforms are expressed at different times during embryonic chick skeletal muscle development. J Cell Biol. 1991 May;113(4):793–803. doi: 10.1083/jcb.113.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
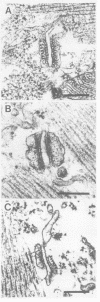
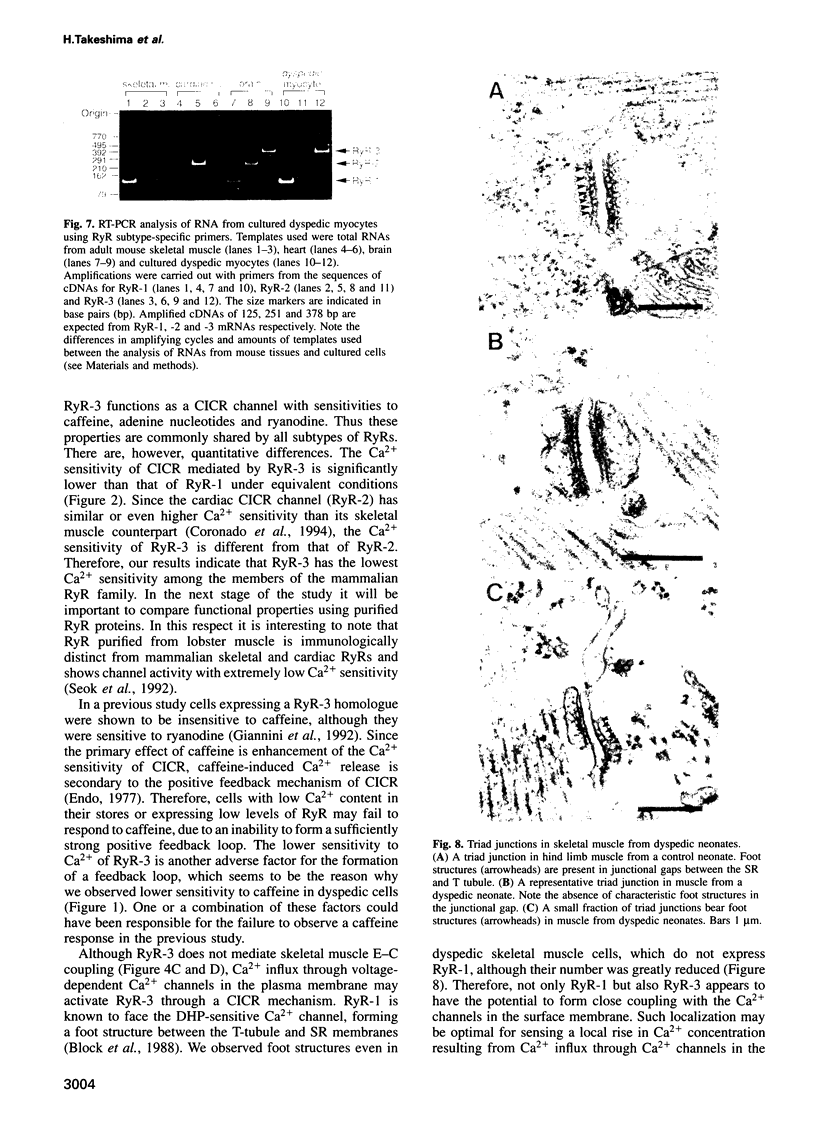
- Takekura H., Nishi M., Noda T., Takeshima H., Franzini-Armstrong C. Abnormal junctions between surface membrane and sarcoplasmic reticulum in skeletal muscle with a mutation targeted to the ryanodine receptor. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3381–3385. doi: 10.1073/pnas.92.8.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshima H., Iino M., Takekura H., Nishi M., Kuno J., Minowa O., Takano H., Noda T. Excitation-contraction uncoupling and muscular degeneration in mice lacking functional skeletal muscle ryanodine-receptor gene. Nature. 1994 Jun 16;369(6481):556–559. doi: 10.1038/369556a0. [DOI] [PubMed] [Google Scholar]
- Takeshima H., Nishimura S., Matsumoto T., Ishida H., Kangawa K., Minamino N., Matsuo H., Ueda M., Hanaoka M., Hirose T. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature. 1989 Jun 8;339(6224):439–445. doi: 10.1038/339439a0. [DOI] [PubMed] [Google Scholar]
- Takeshima H., Nishimura S., Nishi M., Ikeda M., Sugimoto T. A brain-specific transcript from the 3'-terminal region of the skeletal muscle ryanodine receptor gene. FEBS Lett. 1993 May 10;322(2):105–110. doi: 10.1016/0014-5793(93)81547-d. [DOI] [PubMed] [Google Scholar]
- Zorzato F., Fujii J., Otsu K., Phillips M., Green N. M., Lai F. A., Meissner G., MacLennan D. H. Molecular cloning of cDNA encoding human and rabbit forms of the Ca2+ release channel (ryanodine receptor) of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1990 Feb 5;265(4):2244–2256. [PubMed] [Google Scholar]