Abstract
Endocrine neoplasia syndromes feature a wide spectrum of benign and malignant tumors of endocrine and non-endocrine organs associated with other clinical manifestations. This study outlines the main clinical features, genetic basis, and molecular mechanisms behind two multiple endocrine neoplasia syndromes that share quite a bit of similarities, but one can be inherited whereas the other is always sporadic, Carney complex (CNC) and McCune-Albright (MAS), respectively. Spotty skin pigmentation, cardiac and other myxomas, and different types of endocrine tumors and other characterize Carney complex, which is caused largely by inactivating Protein Kinase A, Regulatory subunit, type I, Alpha (PRKAR1A) gene mutations. The main features of McCune-Albright are fibrous dysplasia of bone (FD), café-au-lait macules and precocious puberty; the disease is caused by activating mutations in the Guanine Nucleotide-binding protein, Alpha-stimulating activity polypeptide (GNAS) gene which are always somatic. We review the clinical manifestations of the two syndromes and provide an update on their molecular genetics
Keywords: Carney Complex, PRKAR1A, McCune-Albright, GNAS, endocrine neoplasia
1. Carney complex (CNC)
Carney complex (CNC, OMIM# 160980, 608837) was originally described in 1985 by J. Aidan Carney (Carney, Gordon et al. 1985) as “the complex of myxomas, spotty skin pigmentation and endocrine over activity”. The most common type of endocrine tumors in Carney complex is the primary pigmented nodular adrenocortical disease (PPNAD) (present in two-thirds of the patients), a form of bilateral adrenal hyperplasia that leads to adrenocorticotropic hormone (ACTH)-independent Cushing syndrome. Carney complex patients also display growth hormone (GH)- or growth hormone and prolactin (GH-PRL)-secreting (mammosomatroph) pituitary adenomas. Other endocrine glands that can be affected in the Carney complex include (1) the thyroid with thyroid nodules present in up to 75% of the patients and cancer developing rarely; and (2) the gonads: large-cell calcifying Sertoli cell tumors (LCCSCT), adrenal rest tumors (composed of ectopic primary pigmented nodular adrenocortical disease) and Leydig cell tumors may be found in male Carney complex patients (Stratakis, Kirschner et al. 2001; Burton, McDermott et al. 2006). Female Carney complex patients often develop ovarian cystadenomas and rarely cancer (Stratakis, Papageorgiou et al. 2000).
Among the non-endocrine tumors of Carney complex cardiac myxomas (present in 30–40% of Carney complex patients) are the most significant because although benign, due to their location, they cause significant mortality (Stratakis, Kirschner et al. 2001; Horvath and Stratakis 2009; Yin and Kirschner 2009; Espiard and Bertherat 2013). Skin lesions are, on the other hand, the most common Carney complex manifestation: lentigines are present in 70 to 75% of patients with Carney complex. Lentigines are hamartomatous melanocytic lesions that appear in a characteristic distribution in patients with Carney complex, on the vermillion border of the lips, on the eyelids and elsewhere on the face, the ears and the genital area. Blue nevi (present in 40% of Carney complex patients) are also typical of Carney complex, whereas other pigmented lesions (compound nevi, café-au-lait spots, variably pigmented areas) are less common. Cutaneous myxomas (present in up to one third of Carney complex patients) may appear anywhere but have particular predilection for locations such as on the eyelids, breast nipples, external ear canal and ear lobes, and the perineum (Carney, Gordon et al. 1985; Carney 1995; Stratakis, Kirschner et al. 2001; Horvath and Stratakis 2008; Mateus, Palangie et al. 2008; Espiard and Bertherat 2013). Breast myxomas also develop in females with Carney complex subjects (in up to 20% of the cases) and they are usually bilateral (Stratakis, Kirschner et al. 2001). Psammomatous melanotic schwannomas can be found rarely (in up to 8% of the subjects) anywhere, but mostly in the central nervous system, the gastrointestinal tract and along the paraspinal sympathetic chain. Recently, adrenal, hepatocellular and pancreatic cancer, as well as bone tumors (mostly osteochondromyxomas) have been reported as being part of Carney complex but are seen only rarely (Stratakis, Kirschner et al. 2001; Horvath and Stratakis 2008; Horvath and Stratakis 2009; Anselmo, Medeiros et al. 2012; Morin, Mete et al. 2012).
Carney complex may be inherited as an autosomal dominant trait but in a significant number of patients the disease is sporadic, presumably due to de novo mutations (Bertherat, Horvath et al. 2009). As an autosomal dominant multiple endocrine neoplasia, Carney complex shares common clinical features with McCune-Albright syndrome (MAS, OMIM# 174800), as well as with multiple endocrine neoplasias type 1 and 2 (MEN 1, OMIM# 131100 and MEN 2, OMIM# 171400, respectively). It also shares features with Peutz-Jeghers syndrome (PJS, OMIM#175200) (Oberg, Skogseid et al. 1989; Dumitrescu and Collins 2008; Beggs, Latchford et al. 2010), especially its skin pigmentation defects.
1.1 Genetics of Carney complex (CNC)
To date, two genetic loci associated with a predisposition to Carney Complex have been reported. The 17p22–24 locus referred as CNC1 and the 2p16 locus referred as CNC2 (Matyakhina, Pack et al. 2003; Bertherat, Horvath et al. 2009; Horvath, Bertherat et al. 2010). The Protein Kinase A, Regulatory, type I, Alpha (PRKAR1A) gene is located in CNC1. The gene(s) responsible for Carney complex at the CNC2 locus remain(s) unknown.
1.1.1 PRKAR1A mutations in Carney Complex (CNC1)
The regulatory subunit type I alpha of protein kinase A (PRKAR1A) gene has a total genomic length of 21kB and is situated at the band 24.2–24.3 of the short arm of chromosome 17. It consists of 11 exons, 10 of which are coding (exon 2–11). PRKAR1A has three transcript isoforms (NM_212471.1, NM_212472.1 and NM_002734.3) that differ at the 5′-untranslated region but share the same coding sequence (1143bp coding region, 384 amino acids) (Zawadzki and Taylor 2004).
To date, more than 120 disease-causing PRKAR1A mutations have been reported in Carney complex patients (Kirschner, Sandrini et al. 2000; Veugelers, Wilkes et al. 2004; Bertherat, Horvath et al. 2009; Horvath, Bertherat et al. 2010). Most of these mutations include single base substitutions and small deletions that do not exceed 15 kb. Small insertions and rearrangements have also been detected throughout the whole coding region of the gene (Blyth, Huang et al. 2008). Recently large PRKAR1A deletions up to 4Kb in size were also reported (Blyth, Huang et al. 2008; Horvath, Bossis et al. 2008).
PRKAR1A mutations are present in up to 70% of the patients diagnosed with Carney complex; the percentage of PRKAR1A mutations increases to 80% for those that present with Cushing syndrome due to primary pigmented nodular adrenocortical disease (PPNAD) (Cazabat, Ragazzon et al. 2006; Cazabat, Libe et al. 2007; Bertherat, Horvath et al. 2009).
Among the types of PRKAR1A mutations described above, nonsense substitutions, small indels, variations of splicing sites and other abnormalities that are detected before the end of the last exon, may lead to frame shifts and premature stop codons (PSC). Premature stop codons result in shorter mRNAs that in the case of PRKAR1A are not encoded to protein, but are rather degraded by the nonsense-mediated mRNA decay (NMD) mechanism with a subsequent decrease in the PRKAR1A mRNA and protein levels (Kirschner, Carney et al. 2000; Kirschner, Sandrini et al. 2000; Groussin, Kirschner et al. 2002). Thus, all mutations leading to a premature stop codon, regardless of the location or type of sequence defect, result in PRKAR1A haploinsufficiency.
Missense mutations of the PRKAR1A gene, short in-frame indels and splice variants that do not lead to a frameshift and/or a nonsense mRNA are expressed at the protein level. The cause of disease in these cases is not PRKAR1A haploinsufficiency but a variety of functional defects of the protein that depend on the position of the sequence change (Greene, Horvath et al. 2008; Meoli, Bossis et al. 2008). For example, substitutions within the cAMP-binding domain (i.e. D183Y, A213D), which can affect the affinity of the PRKAR1A protein to cAMP or the binding of the catalytic subunits of protein kinase A (PKA), seem to have the highest impact (Greene, Horvath et al. 2008). Another mutation (R146S) has been shown to affect the catalytic subunit’s binding to the PRKAR1A protein only (Greene, Horvath et al. 2008). A recurrent mutation at the start codon (M1V) leads to an alternate start codon 141bp downstream of the original; the mutant PRKAR1A binds less efficiently both cAMP and the catalytic subunits of the Protein kinase A tetramer (Pereira, Hes et al. 2010).
Multiple splice site mutations in the PRKAR1A gene have been reported and they are all pathogenic. In silico predictions showed either complete or decreased abrogation of the splice junctions. In some heterozygous cases, the expression ratio of wild type and mutant allele varied depending on the mutation and the tissue. For example the mutation c.709-7_709-2del causes a slight effect due to the small proportion that the mutant allele contributes to the total mRNA (Groussin, Horvath et al. 2006).
Another type of PRKAR1A mutations leads to a longer but insufficiently expressed protein: four of these mutations have been detected to date at the last exon of the gene, resulting in loss of the normal stop codon, protein elongation, and the use of a new stop codon downstream within the 3′ untranslated region (Patronas, Horvath et al. 2012). Longer PRKAR1A protein products undergo proteosomal degradation (Patronas, Horvath et al. 2012), and thus the mechanism of disease in these cases, too, is PRKAR1A haploinsufficiency.
1.1.2 The CNC2 locus on 2p16 (CNC 2)
The non-PRKAR1A-associated Carney complex phenotype is less severe, appears later in life, and mostly in a sporadic fashion (Bertherat, Horvath et al. 2009). Initially, linkage analyses in Carney complex patients led to the detection of a 10Mb region at chromosome 2p. The region 2p15–21 was cloned and a yeast artificial chromosome (YAC)-bacterial artificial chromosome (BAC) map was developed to study these sequences (Taymans, Kirschner et al. 1999; Kirschner, Sandrini et al. 2000; Matyakhina, Pack et al. 2003); however, this did not reveal any mutations of interest (Kirschner and Stratakis, unpublished data). Other studies showed amplification of the 2p16–23 in PRKAR1A-negative Carney complex patients, suggesting the existence of a possible oncogene in that region (Matyakhina, Pack et al. 2003) or a copy-number variant that is amplified in Carney complex tumors.
1.1.3 PDE11A, PDE8B and CTNNB1 mutations
Recent genome-wide studies in some sporadic cases of another form of bilateral adrenal hyperplasia (similar to primary pigmented nodular adrenocortical disease but not pigmented) isolated micronodular disease (iMAD) revealed mutations in two genes of the phosphodiesterase family, phosphodiesterase 11A (PDE11A) and phosphodiesterase 8B (PDE8B) (Gunther, Bourdeau et al. 2004; Horvath, Giatzakis et al. 2006; Horvath, Giatzakis et al. 2008; Stratakis 2013). It should be noted that patients with PDE11A and PDE8B defects do not have Carney complex.
Finally, some Carney complex patients that carry germline PRKAR1A defects may also develop somatic activating mutations of the beta-catenin gene (CTNNB1) in their adrenal lesions (Horvath, Mathyakina et al. 2006; Gaujoux, Tissier et al. 2008; Tadjine, Lampron et al. 2008).
1.2 Molecular mechanisms leading to tumor formation in Carney complex (CNC)
PRKAR1A gene encodes for the regulatory subunit type I-alpha (RIα) of protein kinase A (PKA). PKA is a heterotetramer formed by two regulatory (RIα, RIβ, RIIα, or RIIβ) and two catalytic subunits (Cα, Cβ, Cγ, or Cx). Stimulation of adenyl cyclases through G protein subunit (Gs) activation leads to cAMP synthesis and thus activation of Protein kinase A. More specifically, the regulatory subunits bind cAMP, which leads to the dissociation from the catalytic subunits (Figure 1). The catalytic subunits after their dissociation from the Protein kinase A complex phosphorylate many downstream factors such as cAMP response-binding protein (CREB). RIα inactivation thus leads to increased Protein kinase A activity (Kirschner, Carney et al. 2000; Yu, Ragazzon et al. 2012). How increased Protein kinase A activity leads to tumor formation is as much now under investigation, as it was more than a decade ago (Casey, Vaughan et al. 2000; Kirschner, Carney et al. 2000). Certainly activation of other pathways plays a role: Studies in lymphocytes from Carney complex patients with PRKAR1A mutations, for example, showed that higher PKA activity led to increased extracellular signal-regulated kinases 1/2 (ERK1/2) phosphorylation and increased cell proliferation through the activation of the mitogen–activated protein kinase (MAPK) pathway (Robinson-White, Hundley et al. 2003). However, ERK1/2 phosphorylation does not seem to be increased in adrenals of patients with primary pigmented nodular adrenocortical disease. Experiments in the H295 adrenocortical cells showed that PRKAR1A inactivation there leads to decreased expression of mothers against decapentaplegic homolog 3 (SMAD3) gene, which mediates the transforming growth factor beta (TGFβ) receptor signaling. This suggests a possible crosstalk between cAMP and TGFβ signaling in the pathogenesis of tumors in Carney complex (Ragazzon, Cazabat et al. 2009).
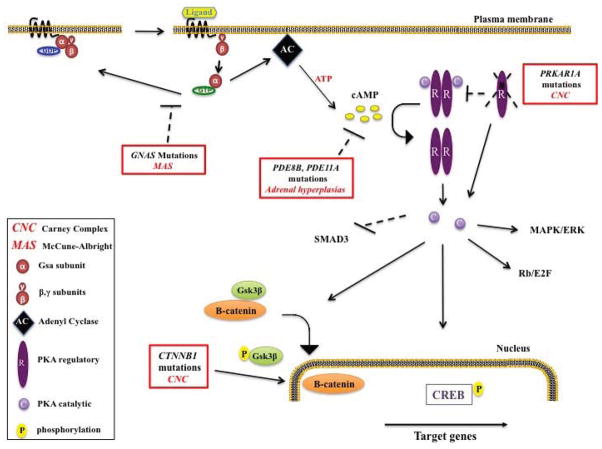
Figure 1.
Molecular mechanisms in Carney complex (CNC) and McCune-Albright syndrome (MAS): Receptor activation (ligand binding) makes Gsα to exchange GDP to GTP, Gsα is then freed from the β-γ dimer and activates adenyl cyclase (AC). Under normal conditions intrinsic turn off takes place through GTP-to-GDP exchange and Gsα gets inactivated. In McCune-Albright syndrome patients, GNAS mutations inhibit Gsa intrinsic turn off and leads to constant Gsa and AC activation. Activated AC produces cAMP, cAMP causes dissociation of the inactive protein kinase A (PKA) tetramer and then the catalytic subunits are freed to mediate serine-threonine phosphorylation of target molecules, including CREB. Phosphorylated CREB and other downstream targets will activate the interacting pathways that control metabolism, growth or proliferation. PRKAR1A inactivating mutations in Carney complex patients will result in less binding of the catalytic to the regulatory subunits and excessive cAMP signaling. PDE8B and PDE11A mutations in patients with adrenal hyperplasias lead to less cAMP degradation and to excess cAMP signaling. Downstream effects of the excessive cAMP/PKA signaling in Carney complex patients include higher CREB phosphorylation, possible MAPK and Rb/E2F pathway activation among others, SMAD3 inactivation, and increased β-catenin nuclear localization (activation of the Wnt pathway) at least in adrenal lesions of patients with Carney complex.
Several studies showed that the Wnt signaling pathway is an important mediator of the defects in proliferation due to increased cAMP and/or Protein kinase A signaling and PRKAR1A haploinsufficiency. It was known that cAMP signaling via Protein kinase A and its target transcription factor CREB (cAMP response-binding protein) are required for Wnt-directed myogenic gene expression (Chen, Ginty et al. 2005). Studies in primary pigmented nodular adrenocortical disease and other forms of adrenal hyperplasia showed overexpression of genes that are part of the Wnt pathway, such as the wingless-type MMTV integration site family, member 1 (WNT1)-inducible signaling pathway protein 2 (WISP2), Glycogen synthase kinase 3 beta (GSK3β) and Catenin (cadherin-associated protein) beta 1 (CTNNB1) (Bourdeau, Antonini et al. 2004; Horvath, Mathyakina et al. 2006; Stratakis 2013). The role of Wnt pathway in Carney complex was also further supported by the detection of somatic activating mutations of CTNNB1 in the adrenal lesions of Carney complex patients (Horvath, Mathyakina et al. 2006; Gaujoux, Tissier et al. 2008; Tadjine, Lampron et al. 2008). This is a rare finding: somatic CTNNB1 mutations in Carney complex have only been reported in adrenal adenomas developing in the context of primary pigmented adrenocortical disease. Thus, they may only be suspected in patients with rapid advancement of adrenocorticotropic (ACTH)-independent Cushing syndrome who had been previously diagnosed with the insidious form of Cushing syndrome associated with Carney complex; if an imaging study, shows growth of an adenoma, it is likely that this tumor contains a CTNNB1 somatic mutation.
Other pathways may also be involved: experiments in prkar1a−/− mouse fibroblast immortalized cell lines and human adrenal transfected PRKAR1A-haploinsufficient cells showed subsequent deregulation of some cyclins and of the E2-promoter binding transcription factor (E2F) (Nadella and Kirschner 2005; Nesterova, Bossis et al. 2008).
Primary pigmented adrenocortical disease is the most common manifestation in Carney complex and familial and isolated (sporadic) PPNAD may be caused by the inactivating mutations in PRKAR1A gene. Primary pigmented nodular adrenocortical disease (PPNAD) is a pigmented form of micronodular adrenocortical disease (MAD, when isolated iMAD) which is a distinct type of adrenocorticotropic hormone (ACTH)-independent bilateral adrenocortical hyperplasia characterized by multiple nodules, resulting in extra-adrenal cortical excrescences and hyperplasia of the cortex. Genome wide studies in sporadic both pigmented and non-pigmented adrenocortical hyperplasia cases (isolated micronodular disease, iMAD), that do not have Carney complex, revealed inactivating mutations of the phosphodiesterase gene 11A (PDE11A). PDE11A inactivation leads to increased cAMP levels and increased protein kinase A signaling, an effect similar to that of PRKAR1A inactivating mutations (Gunther, Bourdeau et al. 2004; Horvath, Boikos et al. 2006; Stratakis 2009; Vezzosi, Libe et al. 2012; Stratakis 2013). Finally, a germline Phosphodiesterase 8B (PDE8B) missense substitution (c.914A>C/H305P) has been described in a patient with pigmented isolated micronodular disease unassociated with Carney complex (Horvath, Giatzakis et al. 2008; Stratakis 2009).
2. McCune Albright syndrome (MAS)
McCune Albright syndrome is a rare disease that initially was defined as the triad of polyostotic fibrous dysplasia of bone (FD), café au-lait skin pigmentation, and precocious puberty (PP). Later, hyperthyroidism, growth hormone excess, renal phosphate wasting with or without rickets/osteomalacia and Cushing syndrome were also described as part of the condition (Danon and Crawford 1987; Sherman and Ladenson 1992; Mastorakos, Mitsiades et al. 1997; Kirk, Brain et al. 1999; Collins, Chebli et al. 2001; Akintoye, Chebli et al. 2002; Collins, Singer et al. 2012). More recently, hepatic and cardiac involvement in McCune-Albright syndrome has also been reported (Weinstein, Shenker et al. 1991; Silva, Lumbroso et al. 2000; Dumitrescu and Collins 2008).
Fibrous dysplasia (FD) is one of the initial symptoms of the disease. The areas that most commonly display fibrous dysplasia are the proximal femurs and the skull base. The bone in Fibrous dysplasias is characterized by expansive lesions with endosteal scalloping, thin cortex, and an intramedullary tissue matrix showing a “ground class” appearance. In some cases (less than 1%), malignancy (a sarcoma) may develop from within fibrous dysplasia (Ruggieri, Sim et al. 1994; Leet, Chebli et al. 2004; Hart, Kelly et al. 2007; Dumitrescu and Collins 2008; Collins, Singer et al. 2012).
Café au-lait spots are present in McCune-Albright syndrome patients and usually appear shortly after birth. There is no correlation between size of the spots and the severity of the disease (Dumitrescu and Collins 2008; Collins, Singer et al. 2012).
Beyond fibrous dysplasia and the skin lesions, precocious puberty (PP) is the most common endocrine manifestation of McCune-Albright syndrome. It is more common in girls than in boys. Typically vaginal bleeding or spotting is followed by the development of breast tissue. Precocius puberty in females is caused by elevated serum estradiol levels due to intermittent autonomous activation of the ovaries (Kim, Kim et al. 1999; Dumitrescu and Collins 2008; Collins, Singer et al. 2012). In male McCune-Albright syndrome patients, bilateral or unilateral testicular tumors are associated with penile enlargement due to excess testosterone. Leydig cell and/or Sertoli cell tumors or hyperplasia are common (Dumitrescu and Collins 2008; Collins, Singer et al. 2012). Besides the gonads, thyroid is another endocrine gland with manifestations in McCune-Albtright syndrome: hyperthyroidism is present in 38% of McCune-Albright syndrome cases. More commonly, in up to 63% of McCune-Albright syndrome patients, one sees suppressed levels of thyroid stimulating hormone (TSH) accompanied by elevated triiodothyronine (T3+) and abnormal thyroid gland morphology without overt hyperthryoidism. Thyroid cancer develops rarely (Feuillan, Shawker et al. 1990; Collins, Sarlis et al. 2003; Dumitrescu and Collins 2008; Collins, Singer et al. 2012). Renal phosphate wasting is also detected, as part of a tubulopathy, with or without hypophosphatemia and/or rickets/osteomalacia. These symptoms are caused due to the release of fibroblast growth factor-23 (FGF23) by bone tissue affected by fibrous dysplasia (Collins, Chebli et al. 2001; Riminucci, Collins et al. 2003).
The pituitary gland is also affected in McCune-Albright syndrome cases since growth hormone (GH) and prolactin (PRL) excess are common: up to 21% of the patients have abnormal growth hormone and/or prolactin levels. These findings are important since the growth hormone excess can worsen craniofacial bone disease (Akintoye, Chebli et al. 2002). Cushing syndrome by nodular adrenal hyperplasia also occurs but only in neonatal McCune-Albright syndrome patients (Kirk, Brain et al. 1999). In a recent study a Primary Bimorphic Adrenocortical Disease (PBAD) was described causing hypercortisolism in McCune-Albright syndrome patients (Carney, Young et al. 2011).
2.1 Genetics of McCune Albright syndrome (MAS)
McCune-Albright Syndrome (MAS) is caused by mosaicism for a mutation in the Guanine Nucleotide-binding protein, Alpha-Stimulating activity polypeptide (GNAS) gene (Table 1). The GNAS mutated alleles are always in equal or less abundance than the wild type allele, proving that they act in a dominant way.
TABLE 1.
Clinical and genetic data in CNC and MAS
| Syndrome | Clinical manifestations | Mutations |
|---|---|---|
| Carney complex (CNC) | Skin pigmented lesions (lentigines, blue nevi, other) Myxomas (cutaneous, mucosal, cardiac, other) PPNAD (Cushing Syndrome) GH-PRL-producing adenomas (acromegaly) Leydig or Sertoli cell tumors Ovarian cystadenoma or cancer Thyroid adenoma or cancer Psammomatous melanotic schwannoma Breast ductal adenoma Osteochondromyxoma Cancers (liver, pancreas, adrenal) |
PRKAR1A: Small substitutions, indels (result in alternative splicing sites or stop codon due to frame shift) Missense mutations (altered RIa protein) (Horvath et al., 2010) 2p16 amplification PDE11A, PDE8B, CTNNB1 (Horvath et al., 2008, Tadjine et al., 2008, Horvath et al., 2006) |
|
| ||
| McCune-Albright (MAS) | Fibrous Dysplasia (FD) Precocious Puberty (PP) Hyperthyroidism Renal phosphate wasting GH-producing adenomas Cushing Syndrome |
GNAS Missense mutations (R201C or R201H or R201S or R201G or Q227R or Q227K) |
PPNAD: Primary Pigmented Nodular Adrenocortical Disease; GH: Growth Hormone; PRL: Prolactin
GNAS maps in chromosome 20q13 and encodes the ubiquitously expressed stimulatory subunit alpha of the G protein (Gsa). Gsa activates the adenyl cyclase and leads to the generation of cAMP. Mutations in GNAS were initially detected in growth hormone (GH)-producing tumors and later the same genetic defect was identified in McCune-Albright syndrome. The most common GNAS mutations in McCune-Albright syndrome are the ones who lead to the amino acid substitutions at Arg 201 (to Cys or His or Ser or Gly) and rarely at Gln 227 (to Arg or Lys). These mutations cause a constitutively activated form of Gsa which lead to high adenyl cyclase activity and cAMP levels (Vallar, Spada et al. 1987; Landis, Masters et al. 1989; Lyons, Landis et al. 1990; Weinstein, Shenker et al. 1991; Yang, Park et al. 1996; Riminucci, Fisher et al. 1999; Vortmeyer, Glasker et al. 2012).
Patients with McCune-Albright syndrome present sporadically because constitutive activation of the GNAS gene in the germline is incompatible with life. Each case represents a de novo defect and the mutation is present in multiple tissues in variable abundance (Weinstein, Shenker et al. 1991; Schwindinger, Francomano et al. 1992; Malchoff, Reardon et al. 1994; Shenker, Weinstein et al. 1994; Alman, Greel et al. 1996; Kim, Kim et al. 1999; Bianco, Riminucci et al. 2000).
The GNAS gene in a number of tissues, including the pituitary and thyroid glands, renal proximal tubes and the ovary is transcribed mainly from the maternal allele due to imprinting (Hayward, Barlier et al. 2001; Mantovani, Ballare et al. 2002; Linglart, Maupetit-Mehouas et al. 2013). For example, McCune-Albright syndrome patients with growth hormone (GH)-secreting pituitary adenomas bear their GNAS mutations on the maternal allele; allele-biased mutations are not seen in other tissues where the GNAS gene is not subject to imprinting (Hayward, Barlier et al. 2001; Mantovani, Ballare et al. 2002; Linglart, Maupetit-Mehouas et al. 2013).
2.2 Molecular mechanisms of tumor formation in McCune-Albright syndrome
The Guanine Nucleotide-binding protein, Alpha-stimulating activity polypeptide (GNAS) gene encodes for the ubiquitously expressed stimulatory subunit alpha of the G protein (Gsα). G protein couples hormone receptors to adenyl cyclase, which is necessary for the generation of intracellular cAMP that mediates G-protein-coupled hormone signaling. The reported GNAS mutations in Arg 201 and Gln 227 in McCune-Albright syndrome inhibit the guanosine triphosphate hydrolase (GTPase) catalytic ability of Gsa making impossible to control the Gsa activation and as a result there is excessive cAMP production, even in the absence of stimulating hormones (Figure 1) (Landis, Masters et al. 1989; Lyons, Landis et al. 1990; Horvath and Stratakis 2008; Xekouki, Azevedo et al. 2010). Persistently high levels of cAMP activate PKA, which explains the similarities between Carney complex and McCune-Albright syndrome. For example, the pathology of the pituitary in McCune-Albright syndrome patients that carry GNAS mutations and in Carney complex patients that carry PRKAR1A mutations is almost identical (Spada, Arosio et al. 1990; Bertherat, Chanson et al. 1995; Picard, Silvy et al. 2007; Xekouki, Azevedo et al. 2010). Likewise, the skin manifestations in the two diseases are due to excess melanin production by the affected melanocytes (Kim, Kim et al. 1999).
Like in Carney complex, other pathways are implicated downstream of excess cAMP in the manifestations in the various tissues. In the bone, Gsα/cAMP activation increases cellular oncogene fos (c-fos gene) expression and this has been shown in fibrous dysplasia lesions from patients with McCune-Albright syndrome (Ruther, Garber et al. 1987; Stein and Lian 1993; Gaiddon, Boutillier et al. 1994; Candeliere, Glorieux et al. 1995; Sassone-Corsi 1995). In the heart, cardiac hypertrophy in McCune-Albright syndrome patients may be through activation of mitogen-activated protein kinase (MAPK), as is the case in β-adrenergic stimulation of cardiomyocytes (Bianco and Robey 1999; Kim, Kim et al. 1999; Bianco, Riminucci et al. 2000).
3. Summary
Carney complex and McCune-Albright syndrome share clinical manifestations and molecular defects (Table 1) in the same signaling pathway (Figure 1). Their clinical and histological differences are harder to explain but almost certainly hold the key to understanding the downstream pathways involved in tumor formation in the two conditions. Ongoing work in our and other laboratories and the use of animal models are aiming at the elucidation of the molecular etiology of tumor formation in these two very interesting conditions.
Highlights.
An outline of the main clinical features behind two multiple endocrine neoplasia syndromes: Carney complex (CNC) and McCune-Albright syndrome (MAS)
An update on the genetic defects associated with CNC and MAS
A description of the molecular mechanisms and common features underlying CNC and MAS
Abbreviations
- CNC
Carney complex
- MAS
McCune-Albright syndrome
- FD
fibrous dysplasia
- PPNAD
primary pigmented nodular adrenocortical disease
- ACTH
adrenocorticotropic hormone
- GH
growth hormone
- PRL
prolactin
- LCCSCT
large-cell calcifying Sertoli cell tumors
- MEN
multiple endocrine neoplasia
- PKA
protein kinase A
- PRKAR1A
regulatory subunit type I alpha of protein kinase A
- PSC
premature stop codon
- NMD
nonsense-mediated decay
- YAC
yeast artificial chromosome
- BAC
bacterial artificial chromosome
- PBAD
primary bimorphic adrenocortical disease
- PDE
phosphodiesterase
- CREB
cAMP response element (CRE)-binding protein
- GNAS
guanine nucleotide binding protein (G protein), alpha stimulating activity
- PP
precocious puberty
Footnotes
Disclosure statement: The authors have nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akintoye SO, Chebli C, et al. Characterization of gsp-mediated growth hormone excess in the context of McCune-Albright syndrome. J Clin Endocrinol Metab. 2002;87(11):5104–5112. doi: 10.1210/jc.2001-012022. [DOI] [PubMed] [Google Scholar]
- Alman BA, Greel DA, et al. Activating mutations of Gs protein in monostotic fibrous lesions of bone. J Orthop Res. 1996;14(2):311–315. doi: 10.1002/jor.1100140221. [DOI] [PubMed] [Google Scholar]
- Anselmo J, Medeiros S, et al. A large family with Carney complex caused by the S147G PRKAR1A mutation shows a unique spectrum of disease including adrenocortical cancer. J Clin Endocrinol Metab. 2012;97(2):351–359. doi: 10.1210/jc.2011-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs AD, Latchford AR, et al. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut. 2010;59(7):975–986. doi: 10.1136/gut.2009.198499. [DOI] [PubMed] [Google Scholar]
- Bertherat J, Chanson P, et al. The cyclic adenosine 3′,5′-monophosphate-responsive factor CREB is constitutively activated in human somatotroph adenomas. Mol Endocrinol. 1995;9(7):777–783. doi: 10.1210/mend.9.7.7476961. [DOI] [PubMed] [Google Scholar]
- Bertherat J, Horvath A, et al. Mutations in regulatory subunit type 1A of cyclic adenosine 5′-monophosphate-dependent protein kinase (PRKAR1A): phenotype analysis in 353 patients and 80 different genotypes. J Clin Endocrinol Metab. 2009;94(6):2085–2091. doi: 10.1210/jc.2008-2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P, Riminucci M, et al. Mutations of the GNAS1 gene, stromal cell dysfunction, and osteomalacic changes in non-McCune-Albright fibrous dysplasia of bone. J Bone Miner Res. 2000;15(1):120–128. doi: 10.1359/jbmr.2000.15.1.120. [DOI] [PubMed] [Google Scholar]
- Bianco P, Robey P. Diseases of bone and the stromal cell lineage. J Bone Miner Res. 1999;14(3):336–341. doi: 10.1359/jbmr.1999.14.3.336. [DOI] [PubMed] [Google Scholar]
- Blyth M, Huang S, et al. A 2.3Mb deletion of 17q24.2-q24.3 associated with ‘Carney Complex plus’. Eur J Med Genet. 2008;51(6):672–678. doi: 10.1016/j.ejmg.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Bourdeau I, Antonini SR, et al. Gene array analysis of macronodular adrenal hyperplasia confirms clinical heterogeneity and identifies several candidate genes as molecular mediators. Oncogene. 2004;23(8):1575–1585. doi: 10.1038/sj.onc.1207277. [DOI] [PubMed] [Google Scholar]
- Burton KA, McDermott DA, et al. Haploinsufficiency at the protein kinase A RI alpha gene locus leads to fertility defects in male mice and men. Mol Endocrinol. 2006;20(10):2504–2513. doi: 10.1210/me.2006-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candeliere GA, Glorieux FH, et al. Increased expression of the c-fos proto-oncogene in bone from patients with fibrous dysplasia. N Engl J Med. 1995;332(23):1546–1551. doi: 10.1056/NEJM199506083322304. [DOI] [PubMed] [Google Scholar]
- Carney JA. The Carney complex (myxomas, spotty pigmentation, endocrine overactivity, and schwannomas) Dermatol Clin. 1995;13(1):19–26. [PubMed] [Google Scholar]
- Carney JA, Gordon H, et al. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore) 1985;64(4):270–283. doi: 10.1097/00005792-198507000-00007. [DOI] [PubMed] [Google Scholar]
- Carney JA, Young WF, et al. Primary bimorphic adrenocortical disease: cause of hypercortisolism in McCune-Albright syndrome. Am J Surg Pathol. 2011;35(9):1311–1326. doi: 10.1097/PAS.0b013e31821ec4ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey M, Vaughan CJ, et al. Mutations in the protein kinase A R1alpha regulatory subunit cause familial cardiac myxomas and Carney complex. J Clin Invest. 2000;106(5):R31–38. doi: 10.1172/JCI10841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazabat L, Libe R, et al. Germline inactivating mutations of the aryl hydrocarbon receptor-interacting protein gene in a large cohort of sporadic acromegaly: mutations are found in a subset of young patients with macroadenomas. Eur J Endocrinol. 2007;157(1):1–8. doi: 10.1530/EJE-07-0181. [DOI] [PubMed] [Google Scholar]
- Cazabat L, Ragazzon B, et al. PRKAR1A mutations in primary pigmented nodular adrenocortical disease. Pituitary. 2006;9(3):211–219. doi: 10.1007/s11102-006-0266-1. [DOI] [PubMed] [Google Scholar]
- Chen AE, Ginty DD, et al. Protein kinase A signalling via CREB controls myogenesis induced by Wnt proteins. Nature. 2005;433(7023):317–322. doi: 10.1038/nature03126. [DOI] [PubMed] [Google Scholar]
- Collins MT, Chebli C, et al. Renal phosphate wasting in fibrous dysplasia of bone is part of a generalized renal tubular dysfunction similar to that seen in tumor-induced osteomalacia. J Bone Miner Res. 2001;16(5):806–813. doi: 10.1359/jbmr.2001.16.5.806. [DOI] [PubMed] [Google Scholar]
- Collins MT, Sarlis NJ, et al. Thyroid carcinoma in the McCune-Albright syndrome: contributory role of activating Gs alpha mutations. J Clin Endocrinol Metab. 2003;88(9):4413–4417. doi: 10.1210/jc.2002-021642. [DOI] [PubMed] [Google Scholar]
- Collins MT, Singer FR, et al. McCune-Albright syndrome and the extraskeletal manifestations of fibrous dysplasia. Orphanet J Rare Dis. 2012;7(Suppl 1):S4. doi: 10.1186/1750-1172-7-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danon M, Crawford JD. The McCune-Albright syndrome. Ergeb Inn Med Kinderheilkd. 1987;55:81–115. doi: 10.1007/978-3-642-71052-0_3. [DOI] [PubMed] [Google Scholar]
- Dumitrescu CE, Collins MT. McCune-Albright syndrome. Orphanet J Rare Dis. 2008;3:12. doi: 10.1186/1750-1172-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espiard S, Bertherat J. Carney complex. Front Horm Res. 2013;41:50–62. doi: 10.1159/000345669. [DOI] [PubMed] [Google Scholar]
- Feuillan PP, Shawker T, et al. Thyroid abnormalities in the McCune-Albright syndrome: ultrasonography and hormonal studies. J Clin Endocrinol Metab. 1990;71(6):1596–1601. doi: 10.1210/jcem-71-6-1596. [DOI] [PubMed] [Google Scholar]
- Gaiddon C, Boutillier AL, et al. Genomic effects of the putative oncogene G alpha s. Chronic transcriptional activation of the c-fos proto-oncogene in endocrine cells. J Biol Chem. 1994;269(36):22663–22671. [PubMed] [Google Scholar]
- Gaujoux S, Tissier F, et al. Wnt/beta-catenin and 3′,5′-cyclic adenosine 5′-monophosphate/protein kinase A signaling pathways alterations and somatic beta-catenin gene mutations in the progression of adrenocortical tumors. J Clin Endocrinol Metab. 2008;93(10):4135–4140. doi: 10.1210/jc.2008-0631. [DOI] [PubMed] [Google Scholar]
- Greene EL, Horvath AD, et al. In vitro functional studies of naturally occurring pathogenic PRKAR1A mutations that are not subject to nonsense mRNA decay. Hum Mutat. 2008;29(5):633–639. doi: 10.1002/humu.20688. [DOI] [PubMed] [Google Scholar]
- Groussin L, Horvath A, et al. A PRKAR1A mutation associated with primary pigmented nodular adrenocortical disease in 12 kindreds. J Clin Endocrinol Metab. 2006;91(5):1943–1949. doi: 10.1210/jc.2005-2708. [DOI] [PubMed] [Google Scholar]
- Groussin L, Kirschner LS, et al. Molecular analysis of the cyclic AMP-dependent protein kinase A (PKA) regulatory subunit 1A (PRKAR1A) gene in patients with Carney complex and primary pigmented nodular adrenocortical disease (PPNAD) reveals novel mutations and clues for pathophysiology: augmented PKA signaling is associated with adrenal tumorigenesis in PPNAD. Am J Hum Genet. 2002;71(6):1433–1442. doi: 10.1086/344579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther DF, Bourdeau I, et al. Cyclical Cushing syndrome presenting in infancy: an early form of primary pigmented nodular adrenocortical disease, or a new entity? J Clin Endocrinol Metab. 2004;89(7):3173–3182. doi: 10.1210/jc.2003-032247. [DOI] [PubMed] [Google Scholar]
- Hart ES, Kelly MH, et al. Onset, progression, and plateau of skeletal lesions in fibrous dysplasia and the relationship to functional outcome. J Bone Miner Res. 2007;22(9):1468–1474. doi: 10.1359/jbmr.070511. [DOI] [PubMed] [Google Scholar]
- Hayward BE, Barlier A, et al. Imprinting of the G(s)alpha gene GNAS1 in the pathogenesis of acromegaly. J Clin Invest. 2001;107(6):R31–36. doi: 10.1172/JCI11887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath A, Bertherat J, et al. Mutations and polymorphisms in the gene encoding regulatory subunit type 1-alpha of protein kinase A (PRKAR1A): an update. Hum Mutat. 2010;31(4):369–379. doi: 10.1002/humu.21178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath A, Boikos S, et al. A genome-wide scan identifies mutations in the gene encoding phosphodiesterase 11A4 (PDE11A) in individuals with adrenocortical hyperplasia. Nat Genet. 2006;38(7):794–800. doi: 10.1038/ng1809. [DOI] [PubMed] [Google Scholar]
- Horvath A, Bossis I, et al. Large deletions of the PRKAR1A gene in Carney complex. Clin Cancer Res. 2008;14(2):388–395. doi: 10.1158/1078-0432.CCR-07-1155. [DOI] [PubMed] [Google Scholar]
- Horvath A, Giatzakis C, et al. Adrenal hyperplasia and adenomas are associated with inhibition of phosphodiesterase 11A in carriers of PDE11A sequence variants that are frequent in the population. Cancer Res. 2006;66(24):11571–11575. doi: 10.1158/0008-5472.CAN-06-2914. [DOI] [PubMed] [Google Scholar]
- Horvath A, Giatzakis C, et al. A cAMP-specific phosphodiesterase (PDE8B) that is mutated in adrenal hyperplasia is expressed widely in human and mouse tissues: a novel PDE8B isoform in human adrenal cortex. Eur J Hum Genet. 2008;16(10):1245–1253. doi: 10.1038/ejhg.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath A, Mathyakina L, et al. Serial analysis of gene expression in adrenocortical hyperplasia caused by a germline PRKAR1A mutation. J Clin Endocrinol Metab. 2006;91(2):584–596. doi: 10.1210/jc.2005-1301. [DOI] [PubMed] [Google Scholar]
- Horvath A, Stratakis CA. Clinical and molecular genetics of acromegaly: MEN1, Carney complex, McCune-Albright syndrome, familial acromegaly and genetic defects in sporadic tumors. Rev Endocr Metab Disord. 2008;9(1):1–11. doi: 10.1007/s11154-007-9066-9. [DOI] [PubMed] [Google Scholar]
- Horvath A, Stratakis CA. Carney complex and lentiginosis. Pigment Cell Melanoma Res. 2009;22(5):580–587. doi: 10.1111/j.1755-148X.2009.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IS, Kim ER, et al. Activating mutation of GS alpha in McCune-Albright syndrome causes skin pigmentation by tyrosinase gene activation on affected melanocytes. Horm Res. 1999;52(5):235–240. doi: 10.1159/000023467. [DOI] [PubMed] [Google Scholar]
- Kirk JM, Brain CE, et al. Cushing’s syndrome caused by nodular adrenal hyperplasia in children with McCune-Albright syndrome. J Pediatr. 1999;134(6):789–792. doi: 10.1016/s0022-3476(99)70302-1. [DOI] [PubMed] [Google Scholar]
- Kirschner LS, Carney JA, et al. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet. 2000;26(1):89–92. doi: 10.1038/79238. [DOI] [PubMed] [Google Scholar]
- Kirschner LS, Sandrini F, et al. Genetic heterogeneity and spectrum of mutations of the PRKAR1A gene in patients with the carney complex. Hum Mol Genet. 2000;9(20):3037–3046. doi: 10.1093/hmg/9.20.3037. [DOI] [PubMed] [Google Scholar]
- Landis CA, Masters SB, et al. GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature. 1989;340(6236):692–696. doi: 10.1038/340692a0. [DOI] [PubMed] [Google Scholar]
- Leet AI, Chebli C, et al. Fracture incidence in polyostotic fibrous dysplasia and the McCune-Albright syndrome. J Bone Miner Res. 2004;19(4):571–577. doi: 10.1359/JBMR.0301262. [DOI] [PubMed] [Google Scholar]
- Linglart A, Maupetit-Mehouas S, et al. GNAS-Related Loss-of-Function Disorders and the Role of Imprinting. Horm Res Paediatr. 2013:119–129. doi: 10.1159/000348516. [DOI] [PubMed] [Google Scholar]
- Lyons J, Landis CA, et al. Two G protein oncogenes in human endocrine tumors. Science. 1990;249(4969):655–659. doi: 10.1126/science.2116665. [DOI] [PubMed] [Google Scholar]
- Malchoff CD, Reardon G, et al. An unusual presentation of McCune-Albright syndrome confirmed by an activating mutation of the Gs alpha-subunit from a bone lesion. J Clin Endocrinol Metab. 1994;78(3):803–806. doi: 10.1210/jcem.78.3.8126161. [DOI] [PubMed] [Google Scholar]
- Mantovani G, Ballare E, et al. The gsalpha gene: predominant maternal origin of transcription in human thyroid gland and gonads. J Clin Endocrinol Metab. 2002;87(10):4736–4740. doi: 10.1210/jc.2002-020183. [DOI] [PubMed] [Google Scholar]
- Mastorakos G, Mitsiades NS, et al. Hyperthyroidism in McCune-Albright syndrome with a review of thyroid abnormalities sixty years after the first report. Thyroid. 1997;7(3):433–439. doi: 10.1089/thy.1997.7.433. [DOI] [PubMed] [Google Scholar]
- Mateus C, Palangie A, et al. Heterogeneity of skin manifestations in patients with Carney complex. J Am Acad Dermatol. 2008;59(5):801–810. doi: 10.1016/j.jaad.2008.07.032. [DOI] [PubMed] [Google Scholar]
- Matyakhina L, Pack S, et al. Chromosome 2 (2p16) abnormalities in Carney complex tumours. J Med Genet. 2003;40(4):268–277. doi: 10.1136/jmg.40.4.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meoli E, Bossis I, et al. Protein kinase A effects of an expressed PRKAR1A mutation associated with aggressive tumors. Cancer Res. 2008;68(9):3133–3141. doi: 10.1158/0008-5472.CAN-08-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin E, Mete O, et al. Carney complex with adrenal cortical carcinoma. J Clin Endocrinol Metab. 2012;97(2):E202–206. doi: 10.1210/jc.2011-2321. [DOI] [PubMed] [Google Scholar]
- Nadella KS, Kirschner LS. Disruption of protein kinase a regulation causes immortalization and dysregulation of D-type cyclins. Cancer Res. 2005;65(22):10307–10315. doi: 10.1158/0008-5472.CAN-05-3183. [DOI] [PubMed] [Google Scholar]
- Nesterova M, Bossis I, et al. An immortalized human cell line bearing a PRKAR1A-inactivating mutation: effects of overexpression of the wild-type Allele and other protein kinase A subunits. J Clin Endocrinol Metab. 2008;93(2):565–571. doi: 10.1210/jc.2007-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg K, Skogseid B, et al. Multiple endocrine neoplasia type 1 (MEN-1). Clinical, biochemical and genetical investigations. Acta Oncol. 1989;28(3):383–387. doi: 10.3109/02841868909111211. [DOI] [PubMed] [Google Scholar]
- Patronas Y, Horvath A, et al. In vitro studies of novel PRKAR1A mutants that extend the predicted RIalpha protein sequence into the 3′-untranslated open reading frame: proteasomal degradation leads to RIalpha haploinsufficiency and Carney complex. J Clin Endocrinol Metab. 2012;97(3):E496–502. doi: 10.1210/jc.2011-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AM, Hes FJ, et al. Association of the M1V PRKAR1A mutation with primary pigmented nodular adrenocortical disease in two large families. J Clin Endocrinol Metab. 2010;95(1):338–342. doi: 10.1210/jc.2009-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard C, Silvy M, et al. Gs alpha overexpression and loss of Gs alpha imprinting in human somatotroph adenomas: association with tumor size and response to pharmacologic treatment. Int J Cancer. 2007;121(6):1245–1252. doi: 10.1002/ijc.22816. [DOI] [PubMed] [Google Scholar]
- Ragazzon B, Cazabat L, et al. Inactivation of the Carney complex gene 1 (protein kinase A regulatory subunit 1A) inhibits SMAD3 expression and TGF beta-stimulated apoptosis in adrenocortical cells. Cancer Res. 2009;69(18):7278–7284. doi: 10.1158/0008-5472.CAN-09-1601. [DOI] [PubMed] [Google Scholar]
- Riminucci M, Collins MT, et al. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest. 2003;112(5):683–692. doi: 10.1172/JCI18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riminucci M, Fisher LW, et al. A novel GNAS1 mutation, R201G, in McCune-albright syndrome. J Bone Miner Res. 1999;14(11):1987–1989. doi: 10.1359/jbmr.1999.14.11.1987. [DOI] [PubMed] [Google Scholar]
- Robinson-White A, Hundley TR, et al. Protein kinase-A activity in PRKAR1A-mutant cells, and regulation of mitogen-activated protein kinases ERK1/2. Hum Mol Genet. 2003;12(13):1475–1484. doi: 10.1093/hmg/ddg160. [DOI] [PubMed] [Google Scholar]
- Ruggieri P, Sim FH, et al. Malignancies in fibrous dysplasia. Cancer. 1994;73(5):1411–1424. doi: 10.1002/1097-0142(19940301)73:5<1411::aid-cncr2820730516>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Ruther U, Garber C, et al. Deregulated c-fos expression interferes with normal bone development in transgenic mice. Nature. 1987;325(6103):412–416. doi: 10.1038/325412a0. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P. Signaling pathways and c-fos transcriptional responses--links to inherited diseases. N Engl J Med. 1995;332(23):1576–1577. doi: 10.1056/NEJM199506083322311. [DOI] [PubMed] [Google Scholar]
- Schwindinger WF, Francomano CA, et al. Identification of a mutation in the gene encoding the alpha subunit of the stimulatory G protein of adenylyl cyclase in McCune-Albright syndrome. Proc Natl Acad Sci U S A. 1992;89(11):5152–5156. doi: 10.1073/pnas.89.11.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenker A, Weinstein LS, et al. An activating Gs alpha mutation is present in fibrous dysplasia of bone in the McCune-Albright syndrome. J Clin Endocrinol Metab. 1994;79(3):750–755. doi: 10.1210/jcem.79.3.8077356. [DOI] [PubMed] [Google Scholar]
- Sherman SI, Ladenson PW. Octreotide therapy of growth hormone excess in the McCune-Albright syndrome. J Endocrinol Invest. 1992;15(3):185–190. doi: 10.1007/BF03348702. [DOI] [PubMed] [Google Scholar]
- Silva ES, Lumbroso S, et al. Demonstration of McCune-Albright mutations in the liver of children with high gammaGT progressive cholestasis. J Hepatol. 2000;32(1):154–158. doi: 10.1016/s0168-8278(00)80202-0. [DOI] [PubMed] [Google Scholar]
- Spada A, Arosio M, et al. Clinical, biochemical, and morphological correlates in patients bearing growth hormone-secreting pituitary tumors with or without constitutively active adenylyl cyclase. J Clin Endocrinol Metab. 1990;71(6):1421–1426. doi: 10.1210/jcem-71-6-1421. [DOI] [PubMed] [Google Scholar]
- Stein GS, Lian JB. Molecular mechanisms mediating proliferation/differentiation interrelationships during progressive development of the osteoblast phenotype. Endocr Rev. 1993;14(4):424–442. doi: 10.1210/edrv-14-4-424. [DOI] [PubMed] [Google Scholar]
- Stratakis CA. New genes and/or molecular pathways associated with adrenal hyperplasias and related adrenocortical tumors. Mol Cell Endocrinol. 2009;300(1–2):152–157. doi: 10.1016/j.mce.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratakis CA. cAMP/PKA signaling defects in tumors: genetics and tissue-specific pluripotential cell-derived lesions in human and mouse. Mol Cell Endocrinol. 2013;371(1–2):208–220. doi: 10.1016/j.mce.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratakis CA, Kirschner LS, et al. Clinical and molecular features of the Carney complex: diagnostic criteria and recommendations for patient evaluation. J Clin Endocrinol Metab. 2001;86(9):4041–4046. doi: 10.1210/jcem.86.9.7903. [DOI] [PubMed] [Google Scholar]
- Stratakis CA, Papageorgiou T, et al. Ovarian lesions in Carney complex: clinical genetics and possible predisposition to malignancy. J Clin Endocrinol Metab. 2000;85(11):4359–4366. doi: 10.1210/jcem.85.11.6921. [DOI] [PubMed] [Google Scholar]
- Tadjine M, Lampron A, et al. Detection of somatic beta-catenin mutations in primary pigmented nodular adrenocortical disease (PPNAD) Clin Endocrinol (Oxf) 2008;69(3):367–373. doi: 10.1111/j.1365-2265.2008.03273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taymans SE, Kirschner LS, et al. Radiation hybrid mapping of chromosomal region 2p15-p16: integration of expressed and polymorphic sequences maps at the Carney complex (CNC) and Doyne honeycomb retinal dystrophy (DHRD) loci. Genomics. 1999;56(3):344–349. doi: 10.1006/geno.1998.5720. [DOI] [PubMed] [Google Scholar]
- Vallar L, Spada A, et al. Altered Gs and adenylate cyclase activity in human GH-secreting pituitary adenomas. Nature. 1987;330(6148):566–568. doi: 10.1038/330566a0. [DOI] [PubMed] [Google Scholar]
- Veugelers M, Wilkes D, et al. Comparative PRKAR1A genotype-phenotype analyses in humans with Carney complex and prkar1a haploinsufficient mice. Proc Natl Acad Sci U S A. 2004;101(39):14222–14227. doi: 10.1073/pnas.0405535101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzosi D, Libe R, et al. Phosphodiesterase 11A (PDE11A) gene defects in patients with acth-independent macronodular adrenal hyperplasia (AIMAH): functional variants may contribute to genetic susceptibility of bilateral adrenal tumors. J Clin Endocrinol Metab. 2012;97(11):E2063–2069. doi: 10.1210/jc.2012-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vortmeyer AO, Glasker S, et al. Somatic GNAS mutation causes widespread and diffuse pituitary disease in acromegalic patients with McCune-Albright syndrome. J Clin Endocrinol Metab. 2012;97(7):2404–2413. doi: 10.1210/jc.2012-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein LS, Shenker A, et al. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991;325(24):1688–1695. doi: 10.1056/NEJM199112123252403. [DOI] [PubMed] [Google Scholar]
- Xekouki P, Azevedo M, et al. Anterior pituitary adenomas: inherited syndromes, novel genes and molecular pathways. Expert Rev Endocrinol Metab. 2010;5(5):697–709. doi: 10.1586/eem.10.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang I, Park S, et al. Characteristics of gsp-positive growth hormone-secreting pituitary tumors in Korean acromegalic patients. Eur J Endocrinol. 1996;134(6):720–726. doi: 10.1530/eje.0.1340720. [DOI] [PubMed] [Google Scholar]
- Yin Z, Kirschner LS. The Carney complex gene PRKAR1A plays an essential role in cardiac development and myxomagenesis. Trends Cardiovasc Med. 2009;19(2):44–49. doi: 10.1016/j.tcm.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Ragazzon B, et al. Protein kinase A alterations in endocrine tumors. Horm Metab Res. 2012;44(10):741–748. doi: 10.1055/s-0032-1316292. [DOI] [PubMed] [Google Scholar]
- Zawadzki KM, Taylor SS. cAMP-dependent protein kinase regulatory subunit type IIbeta: active site mutations define an isoform-specific network for allosteric signaling by cAMP. J Biol Chem. 2004;279(8):7029–7036. doi: 10.1074/jbc.M310804200. [DOI] [PubMed] [Google Scholar]